- 1Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2School of Medicine, South China University of Technology, Guangzhou, China
- 3Department of Cardiac Rehabilitation, Guangdong Cardiovascular Institute, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
Objective: To determine the association of perceived stress with coagulation function and their predictive values for clinical outcomes.
Methods: This prospective cohort study derived from a cross-sectional study for investigating the psychological status of inpatients with suspicious coronary heart disease (CHD). In this study, the 10-item Perceived Stress Scale (PSS-10) as an optional questionnaire was used to assess the severity of perceived stress. Coagulation function tests, such as activated partial thromboplastin time (APTT), prothrombin time (PT), and fibrinogen were measured within 1 h after admission. Furthermore, 241 patients with CHD out of 705 consecutive inpatients were included in the analyses and followed with a median of 26 months for the clinical outcomes.
Results: The patients in high perceived stress status (PSS-10 score > 16) were with shorter APTT (36.71 vs. 38.45 s, p = 0.009). Shortened APTT ( ≤ 35.0 s) correlated with higher PSS-10 score (14.67 vs. 11.22, p = 0.003). The association of APTT with depression or anxiety was not found. Multiple linear models adjusting for PT estimated that every single point increase in PSS-10 was relevant to approximately 0.13 s decrease in APTT (p = 0.001) regardless of the type of CHD. APTT (every 5 s increase: hazard ratio (HR) 0.68 [0.47–0.99], p = 0.041) and perceived stress (every 5 points increase: HR 1.31 [1.09–1.58], p = 0.005) could predict the cardiovascular outcomes. However, both predictive values would decrease when they were simultaneously adjusted. After adjusting for the physical clinical features, the associated of perceived stress on cardiac (HR 1.25 [1.04–1.51], p = 0.020) and composite clinical outcomes (HR 1.24 [1.05–1.47], p = 0.011) persisted.
Conclusions: For the patients with CHD, perceived stress strongly correlates with APTT. The activation of the intrinsic coagulation pathway is one of the mechanisms that high perceived stress causes cardiovascular events. This hints at an important role of the interaction of mental stress and coagulation function on cardiovascular prognosis. More attention needs to be paid to the patients with CHD with high perceived stress.
Introduction
Although several large observational studies focusing general population have demonstrated that high perceived stress is associated with increased incidence of coronary heart disease (CHD) and risk for cardiovascular events (1–3), the underlying mechanisms of the negative influence of perceived stress on the cardiovascular system are rarely studied. Coagulation activation has undoubtedly played an important role in the pathogenesis of intracoronary thrombi, which further leads to the occurrence of cardiovascular events. However, although several possible mechanisms (altered automatic nervous system activity, elevated inflammation activity, etc.) have been reported to explain the linkage of psychological distress on the increased risk of cardiovascular events (4), the involvement of activated coagulation and fibrinolysis system has only been mentioned in a few articles (5, 6).
As is known, coagulation can be activated by the two fundamental approaches, the intrinsic pathway reflected by activated partial thromboplastin time (APTT), and the extrinsic pathway reflected by prothrombin time (PT), which converge to a common pathway of thrombosis. Evidence has emerged that in an animal experiment, shortened APTT, PT, and elevated fibrinogen could be induced by chronic localized cold stress (7). In human studies, the acute stressful laboratory tasks can cause a transient activation of coagulation and fibrinolysis in the general population and patients with major depression disorder (8–10). Besides, a shortened APTT more frequently occurred in patients with acute coronary syndromes (ACSs) (11). Outbursts of anger have been demonstrated to be related to the occurrence of acute myocardial infarction (AMI) and ischemic stroke (12). Research on long-term stress has found a heightened level of fibrinogen in subjects with job strain or mood disorders (8). One prior study observed a significant negative correlation between depression and APTT in the participants with low perceived social support (13). Therefore, it is reasonable to hypothesize that high perceived stress could through activating coagulation system to promote the occurrence of cardiovascular events. However, to the best of our knowledge, the studies concerning the relationships among the perceived stress, coagulation, and cardiac outcomes have previously not been published.
Thus, this study aimed to investigate the association between perceived stress and coagulation function, especially the intrinsic pathway coagulation function, and to explore the predictive effect of both on the cardiac and composite clinical outcomes.
Methods
Patient and Public Involvement
This is an exploratory analysis of a perspective cohort based on a cross-sectional study for investigating the psychological status of inpatients with admitted diagnose of CHD. The detailed protocol has been published (14). In brief, 705 consecutive inpatients with a main admitting diagnosis of CHD and without the urgency for emergency percutaneous coronary intervention (PCI) or intensive care, in the Guangdong Provincial People's Hospital were surveyed by the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Questionnaire-7 Scale (GAD-7), and 10-item Perceived Stress Scale (PSS-10) between October 2017 and January 2018 under the supervision of a well-trained psycho-cardiologist on the day before coronary angiography (CAG). The patients were categorized based on the outcomes of CAG and discharge diagnosis, and further classified as a chronic coronary syndrome (CCS) or ACS according to the European Society of Cardiology (ESC) guidelines (15). Since PSS-10 was an optional item, 252 obstructive patients with CHD finally finished the questionnaire. We further excluded the patients with prehospital use of anticoagulants and incomplete data for coagulation function, leaving 241 subjects into the analyses (Figure 1). Follow-up data were collected at the 6th month and later yearly through a scripted telephone interview. The participants were queried for the occurrence of cardiac and non-cardiac rehospitalization, myocardial infarction, stroke, revascularization, and death. Major adverse cardiovascular event (MACE) was defined as a composite of cardiac revascularization, cardiovascular death, non-fatal AMI, non-fatal stroke, or urgent coronary revascularization. Composite endpoint was defined as any occurrence of events mentioned above. The patients were not involved in the recruitment and conduct of the study.
