
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 24 November 2021
Sec. Lipids in Cardiovascular Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.777131
This article is part of the Research TopicStatin Therapy: Controversial issuesView all 8 articles
Non-alcoholic fatty liver disease (NAFLD) is the primary cause of chronic liver disease. The range is extensive, including hepatocellular carcinoma, cirrhosis, fibrosis, fatty liver, and non-alcoholic steatohepatitis (NASH). NASH is a condition related to obesity, overweight, metabolic syndrome, diabetes, and dyslipidemia. It is a dynamic condition that can regress to isolated steatosis or progress to fibrosis and cirrhosis. Statins exert anti-inflammatory, proapoptotic, and antifibrotic effects. It has been proposed that these drugs could have a relevant role in NASH. In this review, we provide an overview of current evidence, from mechanisms of statins involved in the modulation of NASH to human trials about the use of statins to treat or attenuate NASH.
Beyond cholesterol-lowering effects, statins exert anti-inflammatory, proapoptotic, and antifibrotic activities (1, 2) thus it's has been proposed that statins could have a relevant role in non-alcoholic steatohepatitis (NASH). In this review, we provide an overview of current evidence about the use of statins to treat or attenuate NASH.
Non-alcoholic fatty liver disease (NAFLD) is a primary cause of chronic liver disease. The range is extensive, including hepatocellular carcinoma, cirrhosis, fibrosis, fatty liver, and NASH. Specifically, NASH can be classified as inflammation and steatosis without or with fibrosis (3) and it is well established that this condition is related to obesity, overweight, metabolic syndrome, diabetes, and dyslipidemia (4). At this point is important to note that NASH is a dynamic condition that can regress to isolated steatosis, remain at a constant level of activity, or progress to fibrosis and cirrhosis (3).
When signaling between hepatocyte, adipose tissue, and microbiome is not altered, the hepatocyte homeostasis is preserved. However, the presence of insulin resistance, disruption in lipid metabolism, inflammation, and oxidative stress results in disruption of the protective mechanisms of hepatocytes and finally these cells die. After the death of hepatocytes, a process for the repair of damage is initiated by the release of signals that activate immune cells, sinusoidal endothelium, stellate cells, and ductal cells that results in fibrogenesis and endothelial remodeling (5).
Is important to note that inherited and environmental factors are involved in the physiopathology of NASH. On the one hand, genetic factors such as polymorphisms in patatin-like phospholipase domain–containing 3 (PNPLA3) and transmembrane 6 superfamily, member 2 (TM6SF2) promote the development of NASH. The first one is related to hepatic steatosis and cancer, and the second one modulates hepatocytes lipids content (6, 7). On the other hand, environmental factors may modulate NASH and evidence suggests that the gut-liver axis modifications are bidirectional: gut microbiota affects host obesity and hepatic diseases (including hepatic steatosis, NASH or cancer) and host factors (among others diet, sleep, shift-work, travel or feeding habit) influence the gut microbiota (8–10). An overview of the mechanisms involved in the pathogenesis of NASH that can be modified by statins is discussed below (see “Mechanisms of statins that can modulate NASH” and Figure 1).
