
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 06 December 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.769472
This article is part of the Research Topic Arteriogenesis and Collateral Remodelling in Ischaemic Disease View all 7 articles
 Xiaolang Jiang1†
Xiaolang Jiang1† Hao Liu1†
Hao Liu1† Tianyue Pan1†
Tianyue Pan1† Shiyang Gu2
Shiyang Gu2 Yuan Fang1
Yuan Fang1 Zheng Wei2
Zheng Wei2 Gang Fang1
Gang Fang1 Bin Chen1
Bin Chen1 Junhao Jiang1
Junhao Jiang1 Yun Shi1
Yun Shi1 Peng Liu2
Peng Liu2 Weiguo Fu1*
Weiguo Fu1* Zhihui Dong1*
Zhihui Dong1*Background: Peripheral blood mononuclear cells (PBMNCs) showed encouraging short outcomes in the treatment of angiitis-induced no-option critical limb-threatening ischemia (AICLTI) in the pilot study. This study aimed to demonstrate the long-term outcomes of this treatment.
Methods: From May 2014 to December 2018, patients diagnosed with AICLTI and treated by autotransplantation of PBMNCs in our center were enrolled and analyzed. The primary endpoint was major amputation-free survival (MAFS), the secondary endpoints included peak pain-free walking time (PPFWT), Wong-Baker FACES pain rating scale score (WFPRSS), labor recovery, ankle-brachial index (ABI), transcutaneous partial oxygen pressure (TcpO2), and SF-36v2 scores.
Results: A total of 58 patients were enrolled. During a minimal follow-up of 36 months, the MAFS was 93.1% and the labor competence restored rate was 62.1%. The WFPRSS was decreased from 8.7 ± 1.6 to 1.6 ± 3.2, and PPFWT was significantly improved from 2.9 ± 4.2 min to 16.6 ± 6.9 min. The quality of life was also significantly improved at each follow-up point. Perfusion evaluating parameters, such as ABI and TcPO2, were also significantly improved. No critical adverse event was observed during the treatment and follow-up period.
Conclusions: The treatment of AICLTI by autotransplantation of PBMNCs demonstrated encouraging long-term results. It could not only restore labor competence, improve the quality of life, but also significantly reduce the major amputation rate.
Angiitis-induced critical limb-threatening ischemia (AICLTI) was identified to be different with atherosclerosis obliterans (ASO). Patients with AICLTI were usually younger, involved the middle and distal vessels, and the angiitis was the main pathogeny (1, 2), which made the mainstream vascular reconstruction difficult to be performed or with poor outcomes. Thromboangiitis obliterans (TAO) accounted for more than 90% of the patients with AICLTI, the primary and secondary patencies after bypass procedure were 41 and 54% at 1 year, 32 and 47% at 5 years, and 30 and 39% at 10 years, respectively (3). Studies, such as peripheral blood mononuclear cells (PBMNCs), bone marrow-derived stem cells (BMDSCs), and purified CD34+ cells (PCCs) in the treatment of AICLTI have been reported (4). However, most of the previous studies focused on the patients with ASO or diabetes mellitus and were absent from long-term outcomes (5, 6). Of note is the fact that most of the previous studies merely involved a few to a dozen patients with AICLTI (7–11). In our previous randomized controlled trial (RCT), comparing the PBMNCs with PCCs in the treatment of AICLTI, the 3-year outcomes of PBMNCs also have been reported (12). Compared with BMDSCs and PCCs, the extraction and collection of PBMNCs were simpler and more cost-effective. Despite this, the sample size of this RCT was too small and the long-term outcome of PBMNCs needed to be further elucidated with a larger sample size. We herein conducted this study to report the long-term clinical outcome of AICLTI treated by PBMNCs with more patients and a minimal follow-up of 36 months.
From May 2014 to July 2018, patients diagnosed with AICLTI and treated by autotransplantation of PBMNCs in our center were enrolled and analyzed. The definition of TAO was according to Olin (2). The study was carried out according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Zhongshan Hospital, Fudan University. Informed consent was obtained from all eligible patients, and their medical records were reviewed. The inclusion and exclusion criteria had been elucidated previously (4). Patients should meet all the following criteria: (1) aged 18–80 years; (2) stenotic or occlusive lesions in the limb arteries confirmed by CT angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA); (3) Rutherford classification four or five unsuitable for open surgery or endovascular therapy; (4) no improvement for at least 3 months after open surgery or endovascular treatment, or at least 1 month of conservative treatment, such as smoking cessation, drug therapy, and exercise therapy; and (5) an unhealing ulcer at least 1 month after optimal care. Exclusion criteria included (1) serious health events that occurred <3 months before admission, including but not limited to myocardial infarction, cerebral apoplexy, pulmonary embolism, severe hepatic dysfunction, and renal dysfunction; (2) or contraindications for the administration of the granulocyte colony-stimulating factor (G-CSF); and (3) no informed consent signed.