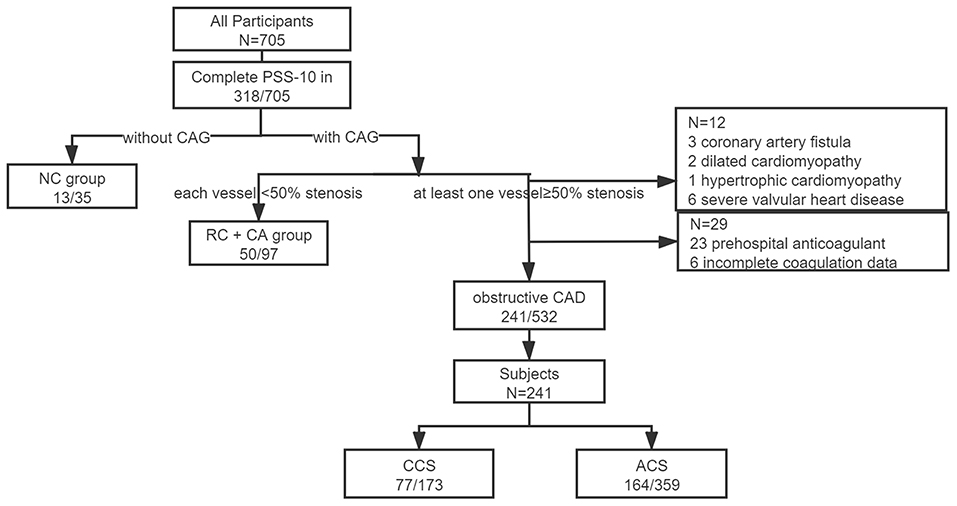
Figure 1. Patient recruitment. Obstructive coronary artery disease (CAD) indicated with at least one obstructive vessel (≥50%) presented in coronary angiography during this hospitalization or with a history of coronary artery bypass grafting or coronary stent implantation. NC, without coronary angiography; RC, rule out of CAD; CA, coronary atherosclerosis; CCS, chronic coronary syndrome; ACS, acute coronary syndrome; and CAD, coronary artery disease.
The study complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Guangdong Provincial People's Hospital with the following reference number: No.GDREC2017203H. Clinical data were obtained from the medical records. All the participants gave written informed consent before inclusion.
Coagulation Function Test
To avoid the interference of possible prescription of low molecular weight heparin (LMWH) to the patients with ACS, the samples of venous blood were drawn within 1 h after admission for coagulation function test and other biochemical analyses. A comparatively calm state of subjects was warranted before blood drawing and psychological assessment.
Activated partial thromboplastin time, PT, TT, fibrinogen, and D-dimer were assayed on the STA-R Evolution® System (Beijing Stago Diagnosis Trading Co., Ltd., Beijing, China) with the accompanying reagents of the instrument (Diagnostica Stago, Taverny, France). The reference ranges of APTT were 30.0–45.0 s given by the Clinical Laboratory and the mean value of APTT in our study was 37.95 s. A shortened APTT was defined as a specimen exhibiting an APTT of <35.0 s (lower quartile).
Assessment of Perceived Stress
Perceived stress status was evaluated with the Chinese version of PSS-10 (as shown in Additional File 1), which as a short form of PSS-14 (16) has been validated in the Chinese cardiac patients (17). The patients were asked about how often in the last month, they felt (1) upset by something happening unexpectedly; (2) unable to control important things in life; (3) nervous and stressed; (4) confident about the ability to handle personal problems; (5) that things were going as expected; (6) unable to cope with all the things had to do; (7) able to deal with irritations; (8) on top of things; (9) angered because of things that happened out of control; and (10) unable to overcome piled up difficulties. Each item was scored on a 5-point scale ranging from 0 = never to 4 = very often. Higher score represented higher stress level. A well-acknowledged cutoff for PSS-10 did not exist, however, to differentiate, we defined the upper quartile as the threshold (PSS-10 > 16) for binary classification.
Assessment of Depression and Anxiety
The Chinese version of PHQ-9 (18) and GAD-7 (19) were applied for evaluating the depression and anxiety symptoms of patients. Each questionnaire, rated on a 0–3 Likert-type scale with a higher score on each item representing more frequently being bothered by the symptom in the last 2 weeks, has demonstrated good validity and reliability among the Chinese cardiac patients (20, 21).
Creatinine Clearance (CCR) and Coronary Artery Stenosis
Creatinine clearance (CCR) was estimated by using the Cockcroft-Gault formula with the value of serum creatinine tested at admission.
Coronary artery stenosis was assessed by CAG during this hospitalization. A 3-vessel obstructive coronary artery disease (CAD) referred to the presence of obstructive stenosis (≥50%) in CAG or history of coronary artery bypass grafting or stent implantation, in all the three main epicardial coronary arteries.
Other Outcomes
The results of other laboratory examinations, echocardiography, and medical history were acquired from the medical records.
Statistical Analysis
The clinical characteristics were reported as rate (percentages) for the categorical variables and mean ± SD or median (interquartile range [IQR]) for continuous variables. The homogeneity of clinical characteristics between those who completed and did not complete the questionnaire was first analyzed. The differences in baseline characteristics from the perspective of perceived stress status (PSS ≤ 16 or >16) and APTT (APTT ≤ 35.0 s or >35.0 s) were both compared with Student's t-test or Wilcoxon rank-sum test for comparing the continuous variables, and the chi-square test or Fisher's exact test for categorical variables as appropriate. The risk ratio of a high perceived stress status on shortened APTT was analyzed using a forward stepwise logistic regression model, such as covariates with age, sex, body mass index (BMI), CCR, level of low-density lipoprotein cholesterol (LDLC), PT, type of CHD, comorbid hypertension, and diabetes. Spearman's correlation was applied to describe the association between APTT and perceived stress, depression, or anxiety score. Multiple linear analyses for each APTT parameter with a PSS-10 score were analyzed, taking PT as another confounding variable after the selection using a forward stepwise method. To avoid the risk of prolonged APTT caused by unrecorded prior intake of coagulants, four cases with large-standardized residuals (>+3) were not included in multiple linear models. The Kaplan–Meier survival analyses were conducted to determine the influence of high perceived stress and shortened APTT status on the clinical outcomes. Cox regression analyses were used with APTT and PSS-10 score individually and simultaneously being taken into the models. In addition, we testify the stability of the outcomes through the stepwise addition of confounding factors. Statistical analysis was performed using SAS 9.4 software. A value of p < 0.05 was considered statistically significant.
