- 1Department of Cardiovascular Surgery, The Second Xiangya Hospital of Central South University, Central South University, Changsha, China
- 2State Key Laboratory of Developmental Biology of Freshwater Fish, Hunan Normal University, Changsha, China
- 3College of Life Sciences, Hunan Normal University, Changsha, China
- 4The Clinical Center for Gene Diagnosis and Therapy of The State Key Laboratory of Medical Genetics, The Second Xiangya Hospital of Central South University, Central South University, Changsha, China
Background: The dihydrofolate reductase (DHFR) gene is imperative in development, therefore it is essential to explore its effects on heart development. Thus, here a dhfr zebrafish knock-in (KI) strain was constructed.
Methods: CRISPR/Cas9 technology was used to establish the dhfr KI zebrafish strain. This strain was hybridized with TgG fluorescent strain zebrafish to observe the phenotypes of heart shape, size, and circularization direction. Wild-type (WT) and KI zebrafish were then dissected and histologically stained to observe pathological changes. Western blot analysis was used to verify the increased expressions of zebrafish genes after KI. Hybridization experiments were used to confirm the presence of abnormal gonadal dysplasia.
Results: The zebrafish dhfr KI strain was successfully constructed through CRISPR/Cas9 technology. At 6 days post fertilization (dpf), microscopic examinations of KI (homozygous) specimens revealed pericardial effusions, heart compressions, and curled tails. Compared with WT, the Hematoxylin and Eosin (H&E) tissue sections of KI-homozygous zebrafish showed defects such as reduced atria and ventricles. Western blot analysis indicated that the expression of the DHFR protein increased in both heterozygotes and homozygotes of dhfr KI zebrafish. Hybridization experiments revealed that dhfr KI may affect gonadal function.
Conclusion: The DHFR gene plays an important regulatory role in the process of heart development, and copy number variations (CNVs) of this gene may constitute a new pathogenic mechanism of congenital heart disease (CHD).
Introduction
Congenital heart disease (CHD) is one of the most common congenital malformations in fetuses. Its development process is regulated by the expressions of a variety of genes. The abnormal expressions of these genes can lead to different types of heart defects (1). Pulmonary Atresia (PA) is an extreme type of right ventricular system CHD; it is a kind of conical malformation. The anatomical lesions of PA can affect almost all right heart structures, including the atrioventricular valve, ventricular outflow tract, pulmonary valve, and pulmonary artery. The natural prognosis for children with PA is extremely poor (2). Most patients require multiple operations or interventions, and their long-term prognoses may involve developmental delays, developmental delay, and an overall unsatisfactory quality of life and life span. This can become a severe burden on the patient's family, and on society (3, 4). Identifying the regulatory genes in heart development will help to better understand the pathogenesis of PA and aid investigations into its treatment options.
With the continuous advancements in genetic testing and editing technologies, more and more disease-causing genes and their mechanisms are becoming familiar to researchers. Copy number variations (CNVs) are defined as an important genetic mechanism of human phenotypic heterogeneity; their deoxyribose nucleic acid (DNA) variations range from >1 Kbp to several Mbp (5, 6). Recently, some studies have reported that the mutation of the dihydrofolate reductase (DHFR) gene (OMIM#126060) mainly causes megaloblastic anemia caused by dihydrofolate reductase deficiencies (7). At the same time, some studies have shown that gene CNVs may also cause cell and animal resistance to the anti-cancer drug methotrexate (8–10). In addition, studies have shown that DHFR affects cell proliferation and apoptosis by regulating gene transcription, and that it plays a key role in the development of the hearts and outflow tracts of zebrafish (Danio rerio) (11). A previous study performed array detection on 82 PA patients, revealing that one patient had a DHFR gene CNV (12). This DHFR gene CNV could potentially play an important role during heart development, and thus contribute in the pathogenicity mechanism of PA. Therefore, the present study used CRISPR/Cas9 gene editing technology to construct a dhfr knock-in (KI) model for zebrafish, which are model organisms, to verify this hypothesis and preliminarily explore the pathogenesis of PA.
Materials and Methods
Ethics Review
The animal study was reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (2021123).
Target Construction
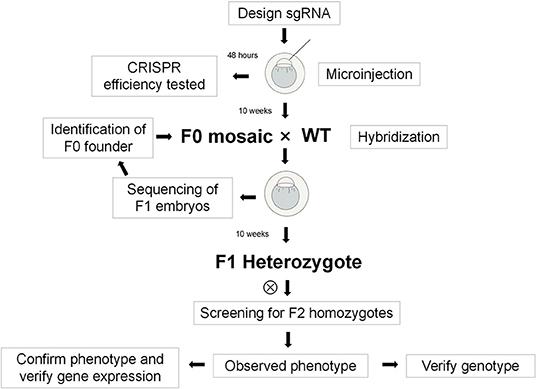
According to the provided species, gene name, or gene identification (ID), the National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/gene/81882) and Ensemble databases were used to search for related genes, species information, and coding sequence (CDS) gene areas. The corresponding genome structures were analyzed to confirm the exon part of each CDS. According to the nature of each gene, the exon region contained in the statistical gene was identified as the candidate KI site. After analyzing the nature of the gene itself and the encoded protein structure, the exon was placed after the initiation codon ATG and the exon region was placed before the selection. Finally, select sites with low off-target rates were identified as targets for gene KI. The entire process diagram is shown in Figure 1.
Design of Guide Ribose Nucleic Acid and Identification Primer
According to the protein-conserved functional domain and genetic structure, the target sequence to be knocked-in comprised a small guide RNA (sgRNA) recognition sequence on coding exon 6 that was designed to realize KI. GuideRNA: 5′-TGATGCAATGGTCAGAGATGTGG-3′ (DHFR-sgRNA5; Figure 2). The following polymerase Chain Reaction (PCR) primers were designed for the target region: 5′-GGCACCCTATCAGACTGAGC-3′ (dhfr-trans-F1) and 5′-CCTGCATTGGACACACCAAAAT-3′ (dhfr-3'KI-R1).