The detailed treatment procedure had been described previously (4, 13, 14), and it was summarized as follows: the G-CSF was mobilized for 5 days, during which low molecular weight heparin (4100 IU) was injected subcutaneously each day. Meanwhile, the blood test was performed every single day to check the count of white blood cells (WBC) and CD34+ cells. On the fifth day, apheresis (COM.TEC; Fresenius HemoCare, Friedberg, Germany) was performed. The final cell products were assessed by leukocyte counting and flow cytometry using CD34 antibodies. The implanted CD34+ cell doses were ensured ranged from 105 to 106 per kg body weight. Cell transplantation was performed by intramuscular injection under general anesthesia with laryngeal mask anesthesia. A total of 80 and 160 sites were each injected with 0.5 ml. In addition, the injections were distributed in the calf and foot or the forearm and hand of the ischemic limbs evenly. Debridement and minor amputation were not routinely performed unless the patient was complicated with wet gangrene or serious infection. Patients with autoimmunological diseases were administered regular immunological treatment and follow-up by rheumatologists. The patients were given aspirin (100 mg/d), cilostazol (400 mg/d), and anplag (300 mg/d) for at least 1 year after the operation. In addition, analgesic therapy was not usually recommended because most of the pain would alleviate within 1 month.
All patients were followed once a month for the first 3 months, and every 3 months for the rest of the first year, and once a year thereafter. The primary endpoint was major amputation-free survival (MAFS), which was defined as amputation above the joint. The secondary endpoints included (1) peak pain-free walking time (PPFWT), which was measured on a treadmill at 2.5 km/h and 10% incline at 0, 3, 6, 12, and 36 months, (2) Wong-Baker FACES pain rating scale score (WFPRSS), which was used to test the pain of the patients at 0, 1, 3, 6, 12, and 36 months, (3) labor recovery, which was defined as returning to original work of patients who were unemployed or suspended, (4) ankle-brachial index (ABI) and transcutaneous partial oxygen pressure (TcPO2) of the treated limb were recorded at 1, 3, 6, 12, and 36 months, and 36-item Short-Form Health Survey version 2 (SF-36v2) score (15), which was used the evaluated the quality of life (QoL) at 0, 12, 24, and 36 months. Ulcer healing is confirmed by a clinician who observed and photographed the limbs of the patients. The definitions of recurrence and ischemia onset were detailed in the previous article (13).
Data were collected using Microsoft Excel (Microsoft, Redmond, WA, USA) and analyzed by GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). Continuous variables were presented as mean ± SD, and categorical variables were presented as frequencies and percentages. The Student's t-test or Mann-Whitney U-test was applied to test the differences at different follow-up points for continuous variables depending on the distribution of the data. MAFS was calculated by Kaplan-Meier analysis.
All P-values were two-tailed, and a P < 0.05 was defined as statistically significant.
From May 2014 to July 2018, a total of 58 patients underwent transplantation of PBMNCs in our center. The mean age was 41.9 ± 13.0 years with males accounting for 100%. The etiology was TAO in 56 (96.6%), systemic lupus erythematosus (SLE) in 1 (1.7%), and eosinophilia in 1 (1.7%) patient. The mean follow-up time was 56.4 ± 16.0 months, and the median numbers of CD34+ cells were 8.5 × 105 cells/kg [the interquartile range (IQR): 5.1–11.7 × 105 cells/kg] (Table 1).
One patient died 18 months after transplantation, which was identified to be irrelevant to the intervention. Major amputation occurred in four (6.9%) patients. Patient one was a 44-year-old male with a more than 10-year history of CLTI in the right lower extremity. He underwent lumbar sympathectomy and bypass procedure before treatment of PBMNCs; however, the symptom was not alleviated. Although the pain was significantly relieved after transplantation, the ulcer in the foot was unhealed. He underwent amputation above the knee 6 months later. Patients two and three were all Rutherford classification 5, and they underwent amputation above the knee at 3 and 2 months after transplantation due to unbearable pain, respectively. Patient four was a 50-year-old man who underwent amputation above the knee 3 months later due to an unhealed ulcer. Amputation of toes was performed in five (8.6%) patients. Among them, three patients were performed along with the transplantation, and the other patients underwent the amputation within 1 month. The 36-month MAFS was 93.1% (Figure 1A).
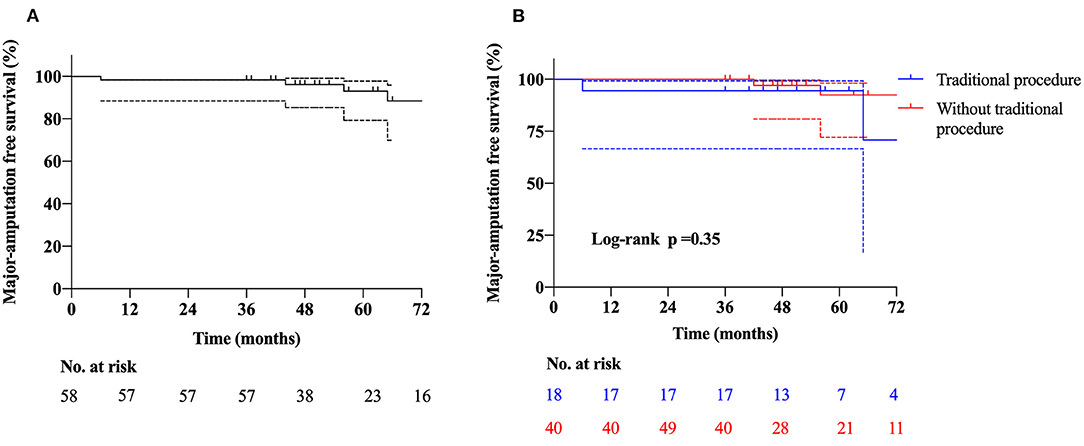
Figure 1. Kaplan-Meier curves show the rates of (A) major amputation free survival, (B) major amputation free survival in patients with and without traditional procedures.