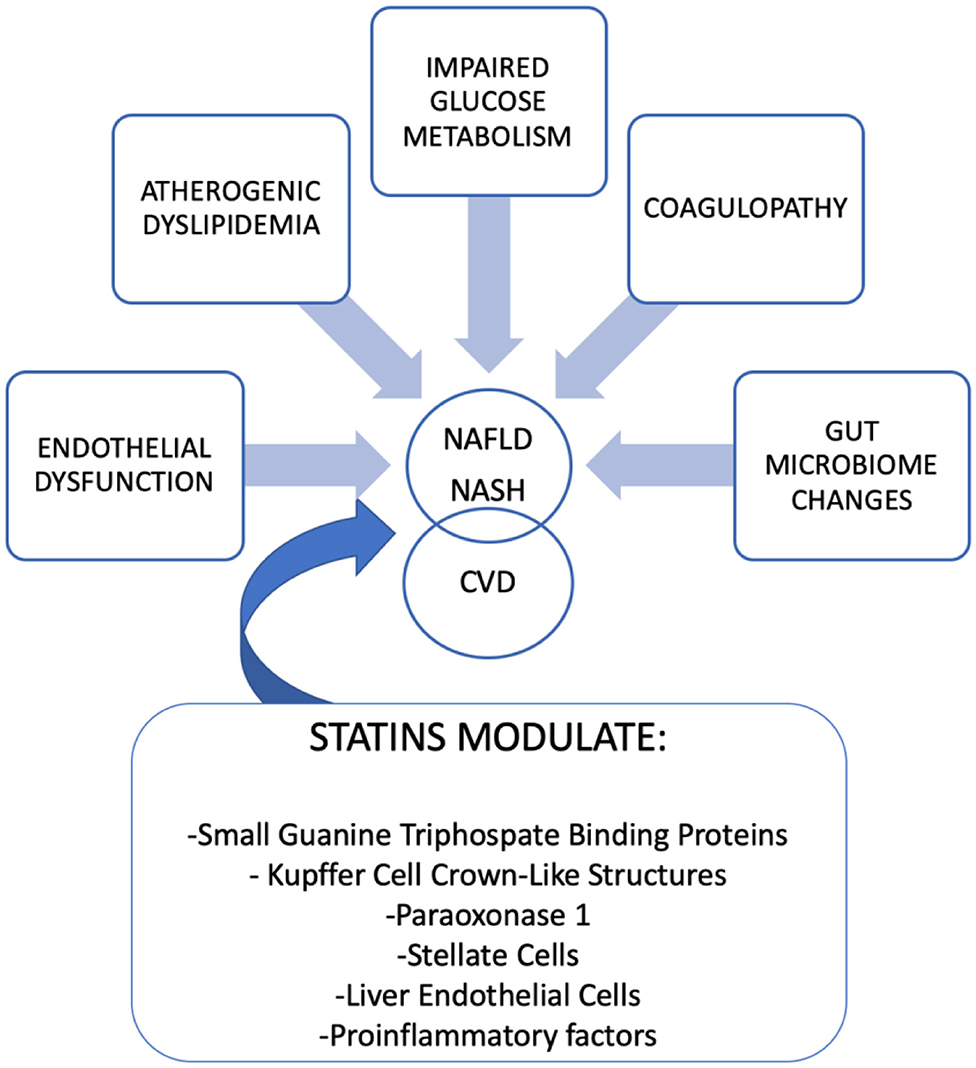
Figure 1. Physiopathology of NAFLD/NASH and link between Cardiovascular Disease (CVD). Mechanism of statins that modulate NAFLD/NASH.
The mechanisms linking NASH/NAFLD to cardiovascular disease (CVD) are complex, varied, and include different pathways (Figure 1). As we previously mentioned, environmental factors, including sedentary lifestyle, diet rich in saturated fat, smoking, sleep disorders, the increase of visceral fat, and high body weight are key factors involved in NAFLD and NASH. Moreover, genetic variants of PNPLA3 and TM6SF2 are related to NASH and liver cancer but also to triglycerides, LDL cholesterols serum concentrations, and coronary heart disease (11). CVD is characterized by endothelial dysfunction and is typically associated with a decreased bioavailability of nitric oxide (NO). At this point, it is important to highlight that the presence of high levels of an antagonist of NO synthase, the asymmetric dimethylarginine (ADMA) is present in patients with NAFLD/NASH (12). In addition to endothelial dysfunction, a procoagulant state in these patients, characterized by elevated coagulation factor FXII to FVIII and fibrinogen, increases the risk of CVD (13). Alterations in glucose metabolism, diabetes, and hepatic insulin resistance are risk factors shared by NAFLD/NASH and atherosclerotic diseases (14). The imbalance in lipid metabolism that appears in NAFLD/NASH results in increased VLDL, IDL, LDL, triglycerides, and their remnants that develops atherogenic dyslipidemia (15).
Lifestyle modifications and specifically weight loss, along with physical activity, is one of the best treatments (16), but lifestyle modifications are a challenge for patients and physicians because they are often difficult to maintain over time. This is the reason for the need to search for therapeutic targets to treat NASH/NAFLD (17) (Figure 2). The pharmacotherapies evaluated to treat NASH/NAFLD include “classic” therapies: metformin (18), thiazolidinediones (3), vitamin E(3), omega-3 fatty acids (19), and statins. Some of them only reported improvement in aminotransferase elevations (metformin and omega-3 fatty acids) and other improved histology (vitamin E, thiazolidinediones). The specific impact on aminotransferase and histology with the use of statins is discussed separately (see “the role of statins as therapeutic strategy in NASH/NAFLD patients”). On the other hand, there are novel therapies under investigation (20–23) alone or in combination (24, 25): Glucagon-Like Peptide 1(GLP1) Agonist, Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Peroxisome Proliferator-Activated-Receptor (PPAR) agonist, Caspase inhibitors, Acetyl-CoA inhibitors, Apoptosis Signal-Regulating Kinase 1 (ASK1) Inhibitors, and Farnesoid X Receptor (FXR) agonists. Some of these therapies increase serum lipids and the combination of statins with them will mitigate this effect (26). The results of phase 3 trials with these drugs will shed light on future treatment of NASH/NAFLD.