Figure 2. Design of guide RNA and identification primer. (A) Guide #5 TGATGCAATGGTCAGAGATGTGG; (B) the sequencing result of the activity verification; (C) the optimized amino acid sequence alignment (the lowercase font is the optimized base).
KI Donor Design
The dhfr zebrafish genome sequence (dhfr-201) was downloaded from the Ensembl online database. The dhfr CDS codon was merged and optimized through the Codon Usage Database to avoid the appearance of repetitive sequences (http://www.kazusa.or.jp/codon/cgi-bin/showcodon.cgi?species=7955).
In vitro Transcription of SgRNA and Cas9mRNA
2 × Mastermix (10 μL), ultrapure water (7 μL), forward and reverse primers (F1 and R1) 1 μl (5 μM), and zebrafish genomic DNA (50 ng·μL−1) were used to amplify the guide RNA; the amplified product was used for gene sequencing. A T7 in vitro transcription kit was used to perform the in vitro transcription of sgRNA, using the product as a template. RNA was precipitated with absolute ethanol, following which ultrapure water treated with diethylpyrocarbonate was used for resuspension. The resuspended liquid was stored in a refrigerator at −80°C for later use.
The T7-driven humanized Cas9 coding sequence was used as a template and T7 RNA polymerase was used for Cas9 in vitro transcription. It was then capped and tailed. RNA was precipitated with absolute ethanol and resuspended in ultrapure water treated with diethylpyrocarbonate. The resuspension liquid was stored in a refrigerator at −80°C for later use.
Microinjection
A semi-automatic microinjector was used to inject the mixed liquid of the above-mentioned sgRNA (final concentration of 50 ng·μL−1) and Cas9mRNA (final concentration of 250 ng·μL−1) into the zebrafish zygotes. At the same time, some wild-type (WT) embryos of the same batch were collected as a control group. The embryos were then placed in an incubator (temperature = 28°C) and incubated for 48 h. When the embryos developed to 24 h post-fertilization (hpf), they were taken to prepare a DNA template. The PCR products were sub-cloned into the pGEM-TEasy vector, via amplification with forward and reverse primers. The single transformed clones were subjected to PCR and then sequenced. Finally, the obtained sequence was compared with the WT sequence provided by the NCBI Basic Local Alignment Search Tool (BLAST) to determine the specific site of the mutation.
Screening and Identification of Founder Generation Zebrafish
The same batch of embryos that tested positive for the effectiveness of the injection was grown to 3 months of age. Part of the tail fin (1–2 mm) of each Founder generation zebrafish was clipped. Lysate was used to extract genomic DNA. The above-designed positive and negative primers were used for PCR amplification, following which the PCR products were subjected to Sanger sequencing. The single peak identified in the sequencing results showed that the corresponding Founder generation zebrafish had hit the target, confirming that the construction was successful.
Establishment of F1 Generation Zebrafish Strain
Founder generation zebrafish were crossed with WT zebrafish to obtain the F1 generation. Tail trimming was performed on F1 generation zebrafish to identify their genotypes. The birth of a positive zebrafish indicated that the transgene has been integrated into the germ cells, marking the establishment of a zebrafish line with the dhfr KI.
Identification and Dissection of F2 Generation Zebrafish
The F2 generation was obtained from the heterozygous F1 generation. The embryonic heart development and phenotype were observed at 6 days post-fertilization (dpf), and the F2 generation zebrafish were tail-cut and sequenced. The 6-dpf zebrafish were stained with Hematoxylin and Eosin (H&E), and the morphologies, sizes, and circularization directions of their hearts were observed under a microscope. The identification primers were as follows: forward primer, 5′-AGGACAATGGTGTTCAGTATG-3′ (DHFR-F); reverse primer, 5′-GCATGGGAGATAGGTTGAAG-3′ (DHFR-R).
Observation of Phenotypes of the Homozygous dhfr of the TgG Fluorescent Strain
F1 generation heterozygous females and WT TgG strains of fluorescent zebrafish males were crossed to obtain the F2 generation. After screening and identifying genotypes via fluorescence microscopy, F2 generation fluorescent heterozygous zebrafish were screened out. F2 generation zebrafish underwent heterosexual mating to obtain F3 generation fluorescent zebrafish offspring, the phenotypes of which were observed.
Western Blot Analysis
A 3-month-old zebrafish was dissected to obtain a heart sample. The heart was washed with cold phosphate-buffered saline (PBS), and then lysed with radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Scientific, 89901) containing a mixture of 1% protease and phosphatase inhibitor (Thermo Fisher Scientific, 78442). According to the manufacturer's protocols, the protein concentration of the lysate was measured using a total protein quantification (BCA) kit (Thermo Fisher Scientific, 23227). The protein sample (30 μg) was separated via 10% sodium lauryl sulfate-polyacrylamide gel electrophoresis, and then transferred to a polyvinylidene fluoride membrane. The membrane was sealed with 5% skimmed milk for 1.5 h, before being incubated with the specific primary antibodies DHFR (Proteintech, 15194-1-AP) and GAPDH (Proteintech, 10494-1-AP) at 4°C overnight. The next day, after being washed thrice with Tris-Buffered Saline and Tween 20 (TBST), the membrane was incubated with IgG secondary antibodies (Abcam, ab97051) at 37°C for 1 h, and then washed again with TBST thrice. Enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific, 34580) was used to detect antibody-binding proteins. ImageJ software was used to quantitatively analyze the gray scale of each band.
