
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 15 October 2021
Sec. Thrombosis and Haemostasis
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.757188
This article is part of the Research Topic Anticoagulation in Cardiovascular Diseases: Evolving role, unmet needs and grey areas View all 26 articles
Background: Recent observational studies have compared effectiveness and safety profiles between non-vitamin K antagonist oral anticoagulants (NOACs) and warfarin in patients with atrial fibrillation (AF). Nevertheless, the confounders may exist due to the nature of clinical practice-based data, thus potentially influencing the reliability of results. This systematic review and meta-analysis were conducted to compare the effect of NOACs with warfarin based on the propensity score-based observational studies vs. randomized clinical trials (RCTs).
Methods: Articles included were systematically searched from the PubMed and EMBASE databases until March 2021 to obtain relevant studies. The primary outcomes were stroke or systemic embolism (SSE) and major bleeding. Hazard ratios (HRs) and 95% confidence intervals (CIs) of the outcomes were extracted and then pooled by the random-effects model.
Results: A total of 20 propensity score-based observational studies and 4 RCTs were included. Compared with warfarin, dabigatran (HR, 0.82 [95% CI, 0.71–0.96]), rivaroxaban (HR, 0.80 [95% CI, 0.75–0.85]), apixaban (HR, 0.75 [95% CI, 0.65–0.86]), and edoxaban (HR, 0.71 [95% CI, 0.60–0.83]) were associated with a reduced risk of stroke or systemic embolism, whereas dabigatran (HR, 0.76 [95% CI, 0.65–0.87]), apixaban (HR, 0.61 [95% CI, 0.56–0.67]), and edoxaban (HR, 0.58 [95% CI, 0.45–0.74]) but not rivaroxaban (HR, 0.92 [95% CI, 0.84–1.00]) were significantly associated with a decreased risk of major bleeding based on the observational studies. Furthermore, the risk of major bleeding with dabigatran 150 mg was significantly lower in observational studies than that in the RE-LY trial, whereas the pooled results of observational studies were similar to the data from the corresponding RCTs in other comparisons.
Conclusion: Data from propensity score-based observational studies and NOAC trials consistently suggest that the use of four individual NOACs is non-inferior to warfarin for stroke prevention in AF patients.
Atrial fibrillation (AF), the most common arrhythmia in clinical practice, increases the five-fold risk of ischemic stroke and two-fold for all-cause mortality (1, 2). Before 2010, warfarin was primarily used to prevent stroke in AF patients, but there is a limited range for treatment due to the regular monitoring of the international normalized ratio (INR), and the dosage is adjusted frequently (3). Subsequently, non-vitamin K oral anticoagulants (NOACs), including direct thrombin inhibitor (dabigatran) and factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) are recommended as the preferred drugs for stroke prevention among nonvalvular AF patients (4–6). Compared with warfarin, NOACs do not require anticoagulation monitoring, have easier dosing regimens, and have fewer food and drug interactions (7).
Previous randomized clinical trials (RCTs) have shown that the efficacy and safety of the NOACs are superior or non-inferior to warfarin in AF patients. Specifically, compared with warfarin, dabigatran is associated with lower rates of stroke and systemic embolism (SSE) and a similar rate of major bleeding (8), apixaban has decreased rates of SSE and MB (9), rivaroxaban has non-inferior rates of SSE and a similar rate of major bleeding (10), and edoxaban has non-inferior rates of SSE and a lower rate of major bleeding (11). Although RCTs could ensure the balance of results between different patient groups and get a fair evaluation of the trial treatment effect, they limit the assessment of the risks and benefits of interventions for all the populations when these interventions are used in real-world settings. By contrast, observational studies could infer a wider range of patient characteristics and evaluate a broader range of outcomes over a more extended period (12, 13). More recently, many observational studies have been published to compare the effectiveness and safety of NOACs vs. warfarin in AF patients. However, the obvious confounders and significant biases may exist in several observational studies due to the nature of clinical practice-based data, thus potentially influencing the reliability of findings.