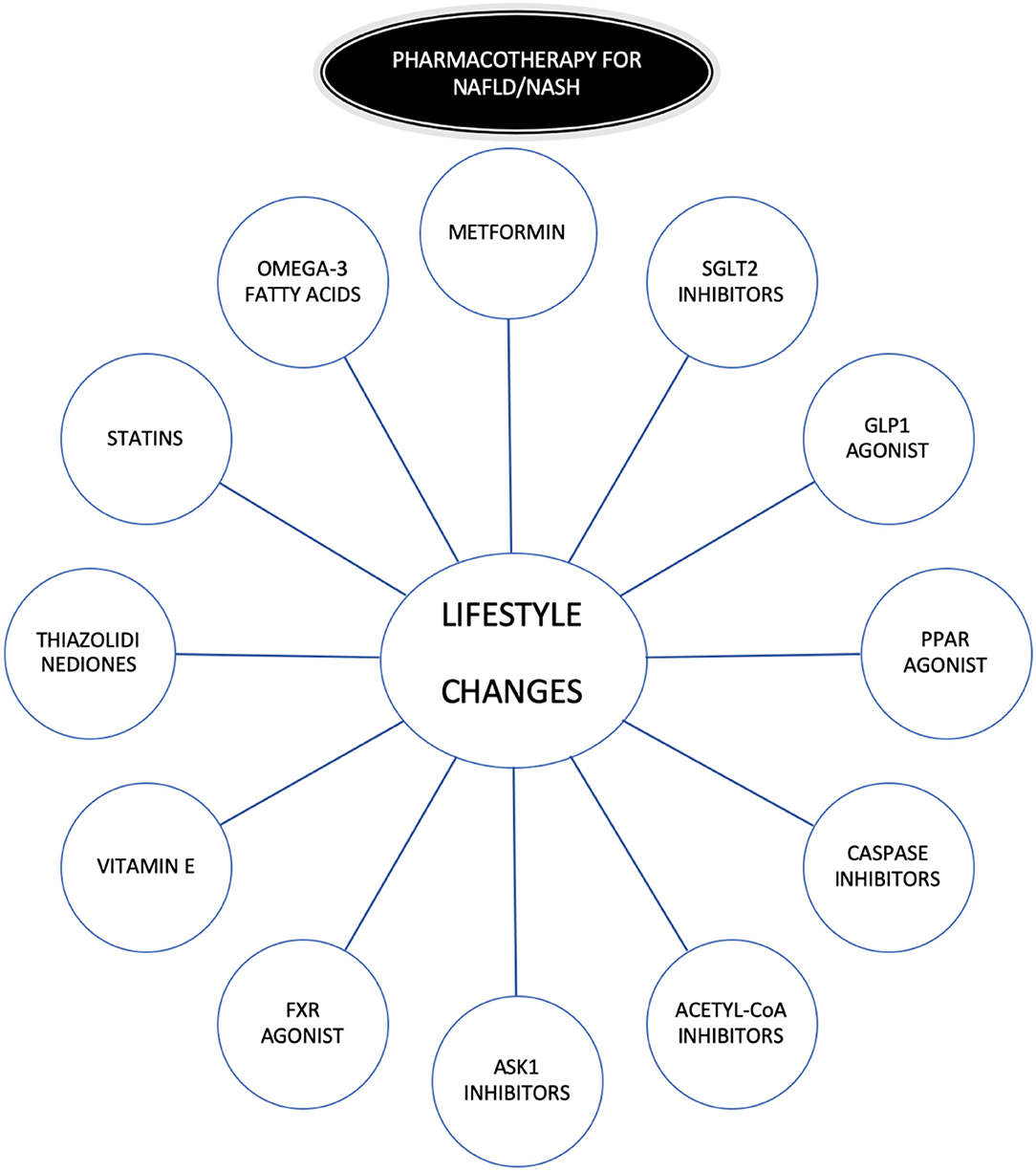
Figure 2. Pharmacotherapy in NAFLD/NASH. GLP1, Glucagon-Like Peptide 1; SGLT2, Sodium-Glucose Cotransporter-2; PPAR, Peroxisome proliferator-activated-receptor; ASK1, Apoptosis Signal-Regulating Kinase 1; FXR, inhibitors and Farnesoid X Receptor.
Statins may protect against NASH by inhibiting small GTPases. GTPases have diverse functions such as signal transduction, modulate protein synthesis, cell differentiation processes, or intracellular vesicle transport (27). In this line, a work developed by Schierwagen et al. (28) showed that treatment with simvastatin reduced liver inflammation and fibrosis in mice with NAFLD through inhibition of mice sarcoma protein. This work suggests that statins can change intracellular signaling. This mechanism may be responsible for the prospective liver effects in NAFLD/NASH.