Results
Clinical Characteristics
After ruling outpatients with incomplete data or with prehospital use of anticoagulants, 241 out of 532 inpatients with a mean age of 62.12 ± 10.47 years were included in the analyses. The median (IQR) score of PSS-10 was 11 (7–16) points. The prevalence of concurrent hypertension and concurrent diabetes were 63.5 and 34.4%. Additionally, 68.0% of these patients were diagnosed with ACS. During a median follow-up of 26 months, 49 major adverse cardiovascular events, 21 non-cardiac rehospitalization, and 67 cases of composite endpoint happened in 208 patients (follow-up rate 86.3%).
Compared to the ones who did not finish the questionnaire, those who completed PSS-10 were younger, with better renal function, lower LDL-C, and higher PHQ-9 and GAD-7 scores (as shown in Supplementary Table 1). Since PSS-10 was an optional item for the patients, the patients with less difficulties in reading and writing and more psychological disturbance might be more willing to complete the survey. However, no significant difference was found in coronary artery condition, ejection fraction, comorbid diabetes or hypertension, and coagulation function. The ratio of completers over the participants maintained around 45% regardless of the categorization of patients (CCS: 44.5%; ACS: 45.7%).
Perceived Stress and APTT
The comparisons of the baseline characteristics between the groups categorized by PSS > 16 vs. PSS ≤ 16, and APTT ≤ 35.0 s vs. >35.0 s are presented in Tables 1, 2. The patients with a high perceived stress status had shorter APTT (36.71 vs. 38.45, p = 0.009), accompanied by elevated D-dimer level (p = 0.032) and depression (p < 0.001) and anxiety symptom scores (p < 0.001). No significant difference was found in the comparisons in PT, TT, fibrinogen, and the other clinical features, such as renal and hepatic function. Simultaneously, shortened APTT status was significantly associated with increased PSS-10 score (14.67 vs. 11.22, p = 0.003). Interestingly, a closer linkage of APTT with perceived stress rather than with depression (p = 0.012) or anxiety (p = 0.29) was observed. Furthermore, Spearman's correlation coefficient showed that the correlation of APTT with perceived stress score (rs = −0.20, p = 0.002) was much stronger than with depression (rs = −0.13, p = 0.054) or anxiety symptom score (rs = −0.037, p = 0.57).
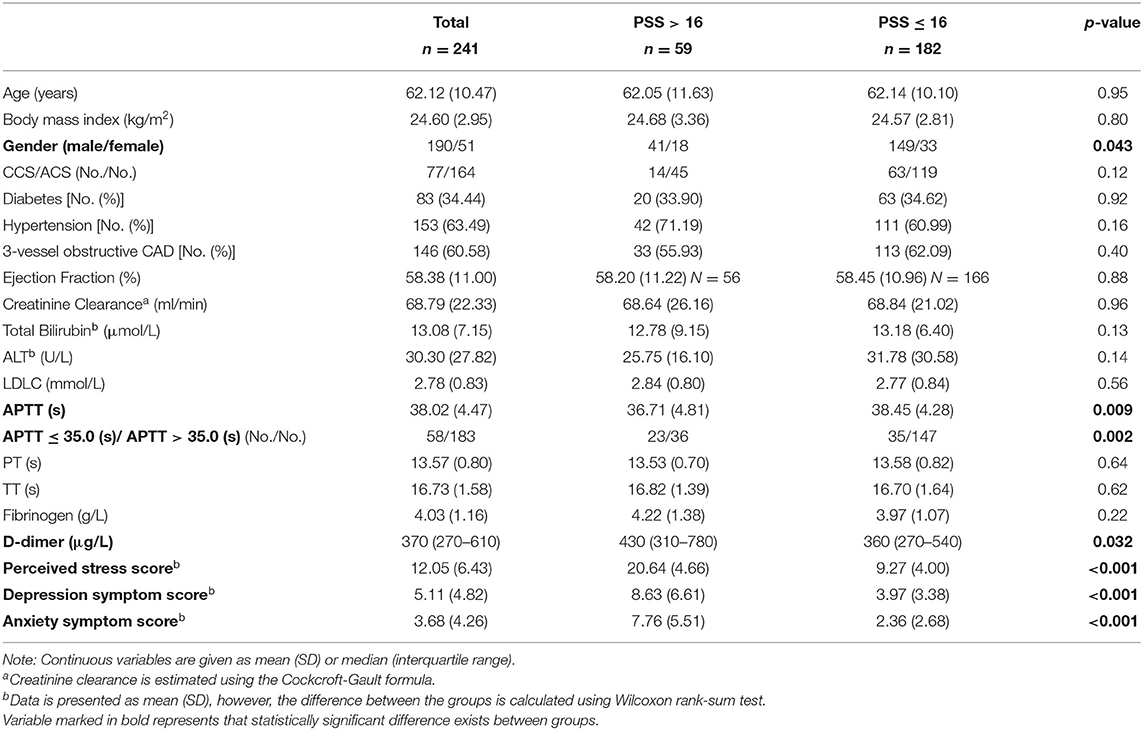
Table 1. Comparisons of the baseline characteristics between the groups categorized by perceived stress status.
