- 1Department of Epidemiology, School of Public Health, Southern Medical University, Guangzhou, China
- 2Department of Nutritional Sciences, Pennsylvania State University, University Park, PA, United States
- 3Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 4Division of Rheumatology, Department of Medicine, Duke University School of Medicine, Duke Molecular Physiology Institute, Durham, NC, United States
- 5Division of Laboratory Medicine, Microbiome Medicine Center, Zhujiang Hospital, Southern Medical University, Guangzhou, China
Background: Hypertension is a leading contributor to the global burden of disease and to mortality. The combined effects of sleep factors on the risk of hypertension are unclear. We aimed to evaluate the effect of combined sleep factors on the risk of hypertension and to explore whether this association is independent of genetic risk.
Methods: This population-based prospective cohort study included 170,378 participants from the UK Biobank study. We conducted a healthy sleep score based on a combination of major five sleep factors and a genetic risk score based on 118 risk variants. Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: A total of 170,378 participants were included. Compared to participants with a healthy sleep score of 0–1, those with healthy sleep scores of 2 (HR, 0.90; 95% CI, 0.83–0.98), 3 (HR, 0.81; 95% CI, 0.75–0.88), 4 (HR, 0.74; 95% CI, 0.68–0.81), or 5 (HR, 0.67; 95% CI, 0.59–0.77) had increasingly lower risks of hypertension (P for trend <0.001). Participants with high genetic risk and an unfavorable sleep pattern had a 1.80-fold greater risk of hypertension than participants with low genetic risk and a favorable sleep pattern. The association between sleep patterns and hypertension persisted in subgroup analysis, stratified by the genetic risk. Nearly 18.2% of hypertension events in this cohort could be attributed to unfavorable sleep pattern.
Conclusions: Favorable sleep pattern was associated with a low risk of hypertension, regardless of genetic risk. These findings highlight the potential of sleep interventions to reduce risk of hypertension across entire populations.
Introduction
Hypertension is a leading contributor to the global burden of disease and to mortality (1). It is a complex disease driven by both environmental and genetic factors (2–6). Sleep factor is an important modifiable risk factor for hypertension (7, 8). A rich body of evidence shows that healthy sleep factors, including no excessive daytime sleepiness (9), adequate sleep duration (10, 11), no insomnia (12, 13), no snoring (14, 15), and early chronotype (16), were associated with low risk of hypertension. Although those sleep factors have been independently correlated to increased risk of hypertension, a combination of sleep factors may have synergistic effects as these sleep factors are highly associated with each other (17–20). Bathgate et al. (21) have assessed sleep factors jointly and indicated the highest risk of hypertension in association with a joint effect of short sleep duration (<6 h) with insomnia. Therefore, a composite variable may better aid investigation of how sleep factors act synergistically to affect hypertension risk. However, few studies have investigated the combined impact of sleep factors, let alone all the aforementioned sleep factors jointly, on hypertension risk.
Early evidence supporting a role for genetics in the risk of hypertension came from twin studies and the Framingham Study (5, 22–24). Further evidence has emerged from genome-wide association studies (GWASs), which have identified genetic variants associated with the risk of hypertension (2, 25). These risk alleles, when aggregated into a polygenic risk score, are predictive of incident hypertension and provide a quantitative measure of the genetic risk for hypertension. It might be hypothesized that adhering to a healthy sleep pattern could attenuate the effect of genetics on the risk of hypertension. A previous study on cerebrovascular disease, a condition closely related to hypertension, found a statistically significant role of the interplay between sleep factors and genetics in the risk of cerebrovascular disease (17). However, whether a healthy sleep pattern, which integrates several modifiable sleep factors, can modify the effect of genetic predisposition on hypertension remains less certain; moreover, no study to date has investigated the risk of hypertension with regard to sleep patterns and genetics.
Therefore, in a large population-based cohort study, we prospectively investigated the association of a healthy sleep score based on a combination of major sleep factors with the risk of incident hypertension. We further explored whether the association between sleep pattern and the risk of incident hypertension was independent of genetic risk.
Methods
Study Population
The UK Biobank study is a large, population-based prospective cohort study that recruited >500,000 individuals aged between 40 and 70 years from across the UK (Scotland, England, and Wales) between 2006 and 2010. The population and design of the UK Biobank study have been described in detail in previous reports (26–29). The study collected extensive data, including demographic, health, and lifestyle (e.g., sleep factors) data, from questionnaires, interviews, physical measurements, and health records. Blood samples were also obtained and used for genotyping (30). Approval for this research was obtained from the North West Multicenter Research Ethics Committee (11/NW/0382), and all participants provided informed consent.
In this study, after excluding participants with hypertension or cardiovascular diseases at baseline, those with missing data for any of the five sleep factors, or those without genetic data, 170,378 participants were finally included in the present analyses. A flowchart depicting the selection of the study participants is presented in Supplementary Figure 1.
