
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cardiovasc. Med. , 17 November 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.764478
The gastrocardiac syndrome was coined originally at the beginning of the 19th century to describe an alleged gastric-cardiopathy with reflux heartburn mimicking cardiac chest pain. Today, a wider perspective of gastrocardiac syndrome has emerged. First, the cardiovascular risk factor chronic systemic inflammation may reflect gastroenterological inflammatory conditions, such as inflammatory bowel disease and gastrointestinal infections, in particular, chronic Helicobacter pylori infection. Furthermore, since contemporary treatment of cardiovascular disease commonly includes potent antithrombotic medications, the cardiovascular benefit in terms of a decrease in the incidence of recurrent ischemic events and death needs to be carefully balanced with an increased risk of gastrointestinal bleeding. Several strategies to target chronic gastrointestinal inflammation and to diagnose and treat Helicobacter pylori to reduce the risk of cardiovascular events and gastrointestinal bleeding are available but residual controversy remains and large-scale gastro-cardiology trials are needed to determine the optimal treatment approaches. In perspective, the centennial gastrocardiac syndrome is more relevant than ever in a contemporary gastroenterology and cardiology setting. A collaborative subspecialty, namely Gastro-cardiology, would introduce novel unique means to study, diagnose and treat gastrocardiac conditions with the aim to reduce the risk of cardiovascular and bleeding events to improve the prognosis for gastro-cardiology patients.
The gastrocardiac syndrome was coined by Ludwig von Roemheld in 1913 to describe an alleged gastric-cardiopathy (1). Contemporary cardiology did however not adopt this diagnosis and reduced the gastrocardiac syndrome to define reflux heartburn mimicking cardiac chest pain. Today, modern cardiology is rapidly developing a close relation to other medical specialties. Cardio-oncology has been formalized, and for example, cardio-rheumatology and cardio-nephrology emerge as subspecialties. The frequent intersections of the roads of gastroenterologists and cardiologists in the centennium following the coining of Roemheld of gastrocardiac provide the perspective to debouch in gastro-cardiology today.
Chronic systemic inflammation is a well-established cardiovascular risk factor with an underlying immune activation as a major pathophysiological driver in atherosclerosis (2). Chronic inflammation of intestinal origin from Crohn's disease and ulcerative colitis is associated with an increased risk of coronary, cerebrovascular, and peripheral artery disease (3). Trials of anti-inflammatory treatments for optimized cardiovascular prevention and controlled inflammatory bowel disease however differ substantially in terms of the putative therapeutic immune targets (4, 5). Common trial planning, follow-up, analysis, and performance are required to align the research for the optimal gastro-intestinal anti-inflammatory targets. In addition to inflammatory bowel disease, the centenarian gastrocardiac syndrome can today be extended to chronic inflammation through Helicobacter pylori (H. pylori) infection as an additional ventriculo-duodenal causal factor for atherosclerosis progression and cardiovascular events (6). An almost doubled atherothrombotic cardiovascular risk by H. pylori has been established over the last two decades (7). The possible mechanism linking H. pylori and atherosclerosis could relate to two pathways: (1) chronic inflammation caused by direct colonization of the vascular wall enhancing and disrupting atherosclerotic lesions, and (2) a systemic inflammatory response in reaction to the colonization of the gastric mucosa (6). Where the first mechanism drives the inflammatory cascade by local and humoral processes toward plaque progression and instability, the latter is influenced by the host health status (e.g., comorbidities such as diabetes) including socioeconomic factors, and host exposure to environmental factors such as smoking and alcohol, all recognized independent predictors of poor cardiovascular outcomes (Figure 1). A common gastro-cardiology fight to dampen inflammation can be further encouraged by H. pylori being one of the most common chronic infections in the world with an estimated global prevalence of 50% (ranging from 10% in Northern Europe to 80% in Africa) (8) and the potential that lowering inflammation reduces the burden of the most common mortality cause from cardiovascular disease.