Fertility Evaluation
Adult age-matched WT Tuebingen (TU) strain (n = 12) and homozygous zebrafish (n = 12) were crossed regularly (five crosses in total) to explore the fertility of homozygotes with dhfr KI. The following crosses (three pairs per condition) were performed: WT incross, KI incross, WT female crossed with KI male, and KI female crossed with WT male. The sample size was determined as the maximum number of crossings actually possible. The numbers of eggs per fertilization event were assessed for each genotype. The inclusion criteria for fertility analysis were as follows: a successful cross was defined as an event where each paired mating produced >10 fertilized eggs. Infertility was defined as an event in which there was no fertilized egg after three repetitions with the same result. This standard was established before the start of the experiment.
Statistical Analyses
Independent t-tests (two-tailed) were used to evaluate the statistical significances of the differences between two groups, and one-way analysis of variance (ANOVA) was used to evaluate the statistical significances of the differences between two or more groups. For all tests, p < 0.05 were considered to be statistically significant.
Results
The edited Founder generation zebrafish were crossed with WT zebrafish using CRISPR/Cas9 gene-editing technology, and the Founder generation zebrafish were mated. F1 generation KI zebrafish organisms were identified, confirming that the dhfr KI zebrafish model organisms and strains were successfully constructed.
Screening and Identification of F1 Generation Zebrafish
Through the DNA extraction, PCR amplification, electrophoresis, and gene identification of F1 generation zebrafish, WT individuals were found to have a 150 bp band, heterozygotes had two bands at 750 and 150 bp, and homozygotes had one band at 750 bp. Eleven candidate dhfr KI F1 generations were identified, 10 of which were confirmed as dhfr KI (two females and eight males; Figure 3A).
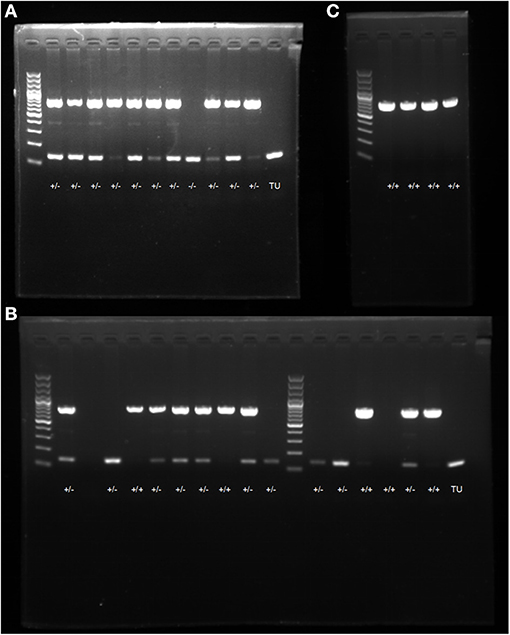
Figure 3. PCR electrophoresis diagram. (A) screening and genotype identification of F1 generation zebrafish; (B) identification of positive expressions of genotypes in zebrafish; (C) self-progeny genotype identification of F1 generation heterozygous zebrafish.
Morphological Observations of F2 Generation Zebrafish
F2 generation zebrafish were mated to obtain the F3 generation. The genotype identification results of 16 embryos at 48 hpf showed that the hybridization results were in line with Mendelian inheritance (Figure 3B). At 6 dpf, F2 generation zebrafish KI homozygotes showed abnormal phenotypes such as severe heart compressions, deformations, pericardial edemas, and slow blood flow. Normal-sized atria and ventricles were observed in the WT zebrafish heart areas, as were normal-sized pericardial cavities and blood circulation. However, the transparency around the heart areas of individuals with KI increased significantly, and no normal-sized atria and ventricles were seen. The enlarged peripheral cavity and the heart were deformed under pressure, and the blood circulation in the visual field was abnormal (it decreased or disappeared; Figure 4). The genotyping results showed that all fishes with cardiac abnormalities were homozygous (Figure 3C).

Figure 4. F2 generation zebrafish KI homozygotes at 6 dpf. (A,C,E,F) F1 heterozygous self-progeny. (B,D,G,H) self-progeny of TU.
Gene Sequencing and Identification of F2 Generation Zebrafish
Sanger sequencing and sequence comparison analysis showed that both WT zebrafish and homozygous KI individuals exhibited a “single peak” waveform, with a 639 bp base sequence knocked in; this is an optimized CDS sequence. The sequence specific KI was as follows: 5′-GGAAGCGGAGCTACTAACTTCAGCCTGCTGAAGCAGGCTGGAGACGTGGAGGAGAACCCTGGACCTATGAGCAGAATCCTGAACTGTATCGTGGCTGTGTGTCCTGACATGGGAATCGGGAAAAACGGAAACCTGCCTTGGCACCCTATCAGACTGAGCAACGAGCTGAAACACTTCCAGAAAATGACAATGACACCTAGCGACGAGGGAAAAAAAAACGTGGTGATCATGGGAAGAAAAACATGGTTCAGCATCCCTGCTGCTCACAGACCTCTGAAAAACAGAATCAACATCGTGCTGAGCAGAGAGCTGAAAACAGCTCCTGAGGGAGCTCACTACCTGGC TAGCGACTTCAGCAGCGCTCTGCACCTGCTGGACAGCGGAGAGCTCGAGAAACTGGTGGACCAGGTGTGGATCATCGGAGGAAGCAGCCTGTACAAAGAGGTGATGGAGAGAAGCGGACACAGAAGACTGTTCGTGACAAGAATCCTGAAACAGTTCGACTGTGACACATTCATCCCTAACTTCGACATGG ACAAATACAAACTGCTGCCTGAGTTCCCTGGAGTGCCTGTCGGACTGCAGGAGGACAACGGAGTCCAGTACGTGTTCGAGGTGTACGAGAGCATCGAACACTAA-3′ (Figure 5).