Measure
Healthy Sleep Score and Sleep Pattern
The UK Biobank participants completed a self-reported touchscreen questionnaire on their usual sleep factors. To investigate the association between the combination of sleep factors and hypertension, we constructed a healthy sleep score based on the recommendations regarding five potentially modifiable sleep factors, including daytime sleepiness, sleep duration, insomnia, snoring, and chronotype, on the basis of previous studies (13, 17, 18). The methods of assessment of the sleep factors is described in Appendix. We dichotomized each sleep factor based on previous knowledge (17). Participants were assigned one point for each of five low-risk sleep factors defined as follows: sleep 7–9 h per day, no insomnia (“never/rarely” having insomnia symptoms), no snoring, early chronotype (“morning” or “morning more than evening” person), and no frequent daytime sleepiness (“never/rarely” or “sometimes”). The points for the five sleep factors were summed to obtain the healthy sleep score, which ranged from 0 (least healthy) to 5 (most healthy), and was subsequently categorized as a favorable (score of 4 or 5), intermediate (score of 2 or 3), or unfavorable (score of 0 or 1) sleep pattern, as described previously (17).
A weighted standardized healthy sleep score was then derived based on the five sleep factors with the following equation: weighted sleep score = (β1×sleep factor1 + β2×sleep factor2 +…+ β5×sleep factor5) × (5/sum of the β coefficients). This weighted sleep score also ranged from 0 to 5 points but took into account the magnitudes of the adjusted risk for each behavior in each sleep pattern and in the combination of the five sleep factors (17).
Genotyping and Polygenic Risk Score
The genotyping process in the UK Biobank study has been reported in detail elsewhere (30). The polygenic risk score for hypertension was based on a recent GWAS of individuals of European ancestry (31). Therefore, the study considered only individuals who self-reported as British or other white backgrounds. We excluded SNPs that were missing from the UK Biobank study. Independent SNPs were selected based on the P-value by using the linkage disequilibrium (LD) clumping procedure (at R2 < 0.01) conducted in PLINK version 2.0 (https://www.cog-genomics.org/plink2). The polygenic risk score was calculated across all selected SNPs associated with hypertension totaling 118 (Supplementary Table 1) (31). For each individual in the UK Biobank sample, we calculated polygenic risk scores, defined as the sum of the number of risk alleles (0, 1, or 2) present at each locus weighted by the natural logarithm of the estimated odds ratio for that locus. Polygenic risk scores were then z-standardized based on values for all individuals and categorized into low (lowest quintile), intermediate (quintiles 2–4), or high (highest quintile) risk (32).
Incident Hypertension
Data on incident hypertension in the UK Biobank were based on medical history and linked to data on hospital admissions and mortality. The linkage procedure can be found on the website (http://content.digital.nhs.uk/services) in detail. We defined participants with hypertension according to the International Classification of Diseases edition 10 (ICD-10): I10 for hypertension.
Covariates
The covariates included in the present study were as follows: age, sex (male or female), education (degree [college/university degree] or no degree), Townsend deprivation index (TDI) (33), race (white or other), physical activity, smoking status (current, previous, or never), alcohol consumption (current, previous, or never), body mass index [BMI: was calculated by dividing an individual's weight (kg) by the square of height in meter (m)], family history of hypertension, and medical history (physician diagnosis of diabetes, depression, and cancer), obtained from the self-completed baseline questionnaire. Details of these measurements can be found on the website of the UK Biobank (www.ukbiobank.ac.uk).
Statistical Analysis
We imputed the missing covariate values (all covariates had <3% of values missing) through multiple imputation by chained equations (34). The mean and standard deviation (SD) (continuous variables) or number and percentage (categorical variables) were used to describe the participants' baseline characteristics.
We used Cox proportional hazard regression models to test the association of sleep factors with the incident hypertension risk. The duration of follow-up was calculated as the time between the date of attendance and the date of first diagnosis, date of death, or February 28, 2017, for Scotland, and February 25, 2018, for Wales and England, whichever occurred first. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated. The multivariable-adjusted models were adjusted for age, sex, education, TDI, race, physical activity, smoking status, alcohol consumption, BMI, and family history of hypertension, depression, cancer, and diabetes. The proportionality of hazards assumption was assessed using the Schoenfeld residuals and was satisfied (35). We included an interaction term in the regression model to test for the statistical interaction between the sleep pattern and genetic risk categories. In addition, adjusted population attributable fractions (PAFs) and 95% CIs were calculated to estimate the proportion of hypertension cases that theoretically would not have occurred if all participants had healthy sleep factor.