PPARs are receptors that bind fatty acids and regulate inflammatory and metabolic pathways. Specifically, PPAR α acts mainly in the hepatocyte modulating lipid transport, β-oxidation and gluconeogenesis genes (29). This PPARs receptor became an attractive target to treat NAFLD/NASH and clinical trials are ongoing to evaluate the effect of PPAR agonists in these diseases (21). Thus, the mitochondria and peroxisome have an important role in the degradation of fatty acids and in steatohepatitis by the accumulation of cholesterol: specifically, it has been suggested that mitochondrial and peroxisomal activity can be increased by statins, thus, a study conducted on mice (30) after the consumption of standard chow or a diet with a reduced content in choline and methionine without or with different statins improved hepatic fatty acid oxidation via PPARα and its target genes. Moreover, in this study, steatosis, inflammation, and fibrosis improved with the use of statins. Interestingly, these findings were not associated with the intensity of statins (atorvastatin, fluvastatin, rosuvastatin, pravastatin, and simvastatin). In summary, these findings are relevant: they suggest that statins treatment reduce hepatic steatohepatitis.
PON 1 is an antioxidant enzyme from the liver and is related to a reduction in inflammation and antiatherogenic effects. The reduction of PON1 is a marker of lipid peroxidation that is improved by the use of statins (31). A trial conducted by Samy et al. (32) evaluated the use of statins in patients with NAFLD. 25 participants received 40 mg of atorvastatin daily for a period of 8 months and 25 received a placebo. The investigators evaluated, among others, liver ultrasonography, liver tests, lipids, and serum PON1 activity. After intervention, participants that received therapy with atorvastatin improved serum PON1 activity. KCs with crown-like structures and cholesterol are involved in inflammation, lipotoxicity, and fibrosis (33). In this context, a study in animal models (mice) investigator evaluated the effect of different interventions (34) including one group with ezetimibe, one group with atorvastatin, one group with atorvastatin plus ezetimibe, and one control group. Interestingly, animals that received atorvastatin and ezetimibe resolved cholesterol crystals, crown-like structures, and reduced fibrosis. In contrast, atorvastatin or ezetimibe alone developed a mild effect on these outcomes. This study provided evidence showing that the use of lipid-lowering drugs (including statins) reduces cholesterol crystals and modulate KC crown-like structures reducing NASH.
Hepatic stellate cells can be activated via paracrine of the hepatocyte and this activation results in fibrogenesis. This activation may be suppressed by statins treatment. Specifically, a study conducted using fluvastatin (35) in animal models showed that this treatment reduced profibrogenic pathways, showing the potential role of fluvastatin as an antifibrotic agent that could be used in NASH. Similar findings were demonstrated by simvastatin with in vivo and in vitro studies (1).
Liver endothelial cell function with reduced NO and stellate cells activation develop fibrosis. This could be modulated by statins. A study conducted in rats treated with simvastatin or atorvastatin (36) showed that statins reduced sinusoidal endothelial dysfunction, exerting the vasoprotective effects of these drugs.
Moreover, statins have anti-inflammatory and anti-fibrogenic effects. This was evaluated in a study that was developed to test the effect of rosuvastatin over different proinflammatory and profibrogenic factors (37). The rosuvastatin group showed a decrease in proinflammatory cytokines, including IL-6, TNF-α, IL-1β, and other angiogenic and profibrogenic factors. In this line, a trial with atorvastatin, vitamin E, and dietary intervention reduced the expression of fibrotic genes (38). All these findings support that statins could be protective against hepatic inflammation associated with NASH.
To sum up, statins improved liver endothelial cells dysfunction, receded activation of hepatic stellate cells, and exerted anti-fibrogenic effects.
The etiology of NASH/NAFLD is influenced by inflammation and oxidative stress. Statins by their pleiotropic effects might improve liver histology. As previously mentioned, animal models showed how statins reduce the progression of fibrosis by antioxidant and antioxidant effects (1, 39).