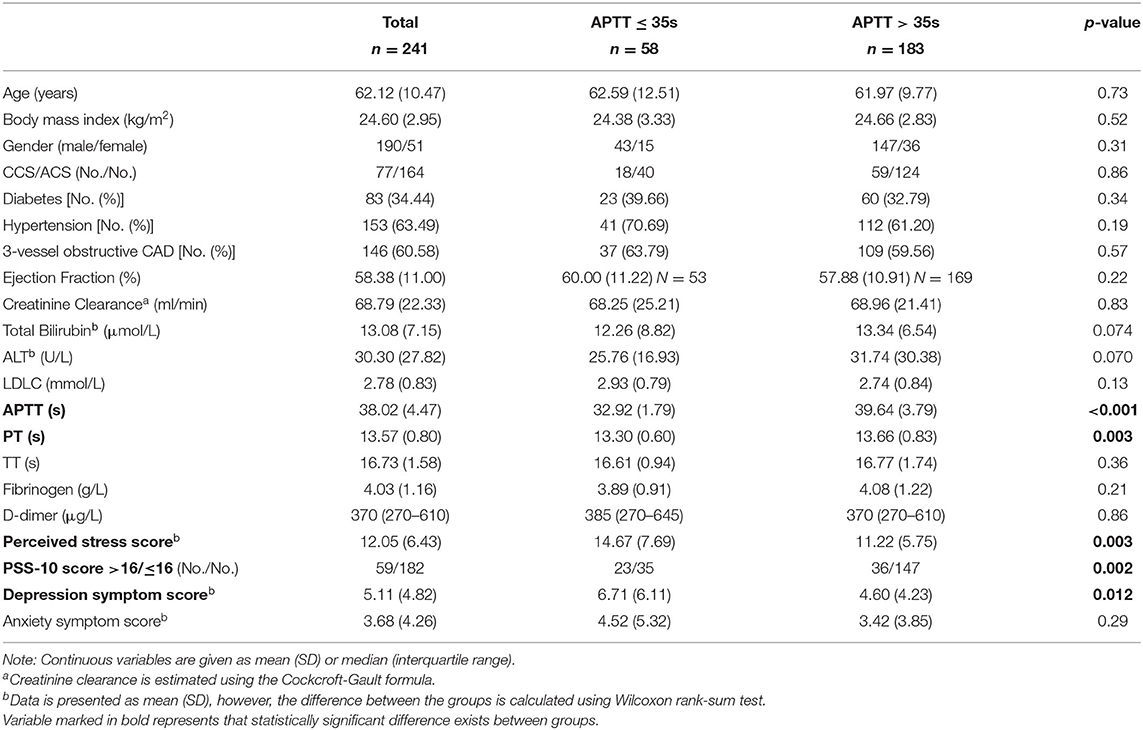
Table 2. Comparisons of the baseline characteristics between the groups categorized by activated partial thromboplastin time (APTT).
The multivariate analyses using a forward stepwise logistic regression model taking all the physical features into analysis revealed that PT and perceived stress status (odds ratio [OR] 2.75 (95% CI 1.42–5.33), p = 0.003) were the only two remaining factors that associated with shortened APTT. This association kept marginal significant [OR 2.05 (95% CI 0.98–4.30), p = 0.056] after adding PHQ-9 score into adjustment. Multiple linear regression models indicated that every single point increase in PSS-10 was relevant to approximately 0.13 s decrease in APTT, regardless of the type of coronary heart disease (for all p = 0.001: CCS: 0.16 s, p = 0.021; ACS: 0.12 s, p = 0.011) (Table 3).
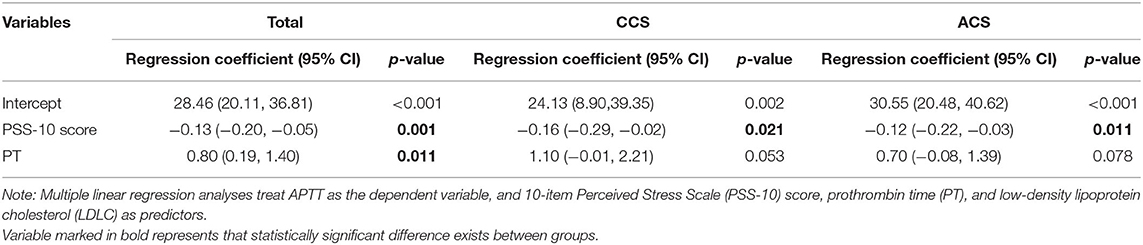
Table 3. Multiple linear regression models of APTT in the patients with the chronic coronary syndrome (CCS) and acute coronary syndrome (ACS).
Perceived Stress, APTT, and Prognosis
From the perspective of clinical outcome (Table 4), the patients with MACE had significantly shorter APTT (p = 0.041), higher PSS-10 (p = 0.051), and PHQ-9 scores (p = 0.030). The occurrence of composite endpoint was associated with PSS-10 (p = 0.021) and depression symptom scores (p = 0.017). Non-cardiac rehospitalization was irrelevant to both perceived stress and APTT. Similarly, the Kaplan–Meier survival analyses (as shown in Supplementary Figures 1, 2) exhibited an obvious tendency of a negative influence of high perceived stress status on MACE (p = 0.095) and composite outcomes (p = 0.049).