We performed subgroup analyses stratified by age (<60 or ≥60 years), sex (male or female), current smoking status (yes or no), current alcohol consumption status (yes or no), physical activity (inactive [ <400 MET-h/week] or active[≥400 MET-h/week]), BMI (non-obese [ <30 kg/m2] or obese [≥30 kg/m2]), and family history of hypertension (yes or no). In addition, we conducted several sensitivity analyses. To minimize the influence of reverse causation, we performed a sensitivity analysis by excluding participants who experienced hypertension events within the first 2 years of follow-up. Moreover, the risk of incident hypertension was investigated in sensitivity analyses using non-imputed data. All analyses were performed using R software, version 4.0.0 (R Development Core Team, Vienna, Austria). P-values were two-sided, with statistical significance set at <0.05.
Results
Baseline Characteristics
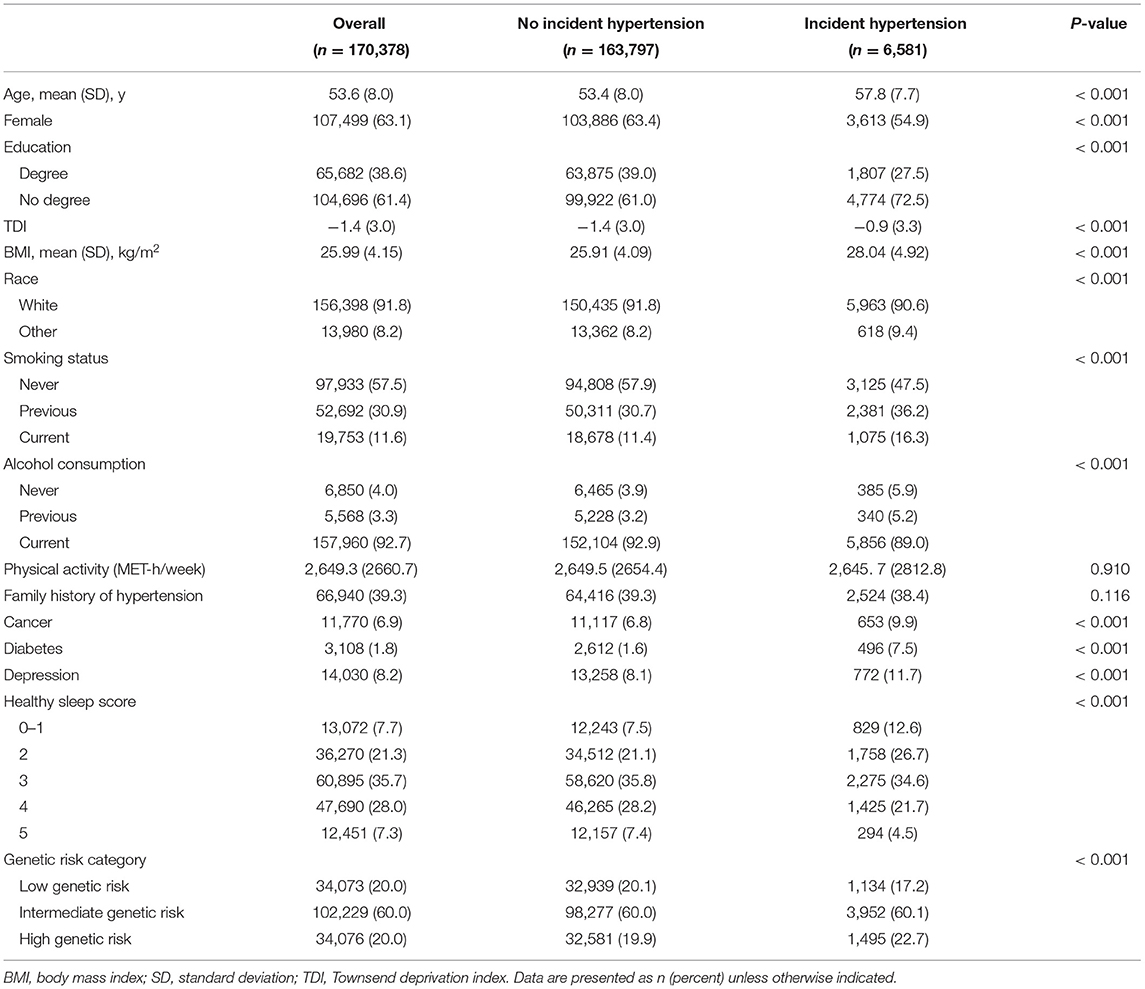
This analysis included 170,378 (mean [SD] age: 53.6 [8.0] years) participants, of whom 107,499 (63.1%) were female and 156,398 (91.8%) were white (Table 2). Participants with hypertension were more likely to be older, to have a lower level of education, to have a higher BMI, to smoke, to have higher prevalence rates of cancer, diabetes, and depression, and to have a lower healthy sleep score compared with those without incident hypertension (Table 1).