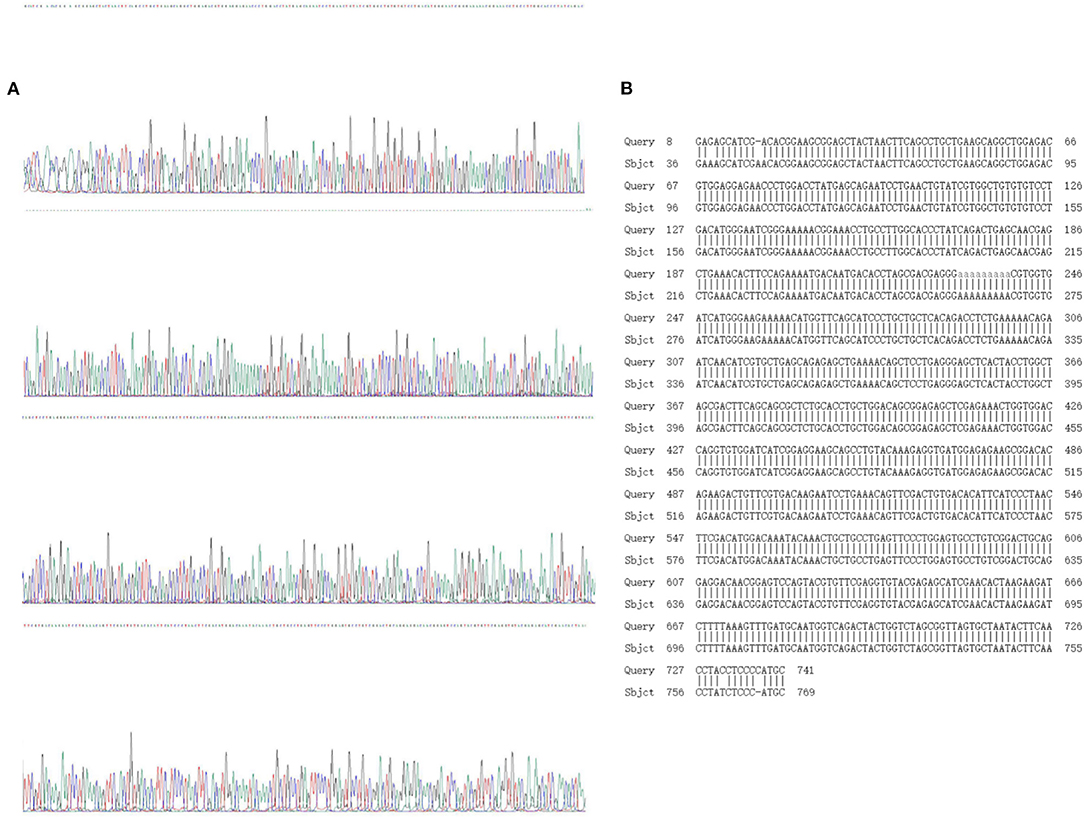
Figure 5. Sanger sequencing and sequence alignment analysis results. (A) Sanger sequencing results; (B) design sequence and sequencing alignment results.
Observation of the Phenotype of dhfr Homozygous TgG Fluorescent Strain
At 6 dpf, the F2 generation zebrafish KI homozygotes of the TgG fluorescent strain showed abnormal phenotypes such as severe heart compressions, deformations, pericardial edema, and blood flow slowdown; these were the same as the phenotypes of the TU dhfr KI strain (Figure 6).
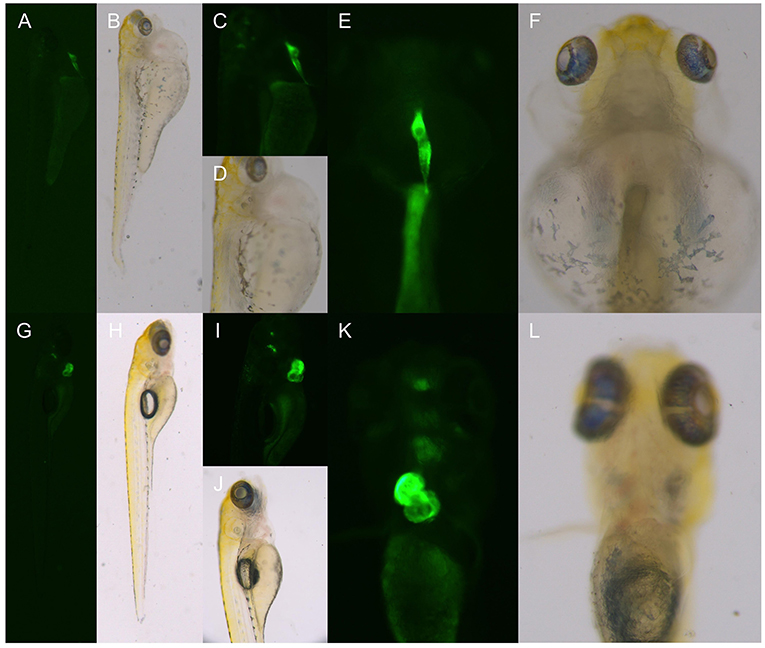
Figure 6. The phenotype of the hybrid offspring of the TgG fluorescent line. (A–F) TgG homozygous fluorescent strain dhfr. (G–L) TgG fluorescent strain TU.
Pathological Examination of Zebrafish
The H&E stained pathological section results showed that the individual hearts of KI the zebrafish, were relatively small and contained linear malformations, pericardial edema, and other defective phenotypes (Figures 7A,B).