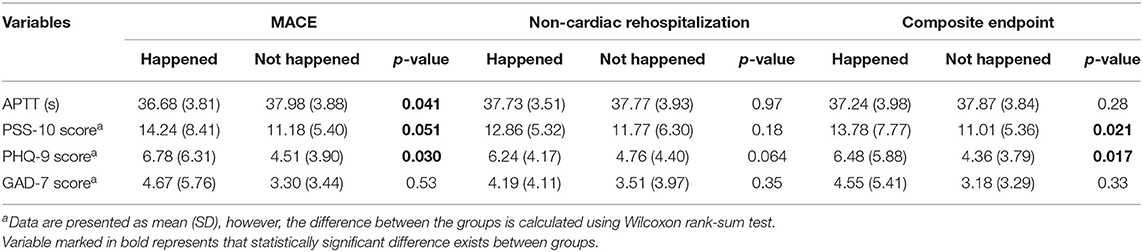
Table 4. Comparison of APTT, PSS-10, Patient Health Questionnaire (PHQ-9), Generalized Anxiety Questionnaire-7 Scale (GAD-7) scores for different clinical outcomes.
We tried taking APTT and PSS-10 scores individually or simultaneously into the different Cox proportional hazard models (Table 5) and found that in the univariate analyses both APTT (every 5 s increase: hazard ratio [HR] 0.68 (95% CI 0.47–0.99), p = 0.041) and PSS-10 score (every 5 points increase: HR 1.31 (95% CI 1.09–1.58), p = 0.005) could predict the cardiovascular prognosis. Only PSS-10 score was associated with composite outcomes [every 5 points increase: HR 1.26 (95% CI 1.07–1.48), p = 0.006]. Different adjustments for other clinical variables proved that the statistical results were stable. Although the significance of the PSS-10 score was retained, the predictive values of APTT were to some extend decreased. Both the correlations of APTT and PSS-10 score with the clinical outcomes weakened when they were simultaneously incorporated into the models. After taking APTT and other physical characteristics into adjustment, PSS-10 score was still associated with increased risk for MACE [every 5 points increase: HR 1.25 (95% CI 1.04–1.51), p = 0.020] and composite endpoint [every 5 points increase: HR 1.24 (95% CI 1.05–1.47), p = 0.011], however, the statistical significance of APTT for MACE disappeared.
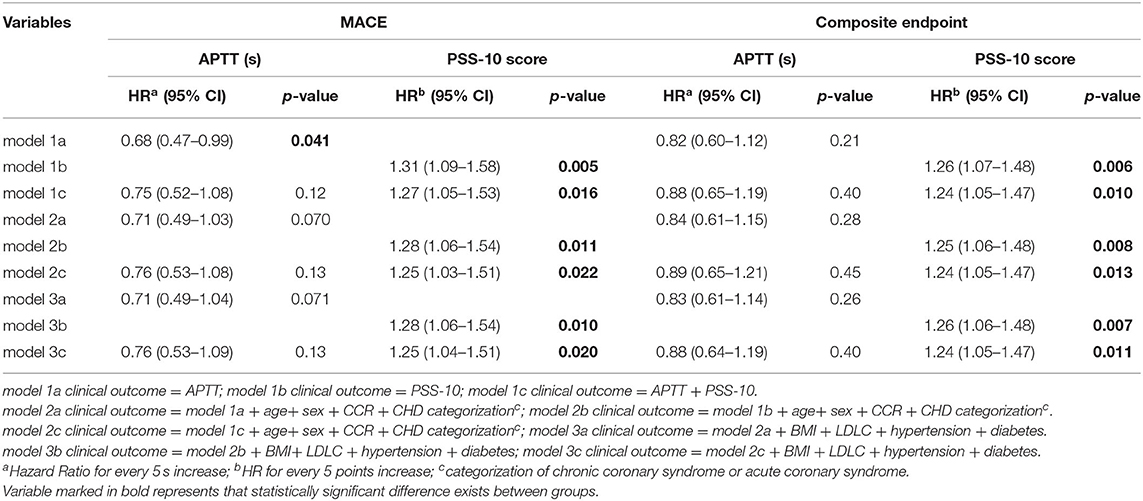
Table 5. Comparison of hazard ratios (HRs) of APTT, PSS-10, the score for a major adverse cardiovascular event (MACE), and composite endpoint among the different Cox proportional hazard models.
Discussion
Our research through both the univariate and multivariate analyses demonstrated that high perceived stress was associated with shortened APTT in patients with CHD. The classification of CCS or ACS did not affect the establishment of the conclusion. As compared with depression or anxiety, perceived stress exhibited a closer linkage to APTT. Shortened APTT and increased perceived stress were both individually related to worse cardiovascular prognosis, however, the effect of APTT on MACE could be diluted when adjusting for perceived stress. This hinted that the negative impact of perceived stress on cardiovascular outcomes might act partially through the mechanism of a hypercoagulable state in the intrinsic pathway. After adjusting for all clinical features, high perceived stress remained significantly associated with MACE and composite outcomes.
Though contradicted by few articles (13), APTT was reported irrelevant with depression or anxiety (5, 22), which was demonstrated by our research. Considering the strong correlation between mood disturbance and perceived stress, it was interesting to reveal an intensive linkage between APTT and perceived stress. As compared with mood disturbance, perceived stress measured by PSS-10 could be a more accurate indicator representing the stress received by the human body. Although to the best of our knowledge, the linkage between APTT and perceived stress has not been reported before, the findings that shortened APTT and platelet hyperreactivity in the patients with mood disorders would be reversed after the improvement of psychiatric symptoms (23, 24) might confirm the rationality.
The mechanisms that underlie the association between perceived stress and APTT are still poorly understood. Previous research toward the Chinese population reported a linkage between the PSS-14 score and the level of epinephrine and norepinephrine (25). It is hypothesized that catecholamine and cortisol under the control of perceived stress may exert an important influence on hemostasis (26–28). Another possible explanation is the elevation of intrinsic coagulation factors. It was revealed by Doulalas et al. (29) that more coagulation factors VII and X were expressed in the healthy individuals with depressed moods. Similarly, elevated factors VII and VIII were observed closely correlated with job strain among Korean male workers (30).