Before transplantation, three and 55 patients were categorized as Rutherford classification four and five, respectively. Fifty-five (94.8%) patients had ulcers on the feet, and 48 (87.3%) of them healed during the follow-up period (Figure 2). The mean time of healing was 6.1 ± 3.7 months. The mean time of pain alleviation was 1.7 ± 1.2 months. The WFPRSS showed significant pain alleviation at each follow-up point and remained persisted until a minimal follow-up of 36 months. The WFPRSS was decreased from 8.7 ± 1.6 at baseline to 1.6 ± 3.2 at 36 months (Figure 3A).
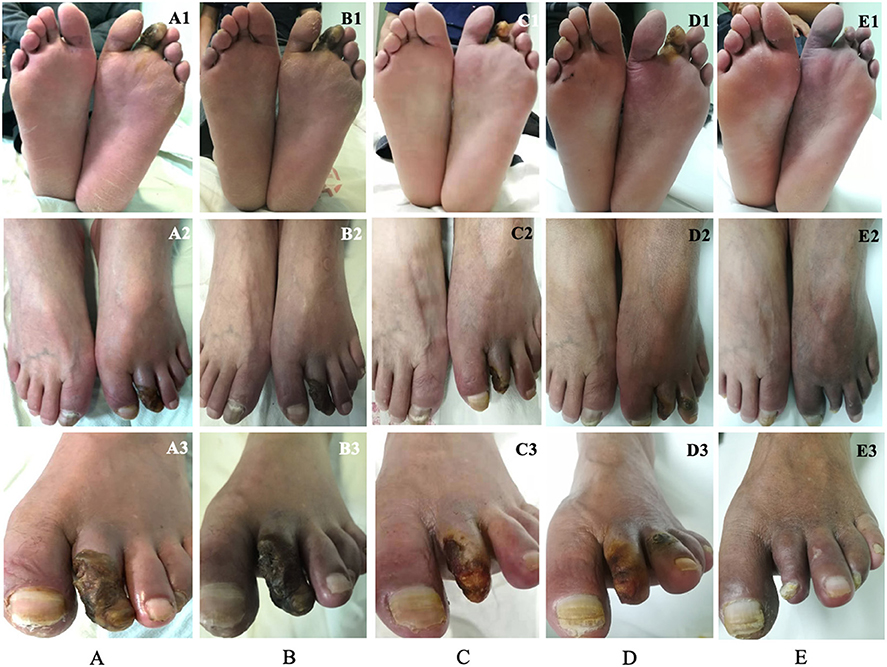
Figure 2. A 55-year-old man was diagnosed with TAO and the progress of ulcer healing during the 12-month follow-up, which was photographed in three different angles. Before transplantation of PBMNCs, this patient presented gangrene in the second toe of the left foot (A). The swelling around the gangrene and the rest pain decreased significantly at 1 month (B). The pain disappeared, the necrotic tissue, and the toenail of the toe dropped at 3 months, only a little ulcer remained (C). At 6 months, the remaining ulcer in the second toe was completely healed, and the patient returned to work. However, the ulcer appeared in the third toe (D). The toenail of the second toe grew in again and the ulcer in the third healed completely (E). TAO, thromboangiitis obliterans; PBMNCs, peripheral blood mononuclear cells.
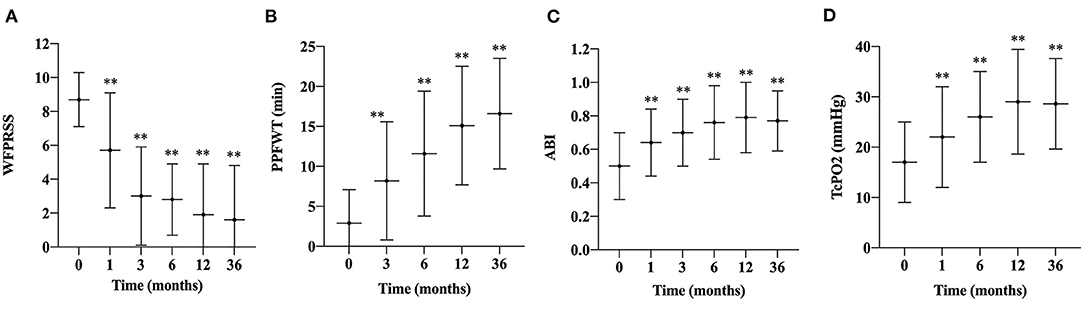
Figure 3. Changes in WFPRSS, PPFWT, ABI, and TcPO2 during the follow-up. Changes in the WFPRSS (A), PPFWT (B), ABI (C), TcPO2, (D) in patients after treatment of PBMNCs are depicted as linear graphs with mean values and the SD bars. (**, P < 0.01). WFPRSS, Wong-Baker FACES pain rating scale score; PPFWT, peak pain-free walking time; ABI, ankle-brachial index; TcPO2, transcutaneous partial oxygen pressure; SD, standard deviation.
After transplantation, the PPFWT was significantly improved at each follow-up time point compared with baseline measurement (2.9 ± 4.2 min) and remained persisted till 36 months (16.6 ± 6.9 min) (Figure 3B).