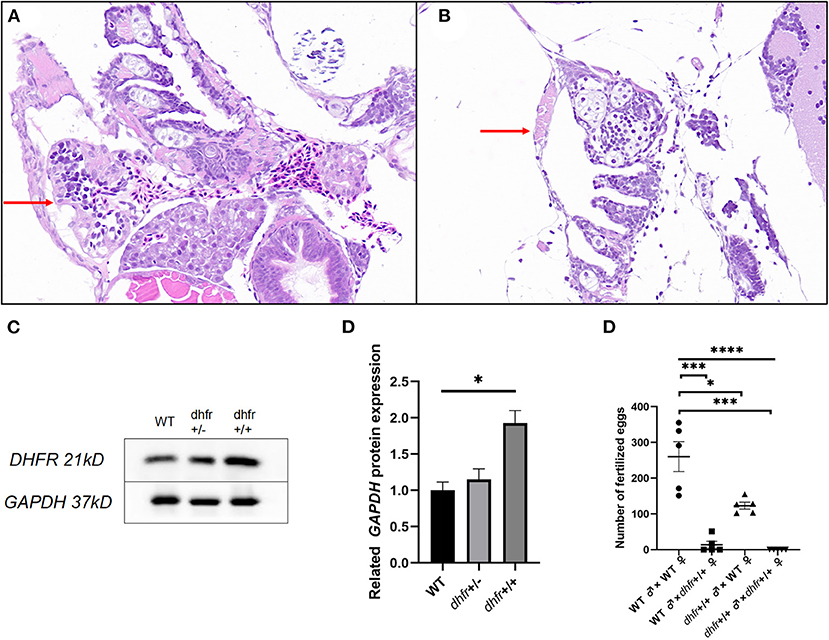
Figure 7. Results of pathology, western blot, and hybridization experiments. (A) TU zebrafish; (B) F1 dhfr heterozygous self-progeny (the red arrow points to the heart area). (C,D) WT, dhfr KI heterozygote, and dhfr KI homozygous DHFR protein expression analysis (GAPDH was used as an internal control). E: impaired fertility in zebrafish knocked in by dhfr (*P < 0.05, ***P < 0.001, ****P < 0.0001).
Dhfr Protein Expression in dhfr KI Strain Zebrafish
To detect the changes in zebrafish heart protein expressions after in vitro dhfr gene KI, and to determine whether KI was successful, western blot experiments were used to verify the expression of the dhfr gene in the heart. The expression of the DHFR protein in the hearts of homozygous zebrafish with dhfr KI was significantly higher (p = 0.024) than in those of WT zebrafish, with an average expression increase of 1.93 times. Heterozygous and WT zebrafish were found to be not statistically different, but the average expression increased by 1.15 times (Figures 7C,D).
Fertility of dhfr KI Zebrafish
Compared with WT pairs, WT females/KI males and KI females/WT males produced lower numbers of eggs per mating, and KI pairs did not produce any fertilized eggs at all. There were statistically significant differences between the fertilized eggs laid by each group (p < 0.0001). The number of crosses indicated that there were statistically significant differences between KI, KI male/WT female, KI female/WT male, and WT pairs (p = 0.0004, p = 0.0127, and p = 0.0003, respectively). Whenever KI females were involved, the number of fertilized eggs was significantly lower (Figure 7E).
Discussion
At present, it is believed that CHDs are complex diseases with abnormal cardiovascular developments caused by multiple factors (such as genetics and the environment), among which genetic factors are thought to play a major role. CHDs caused by genetic factors are generally divided into three subtypes: Mendelian single gene-diseases (such as splicing sites or exon base changes; the size is generally one base) such as GATA4, NKX2-5, TBX1, JAG1, FOG2, ALK2, GDF1, TDGF1, FOXH1, and ZIC3; and other gene mutations that can cause conical stem deformities (13–15). The second subtype contains chromosomal diseases (generally more than 3 million bases per 3 Mb in size), such as Downs syndrome (16). The last type constitutes genomic diseases (~10–3 million bases per 1 kb−3 Mb in size) (17). Genome rearrangement leads to the duplication or deletion of gene CNVs (18). Studies of genetically engineered mouse models have shown that more than 1,700 genes are involved in heart development, but to date only a few gene mutations have been confirmed to be related to human cardiovascular diseases (19). In recent years, many studies have shown that CNVs play an important role in the genetic pathogeneses of various congenital malformations.
PA is an extreme type of disease in the right heart system disease spectrum of CHDs; it is a conical malformation that occurs at an incidence of ~21.4 cases per 100,000 people (20). PA includes PA with a ventricular septal defect, PA with an intact ventricular septum, and PA with other complex heart malformations. Among them, PA with a ventricular septal defect is the most common type. PA, tetralogy of Fallot, double outlet of the right ventricle, transposition of the great arteries, common trunk, interruption of the aortic arch, and congenitally corrected transposition of the great arteries are all conical trunk malformations. Such malformations are caused by stagnation or interference in the development of the bulbar segment (conus trunk segment) in the embryonic stage (21, 22).
There are various causes of conotruncal defects including PA, but distinct genetic elements responsible for the pathogenesis of conotruncal defects have not yet been elucidated. However, patients with some syndrome, provide a reference to study the etiology of conotruncal defects. For example, ~15% of patients with TOF also suffer from 22q11.2 deletion syndrome (23). The most common congenital heart diseases for 22q11.2 deletion syndrome is conotruncal defects, such as Tetralogy of Fallot (TOF, 20–45%) and PA (10–25%) (24). A recent study found that 15.9% of 251 patients with 22q11.2 deletion syndrome had TOF deletions. This was almost equivalent to the proportion of patients with TOF, having 22q11.2 deletion reported in most studies. Therefore, it is speculated that certain genes are involved in pyramidal stem deformity in this region. Among all the ideal candidates, the primary pathogenic gene is TBX1 (25). Studies have demonstrated that TBX1 expression is essential in pharyngeal mesoderm for the pharyngeal segmentation and the normal outflow tract development. TBX1 promotes increased proliferation and delayed differentiation of the second heart field cells thereby affecting the embryonic development (26). Moreover, some studies have reported that knockout and overexpression of Tbx1 in mouse results in the phenotypes of 22q11.2 deletion syndrome (27, 28). The effect of TBX1 is similar to that of the DHFR gene, and alterations in DHFR dosage may cause the phenotype of PA. Therefore, based on these current findings it could be inferred that the 22q11.2 deletion syndrome is the most common genetic cause of PA. In addition, Down syndrome and Vacterl syndrome have also been shown to be associated with PA (29). Furthermore, in previous reports, we and several other groups have elucidated diverse factors contributing to PA (30).