Associations of Sleep Factors With Incident Hypertension
During a median (interquartile range) follow-up of 9.0 (8.3–9.7) years, 6,581 incident hypertension cases were recorded. In the age- and sex-adjusted model, evening chronotype, long (≥9 h) or short (<7 h) sleep duration, insomnia, snoring, and often/always daytime sleep were significantly associated with an increased incident hypertension risk (Supplementary Table 2). In the multivariable-adjusted model, these associations remained statistically significant except for evening chronotype (Supplementary Table 2).
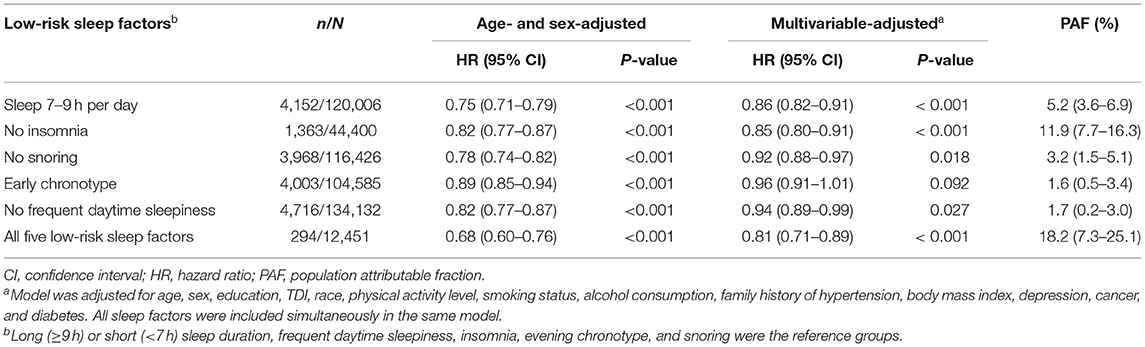
When these five sleep factors were reclassified as either a high (reference) or a low risk, sleep 7–9 h per day (HR, 0.86; 95% CI, 0.82–0.91), no insomnia (HR, 0.85; 95% CI, 0.80–0.91), no snoring (HR, 0.92; 95% CI, 0.88–0.97), and no frequent daytime sleepiness (HR, 0.94; 95% CI, 0.89–0.99) were independently associated with a decreased incident hypertension risk in the multivariable-adjusted model (Table 2).
We also calculated the PAF for each sleep factor separately and the combination of the five sleep factors (Table 2). The estimated PAFs attributable to pre-existing the low-risk sleep factors ranged from 1.6% (for chronotype) to 11.9% (for insomnia). For participants who were adherent to all five of the low-risk sleep factors, the PAF was 18.2% (95% CI: 7.3–25.1%), suggesting that 18.2% of hypertension cases in this cohort would not have occurred if all participants had been in the low-risk group for all five sleep factors.
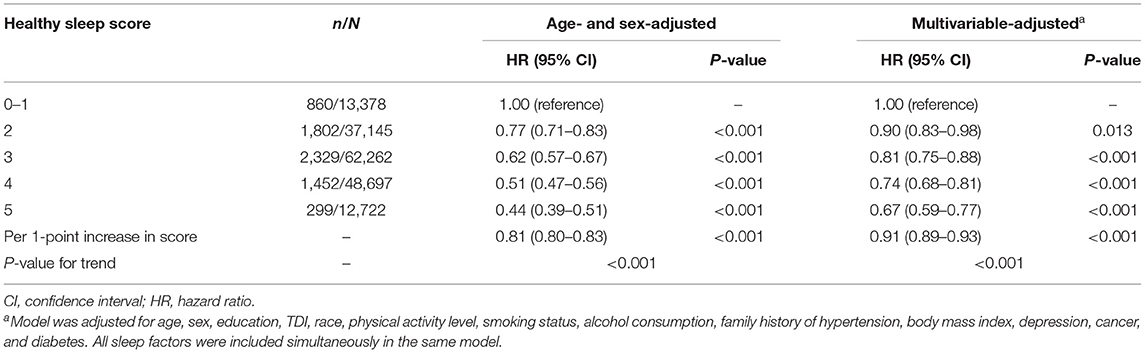
When these five sleep factors were considered jointly by using the healthy sleep score, the risk of hypertension decreased significantly with an increasing healthy sleep score (Table 3; P for trend <0.001). In the multivariable-adjusted model, compared to participants with a healthy sleep score of 0–1, participants with a healthy sleep score of 2 (HR, 0.90; 95% CI, 0.83–0.98), 3 (HR, 0.81; 95% CI, 0.75–0.88), 4 (HR, 0.74; 95% CI, 0.68–0.81), or 5 (HR, 0.67; 95% CI, 0.59–0.77) had increasingly lower risks of hypertension. Each additional healthy sleep factor (per 1-point increase in score) was associated with a 9% (HR, 0.91; 95% CI, 0.89–0.93) lower risk of hypertension (Table 3). The association per 1-point higher score was similar in subgroups that were classified by sex, current smoking status, alcohol consumption status, total physical activity, BMI, or family history of hypertension (all P for interaction >0.05) (Supplementary Table 3). In the sensitivity analyses, the results did not markedly change after using non-imputed data (Supplementary Table 4), or excluding participants who experienced hypertension events within the first 2 years of follow-up (Supplementary Table 5). In addition, the results were not materially different for the weighted healthy sleep score (Supplementary Table 6).