Small uncontrolled investigations in humans used ultrasonography and tomography to assess the effects of statins on hepatic steatosis, and the majority found that they improved this condition. In this sense, it is important to highlight two studies: a randomized trial with atorvastatin in which this drug was more effective than fenofibrate in reducing liver echogenicity (40) and a randomized trial with atorvastatin (10–20 mg daily), where atorvastatin was shown to be as effective as pitavastatin in reducing steatosis (41). If we analyze data on the effect of statins on liver histology, the data are limited but despite this, the use of statins may be justified as an adjunct therapy to treat other conditions associated with NASH/NAFLD (prediabetes/type 2 diabetes) (42, 43). Some retrospective studies report improvement in steatosis without change in inflammation or fibrosis in patients treated with statins and interestingly, progressions in fibrosis in those that did not receive statins (44). In summary, we can conclude that most of the retrospective studies show how statins could reduce hepatic steatosis with improvement inflammation (45–47). The main limitation of these studies is the small number of patients included that may explain the different responses between participants.
It is well established that elevations in serum aminotransferases are usual in patients that receive statins but in contrast (48), hepatic toxicity is not. In fact, the rate of acute liver failure in patients that are taking statins is very close to the general population (49). However, cases have been described of both autoimmune hepatitis associated with statin treatment (50) (Fluvastatin, atorvastatin, and rosuvastatin, among others) and acute liver failure requiring liver transplantation (0.01% of the causes of liver transplantation) (51). Another key point that may explain the alterations in liver function tests is due to the pharmacological interactions of statins rather than the toxic effect of these drugs (52).
Data from clinical trials such as the West of Scotland Coronary Prevention Study (WOSCOPS) (53), the Long-term Intervention with Pravastatin in Ischemic (LIPID) (54) study, and the Cholesterol And Recurrent Events (CARE) (55) suggest that long-term treatment with statins is tolerated without an excess of serious liver adverse events. On the other hand, meta-analyses have been developed to evaluate the effect of statins on liver function tests. One of them (56) analyzed almost 50,000 patients and showed that the use of statins (low or intermediate doses) was not associated with an alteration of liver enzymes. On the other hand, another study showed how comparatively high-dose statins increase the risk of transaminase elevation compared to low doses, especially in the case of hydrophilic statins (57).
Finally, it is important to highlight that the Statin Liver Safety Task Force established some years ago (58) the safety of statin treatment in patients with compensated cirrhosis, NASH, and NAFLD. In contrast, acute liver failure as long as decompensated cirrhosis contraindicate the use of statins.
We have previously described in the first section of this manuscript many in vitro/in vivo studies evaluating the impact of statins in NASH through various mechanisms including their influence on pro-inflammatory factors, hepatic cell activation, sinusoidal endothelial cells, PON1, PPARα, and GTPases modulation.
There are no randomized controlled trials that evaluate the role of statins on NASH. We could only find data from post hoc analyses of prospective studies showing a beneficial effect of statins on NASH (Table 1).
In a subanalysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial, which included coronary heart disease patients, the use of atorvastatin in patients with NASH/NAFLD improved liver enzymes and reduced cardiovascular events in those without alteration in liver tests after 3 years (59, 61).
The Assessing The Treatment Effect in Metabolic Syndrome Without Perceptible diabeTes (ATTEMPT) Study evaluated 1,123 participants with metabolic syndrome without diabetes or cardiovascular disease (60, 62). Participants were randomized into two groups: one with an LDL-C target under 130 mg/dl and the other with a target under 100 mg/dl. In the post hoc analysis liver enzymes and ultrasonography improved during the study.
Other relevant findings are those from a subanalysis from the Incremental Decrease in End Points Through Aggressive Lipid-Lowering (IDEAL) trial, which included >8,500 patients with established cardiovascular disease (60). Specifically, data from the post hoc analysis show that 1,081 have elevated ALT levels, probably due to NASH/ NAFLD. Participants with moderately elevated aminotransferase levels and received atorvastatin 80 mg daily normalized aminotransferase levels in contrast with those who received simvastatin 20–40 mg daily.
Other small trials, described in the previous section “Effects of Statins on Liver Histology” have been developed. These trials suggest the “biopsy-proven” effect of statins on NASH (40, 41, 44–47).
To our knowledge there is only one trial, with 70 participants (NCT04679376) (Table 1), to evaluate the safety and efficacy of statin therapy (atorvastatin 40 mg daily) for the treatment of NASH and hepatic fibrosis. This trial is active but not recruiting yet.
Dyslipidemia is a condition that is associated with NASH/NAFLD (42). Statins are the cornerstone of the treatment of hyperlipidemia. Although all statins seem to be effective to reduce cholesterol in patients with NASH, atorvastatin has a favorable profile to reduce the incidence of cardiovascular events in these patients. In the previous section we analyzed data from trials and effectiveness to treat NASH, but there is one important effect of these drugs in the participants involved in these trials: a reduction of CVD (Table 1). In the GREACE study, the subgroup of patients with NASH/ NAFLD that received statins had reduced CVD events, compared to those who had normal liver tests, and moreover, the investigators observed a reduction of CVD events compared with those with NAFLD without statins (59, 61). In the IDEAL study patients with a high dose of atorvastatin reduced the major CVD events (coronary and cerebrovascular events) compared to participants that received simvastatin (60). On the other hand, in the ATTEMPT study (62), the number of events were too low to establish a conclusion.