In addition to the CNV mechanism, there are other types of genetic mutations that may cause PA. For example, mutations in NKX2-5, GATA4 and TBX5 genes can cause conotruncal defects and are intricately associated with PA (31–33). In addition to these common genes, other important regulatory genes, such as NOTCH1 have recently been reported to be closely related to PA as revealed in Genome wide association study analysis of TOF whole exome sequencing data (23). The association of NOTCH1 with a series of heart defects corroborates its reported role in heart development. Thus, active NOTCH1 expression is observed in the trabecular endocardium. Moreover, global and endothelial-specific knockout of Notch1 in mice results in abnormal trabecular ventricles as well as abnormal patterns of cardiomyocytes (34). With regard to TOF, NOTCH1 plays a crucial role in the tissues of the outflow tract, which requires cellular specification of the neural crest and secondary heart field (35). In addition, NOTCH1 is necessary for endocardial-epithelial-mesenchymal transition, a process that is essential for heart valve formation (36). These findings are of great significance and provide valuable insights which could be useful for further research on conical deformity.
With improvements in genetic testing and editing technologies, more and more candidate pathogenic genes and development mechanisms of genetic diseases have been clarified. The latest gene-editing technology, CRISPR/Cas9, has been widely used in studies of various gene knockout biological models such as mice, zebrafish, and chicken (37). Zebrafish have many of the same characteristics as other vertebrates regarding their anatomy and physiology, and have the advantages of being small individuals that reproduce easily, develop quickly, and produce transparent embryos. As zebrafish have great advantages for the effective study of the genetic development of vertebrate hearts, they have become an ideal model animal for studying genes related to heart development (38).
A previous study used array data analysis to detect that the 5q14.1 micro-repeat segment contained the DHFR gene. This suggested that a CNV of the DHFR gene may be a candidate pathogenic mechanism for PA. Subsequently, the present study used CRISPR/Cas9 technology to establish a zebrafish strain of dhfr KI, so that dhfr was overexpressed in zebrafish. This permitted the investigation of whether dhfr CNVs play a role in heart development. Light microscopy revealed that F3 generation KI zebrafish individuals had severe heart compressions, deformations, pericardial edema, and blood flow slowdown at 6 dpf, indicating that the dhfr gene plays an important regulatory role in the development of the cardiovascular system. Western blot analysis confirmed that the expression of DHFR protein in the hearts of zebrafish homozygous and heterozygous for dhfr KI increased compared with that of WT zebrafish, and the expression of DHFR protein in homozygous and heterozygous dhfr KI also increased. HE staining of adult zebrafish heart sections revealed that the hearts of KI individuals had defective phenotypes such as heart reductions. The shrinkage of the heart has a huge impact on cardiac hemodynamics. Observations also revealed that dhfr gene KI may cause abnormal gonadal function, especially in the ovaries. However, this needs further study. Therefore, the present study of zebrafish gene KI model organisms suggested that the abnormality of the DHFR gene affects the normal development of the heart, leading to abnormal or defective heart development.
The successful establishment of a dhfr KI zebrafish strain could permit the further construction of PA zebrafish model organisms, and be used to verify PA's occurrence and development mechanism, and could allow for the exploration of a new model organism for studying the pathogenic mechanisms of CHDs. As zebrafish embryos and juveniles are transparent, the green fluorescent protein gene can be transfected into zebrafish embryos together with the target gene. Researchers can thus study the expressions and functions of target genes, thereby allowing the exploration and verification of pathogenic signaling pathways. This will lay the foundation for further exploration of the molecular mechanisms of CHDs and their regulatory signal pathways.
The CNV of DHFR the gene studied in this article may cause the molecular pathogenesis of PA. To date there have been no reports on the role of DHFR CNV in heart development. The DHFR gene is located at 5q14.1 of chromosome 5, and the encoded protein is named dihydrofolate reductase. Dihydrofolate reductase has a total of 187 amino acids and is highly conserved. This protein is mainly expressed in mitochondria and converts dihydrofolate into tetrahydrofolate (39). There have been a large number of reports in the literature that DHFR gene mutations mainly cause resistance to antifolate drugs. Sun et al. found that the overexpression of the dhfr gene can partially improve the abnormal development of the heart and blood vessels of zebrafish embryos induced by ethanol. This may have been achieved by the up-regulation of decreases in the expressions of nkx2.5, tbx1, and flk-1, caused by ethanol (40). The regulation mechanisms of the DHFR gene for each exon and dihydrofolate reductase have not yet been fully elucidated. However, here dhfr KI zebrafish showed abnormalities such as cardiac edema and cardiac blood flow slowdown.
Our research has some limitations. First, we only constructed an animal model of zebrafish, and we have not further studied its mechanism. This aspect is also the direction of our future research. We hope to build cell models and conduct research on related mechanisms and pathways. Secondly, about the study of pathological results and sexual function, the amount of data is relatively small because pathological sections of zebrafish juveniles are difficult to cut to the heart, and the number of homozygotes in vivo is relatively small. At the same time, there are fewer homozygous adult fish, so there are fewer repetitions of the western blot results. We will continue to study the changes in the expression of their protein levels in the future. In the future, we will further supplement the data and continue to conduct in-depth research. At the same time, we hope that this copy number variant can be found in other patients in the future to further prove the function and pathogenicity of the alteration. Therefore, we will collect more relevant patients and perform CNV.