Joint Association of Sleep Pattern and Genetic Risk With Incident Hypertension
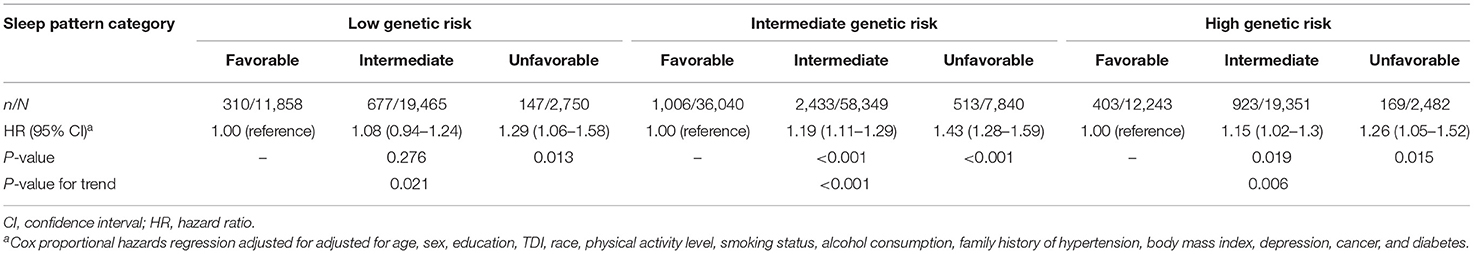
Supplementary Figure 2 shows the cumulative incidence of hypertension according to sleep pattern and genetic risk. We further assessed the joint association of the healthy sleep score and polygenic risk score with the risk of hypertension. We found that participants with an unfavorable sleep pattern and high genetic risk had the highest risk of hypertension, even though there was no statistically significant interaction between the healthy sleep score and genetic susceptibility to hypertension (P for interaction = 0.693) (Figure 1). Participants with an unfavorable sleep pattern and high genetic risk had a 1.80-fold greater risk of hypertension (HR, 1.80; 95% CI, 1.49–2.17) than participants with a favorable sleep pattern and low genetic risk. The results were not materially different for the weighted healthy sleep score (Supplementary Table 7). Moreover, within each genetic risk stratum there was an increase in the strength of the association with a decreasing number of favorable sleep factors (P-value for trend <0.05) (Table 4). In the low genetic risk group, an unfavorable sleep pattern was associated with an increased risk of hypertension (HR, 1.29; 95% CI, 1.06–1.58); the association of hypertension risk with sleep pattern was similar in both the intermediate and high genetic risk groups.
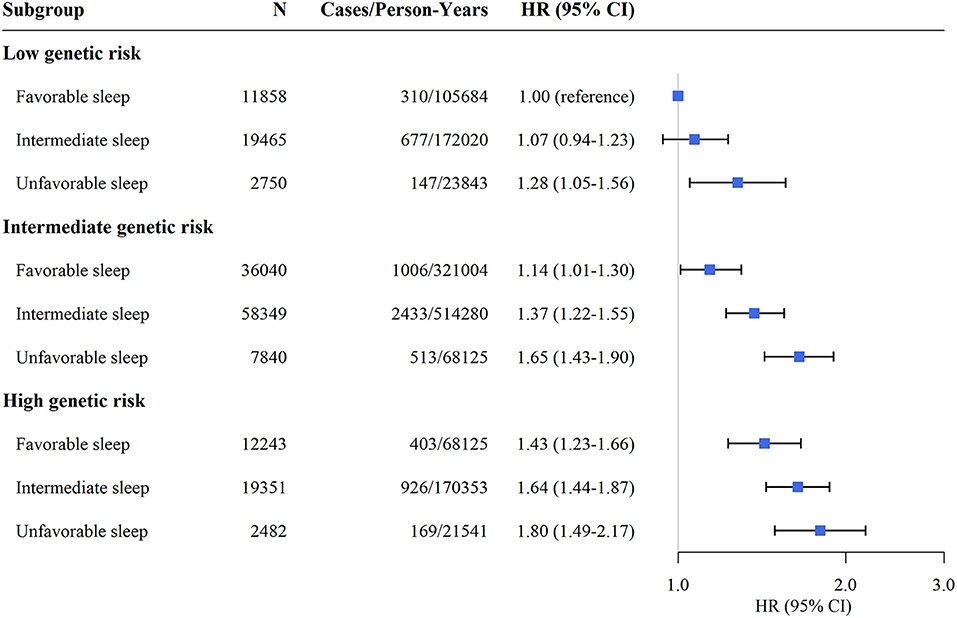
Figure 1. Risk of incident hypertension according to sleep pattern and genetic risk. CI, confidence interval; HR, hazard ratio. The results were obtained after adjusting for age, sex, education, TDI, race, physical activity level, smoking status, alcohol consumption, family history of hypertension, body mass index, depression, cancer, and diabetes.