In accordance with previous literature (31–33), our results demonstrated that a hypercoagulability state predicted a worse cardiovascular prognosis. Although perceived stress has been linked to the increased cardiovascular risk in the general population for a long time (1–3), the research on the influence of perceived stress aiming at the patients with CHD has rarely been reported. Our study revealed that elevated perceived stress was associated with the increased risk of MACE and adverse composite outcomes, which was in line with a previous article reporting that a moderate or high perceived stress status at the time of AMI predicted adverse long-term outcomes (34). As indicated by the results, the negative influence of shortened APTT and elevated perceived stress on cardiovascular prognosis are to some extend overlapped, which means that shortened APTT might be one of the mechanisms that high perceived stress causes cardiovascular events.
As mentioned before, both acute and chronic mental stress can cause a gentle activation of coagulation (35) as well as increases in some coagulation factors, for example, FVIII. Meanwhile, shortened APTT analyzed in this study is mostly within a physiological range. How the minor change leads to a worse cardiovascular prognosis? Emerging data have demonstrated that mental stress only exerted weak effects on the development of CHD in healthy individuals (36), whereas it might play a critical role for a more severe progression and clinical outcome in the patients with a previous cardiovascular disease (34, 37). In our study, the pathological basis of CHD may have contributed to the vulnerability. Recently, a high FVIII level was found causally related to both CHD and venous thrombosis risk (38). Although vulnerable plaques are regarded as the culprit factor for a worse cardiac prognosis, “blood prone to thrombosis” has played an important role (31). Despite the overactivation of the sympathetic nerve system under stress, which might cause the mismatch of oxygen demand and supply for myocardium, mental stress-induced prolonged platelet activation (39, 40) and endothelial dysfunction (41) could be another precipitating factor. With the increased susceptibility of the endothelium to injury, endothelium lesions caused by ischemia or the rupture of plaque, through exposing procoagulant materials to the blood, might catalyze the initialization of thrombosis, and could be further strengthened under the push of high perceived stress through a hypercoagulable state and platelet activation. The alterations in inflammation may be another possible involved mechanism since it can be induced by mental stress (42), coupled with the activation of coagulation function, and is associated with an increased risk of cardiovascular diseases (43).
These findings point out the important role that the interaction of mental stress and coagulation function has played in the occurrence of cardiovascular events in patients with CHD and call for attention to the psychological state of patients in clinical practice. More attention needs to be paid to high perceived stress status due to the close linkage with biological measurements and worse prognosis. In addition, it is worthwhile to explore the benefits of stress management for patients with CHD. Several randomized clinical trials have already proved the effectiveness for improving clinical prognosis (44).
Additionally, mounting evidence has suggested that anticoagulant plays a crucial part in the therapy for ACS especially in the acute phase (45). The combination of low dose anticoagulant plus aspirin can reduce the risk for cardiac events in patients with stable CHD (46), however, increase the possibility of major bleeding events. Whether perceived stress could become an important indicator guiding the anticoagulant strategy needs to be further studied.
Our findings should be considered in light of several potential limitations. First, this is a single center observational study with a limited sample size. Second, the markers for coagulation factors and fibrinolysis and platelet activity were not tested in this study. Fortunately, the values of D-dimer and fibrinogen could at least provide some insights into the changes. Third, smoking could influence coagulation, fibrinolysis system, and platelet activation to promote the thrombi formation (47), but the smoking status was not included in our analyses due to the inaccurate data from medical records. However, based on the inaccurate data, we have roughly estimated the ratio of current smokers between APTT ≤ 35.0 s and >35.0 s, the difference was not significant. Fourth, since the PSS-10 was given to the patients as an optional item to avoid subjects filling out the questionnaire blindly, whether the completers could represent the whole CHD population needs to be explored. However, through the comparisons, a huge difference in physical features was not found.
Conclusions
Perceived stress strongly correlates with APTT regardless of the type of CHD. The negative impact of high perceived stress on cardiovascular prognosis could partially be explained by the activation of the intrinsic coagulation pathway. These findings highlight the importance to recognize high perceived stress status in patients with CHD and indicate a vital role of the interaction between mental stress and coagulation function for cardiovascular events. Further research is needed to explore the deeper mechanisms underlying the phenomenon.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without unduereservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Guangdong Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HY surveyed all the patients at baseline. YL, AL, HW, and FL followed all patients. HY and XC collected and entered data into the database. XC, YL, and AL did the statistical analyses. HY and XC wrote the manuscript. QG, HM, and LG were senior physicians principally responsible for the study. HM and QG revised the manuscript. All the authors read and approved the final manuscript.