This experiment identified important genes of dhfr KI strain zebrafish related to heart development. Based on the results of previous studies, DHFR may be an important regulatory gene for heart development, and so could play an important role in the pathogenesis of CHD. The CNV of this gene may contribute to the pathogenic mechanism of CHD. Here, hybridization experiments revealed that dhfr CNV has an effect on the early heart development of zebrafish. One possible mechanism is that the dhfr CNV changes the expression of dihydrofolate reductase. This leads to abnormal cell folate metabolism, which then leads to abnormal one-carbon metabolism, nitric oxide, folate cycle, and cell cycle signaling pathways. This in turn, results in abnormal heart development and CHD. To further verify this speculation, efforts should be made in the future to construct a model mammalian organism for KI mice and observe the overall development of their hearts. These model organisms could be used to study obstacles that may be caused by one-carbon metabolism, nitric oxide, folate cycle, cell cycle, and other signaling pathways.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), the Second Xiangya Hospital Central South University, China.
Author Contributions
KG, ZT, YY, YD, KC, and LX contributed to conception and design of the study. KG and TX completed the experiment, performed the statistical analysis, and wrote the first draft of the manuscript. KY, TX, YL, and HG wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the National Science Foundation for Young Scientists of China (8150020951), the Natural Science Foundation for Young Scientists of Hunan Province (2016JJ4099), and the National Natural Science Foundation of China (31970504 and 31772548).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the members of the Zebrafish Genetics Laboratory in Hunan Normal University for their assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.763851/full#supplementary-material
Supplementary Figure 1. Ethical review form.
Supplementary Figure 2. Embryo count.
Supplementary Figure 3. Wb.
References
1. Williams K, Carson J, Lo C. Genetics of congenital heart disease. Biomolecules. (2019) 9:20879. doi: 10.3390/biom9120879
2. Limongelli G, Di Salvo G. Natural history of pulmonary atresia: The ACHD clinic perspective. Int J Cardiol. (2019) 280:67–8. doi: 10.1016/j.ijcard.2019.01.057
3. Mishima A, Asano M, Sasaki S, Yamamoto S, Saito T, Ukai T, et al. Long-term outcome for right heart function after biventricular repair of pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg. (2000) 48:145–52. doi: 10.1007/BF03218112
4. Soquet J, Barron DJ, d'Udekem Y. A review of the management of pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Ann Thorac Surg. (2019) 108:601–12. doi: 10.1016/j.athoracsur.2019.01.046
5. Yang Y, Xia C, Zhou Z, Wei D, Xu K, Jia J, et al. A multiplex ligation dependent probe amplification based next generation sequencing approach for the detection of copy number variations in the human genome. Mol Med Rep. (2018) 18:5823–33. doi: 10.3892/mmr.2018.9581
6. Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, et al. Large-scale copy number polymorphism in the human genome. Science. (2004) 305:525–8. doi: 10.1126/science.1098918
7. Cario H, Smith DE, Blom H, Blau N, Bode H, Holzmann K, et al. Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease. Am J Hum Genet. (2011) 88:226–31. doi: 10.1016/j.ajhg.2011.01.007
8. Corin RE, Turner T, Szabo P. Murine erythroleukemia cell variants: isolation of cells that have amplified the dihydrofolate reductase gene and retained the ability to be induced to differentiate. Biochemistry. (1986) 25:3768–73. doi: 10.1021/bi00361a006
9. Domin BA, Grill SP, Cheng Y. Establishment of dihydrofolate reductase-increased human cell lines and relationship between dihydrofolate reductase levels and gene copy. Cancer Res. (1983) 43:2155–8.
10. Hattinger CM, Stoico G, Michelacci F, Pasello M, Scionti I, Remondini D, et al. Mechanisms of gene amplification and evidence of coamplification in drug-resistant human osteosarcoma cell lines. Genes Chromosomes Cancer. (2009) 48:289–309. doi: 10.1002/gcc.20640
11. Sun S, Gui Y, Jiang Q, Song H. Dihydrofolate reductase is required for the development of heart and outflow tract in zebrafish. Acta Biochim Biophys Sin. (2011) 43:957–69. doi: 10.1093/abbs/gmr098
12. Xie L, Chen JL, Zhang WZ, Wang SZ, Zhao TL, Huang, et al. Rare de novo copy number variants in patients with congenital pulmonary atresia. PLoS ONE. (2014) 9:e96471. doi: 10.1371/journal.pone.0096471
13. Karkera JD, Lee JS, Roessler E, Banerjee-Basu S, Ouspenskaia MV, Mez J, et al. Loss-of-function mutations in growth differentiation factor-1 (GDF1) are associated with congenital heart defects in humans. Am J Hum Genet. (2007) 81:987–94. doi: 10.1086/522890
14. Wessels MW, Willems PJ. Genetic factors in non-syndromic congenital heart malformations. Clin Genet. (2010) 78:103–23. doi: 10.1111/j.1399-0004.2010.01435.x
15. Akhirome E, Walton NA, Nogee JM, Jay PY. The complex genetic basis of congenital heart defects. Circ J. (2017) 81:629–34. doi: 10.1253/circj.CJ-16-1343