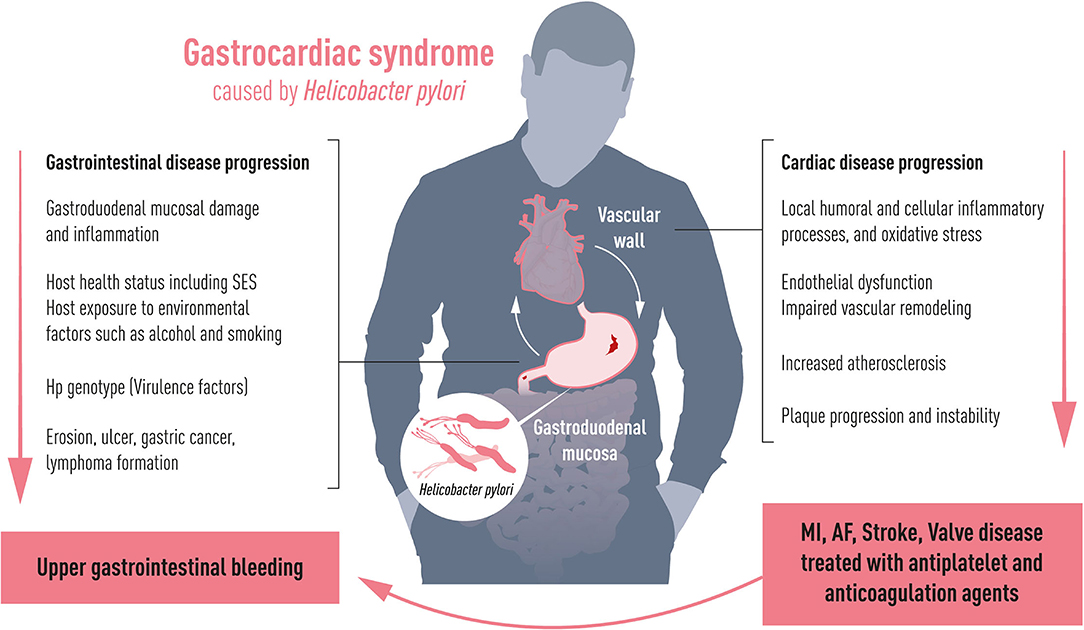
Figure 1. Gastrocardiac syndrome. Depending on host health including socioeconomic status (SES) and virulence factors, Helicobacter pylori affects the gastric mucosa causing gastroduodenal lesions with the risk to develop bleeding complications. Simultaneously, it induces chronic inflammation to the vascular wall affecting progress and degree of cardiovascular disease such as stroke, myocardial infarction (MI), atrial fibrillation (AF) and valvular heart disease. Cardiovascular diseases are commonly treated with antithrombotic drugs, which aggravate the risk of upper gastrointestinal bleeding.
Gastrointestinal bleedings have been a common problem in cardiology starting from the discovery of Heyde's syndrome in the past century. Advances since then have deciphered the mechanisms being that blood passage through a stenotic aortic valve increases shear stress to deplete von Willebrand factor. The acquired von Willebrand factor deficiency represents a reverse causality with the heart causing bleedings from gastrointestinal angiodysplasias in patients with calcific aortic valve stenosis (9). During the last decades, the prognosis for patients with cardiovascular disease, including ischemic heart disease, valvular heart disease, atrial fibrillation, or stroke has remarkably improved. However, the implementation of evidence-based therapies, in particular, the use of antithrombotic treatment presents gastro-cardiological consequences (10). Indeed, the cardiovascular benefit in terms of a decrease in the incidence of recurrent ischemic events and death is counterbalanced by an increase in hemorrhagic complications (11), in particular, from the gastrointestinal tract (12, 13). These typically present as upper gastrointestinal bleedings (UGIB), ranging from a 2-fold increase with low dose aspirin, up to 7-fold with dual anti-platelet treatment, and by a factor of 10 if anticoagulants are co-administered (10). The UGIB complications are not only the direct source for increased morbidity, mortality (14), and medical care costs but may also lead to increased risk of recurrent cardiovascular events due to discontinuation of antithrombotic drugs. Chronic active infection with H. pylori may be a common gastric orchestrator of chronic inflammation (6) and UGIB complications in cardiology (10).