Regarding perfusion evaluating parameters, mean ABI and TcPO2 were also significantly improved from 0.5 ± 0.2 and 22.0 ± 10.0 mmHg at baseline to 0.8 ± 0.2 and 28.6 ± 9.0 mmHg at 36 months, respectively (Figures 3C,D). In addition, the Rutherford classification was also significantly decreased during the follow-up period (Figure 4).
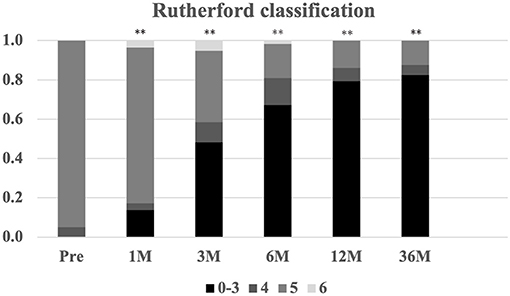
Figure 4. The change in Rutherford classification during the 12-month follow-up. Serial changes in Rutherford classification (0–6) proportions **P < 0.01 vs. baseline.
Quality of life, evaluated by SF-36v2, was significantly improved at each follow-up time (Figure 5). Before PBMNCs transplantation, all of them lost jobs or quitted working temporarily, and 36 of them restored labor competence after transplantation, and the labor competence recovery rate was 62.1% at 36 months.
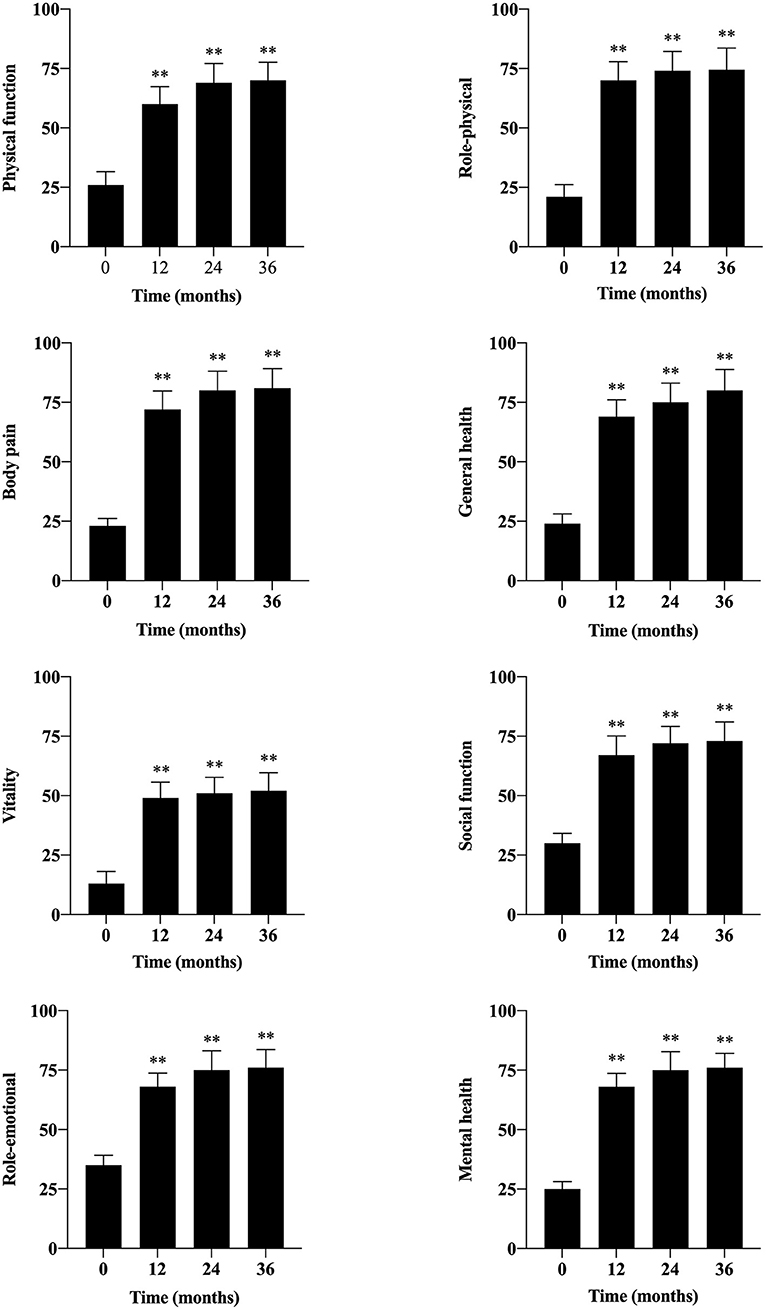
Figure 5. Evaluation of quality of life by SF-36v2 during the follow-up period. The SF-36v2 included eight aspects: physical function, role-physical, body pain, general health, vitality, social function, role-emotional, and mental health. (**, P < 0.01).
No critical adverse events occurred during the treatment and follow-up period. The counts of WBC recovered to a normal level within 1 week in all patients after transplantation. Five patients suffered from adverse events during the mobilization period, such as one patient with a slight fever, two patients with back pain, one patient with transient headache, and one patient with pruritus. All of them completely recovered within 2 weeks after transplantation. Pathological retinal angiogenesis was not observed during the follow-up period.