Discussion
Using data from a large, population-based cohort study, we found that a healthy sleep score based on five potentially modifiable sleep factors, including daytime sleepiness, sleep duration, insomnia, snoring, and chronotype, was inversely associated with the future hypertension risk. Interestingly, this inverse association persisted for all subgroup analyses, including after stratification by genetic risk. Approximately 18.2% of hypertension cases could potentially be prevented if all participants had all five healthy sleep factors. These results provide evidence for the importance of healthy sleep in preventing hypertension and reinforce the tremendous potential of primary prevention.
Existing evidence, together with our result of single sleep factors revealed that healthy sleep factors, such as an early chronotype, no frequent daytime sleepiness, 7–9 h per day sleep duration, no insomnia, and no snoring were independently associated with a decreased incident hypertension risk (9, 13, 14, 36, 37). In our study, out of the five sleep factors, insomnia was component of the healthy sleep score that showed substantially the highest PAF for hypertension. It is reasonable that insomnia is more modifiable and precisely targetable through behavioral therapies (38). Therefore, future clinical trials or community-based intervention studies should be conducted to test whether sleep interventions for insomnia can reduce subsequent incident hypertension risk.
It is important to evaluate the combination of these sleep factors because they are often interconnected. In agreement with our findings, previous studies evaluating other combinations of sleep factors indicated that the combination of insomnia and short sleep duration was strongly associated with the risk of incident hypertension (13, 21). However, despite this, when examining the different combinations of sleep factors, none of the combinations were as protective as the combination of all five sleep factors. More than 18% of hypertension cases could be prevented though modification of the combined five sleep factors. Our study, the largest to date, considered the joint effect of five major sleep factors on the risk of hypertension by constructing a healthy sleep score that reflects a more comprehensive sleep pattern. The reduction in the risk of hypertension was associated with healthy sleep factors in the present study, which highlights the importance of considering sleep factors in the management of blood pressure. The healthy sleep score defined in the present study provides a significant reference for sleep management and the identification of high-risk populations.
The potential mechanism underlying the association between combined sleep factors and the risk of hypertension is not well-understood. However, these sleep factors may individually act through several mechanisms that could operate synergistically to affect the risk of hypertension. For instance, a shortened sleep duration, insomnia symptoms, or excessive daytime sleepiness possibly relates to pathways influencing sympathetic nervous system activity, which lead to blood vessel constriction, increasing blood pressure (39–41). Habitual snoring is thought to be closely related to sleep apnea; repeated apneic episodes cause oxidative stress, accelerate atherosclerosis in the coronary and intracranial arteries, and activate hemodynamics, elevating sympathetic activity and pulmonary artery pressure (42, 43). The circadian shift toward evening causes a longer-term circadian misalignment, which strengthens the association with arterial hypertension (44).
To our knowledge, the present study is the first prospective cohort study to investigate the joint association of sleep pattern and genetic risk with incident hypertension risk. We found that no statistically significant interaction between sleep pattern and genetic risk with regard to hypertension, which suggests that the effect of sleep pattern on the risk of hypertension might be independent of the genetic risk. Interestingly, we found a strong positive association between an unfavorable sleep pattern and the risk of hypertension irrespective of the prevalent genetic risk variants. Although a genetic risk for hypertension among participants from the UK Biobank has been reported previously (31), our results provide evidence that healthy sleep factors may be associated with low risk regardless of the individuals' genetic risk profile. Therefore, our study highlights the fact that adhering to a favorable sleep pattern may be greatly beneficial in the primary prevention of hypertension among the entire population. These results should be used to strengthen the importance of modifiable risk factors in the management of hypertension, as well as to convince individuals of the importance of following healthy sleep recommendations.
Strengths and Limitations
To our knowledge, this is the first prospective cohort study to investigate the associations of five joint sleep factors with risk of incident hypertension. Another strength of this study was the large sample size, which enabled a detailed investigation of the combination of sleep factors and genetic risk. Other strengths include the comprehensive collection of sociodemographic status, medical data, and lifestyle information by self-report, which enabled us to incorporate the most prevalent lifestyle factors convincingly linked to hypertension. However, the present study also has several potential limitations. First, this is an observational study, and the associations between sleep pattern and the risk of hypertension cannot be interpreted as causal. Second, this analysis focused on only five sleep factors. Expanding the range of sleep factors (i.e., rapid eye movement sleep factor and restless legs syndrome) would be of interest in future studies. Third, although a series of confounding factors were adjusted for in the analyses, the possibility of unmeasured or unknown confounding factors may remain. Fourth, the incidence of hypertension might be underestimated by potential underdiagnosed hypertension (45). Finally, the data regarding the sleep factors in the UK Biobank were self-reported, which may have led to some misclassification.