Numerous studies over the last two decades have investigated a possible link between H. pylori infection and atherothrombotic cardiovascular syndromes and found an association with a two-fold increased risk (7, 15).
While previous studies of H. pylori eradication in cardiology aimed to reduce cardiovascular events, the effects of H. pylori eradication on reducing cardiological bleeding complications have been somewhat overlooked (16). Several strategies to reduce the risk of UGIB are available. First, the cardiology perspective is a personalized antithrombotic therapy through shortened duration (17) and/or de-escalation (18) based on clinical features and/or risk scores (19–22). Second, the gastroenterologist perspective complies chronic inhibition of gastric acid secretion by proton pump inhibitors (PPI), which are currently recommended in high-risk individuals (defined as a history of gastric ulcer/bleeding, anticoagulant therapy, chronic non-steroidal anti-inflammatory drugs/corticoid steroid use, or two or more of age ≥65 years, dyspepsia, gastro-esophageal reflux disease, H. pylori infection or chronic alcohol use) (23) to decrease bleeding risk during DAPT post-AMI (24). However, the net benefit of long-term PPI treatment is unresolved. Adverse events include higher rates of chest infections, dementia, cardiovascular events, and chronic kidney disease (24). Further, H. pylori eradication which may achieve similar benefits in infected patients while avoiding the side effects and medication costs associated with long-term PPI use (25), is recommended in guidelines, notably, by expert opinion (8, 26). Concerning the mode of H. pylori detection, both invasive (gastroscopy-based) and non-invasive (serology, urea breath test [UBT], feces antigen) methods are established (8). As invasive tests imply obvious drawbacks in patients with CV disease with concomitant antithrombotic therapy, the majority of previous studies were based on serological testing to detect H. pylori. However, serology does not allow distinction between active and prior H. pylori infection, which encompasses diagnostic difficulties, especially from a clinical perspective regarding eradication therapy. Non-invasive screening for active H. pylori can be performed with high accuracy (sensitivity 96% and specificity 93%) by UBT (27), currently the recommended diagnostic tool (8). No contemporary data using this mode of detection were available until recently when it was shown in a Swedish multicenter prospective cohort study of 310 consecutive AMI patients that active H. pylori diagnosed by UBT were prevalent in 20% of the patients (16), in agreement with previous findings twice as common as in the overall Swedish population with an H. pylori prevalence estimated at 11% (28). Importantly, H. pylori screening and treatment were feasible in the clinical routine during MI hospitalization. Currently, it is still under debate whether eradication therapy alone is sufficient to prevent recurrent UGIB or if long-term PPI treatment nonetheless remains necessary (29). Thus, gastro-cardiology is needed to address the net benefit of long-term treatments, adverse events, and medication costs of antithrombotic and antiacid treatments, and, critically, to determine how to handle H. pylori diagnosis and eradication.
To date, the residual controversy on how to handle the risk of UGIB in patients with cardiovascular disease urges the need for randomized gastro-cardiology trials. Recently, the Helicobacter eradication aspirin trial (HEAT), a double-blind, placebo-controlled, randomized trial of the effects of H. pylori eradication on subsequent ulcer bleeding in infected individuals taking aspirin daily was completed enrolling 30,000 patients (30). The HELicobacter Pylori screening to prevent gastrointestinal bleeding in patients with acute myocardial infarction trial based on the Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated According to Recommended Therapies (HELP-SWEDEHEART, ClinicalTrials.gov Identifier: NCT05024864) is a cluster-randomized, registry-based clinical trial using SWEDEHEART (31) and other national registries as a trial platform for patient enrollment and data collection. The primary objective is to determine whether systematic screening for H. pylori in patients after AMI and subsequent eradication therapy significantly reduces the risk of rehospitalization for UGIB whereas secondary objectives evaluate the incidence of cardiovascular endpoints (rehospitalization for AMI, heart failure, atrial fibrillation, and stroke; cardiovascular and all-cause mortality). Patient enrollment is expected to start in November 2021.