Angiitis-induced no-option critical limb-threatening ischemia has a wide spectrum of etiologies, such as TAO, SLE, eosinophilia, scleroderma, Crohn's disease, and Sjogren's syndrome. TAO is the most common cause accounting for more than 90% of the patients and associated with the major amputation rate of more than 31% with conventional treatment (3, 16). Meanwhile, AICLTI is characterized by a higher incidence in China than that in the West and a higher proportion among no-option patients. Among the 58 patients enrolled, before transplantation, catheter-directed thrombolysis was performed in 20 patients (34.5%), percutaneous balloon angioplasty in 18 patients (31.0%), stenting in five patients (8.6%), embolectomy in three patients (5.2%), a bypass in three patients (5.2%), and lumbar sympathectomy in two patients (3.4%). However, these mainstream treatments, aiming at ASO-induced CLTI, failed to alleviate ischemia of these patients, which also indicated that the differences between AICLTI and ASO-induced CLTI. After treatment of PBMNCs, the MAFS was 93.1% at 3 years, which was not significantly different in the subgroup without previous conventional procedures (Figure 1B). Meanwhile, the PPFWT increased from 2.9 ± 4.2 min at baseline measurement to 16.6 ± 6.9 min at 36 months, and the WFPRSS was significantly decreased from 8.7 ± 1.6 at baseline to 1.6 ± 3.2 at 36 months. In addition, QoL was significantly improved in all aspects. The results demonstrated the efficacy of PBMNCs in the treatment of AICLTI, even for patients having undergone failed traditional therapy.
Therapeutic angiogenesis was demonstrated to be a kind of innovated and promising method for no-option CLTI, and its efficacy had been confirmed by lots of studies (4, 13, 17–19). However, most of the previous studies focused on the patients with ASO or diabetes mellitus and merely included a large sample size of patients with AICLTI (5–11). The most common used methods were vascular endothelial growth factor (VEGF)-related gene transfer (20, 21), bone-marrow mononuclear cells (BMMNCs) (17, 22–25), PBMNCs (6, 26–29), or PCCs transplantation (13, 19). In the initial cell therapy, 400–500 ml bone marrow would be acquired by bone marrow puncture to get the total number of mononuclear cells in (1–3) × 109, and 100–200 ml bone marrow should be extracted even after G-CSF mobilization. Kawamoto confirmed that the number of mononuclear cells mobilized by G-CSF in peripheral blood was similar to that of bone marrow in 2009 (19). After that, plenty of studies concerning G-CSF mobilized PBMNCs have been reported. Horie et al. performed a randomized, large-scale clinical trial of G-CSF mobilized autologous PBMNC transplantation revealed that cell therapy was feasible and tolerable in patients with both mild and severe peripheral artery disease (PAD); however, this study only included nine patients with TAO followed by 12 months (10). In our previous RCT, we firstly compared the effectiveness between PBMNCs and PCCs. Although the 1- and 3-year outcomes demonstrated that PCCs were not inferior to PBMNCs at limb salvage in the treatment of AICLTI and appeared to induce earlier ischemia relief, PCCs were not more cost-effective than PBMNCs (4, 12). Furthermore, PBMNCs were more suitable for patients with two or more critically ischemic limbs, given more CD34+ cells needed, whereas PCC transplantation would lose a substantial number of CD34+ cells during the isolation. Additionally, a smaller number of CD34+ cells (/body weight) was proved to be associated with reduced overall survival in patients with lower limb ischemia (11). Avoidance of the isolation process also made PBMNCs more simple than PCCs. Meanwhile, the separated CD34– cells were also confirmed to contribute to angiogenesis synergistically via paracrine effects. It was unclear whether removal of CD34– fraction could result in beneficial or adverse influence on the efficacy of transplantation of mononuclear cell (30, 31). In this study, the median numbers of CD34+ cells were 8.5 × 105 cells/kg, which was smaller than patients who underwent transplantation of BMMNCs. We enrolled 58 patients with AICLTI, the 3-year MAFS was 93.1%, and 36 (62.1%) patients restored their labor competence, which was significantly higher than reported traditional procedures (3, 16). Based on these advantages, PBMNC was an easy and effective method to develop to treat AICLTI in clinical practice.
As the most common cause of AICLTI, TAO accounted for more than 90% of the patients (3). Moreover, the 5-year cumulative survival rate was about 97%, which was higher than ASO (16). Besides, patients with AICLTI were younger than patients with ASO and 34.8% of them would lose labor competence before 42 years (16, 32). Hence, it is of great significance and social value to find effective and durable new therapies and to avoid major amputation and loss of labor competence, especially in China where there is a relatively large population of patients with AICLTI (33–35). In the current study, 96.6% (56/58) of patients were diagnosed as TAO, and the mean age was only 41.9 ± 13.0 years. Before treatment, all of them lost jobs. Inspiringly, 36 of them restored labor competence after treatment of PBMNCs. Although the other patients were not competent to work, they could take care of themselves in daily life, which was also a great relief for their families.