The latest ASL-EASD-EASO Clinical Practice Guidelines (63) support that the assessment of CVD risk must be developed in patients with NAFLD, and that they should receive treatment with statins if needed. Moreover, in the American Gastroenterological Association update for the treatment of Dyslipidemia in Common Liver Diseases (64), the author suggests that although NASH is not considered a traditional risk factor for the development of cardiovascular disease, it is commonly associated with hyperlipidemia, and statins are safe and well-tolerated in NASH patients to reduce CVD risk.
Beyond cholesterol-lowering effects, statins exert anti-inflammatory, proapoptotic, and antifibrotic activities and could have a relevant role in NASH.
Many in vivo and in vitro studies evaluated the impact of statins in NASH through various mechanisms, including their influence on pro-inflammatory factors, hepatic cells activation, sinusoidal endothelial cells, crown-like structures, PON1, PPARα, and GTPases modulation.
Although there are no randomized controlled trials to evaluate the specific role of statins on NASH, there are post hoc analyses of prospective studies that demonstrate that statins have a positive effect on NASH. In this context, we need more clinical trials investigating the effect of statins on NASH/NAFLD and cardiovascular risk in these patients. However, at the current date, the guidelines support that the use of statins in patients with NAFLD/NASH is safe.
JT-P and FF-J: conceptualization and writing—original draft preparation. JT-P, FF-J, and LM-P: literature review and writing—review and editing. FF-J and LM-P: supervision. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang W, Zhao C, Zhou J, Zhen Z, Wang Y, Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis-related liver fibrosis. PLoS ONE. (2013) 8:e76538. doi: 10.1371/journal.pone.0076538
2. Pramfalk C, Parini P, Gustafsson U, Sahlin S, Eriksson M. Effects of high-dose statin on the human hepatic expression of genes involved in carbohydrate and triglyceride metabolism. J Intern Med. (2011) 269:333–9. doi: 10.1111/j.1365-2796.2010.02305.x
3. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
5. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. (2016) 61:1294–303. doi: 10.1007/s10620-016-4049-x
6. Anstee QM, Seth D, Day CP. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology. (2016) 150:1728–44.e7. doi: 10.1053/j.gastro.2016.01.037
7. Severson TJ, Besur S, Bonkovsky HL. Genetic factors that affect nonalcoholic fatty liver disease: a systematic clinical review. World J Gastroenterol. (2016) 22:6742–56. doi: 10.3748/wjg.v22.i29.6742
8. Federico A, Dallio M, Godos J, Loguercio C, Salomone F. Targeting gut-liver axis for the treatment of nonalcoholic steatohepatitis: translational and clinical evidence. Transl Res. (2016) 167:116–24. doi: 10.1016/j.trsl.2015.08.002
9. Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. (2016) 63:764–75. doi: 10.1002/hep.28356
10. Machado MV, Cortez-Pinto H. Diet, microbiota, obesity, and NAFLD: a dangerous quartet. Int J Mol Sci. (2016) 17:481–481. doi: 10.3390/ijms17040481
11. Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. (2017) 49:1758–66. doi: 10.1038/ng.3977
12. Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. (2016) 65:425–43. doi: 10.1016/j.jhep.2016.04.005
13. Tripodi A, Fracanzani AL, Chantarangkul V, Primignani M, Fargion S. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. (2017) 66:248–50. doi: 10.1016/j.jhep.2016.09.025
14. Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:948–63. doi: 10.1016/j.jacc.2018.11.050
15. Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, et al. High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, atherogenic dyslipidemia, and inflammation. Metab Syndr Relat Disord. (2020) 18:176–85. doi: 10.1089/met.2019.0115
16. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. (2015) 149:367–78.e5. doi: 10.1053/j.gastro.2015.04.005
17. Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. (2017) 37:97–103. doi: 10.1111/liv.13302
18. Musso G, Gambino R, Cassader M, Pagano G A. meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. (2010) 52:79–104. doi: 10.1002/hep.23623
19. Spooner MH, Jump DB. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: where do we stand? Curr Opin Clin Nutr Metab Care. (2019) 22:103–10. doi: 10.1097/MCO.0000000000000539