Conclusion
In conclusion, healthy sleep score combining daytime sleepiness, sleep duration, insomnia, snoring, and chronotype are predictive of incident hypertension. Meanwhile, unfavorable sleep pattern was associated with a higher risk of incident hypertension, regardless of genetic risk. These findings could have important implications for understanding the mechanisms underlying hypertension and provide future opportunities for early intervention.
Data Availability Statement
Data are available in a public, open access repository. The UK Biobank data are available from the UK Biobank on request (www.ukbiobank.ac.uk/).
Ethics Statement
The studies involving human participants were reviewed and approved by North West Multicenter Research Ethics Committee (11/NW/0382). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
CM, Z-HL, and Q-MH designed the research and developed the analytical plan. CM directed the study. Z-HL and Q-MH performed the statistical analyses and had primary responsibility for writing the manuscript. P-DZ, DL, DS, X-RZ, W-FZ, QC, P-LC, and W-QS contributed to data cleaning. CM, VB, XG, X-BW, and VC contributed to the analysis or interpretation of the data. All authors critically reviewed the manuscript for important intellectual content.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 81973109), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (Grant Number: 2019), the Construction of High-level University of Guangdong (Grant Numbers: G820332010, G618339167, G618339164, and G621339832), and the National Institutes of Health/National Institute on Aging (NIH/NIA) (Grant Number: P30AG028716). The funders played no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 43795.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.769130/full#supplementary-material
References
1. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. (2015) 386:801–12. doi: 10.1016/S0140-6736(14)61468-9
2. Warren HR, Evangelou E. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. (2017) 49:403–15. doi: 10.1038/ng.3768
3. Chan Q, Stamler J, Griep LM, Daviglus ML, Horn LV, Elliott P. An update on nutrients and blood pressure. J Atheroscler Thromb. (2016) 23:276–89. doi: 10.5551/jat.30000
4. Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. (2008) 118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410
5. Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, et al. Heritability and risks associated with early onset hypertension: multigenerational, prospective analysis in the Framingham Heart Study. BMJ. (2017) 357:j1949. doi: 10.1136/bmj.j1949
6. Havlik RJ, Feinleib M. Epidemiology and genetics of hypertension. Hypertension. (1982) 4:III121–7. doi: 10.1161/01.HYP.4.5_Pt_2.III121
7. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2019). Hypertens Res. (2019) 42:1235–481. doi: 10.1038/s41440-019-0284-9
8. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
9. Goldstein IB, Ancoli-Israel S, Shapiro D. Relationship between daytime sleepiness and blood pressure in healthy older adults. Am J Hypertens. (2004) 17:787–92. doi: 10.1016/j.amjhyper.2004.05.009
10. Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. Am J Hypertens. (2012) 25:335–41. doi: 10.1038/ajh.2011.201
11. Wang Y, Mei H, Jiang YR, Sun WQ, Song YJ, Liu SJ, et al. Relationship between duration of sleep and hypertension in adults: a meta-Analysis. J Clin Sleep Med. (2015) 11:1047–56. doi: 10.5664/jcsm.5024
12. Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. (2017) 152:435–44. doi: 10.1016/j.chest.2017.01.026
13. Jarrin DC, Alvaro PK, Bouchard MA, Jarrin SD, Drake CL, Morin CM. Insomnia and hypertension: a systematic review. Sleep Med Rev. (2018) 41:3–38. doi: 10.1016/j.smrv.2018.02.003
14. Furukawa T, Nakano H, Yoshihara K, Sudo N. The relationship between snoring sound intensity and morning blood pressure in workers. J Clin Sleep Med. (2016) 12:1601–6. doi: 10.5664/jcsm.6340
15. Kim J, Yi H, Shin KR, Kim JH, Jung KH, Shin C. Snoring as an independent risk factor for hypertension in the nonobese population: the Korean Health and Genome Study. Am J Hypertens. (2007) 20:819–24. doi: 10.1016/j.amjhyper.2007.03.007
16. Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. (2013) 30:470–7. doi: 10.3109/07420528.2012.741171
17. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. (2020) 41:1182–9. doi: 10.1093/eurheartj/ehz849
18. Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. (2019) 16:213–24. doi: 10.1038/s41569-018-0109-6
19. Sivertsen B, Pallesen S, Glozier N, Bjorvatn B, Salo P, Tell GS, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. (2014) 14:720. doi: 10.1186/1471-2458-14-720
20. Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. (2010) 33:1159–64. doi: 10.1093/sleep/33.9.1159
21. Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. (2016) 39:1037–45. doi: 10.5665/sleep.5748
22. Berg K. Twin studies of coronary heart disease and its risk factors. Acta Genet Med Gemellol. (1987) 36:439–53. doi: 10.1017/S0001566000006814
23. Feinleib M, Garrison RJ, Fabsitz R, Christian JC, Hrubec Z, Borhani NO, et al. The NHLBI twin study of cardiovascular disease risk factors: methodology and summary of results. Am J Epidemiol. (1977) 106:284–5. doi: 10.1093/oxfordjournals.aje.a112464
24. Havlik RJ, Garrison RJ, Feinleib M, Kannel WB, Castelli WP, McNamara PM. Blood pressure aggregation in families. Am J Epidemiol. (1979) 110:304–12. doi: 10.1093/oxfordjournals.aje.a112815
25. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. (2011) 478:103–9. doi: 10.1038/nature10405
26. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
27. Palmer LJ. UK Biobank: bank on it. Lancet. (2007) 369:1980–2. doi: 10.1016/S0140-6736(07)60924-6
28. Li ZH, Gao X, Chung VC, Zhong WF, Fu Q, Lv YB, et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Ann Rheum Dis. (2020) 79:829–36. doi: 10.1136/annrheumdis-2020-217176
29. Li ZH, Zhong WF, Liu S, Kraus VB, Zhang YJ, Gao X, et al. Associations of habitual fish oil supplementation with cardiovascular outcomes and all cause mortality: evidence from a large population based cohort study. BMJ. (2020) 368:m456. doi: 10.1136/bmj.m456
30. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
31. Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, et al. Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation. (2018) 137:653–61. doi: 10.1161/CIRCULATIONAHA.117.030898
32. Li ZH, Zhang PD, Chen Q, Gao X, Chung VC, Shen D, et al. Association of sleep and circadian patterns and genetic risk with incident type 2 diabetes: a large prospective population-based cohort study. Eur J Endocrinol. (2021) 185:765–74. doi: 10.1530/EJE-21-0314
34. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. (1999) 8:3–15. doi: 10.1177/096228029900800102
35. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. (1982) 69:239–41. doi: 10.1093/biomet/69.1.239
36. Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. (2007) 50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471
37. Didikoglu A, Maharani A, Payton A, Pendleton N, Canal MM. Longitudinal change of sleep timing: association between chronotype and longevity in older adults. Chronobiol Int. (2019) 36:1285–300. doi: 10.1080/07420528.2019.1641111
38. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. (2015) 163:191–204. doi: 10.7326/M14-2841
39. DiBona GF. Sympathetic nervous system and hypertension. Hypertension. (2013) 61:556–60. doi: 10.1161/HYPERTENSIONAHA.111.00633
40. Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. (2012) 16:47–66. doi: 10.1016/j.smrv.2011.02.005
41. Punjabi NM, Haponik E. Ask about daytime sleepiness! J Am Geriatr Soc. (2000) 48:228–9. doi: 10.1111/j.1532-5415.2000.tb03918.x
42. Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. (2000) 162:566–70. doi: 10.1164/ajrccm.162.2.9908091
43. Rångemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. (1995) 18:188–94. doi: 10.1093/sleep/18.3.188
44. Nguyen J, Wright KP Jr. Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. (2010) 2:9–18. doi: 10.2147/NSS.S7624
Keywords: sleep, genetic risk, hypertension, cohort study, epidemiology
Citation: Li Z-H, Huang Q-M, Gao X, Chung VCH, Zhang P-D, Shen D, Zhang X-R, Zhong W-F, Liu D, Chen P-L, Chen Q, Cai M-C, Cheng X, Yang H-L, Song W-Q, Wu X-B, Kraus VB and Mao C (2021) Healthy Sleep Associated With Lower Risk of Hypertension Regardless of Genetic Risk: A Population-Based Cohort Study. Front. Cardiovasc. Med. 8:769130. doi: 10.3389/fcvm.2021.769130
Received: 01 September 2021; Accepted: 18 October 2021;
Published: 18 November 2021.
Edited by:
Alberto Cordero, Hospital Universitario de San Juan, SpainReviewed by:
Yurii Sviryaev, Almazov National Medical Research Centre, RussiaOxana Rotar, Almazov National Medical Research Centre, Russia
Copyright © 2021 Li, Huang, Gao, Chung, Zhang, Shen, Zhang, Zhong, Liu, Chen, Chen, Cai, Cheng, Yang, Song, Wu, Kraus and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Mao, bWFvY2hlbjlAc211LmVkdS5jbg==
†These authors have contributed equally to this work
 Zhi-Hao Li
Zhi-Hao Li Qing-Mei Huang1†
Qing-Mei Huang1† Vincent C. H. Chung
Vincent C. H. Chung Pei-Dong Zhang
Pei-Dong Zhang Xi-Ru Zhang
Xi-Ru Zhang Dan Liu
Dan Liu Chen Mao
Chen Mao