Funding
This study was supported by the grants of Natural Science Foundation of Guangdong Province (2019A1515011224), Guangdong Medical Science and Technology Research Fund (2019118152336191), Guangdong Provincial Bureau of Traditional Chinese Medicine (20201008), and the High-level Hospital Construction Project of Guangdong Provincial People's Hospital (DFJH201811, DFJH201922, and DFJH2020003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.769857/full#supplementary-material
References
1. Nabi H, Kivimäki M, Batty GD, Shipley MJ, Britton A, Brunner EJ, et al. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: the Whitehall II prospective cohort study. Eur Heart J. (2013) 34:2697–705. doi: 10.1093/eurheartj/eht216
2. Dupre ME, George LK, Liu G, Peterson ED. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch Intern Med. (2012) 172:1731–7. doi: 10.1001/2013.jamainternmed.447
3. Niiyama M, Tanaka F, Nakajima S, Itoh T, Matsumoto T, Kawakami M, et al. Population-based incidence of sudden cardiac and unexpected death before and after the 2011 earthquake and tsunami in Iwate, northeast Japan. J Am Heart Assoc. (2014) 3:e000798. doi: 10.1161/JAHA.114.000798
4. Carney RM, Freedland KE. Depression and coronary heart disease. Nature reviews Cardiology. (2017) 14:145–55. doi: 10.1038/nrcardio.2016.181
5. Geiser F, Urbach AS, Harbrecht U, Conrad R, Pötzsch B, Amann N, et al. Anxiety and depression in patients three months after myocardial infarction: Association with markers of coagulation and the relevance of age. J Psychosom Res. (2017) 99:162–8. doi: 10.1016/j.jpsychores.2017.06.015
6. Geiser F, Meier C, Wegener I, Imbierowicz K, Conrad R, Liedtke R, et al. Association between anxiety and factors of coagulation and fibrinolysis. Psychother psychosom. (2008) 77:377–83. doi: 10.1159/000151518
7. Khatun S, Kanayama N, Belayet HM, Tokunaga N, Sumimoto K, Kobayashi T, et al. (1999). Induction of hypercoagulability condition by chronic localized cold stress in rabbits. Thromb Haemost. (1999) 81:449–55. doi: 10.1055/s-0037-1614493
8. Austin AW, Wissmann T, von Kanel R. Stress and hemostasis: an update. Semin Thromb Hemost. (2013) 39:902–12. doi: 10.1055/s-0033-1357487
9. von Känel R, Merz F, Pfister H, Brückl T, Zimmermann P, Uhr M, et al. Acute stress-induced coagulation activation in patients with remitted major depression versus healthy controls and the role of stress-specific coping. Ann Behav Med. (2020) 54:611–8. doi: 10.1093/abm/kaaa001
10. von Kanel R, Kudielka BM, Haeberli A, Stutz M, Fischer JE, Patterson SM. Prothrombotic changes with acute psychological stress: combined effect of hemoconcentration and genuine coagulation activation. Thromb Res. (2009) 123:622–30. doi: 10.1016/j.thromres.2008.05.014
11. Sotoudeh Anvari M, Tavakoli M, Lotfi-Tokaldany M, Broumand M, Rezahosseini O, Hakki-Kazzazi E, et al. Coronary artery disease presentation and its association with shortened activated partial thromboplastin time. J Tehran Heart Center. (2018) 13:1–5.
12. Mostofsky E, Penner EA, Mittleman MA. Outbursts of anger as a trigger of acute cardiovascular events: a systematic review and meta-analysis. Eur Heart J. (2014) 35:1404–10. doi: 10.1093/eurheartj/ehu033
13. Lukas PS, Neugebauer A, Schnyder S, Biasiutti FD, Krummenacher R, Ferrari ML, et al. Depressive symptoms, perceived social support, and prothrombotic measures in patients with venous thromboembolism. Thromb Res. (2012) 130:374–80. doi: 10.1016/j.thromres.2012.04.011
14. Yin H, Liu Y, Ma H, Liu G, Guo L, Geng Q. Associations of mood symptoms with NYHA functional classes in angina pectoris patients: a cross-sectional study. BMC Psychiatry. (2019) 19:85. doi: 10.1186/s12888-019-2061-3
15. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77.
16. Taylor JM. Psychometric analysis of the Ten-Item Perceived Stress Scale. Psychol Assess. (2015) 27:90–101. doi: 10.1037/a0038100
17. Leung DY, Lam TH, Chan SS. Three versions of perceived stress scale: validation in a sample of Chinese cardiac patients who smoke. BMC Public Health. (2010) 10:513. doi: 10.1186/1471-2458-10-513
18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
19. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
20. Wang L, Lu K, Wang C, Sheng L, Hu D, Ding R. Reliability and validity of GAD-2 and GAD-7 for anxiety screening in cardiovascular disease clinic. Sichuan Mental Health. (2014) 27:3–4.
21. Wang W, Bian Q, Zhao Y, Li X, Wang W, Du J, et al. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. General Hospital Psychiatry. (2014) 36:539–44. doi: 10.1016/j.genhosppsych.2014.05.021
22. Schroeder V, Borner U, Gutknecht S, Schmid JP, Saner H, Kohler HP. Relation of depression to various markers of coagulation and fibrinolysis in patients with and without coronary artery disease. Eur J Cardiovasc Prev Rehabil. (2007) 14:782–7. doi: 10.1097/HJR.0b013e32828622e8
23. Geiser F, Conrad R, Imbierowicz K, Meier C, Liedtke R, Klingmüller D, et al. Coagulation activation and fibrinolysis impairment are reduced in patients with anxiety and depression when medicated with serotonergic antidepressants. Psychiatry Clin Neurosci. (2011) 65:518–25. doi: 10.1111/j.1440-1819.2011.02241.x
24. von Kanel R. Platelet hyperactivity in clinical depression and the beneficial effect of antidepressant drug treatment: how strong is the evidence? Acta psychiatrica Scandinavica. (2004) 110:163–77. doi: 10.1111/j.1600-0447.2004.00308.x
25. Yang Y, Bi M, Xiao L, Chen Q, Chen W, Li W, et al. Perceived stress status and sympathetic nervous system activation in young male patients with coronary artery disease in China. Eur J Intern Med. (2015) 26:726–30. doi: 10.1016/j.ejim.2015.08.005
26. von Kanel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom Med. (2001) 63:531–44. doi: 10.1097/00006842-200107000-00003
27. Tirosh A, Lodish M, Lyssikatos C, Belyavskaya E, Feelders RA, Stratakis CA. Coagulation profile in patients with different etiologies for cushing syndrome: a prospective observational study. Hormone Metab Res. (2017) 49:365–71. doi: 10.1055/s-0043-100113
28. Pinelli A, Trivulzio S, Rossoni G. Activated partial thromboplastin time correlates with methoxyhydroxyphenylglycol in acute myocardial infarction patients: therapeutic implications for patients at cardiovascular risk. In Vivo. (2014) 28:99–104.