Although antiplatelet, anticoagulant, statins, vasodilators, and smoking cessation are widely used in the treatment of patients with TAO, none of these treatments provided noticeable improvement (32, 36). In addition, Fazeli et al. concluded that long-term treatment with angiogenic medication may be a risk factor for dermal gangrene in patients with TAO and ultimately might be a disease management double-edged sword (37). Fang et al. found that smoking cessation does not make a big difference to the symptoms of the patients when they presented CLTI. There was no significant difference between patients with and without smoking cessation in terms of the major amputation rate, the PPFWT, the WFPRSS, the ulcer healing rate, or the recurrence rate after cell therapy (13). All the patients in this study underwent at least 1 month of smoking cessation before transplantation, and no improvement was observed. On the contrary, their ischemic symptoms were significantly alleviated, and ulcer healing was observed in 48 (87.3%) patients. The self-contrast indicated the efficacy of PBMNCs in the treatment of AICLTI. Although repetitive education, quitting smoking was not achieved in all patients. Recurrence and ischemia onset occurred in four (6.9%) and one (1.2%) patients, respectively. All of them were active smokers, indicating smoking may play a critical role in recurrence after transplantation, the difference was not significant probably due to the sample size, however. The mean time from transplantation to recurrence was 14.6 ± 8.5 months. These patients underwent second transplantation and satisfactory outcomes were yielded. Patients with AICLTI definitely benefit from quitting smoking in the long-term follow-up; however, smoking cessation alone is not adequate for the limb salvage in TAO patients with CLTI. The revascularization was mandatory to relieve ischemia, reduce amputation rate, and improve long-term QoL.
Unlike conventional procedure, although the efficacy and validity of PBMNCs in the treatment of AICLTI have been demonstrated by plenty of studies, the potential mechanism still needed to be elucidated. Asahara et al. demonstrated that endothelial-cell progenitors were present as CD34 + hematopoietic stem cells in human peripheral blood and that transplantation of CD34 + instead of CD34– cells promoted angiogenesis in animal models of lower-limb ischemia (31). Miyamoto confirmed that the transplanted endothelial progenitor cells (EPCs) gathered on the outside of the neovessels, meanwhile, the VEGF was also detected (38). The same phenomenon was also confirmed by Kawamoto et al. in an ischemic swine model (39). On the other hand, there were also many studies that reported that EPCs could directly participate in angiogenesis in ischemic limb and myocardial tissues (40, 41). The vasculogenesis induced by EPCs and the angiogenesis stimulated via the paracrine effects of transplanted cells are major contributors to perfusion improvements and limb salvage (42–45). Moreover, the purity of CD34+ cells is directly proportional to their angiogenic efficacy because a higher level of purity promotes angiogenesis. The CD34– cells may promote angiogenesis by paracrine effects, the in?ammatory reaction induced by CD34– cells was also reported, however (45, 46). The question of whether CD34-induced inflammation or promoted angiogenesis remains unclear.
There were several limitations of this study. First, this was a retrospective study and complied with its own nature. Second, although plenty of evaluation index was applied in this study just as in the previous studies (12, 13, 18, 19), there was still a lack of “gold standard” for evaluation of the efficacy of the PBMNCs. Meanwhile, ABI usually reflects the blood flow to the ankle joint, which may not reflect the therapeutic angiogenesis, and the new evaluation method, such as volumetric enhanced CT, maybe more useful. Third, there was no marker indicating the inflammation in these patients, which could forecast the disease progression and clarify the relationship between inflammation and PBMNCs. Despite all these, the current study does provide practical and useful information for the management of AICLTI by PBMNCs.
This study demonstrated that the long-term outcomes of transplantation of PBMNCs in the treatment of AICLTI were encouraging and durable. It could not only reduce the major amputation rate but also significantly restore labor competence, improve the quality of life. Meanwhile, the technical requirements of this operation are relatively low, which allows easier promotion and development in different hospitals. The conclusion needs to be further confirmed by more cases and longer follow-up.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Zhongshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XJ, HL, TP, WF, and ZD: conception and design, writing the manuscript, and overall responsibility. XJ, HL, TP, SG, YF, WF, and ZD: analysis and interpretation. XJ, ZW, GF, BC, JJ, YS, and PL: data collection. ZW, GF, BC, JJ, YS, PL, WF, and ZD: critical revision. XJ, HL, TP, SG, YF, ZW, GF, BC, JJ, YS, PL, WF, and ZD: final approval of the article. XJ, HL, TP, SG, and YF: statistical analysis. WF and ZD: obtained funding. All authors contributed to the article and approved the submitted version.