In perspective, the centennial gastrocardiac syndrome is more relevant than ever in a contemporary gastroenterology and cardiology setting. A collaborative subspecialty to improve diagnosis and treatment of gastrocardiac conditions and to reduce the cardiovascular risk and complications of ischemic-, arrhythmic- and valvular heart diseases would introduce novel unique means to improve the outcomes of gastro-cardiology patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
RH and MB: concept and design, critical revision of the manuscript for important intellectual content, and drafting of the manuscript. All authors contributed to the article and approved the submitted version.
RH was supported by the Swedish Heart-Lung Foundation [grant number, HLF 2018-0187]; the Swedish Research Council [grant number, 2019-00414]; and the Region Stockholm (clinical postdoctoral appointment) [grant number, K 2017-4577]. The funding organizations had no role in the preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roemheld L. Der gastro-kardiale Symptomenkomplex, eine besondere Form sog. Herzneurose Fortschr Med. (1913) 3:57.
2. Back M, Yurdagul A, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. (2019) 16:389–406. doi: 10.1038/s41569-019-0169-2
3. Wu H, Hu T, Hao H, Hill M, Xu C, Liu Z. Inflammatory bowel disease and cardiovascular diseases: a concise review. Eur Heart J Open. (2021). doi: 10.1093/ehjopen/oeab029
4. Sands BE, Colombel JF, Ha C, Farnier M, Armuzzi A, Quirk D, et al. Lipid profiles in patients with ulcerative colitis receiving tofacitinib-implications for cardiovascular risk and patient management. Inflamm Bowel Dis. (2021) 27:797–808. doi: 10.1093/ibd/izaa227
5. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
6. Budzynski J, Kozinski M, Klopocka M, Kubica JM, Kubica, J. Clinical significance of Helicobacter pylori infection in patients with acute coronary syndromes: an overview of current evidence. Clin Res Cardiol. (2014) 103:855–86. doi: 10.1007/s00392-014-0720-4
7. Fang, Y., Fan, C. and Xie, H. Effect of Helicobacter pylori infection on the risk of acute coronary syndrome: a systematic review and meta-analysis. Medicine (Baltimore). (2019) 98:e18348. doi: 10.1097/MD.0000000000018348
8. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. (2017) 66:6–30. doi: 10.1136/gutjnl-2016-312288
9. Pawelzik SC. Back M. Von willebrand factor's vascular crossroad. Cardiovasc Res. (2021). doi: 10.1093/cvr/cvab253. [Epub ahead of print].
10. Hellstrom PM, Benno P, Malfertheiner P. Gastrointestinal bleeding in patients with Helicobacter pylori and dual platelet inhibition after myocardial infarction. Lancet Gastroenterol Hepatol. (2021) 6:684–5. doi: 10.1016/S2468-1253(21)00192-8
11. Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. (2017) 38:804–10. doi: 10.1093/eurheartj/ehw525
12. Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, et al. Characterising and predicting bleeding in high-risk patients with an acute coronary syndrome. Heart. (2015) 101:1475–84. doi: 10.1136/heartjnl-2014-307346
13. Magnani G, Ardissino D, Im K, Budaj A, Storey RF, Steg PG, et al. Predictors, type, and impact of bleeding on the net clinical benefit of long-term ticagrelor in stable patients with prior myocardial infarction. J Am Heart Assoc. (2021) 10:e017008. doi: 10.1161/JAHA.120.017008
14. Sarajlic P, Simonsson M, Jernberg T, Back M, Hofmann R. Incidence, associated outcomes, and predictors of upper gastrointestinal bleeding following acute myocardial infarction: a SWEDEHEART-based nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. (2021). doi: 10.1093/ehjcvp/pvab059. [Epub ahead of print].