16. Antonarakis SE, Skotko BG, Rafii MS, Strydom A, Pape SE, Bianchi DW, et al. Down syndrome. Nat Rev Dis Primers. (2020) 6:9. doi: 10.1038/s41572-019-0143-7
17. Lupski JR. Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. (1998) 14:417–22. doi: 10.1016/S0168-9525(98)01555-8
18. Lauer S, Gresham D. An evolving view of copy number variants. Curr Genet. (2019) 65:1287–95. doi: 10.1007/s00294-019-00980-0
19. Bentham J, Bhattacharya S. Genetic mechanisms controlling cardiovascular development. Ann N Y Acad Sci. (2008) 1123:10–9. doi: 10.1196/annals.1420.003
20. Leonard H, Derrick G, O'Sullivan J, Wren C. Natural and unnatural history of pulmonary atresia. Heart. (2000) 84:499–503. doi: 10.1136/heart.84.5.499
21. Perez M, Kumar TK, Briceno-Medina M, Alsheikh-Ali M, Sathanandam S, Knott-Craig CJ. Common pulmonary vein atresia: report of three cases and review of the literature. Cardiol Young. (2016) 26:629–35. doi: 10.1017/S1047951115002334
22. Kwiatkowski DM, Hanley FL, Krawczeski CD. Right ventricular outflow tract obstruction: pulmonary atresia with intact ventricular septum, pulmonary stenosis, Ebstein's malformation. Pediatr Crit Care Med. (2016) 17:S323–9. doi: 10.1097/PCC.0000000000000818
23. Page DJ, Miossec MJ, Williams SG, Monaghan RM, Fotiou E, Cordell, et al. Whole Exome sequencing reveals the major genetic contributors to nonsyndromic tetralogy of fallot. Circ Res. (2019) 124:553–63. doi: 10.1161/CIRCRESAHA.118.313250
24. Cirillo A, Lioncino M, Maratea A, Passariello A, Fusco A, Fratta F, et al. Clinical manifestations of 22q11.2 deletion syndrome. Heart Fail Clin. (2022) 18:155–64. doi: 10.1016/j.hfc.2021.07.009
25. Goldmuntz E, Clark BJ, Mitchell LE, Jawad AF, Cuneo BF, Reed L, et al. Frequency of 22q11 deletions in patients with conotruncal defects. J Am Coll Cardiol. (1998) 32:492–8. doi: 10.1016/S0735-1097(98)00259-9
26. Parisot P, Mesbah K, Theveniau-Ruissy M, Kelly RG. Tbx1, subpulmonary myocardium and conotruncal congenital heart defects. Birth Defects Res Clin Mol Teratol. (2011) 91:477–84. doi: 10.1002/bdra.20803
27. Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, et al. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. (2004) 13:1577–85. doi: 10.1093/hmg/ddh176
28. Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. (2001) 410:97–101. doi: 10.1038/35065105
29. Chen MY, Chiu SN, Wang JK, Lu CW, Lin MT, Chang CI, et al. Genetic syndromes and outcome after surgical repair of pulmonary atresia and ventricular septal defect. Ann Thorac Surg. (2012) 94:1627–33. doi: 10.1016/j.athoracsur.2012.06.063
30. Gelb BD. Molecular genetics of congenital heart disease. Curr Opin Cardiol. (1997) 12:321–28. doi: 10.1097/00001573-199705000-00014
31. El Bouchikhi I, Belhassan K, Moufid FZ, Houssaini MI, Bouguenouch L, Samri I, et al. GATA4 molecular screening and assessment of environmental risk factors in a Moroccan cohort with tetralogy of Fallot. Afr Health Sci. (2018) 18:922–30. doi: 10.4314/ahs.v18i4.11
32. Baban A, Postma AV, Marini M, Trocchio G, Santilli A, Pelegrini M, et al. Identification of TBX5 mutations in a series of 94 patients with Tetralogy of Fallot. Am J Med Genet. (2014) 164A:3100–7. doi: 10.1002/ajmg.a.36783
33. Wang J, Lu Y, Chen H, Yin M, Yu T, Fu Q. Investigation of somatic NKX2-5, GATA4 and HAND1 mutations in patients with tetralogy of Fallot. Pathology. (2011) 43:322–6. doi: 10.1097/PAT.0b013e32834635a9
34. Grego-Bessa J, Luna-Zurita L, Del Monte G, Bolos V, Melgar P, Arandilla A, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. (2007) 12:415–29. doi: 10.1016/j.devcel.2006.12.011
35. High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, et al. Murine jagged1/notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. (2009) 119:1986–96. doi: 10.1172/JCI38922
36. Berndt S, Clauss H, Dahmer J, Friedel R, Hilfenhaus M, Hubrich K. Didactics in medicine. Basic test of acute gout. 1. Recording tape as teaching medium. Med Welt. (1973) 24:423–7.
37. Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. (2016) 533:125–9. doi: 10.1038/nature17664
38. Staudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu Rev Genet. (2012) 46:397–418. doi: 10.1146/annurev-genet-110711-155646
39. Schweitzer BI, Dicker AP, Bertino JR. Dihydrofolate reductase as a therapeutic target. FASEB J. (1990) 4:2441–52. doi: 10.1096/fasebj.4.8.2185970
Keywords: zebrafish, CRISPR/Cas9, heart development, congenital heart disease, DHFR gene
Citation: Gong K, Xie T, Yang Y, Luo Y, Deng Y, Chen K, Tan Z, Guo H and Xie L (2021) Establishment of a Dihydrofolate Reductase Gene Knock-In Zebrafish Strain to Aid Preliminary Analysis of Congenital Heart Disease Mechanisms. Front. Cardiovasc. Med. 8:763851. doi: 10.3389/fcvm.2021.763851
Received: 24 August 2021; Accepted: 26 November 2021;
Published: 15 December 2021.
Edited by:
Jian Zhuang, Guangdong Provincial People's Hospital, ChinaReviewed by:
Michele Lioncino, University of Campania Luigi Vanvitelli, ItalyGiuseppina Milano, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2021 Gong, Xie, Yang, Luo, Deng, Chen, Tan, Guo and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Xie, eGllbGk1NUBjc3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Ke Gong
Ke Gong Ting Xie1†
Ting Xie1† Zhiping Tan
Zhiping Tan Li Xie
Li Xie