This work was funded by National Nature Science Funds (81970407), the Training Program for Outstanding Academic Leaders of the Shanghai Health and Family Planning System (2018BR40); the Project of Outstanding Academic Leaders of Shanghai Science and Technology Commission (19XD1401200).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Piazza G, Creager MA. Thromboangiitis obliterans. Circulation. (2010) 121:1858–61. doi: 10.1161/CIRCULATIONAHA.110.942383
2. Olin JW. Thromboangiitis obliterans (Buerger's disease). N Engl J Med. (2000) 343:864–9. doi: 10.1056/NEJM200009213431207
3. Kobayashi M, Sugimoto M, Komori K. Endarteritis obliterans in the pathogenesis of Buerger's disease from the pathological and immunohistochemical points of view. Circ J. (2014) 78:2819–26. doi: 10.1253/circj.CJ-14-0656
4. Dong ZH, Pan TY, Fang Y, Wei Z, Gu SY, Fang G, et al. Purified CD34+ cells versus peripheral blood mononuclear cells in the treatment of angiitis-induced no-option critical limb ischaemia: 12-Month results of a prospective randomised single-blinded non-inferiority trial. EBioMedicine. (2018) 35:46–57. doi: 10.1016/j.ebiom.2018.08.038
5. Hoshino J, Ubara Y, Hara S, Sogawa Y, Suwabe T, Higa Y, et al. Quality of life improvement and long-term effects of peripheral blood mononuclear cell transplantation for severe arteriosclerosis obliterans in diabetic patients on dialysis. Circ J. (2007) 71:1193–8. doi: 10.1253/circj.71.1193
6. Ozturk A, Kucukardali Y, Tangi F, Erikci A, Uzun G, Bashekim C, et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J Diabetes Complications. (2012) 26:29–33. doi: 10.1016/j.jdiacomp.2011.11.007
7. Nishida T, Ueno Y, Kimura T, Ogawa R, Joo K, Tominaga R. Early and long-term effects of the autologous peripheral stem cell implantation for critical limb ischemia. Ann Vasc Dis. (2011) 4:319–24. doi: 10.3400/avd.oa.11.00047
8. Huang P, Xiao Z, Li S, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factormobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabets Care. (2005) 28:2155–60. doi: 10.2337/diacare.28.9.2155
9. Ishida A, Ohya Y, Sakuda H, Ohshiro K, Higashiuesato Y, Nakaema M, et al. Autologous peripheral blood mononuclear cell implantation for patients with peripheral arterial disease improves limb ischemia. Circ J. (2005) 69:1260–5. doi: 10.1253/circj.69.1260
10. Horie T, Yamazaki S, Hanada S, Kobayashi S, Tsukamoto T, Haruna T, et al. Outcome from a randomized controlled clinical trial- improvement of peripheral arterial disease by granulocyte colony-stimulating factor-mobilized autologous peripheral-blood-mononuclear cell transplantation (IMPACT). Circ J. (2018) 82:2165–74. doi: 10.1253/circj.CJ-17-1220
11. Horie T, Onodera R, Akamastu M, Ichikawa Y, Hoshino J, Kaneko E, et al. Long-term clinical outcomes for patients with lower limb ischemia implanted with G-CSF-mobilized autologous peripheral blood mononuclear cells. Atherosclerosis. (2010) 208:461–6. doi: 10.1016/j.atherosclerosis.2009.07.050
12. Liu H, Pan T, Fang Y, Fang G, Liu Y, Jiang X, et al. Three-year outcomes of peripheral blood mononuclear cells vs purified CD34+ cells in the treatment of angiitis-induced no-option critical limb ischemia and a cost-effectiveness assessment: A randomized single-blinded noninferiority trial. Stem Cells Transl Med. (2021) 10:647–59. doi: 10.1002/sctm.20-0033
13. Fang Y, Wei Z, Chen B, Pan T, Gu S, Liu P, et al. A five-year study of the efficacy of purified CD34+ cell therapy for angiitis-induced no-option critical limb ischemia. Stem Cells Transl Med. (2018) 7:583–90. doi: 10.1002/sctm.17-0252
14. Dong ZH, Chen B, Fu WG, Wang YQ, Guo DQ, Wei Z, et al. Transplantation of purified CD34 cells in the treatment of critical limb ischemia. J Vasc Surg. (2013) 58:404–11. doi: 10.1016/j.jvs.2013.01.037
15. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
16. Ohta T, Ishioashi H, Hosaka M, Sugimoto I. Clinical and social consequences of Buerger disease. J Vasc Surg. (2004) 39:176–80. doi: 10.1016/j.jvs.2003.08.006
17. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. (2002) 360:427–35. doi: 10.1016/S0140-6736(02)09670-8
18. Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. (2012) 5:821–30. doi: 10.1161/CIRCINTERVENTIONS.112.968321
19. Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. (2009) 27:2857–64. doi: 10.1002/stem.207
20. Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther. (2002) 6:127–33. doi: 10.1006/mthe.2002.0638
21. Mohler ER, Rajagopalan S, Olin JW, Trachtenberg JD, Rasmussen H, Pak R, et al. Adenoviral-mediated gene transfer of vascular endothelial growth factor in critical limb ischemia: safety results from a phase I trial. Vasc Med. (2003) 8:9–13. doi: 10.1191/1358863x03vm460oa
22. Idei N, Soga J, Hata T, Fujii Y, Fujimura N, Mikami S, et al. Autologous bone-marrow mononuclear cell implantation reduces long-term major amputation risk in patients with critical limb ischemia: a comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circ Cardiovasc Interv. (2011) 4:15–25. doi: 10.1161/CIRCINTERVENTIONS.110.955724
23. Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (therapeutic angiogenesis by cell transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. (2008) 156:1010–8. doi: 10.1016/j.ahj.2008.06.025