15. Shindler-Itskovitch T, Chodick G, Shalev V, Muhsen K. Helicobacter pylori infection and prevalence of stroke. Helicobacter. (2019) 24:e12553. doi: 10.1111/hel.12553
16. Warme J, Sundqvist M, Mars K, Aladellie L, Pawelzik SC, Erlinge D, et al. Helicobacter pylori screening in clinical routine during hospitalization for acute myocardial infarction. Am Heart J. (2021) 231:105–9. doi: 10.1016/j.ahj.2020.10.072
17. Wilson SJ, Newby DE, Dawson D, Irving J, Berry C. Duration of dual antiplatelet therapy in acute coronary syndrome. Heart. (2017) 103:573–80. doi: 10.1136/heartjnl-2016-309871
18. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. (2019) 381:2032–42. doi: 10.1056/NEJMoa1908419
19. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. (2009) 119:1873–82. doi: 10.1161/CIRCULATIONAHA.108.828541
20. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. J Am Med Assoc. (2016) 315:1735–49. doi: 10.1001/jama.2016.3775
21. Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. (2017) 389:1025–34. doi: 10.1016/S0140-6736(17)30397-5
22. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. (2019) 40:2632–53. doi: 10.1093/eurheartj/ehz372
23. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa909
24. Sehested TSG, Carlson N, Hansen PW, Gerds TA, Charlot MG, Torp-Pedersen C, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J. (2019) 40:1963–70. doi: 10.1093/eurheartj/ehz104
25. Gisbert JP, Khorrami S, Carballo F, Calvet X, Gene E, Dominguez-Munoz E. Meta-analysis: helicobacter pylori eradication therapy vs. antisecretory non-eradication therapy for the prevention of recurrent bleeding from peptic ulcer. Aliment Pharmacol Ther. (2004) 19:617–29. doi: 10.1111/j.1365-2036.2004.01898.x
26. Chey WD, Leontiadis GI, Howden CW, Moss S. FACG clinical guideline: treatment of helicobacter pylori infection. Am J Gastroenterol. (2017) 112:212–39. doi: 10.1038/ajg.2016.563
27. Gisbert JP, Khorrami S, Carballo F, Calvet X, Gene E, Dominguez-Munoz E. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. (2015) 21:1305–14. doi: 10.3748/wjg.v21.i4.1305
28. Agreus L, Hellstrom PM, Talley NJ, Wallner B, Forsberg A, Vieth M, et al. Towards a healthy stomach? Helicobacter pylori prevalence has dramatically decreased over 23 years in adults in a Swedish community United European. Gastroenterol J. (2016) 4:686–96. doi: 10.1177/2050640615623369
29. Vergara M, Catalan M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. (2005) 21:14114–8. doi: 10.1111/j.1365-2036.2005.02444.x
30. Dumbleton JS, Avery AJ, Coupland C, Hobbs FD, Kendrick D, Moore MV, et al. The helicobacter eradication aspirin trial (HEAT): a large simple randomised controlled trial using novel methodology in primary care. EBioMedicine. (2015) 2:1200–4. doi: 10.1016/j.ebiom.2015.07.012
Keywords: gastrocardiac syndrome, Helicobacter pylori, cardiovascular disease, upper gastrointestinal (GI) bleeding, atherosclerosis, mortality, myocardial infarction, stroke
Citation: Hofmann R and Bäck M (2021) Gastro-Cardiology: A Novel Perspective for the Gastrocardiac Syndrome. Front. Cardiovasc. Med. 8:764478. doi: 10.3389/fcvm.2021.764478
Received: 25 August 2021; Accepted: 15 October 2021;
Published: 17 November 2021.
Edited by:
Lucas Liaudet, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Alessandro Iadanza, University of Siena, ItalyCopyright © 2021 Hofmann and Bäck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Hofmann, cm9iaW4uaG9mbWFubkBraS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.