20. Attia SL, Softic S, Mouzaki M. Evolving role for pharmacotherapy in NAFLD/NASH. Clin Transl Sci. (2021) 14:11–9. doi: 10.1111/cts.12839
21. Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A Randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med. (2021) 385:1547–58. doi: 10.1056/NEJMoa2036205
22. Mahjoubin-Tehran M, De Vincentis A, Mikhailidis DP, Atkin SL, Mantzoros CS, Jamialahmadi T, et al. Non-alcoholic fatty liver disease and steatohepatitis: State of the art on effective therapeutics based on the gold standard method for diagnosis. Mol Metab. (2021) 50:101049. doi: 10.1016/j.molmet.2020.101049
23. Kothari S, Dhami-Shah H, Shah SR. Antidiabetic drugs and statins in nonalcoholic fatty liver disease. J Clin Exp Hepatol. (2019) 9:723–30. doi: 10.1016/j.jceh.2019.06.003
24. Athyros V, Polyzos S, Kountouras J, Katsiki N, Anagnostis P, Doumas M, et al. Non-alcoholic fatty liver disease treatment in patients with type 2 diabetes mellitus; new kids on the block. Curr Vasc Pharmacol. (2019) 5:17. doi: 10.2174/1570161117666190405164313
25. Caldwell S. NASH. Therapy: omega 3 supplementation, vitamin E, insulin sensitizers and statin drugs. Clin Mol Hepatol. (2017) 23:103–8. doi: 10.3350/cmh.2017.0103
26. Connelly MA, Velez Rivera J, Guyton JR, Siddiqui MS, Sanyal AJ. Review article: the impact of liver-directed therapies on the atherogenic risk profile in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. (2020) 52:619–36. doi: 10.1111/apt.15935
27. Goud B, Liu S, Storrie B. Rab proteins as major determinants of the Golgi complex structure. Small GTPases. (2018) 9:66–75. doi: 10.1080/21541248.2017.1384087
28. Schierwagen R, Maybüchen L, Hittatiya K, Klein S, Uschner FE, Braga TT, et al. Statins improve NASH via inhibition of RhoA and Ras. Am J Physiol Gastrointest Liver Physiol. (2016) 311:G724–33. doi: 10.1152/ajpgi.00063.2016
29. Lefere S, Puengel T, Hundertmark J, Penners C, Frank AK, Guillot A, et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages?. J Hepatol. (2020) 73:757–70. doi: 10.1016/j.jhep.2020.04.025
30. Park H, Jang J, Ko M, Woo S, Kim B, Kim H, et al. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab J. (2016) 21:40. doi: 10.4093/dmj.2016.40.5.376
31. Milaciu MV, Vesa ŞC, Bocşan IC, Ciumărnean L, Sâmpelean D, Negrean V, et al. Paraoxonase-1 serum concentration and PON1 gene polymorphisms: relationship with non-alcoholic fatty liver disease. J Clin Med. (2019) 8:2200. doi: 10.3390/jcm8122200
32. Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab J Gastroenterol. (2011) 12:80–5. doi: 10.1016/j.ajg.2011.04.008
33. Ioannou GN, Haigh WG, Thorning D, Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis[S]. J Lipid Res. (2013) 54:1326–34. doi: 10.1194/jlr.M034876
34. Ioannou GN, Van Rooyen DM, Savard C, Haigh WG, Yeh MM, Teoh NC, et al. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J Lipid Res. (2015) 56:277–85. doi: 10.1194/jlr.M053785
35. Chong L-W, Hsu Y-C, Lee T-F, Lin Y, Chiu Y-T, Yang K-C, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. (2015) 15:22. doi: 10.1186/s12876-015-0248-8
36. Bravo M, Raurell I, Hide D, Fernández-Iglesias A, Gil M, Barberá A, et al. Restoration of liver sinusoidal cell phenotypes by statins improves portal hypertension and histology in rats with NASH. Sci Rep. (2019) 9:20183. doi: 10.1038/s41598-019-56366-2
37. Yokohama K, Fukunishi S, Ii M, Nakamura K, Ohama H, Tsuchimoto Y, et al. Rosuvastatin as a potential preventive drug for the development of hepatocellular carcinoma associated with non-alcoholic fatty liver disease in mice. Int J Mol Med. (2016) 38:1499–506. doi: 10.3892/ijmm.2016.2766
38. Klaebel JH, Skjødt M, Skat-Rørdam J, Rakipovski G, Ipsen DH, Schou-Pedersen AMV, et al. Atorvastatin and vitamin E accelerates NASH resolution by dietary intervention in a preclinical guinea pig model. Nutrients. (2019) 11:2834. doi: 10.3390/nu11112834
39. Van Rooyen DM, Gan LT, Yeh MM, Haigh WG, Larter CZ, Ioannou G, et al. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol. (2013) 59:144–52. doi: 10.1016/j.jhep.2013.02.024
40. Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, et al. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Null. (2006) 22:873–83. doi: 10.1185/030079906X104696