24. Miyamoto K, Nishigami K, Nagaya N, Akutsu K, Chiku M, Kamei M, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. (2006) 114:2679–84. doi: 10.1161/CIRCULATIONAHA.106.644203
25. Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo controlled pilot trial (PROVASA). Circ Cardiovasc Interv. (2011) 4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348
26. Kawamura A, Horie T, Tsuda I, Abe Y, Yamada M, Egawa H, et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J Artif Organs. (2006) 9:226–33. doi: 10.1007/s10047-006-0351-2
27. Kawamura A, Horie T, Tsuda I, Ikeda A, Egawa H, Imamura E, et al. Prevention of limb amputation in patients with limbs ulcers by autologous peripheral blood mononuclear cell implantation. Ther Apher Dial. (2005) 9:59–63. doi: 10.1111/j.1774-9987.2005.00218.x
28. Moriya J, Minamino T, Tateno K, Shimizu N, Kuwabara Y, Sato Y, et al. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv. (2009) 2:245–54. doi: 10.1161/CIRCINTERVENTIONS.108.799361
29. Kinoshita M, Fujita Y, Katayama M, Baba R, Shibakawa M, Yoshikawa K, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. (2012) 224:440–5. doi: 10.1016/j.atherosclerosis.2012.07.031
30. Kwon SM, Lee JH, Lee SH., ung SY, Kim DY, Kang SH, et al. Cross talk with hematopoietic cells regulates the endothelial progenitor cell differentiation of CD34 positive cells. PLoS ONE. (2014) 9:e106310. doi: 10.1371/journal.pone.0106310
31. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. (1997) 275:964–7. doi: 10.1126/science.275.5302.964
32. Fazeli B, Rezaee SA, A. review on thromboangiitis obliterans pathophysiology: thrombosis and angiitis, which is to blame? Vascular. (2011) 19:141–53. doi: 10.1258/vasc.2010.ra0045
33. Modaghegh MS, Hafezi S. Endovascular treatment of thromboangiitis obliterans (Buerger's Disease). Vasc Endovascular Surg. (2018) 52:124–30. doi: 10.1177/1538574417744085
34. Stvrtinova V, Mareschova K, Hasakova J. Thromboangiitis obliterans-what do we know 110 years after the description of the disease by Leo Buerger. Bratisl Lek Listy. (2018) 119:670. doi: 10.4149/BLL_2018_120
35. Olin JW. Thromboangiitis obliterans: 110 years old and little progress made. J Am Heart Assoc. (2018) 7:e011214. doi: 10.1161/JAHA.118.011214
36. Le Joncour A, Soudet S, Dupont A, Espitia O, Koskas F, Cluzel P, et al. Long-term outcome and prognostic factors of complications in thromboangiitis obliterans (buerger's disease): a multicenter study of 224 patients. J Am Heart Assoc. (2018) 7:e010677. doi: 10.1161/JAHA.118.010677
37. Fazeli B, Keramat S, Assadi L, Taheri H. Angiogenesis induction in Buerger's disease: a disease management double-edged sword? Orphanet J Rare Dis. (2019) 14:189. doi: 10.1186/s13023-019-1166-6
38. Miyamoto Y, Suyama T, Yashita T, Akimaru H, Kurata H. Bone marrow subpopulations contain distinct types of endothelial progenitor cells and angiogenic cytokine-producing cells. J Mol Cell Cardiol. (2007) 43:627–35. doi: 10.1016/j.yjmcc.2007.08.001
39. Kawamoto A, Asahara T, Losordo DW. Transplantation of endothelial progenitor cells for therapeutic neovascularization. Cardiovasc Radiat Med. (2002) 3:221–5. doi: 10.1016/S1522-1865(03)00082-9
40. Bauer SM, Goldstein LJ, Bauer RJ, Chen H, Putt M, Velazquez OC. The bone marrow-derived endothelial progenitor cell response is impaired in delayed wound healing from ischemia. J Vasc Surg. (2006) 43:134–41. doi: 10.1016/j.jvs.2005.08.038
41. Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. (2006) 113:1605–14. doi: 10.1161/CIRCULATIONAHA.105.553925
42. Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. (2011) 109:724–28. doi: 10.1161/CIRCRESAHA.111.253286
43. Fujita Y, Kinoshita M, Furukawa Y, Nagano T, Hashimoto H, Hirami Y, et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J. (2014) 78:490–501. doi: 10.1253/circj.CJ-13-0864
44. You D, Waeckel L, Ebrahimian TG, Blanc-Brude O, Foubert P, Barateau V, et al. Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation. (2006) 114:328–38. doi: 10.1161/CIRCULATIONAHA.105.589937
45. Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, et al. Synergistic effect of combined intramyocardial CD34 + cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. (2006) 3:S123–8. doi: 10.1038/ncpcardio0430
Keywords: adult stem cells, angiogenesis, autologous stem cell transplantation, CD34+, cell transplantation
Citation: Jiang X, Liu H, Pan T, Gu S, Fang Y, Wei Z, Fang G, Chen B, Jiang J, Shi Y, Liu P, Fu W and Dong Z (2021) Long-Term Outcomes of Peripheral Blood Mononuclear Cells in the Treatment of Angiitis-Induced No-Option Critical Limb-Threatening Ischemia. Front. Cardiovasc. Med. 8:769472. doi: 10.3389/fcvm.2021.769472
Received: 02 September 2021; Accepted: 05 November 2021;
Published: 06 December 2021.
Edited by:
Junxi Wu, University of Strathclyde, United KingdomReviewed by:
Kenji Yanishi, Kyoto Prefectural University of Medicine, JapanCopyright © 2021 Jiang, Liu, Pan, Gu, Fang, Wei, Fang, Chen, Jiang, Shi, Liu, Fu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Dong, ZHpoOTI2QDEyNi5jb20=; Weiguo Fu, ZnUud2VpZ3VvQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.