29. Doulalas AD, Rallidis LS, Gialernios T, Moschonas DN, Kougioulis MN, Rizos I, et al. Association of depressive symptoms with coagulation factors in young healthy individuals. Atherosclerosis. (2006) 186:121–5. doi: 10.1016/j.atherosclerosis.2005.06.030
30. Chang SJ, Koh SB, Cha BS, Park JK. Job characteristics and blood coagulation factors in Korean male workers. J Occup Environ Med. (2002) 44:997–1002. doi: 10.1097/00043764-200211000-00004
31. Campo G, Pavasini R, Pollina A, Tebaldi M, Ferrari R. Coagulation factors and recurrence of ischemic and bleeding adverse events in patients with acute coronary syndromes. Thromb Res. (2013) 132:151–7. doi: 10.1016/j.thromres.2013.06.007
32. Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, et al. D-Dimer Predicts Long-Term Cause-Specific Mortality, Cardiovascular Events, and Cancer in Patients With Stable Coronary Heart Disease: LIPID Study. Circulation. (2018) 138:712–23. doi: 10.1161/CIRCULATIONAHA.117.029901
33. Lin CH, Kuo YW, Kuo CY, Huang YC, Hsu CY, Hsu HL, et al. Shortened activated partial thromboplastin time is associated with acute ischemic stroke, stroke severity, and neurological worsening. J Stroke Cerebrovasc Dis. (2015) 24:2270–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.008
34. Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long-term mortality and health status outcomes. J Am Coll Cardiol. (2012) 60:1756–63. doi: 10.1016/j.jacc.2012.06.044
35. Austin AW, Wirtz PH, Patterson SM, Stutz M, von Kanel R. Stress-induced alterations in coagulation: assessment of a new hemoconcentration correction technique. Psychosom Med. (2012) 74:288–95. doi: 10.1097/PSY.0b013e318245d950
36. Kivimäki M, Jokela M, Nyberg ST, A Singh-Manoux A, Fransson EI, Alfredsson L, et al. Long working hours and risk of coronary heart disease and stroke: a systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet. (2015) 386:1739–46. doi: 10.1016/S0140-6736(15)60295-1
37. Sandrini L, Ieraci A, Amadio P, Zarà M, Barbieri SS. Impact of acute and chronic stress on thrombosis in healthy individuals and cardiovascular disease patients. Int. J Mol Sci. (2020) 21:7818. doi: 10.3390/ijms21217818
38. Sabater-Lleal M, Huffman JE, Vries PSde, Marten J, Mastrangelo MA, Song C, et al. Genome-wide association transethnic meta-analyses identifies novel associations regulating coagulation factor VIII and von Willebrand factor plasma levels. Circulation. (2019) 139:620–35. doi: 10.1161/CIRCULATIONAHA.118.034532
39. Reid GJ, Seidelin PH, Kop WJ, Irvine MJ, Strauss BH, Nolan RP, et al. Mental-stress-induced platelet activation among patients with coronary artery disease. Psychosom Med. (2009) 71:438–45. doi: 10.1097/PSY.0b013e31819cc751
40. Strike PC, Magid K, Brydon L, Edwards S, McEwan JR, Steptoe A. Exaggerated platelet and hemodynamic reactivity to mental stress in men with coronary artery disease. Psychosom Med. (2004) 66:492–500. doi: 10.1097/01.psy.0000130492.03488.e7
41. Sher LD, Geddie H, Olivier L, Cairns M, Truter N, Beselaar L, et al. Chronic stress and endothelial dysfunction: mechanisms, experimental challenges, and the way ahead. Am J Physiol Heart Circ Physiol. (2020) 319:H488–506. doi: 10.1152/ajpheart.00244.2020
42. Jain S, Mills PJ, von Känel R, Hong S, Dimsdale JE. Effects of perceived stress and uplifts on inflammation and coagulability. Psychophysiology. (2007) 44:154–60. doi: 10.1111/j.1469-8986.2006.00480.x
43. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. (2017) 389:834–45. doi: 10.1016/S0140-6736(16)31714-7
44. Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, et al. Enhancing cardiac rehabilitation with stress management training: a randomized clinical efficacy trial. Circulation. (2016) 115.018926. doi: 10.1161/CIRCULATIONAHA.115.018926
45. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.5603/KP.2018.0041
46. Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. (2018) 391:205–18. doi: 10.1016/S0140-6736(17)32458-3
Keywords: perceived stress, coronary heart disease, activated partial thromboplastin time, acute coronary syndrome, depression
Citation: Yin H, Cheng X, Liang Y, Liu A, Wang H, Liu F, Guo L, Ma H and Geng Q (2021) High Perceived Stress May Shorten Activated Partial Thromboplastin Time and Lead to Worse Clinical Outcomes in Patients With Coronary Heart Disease. Front. Cardiovasc. Med. 8:769857. doi: 10.3389/fcvm.2021.769857
Received: 08 September 2021; Accepted: 31 October 2021;
Published: 29 November 2021.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Tsung Neng Tsai, Tri-Service General Hospital, TaiwanDidier Jambou, Laboratoire d'Hématologie - CHU de Nice and UCA, France
Ying Zhao, Zhejiang University, China
Yetti Hernaningsih, Airlangga University, Indonesia
Copyright © 2021 Yin, Cheng, Liang, Liu, Wang, Liu, Guo, Ma and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Ma, bWFodWFuZG9jdG9yQDE2My5jb20=; Qingshan Geng, Z2VuZ3FpbmdzaGFuQGdkcGgub3JnLmNu
†These authors have contributed equally to this work
 Han Yin
Han Yin Xingyu Cheng1†
Xingyu Cheng1† Yanting Liang
Yanting Liang Anbang Liu
Anbang Liu Huan Ma
Huan Ma Qingshan Geng
Qingshan Geng