41. Han KH, Rha SW, Kang H-J, Bae J-W, Choi B-J, Choi S-Y, et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J Clin Lipidol. (2012) 6:340–51. doi: 10.1016/j.jacl.2012.01.009
42. Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. (2013) 12:CD008623. doi: 10.1002/14651858.CD008623.pub2
43. Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Hecht J, Tio F, et al. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: Post hoc analysis of a randomized trial. J Clin Endocrinol Metab. (2017) 102:2950–61. doi: 10.1210/jc.2017-00867
44. Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. (2007) 47:135–41. doi: 10.1016/j.jhep.2007.02.013
45. Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. (2004) 174:193–6. doi: 10.1016/j.atherosclerosis.2004.01.008
46. Kimura Y, Hyogo H, Yamagishi S, Takeuchi M, Ishitobi T, Nabeshima Y, et al. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J Gastroenterol. (2010) 45:750–7. doi: 10.1007/s00535-010-0203-y
47. Kargiotis K, Katsiki N, Athyros V, Giouleme O, Patsiaoura K, Katsiki E, et al. Effect of Rosuvastatin on non-alcoholic steatohepatitis in patients with metabolic syndrome and hypercholesterolaemia: a preliminary report. Curr Vasc Pharmacol. (2014) 31:12. doi: 10.2174/15701611113119990009
48. CHANG CY SCHIANO TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. (2007) 25:1135–51. doi: 10.1111/j.1365-2036.2007.03307.x
49. Onofrei MD, Butler KL, Fuke DC, Miller HB. Safety of statin therapy in patients with preexisting liver disease. Pharmacother J Hum Pharmacol Drug Ther. (2008) 28:522–9. doi: 10.1592/phco.28.4.522
50. Alla V, Abraham J, Siddiqui J, Raina D, Wu GY, Chalasani NP, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. (2006) 40:757–61. doi: 10.1097/00004836-200609000-00018
51. Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplantation. (2004) 10:1018–23. doi: 10.1002/lt.20204
52. Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. (2007) 11:597–613. doi: 10.1016/j.cld.2007.06.010
53. Screening experience and baseline characteristics in the West of Scotland coronary prevention study. Am J Cardiol. 1995 76:485–91. doi: 10.1016/S0002-9149(99)80135-0
54. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. (1998) 339:1349–57. doi: 10.1056/NEJM199811053391902
55. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. (1996) 335:1001–9. doi: 10.1056/NEJM199610033351401
56. de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta-analysis. Pharmacother J Hum Pharmacol Drug Ther. (2004) 24:584–91. doi: 10.1592/phco.24.6.584.34738
57. Alsheikh-Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. (2007) 50:409–18. doi: 10.1016/j.jacc.2007.02.073
58. Bays H, Cohen DE, Chalasani N, Harrison SA. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. (2014) 8:S47–57. doi: 10.1016/j.jacl.2014.02.011
59. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. (2010) 376:1916–22. doi: 10.1016/S0140-6736(10)61272-X
60. Tikkanen MJ, Fayyad R, Faergeman O, Olsson AG, Wun C-C, Laskey R, et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. (2013) 168:3846–52. doi: 10.1016/j.ijcard.2013.06.024
61. Mikhailidis DP, Wierzbicki AS. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) Study. Null. (2002) 18:215–9. doi: 10.1185/030079902125000778
62. Athyros VG, Ganotakis E, Kolovou GD, Nicolaou V, Achimastos A, Bilianou E, et al. Assessing The Treatment Effect in Metabolic Syndrome Without Perceptible Diabetes (ATTEMPT): a prospective-randomized study in middle aged men and women. Curr Vasc Pharmacol. (2011) 9:647–57. doi: 10.2174/157016111797484080
63. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
Keywords: statins, NASH, cardiovascular disease, liver, aminotransferase
Citation: Torres-Peña JD, Martín-Piedra L and Fuentes-Jiménez F (2021) Statins in Non-alcoholic Steatohepatitis. Front. Cardiovasc. Med. 8:777131. doi: 10.3389/fcvm.2021.777131
Received: 14 September 2021; Accepted: 28 October 2021;
Published: 24 November 2021.
Edited by:
M Rosa Bernal-Lopez, Regional University Hospital of Malaga, SpainReviewed by:
Mónica Muñoz Úbeda, Universidad de Málaga, SpainCopyright © 2021 Torres-Peña, Martín-Piedra and Fuentes-Jiménez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco Fuentes-Jiménez, ZmouZnVlbnRlc0B1Y28uZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.