An effective method to evaluate interventions' effectiveness in typical clinical settings can be provided by the propensity score (PS) (14). Observational studies using the PS method may alter the target population by changing the distribution of patient baseline characteristics that facilitate analysis. Therefore, the PS analysis can be used to reduce biases in comparisons between the targeted populations and controls. In the present meta-analysis, we aimed to compare the effectiveness and safety profiles between NOACs and warfarin based on the PS-based observational studies, and further test whether the pooled results of high-quality observational studies were consistent with data from the corresponding RCTs.
This systematic review and meta-analysis were carried out based on the Cochrane Handbook for systemic reviews. The results were presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. Ethical approval was not provided because we only included the published studies.
We performed a systematic search in detail on the PubMed and EMBASE databases until March 2021 to obtain all the relevant studies. To obtain a balanced covariate distribution between groups of NOACs and warfarin, we included observational articles that applied the PS-based methods. In addition, 4 RCTs of NOACs vs. warfarin were also selected (dabigatran [RE-LY], rivaroxaban [ROCKET AF], apixaban [ARISTOTLE], and edoxaban [ENGAGE AF-TIMI 48]). The primary outcomes were SSE and major bleeding. Data extraction was conducted independently by two researchers. The hazard ratios (HR) and 95% confidence intervals (CIs) were considered as the effect sizes, and the pooled by the random-effects model. To test the stability of the results, we re-conducted the analysis using the fixed-effects model, inverse variance heterogeneity (IVhet), and quality effects (QE) models. Detailed information including eligibility criteria, literature search, study selection, and data extraction, quality assessment, and statistical analysis was provided in Supplementary Materials.
All the statistical analyses were carried out by Review Manager 5.3 software (the Cochrane Collaboration 2014. Nordic Cochrane Centre Copenhagen, Denmark), the Stata software (version 16.0, Stata Corp LP, College Station, TX), and MetaXL (version 5.3).
The flow chart of document retrieval is presented in Supplementary Figure 1. A total of 1,139 studies from two electronic databases were under-identification. A total of 782 studies remained after duplication removal, and then 57 studies were left based on the screenings of titles/abstracts. Among the 57 studies undergoing the full-text screenings, 33 of them were excluded due to the following reasons: (1) 23 studies used overlapping databases; (2) 3 studies included single-center patients, and the sample size was less than 1,000; (3) 5 studies reported the comparisons between combined NOACs vs. warfarin, or did not regard warfarin as the reference; (4) 2 studies did not use the PS-based methods to match baseline patient characteristics. Finally, 24 studies (3, 7–11, 15–32) (20 observational cohort studies and 4 RCTs) were included in our current meta-analysis.
The baseline characteristics of the included RCTs are shown in Supplementary Table 1. Detailed information was categorized into different groups based on the dose of NOACs. Baseline characteristics of the 20 observational studies are shown in Table 1. Although some studies extracted data from the same database, they analyzed different kinds of NOACs, included diverse study periods, or included different outcomes for analysis. For instance, both Gupta et al. (25) and Villines et al. (26) obtained data from the US Department of Defense, but the study periods ranged from 2013 to 2015 for Gupta et al., and from 2009 to 2012 for Villines et al. All the included studies applied the PS-based methods to balance the covariates between groups [propensity score matching [PSM], n = 11 (3, 7, 20, 23–27, 29, 31, 32), and inverse probability of treatment weighting [IPTW], n = 9 (15–19, 21, 22, 28, 30)]. For the PS diagnostics, 14 studies used standardized differences, and 6 studies failed to report any further diagnostic use.
The results of the risk of bias assessment for RCTs are shown in Supplementary Table 2, suggesting low risks in biases. The methodological quality assessment of observational cohorts was carried out by the NOS tool (Supplementary Table 3). All articles scored 7 or more points indicating relatively high quality.
Based on the observational studies, the crude event rates and pooled HRs (based on random-effects model) of the outcomes between each NOAC vs. warfarin are summarized in Table 2.
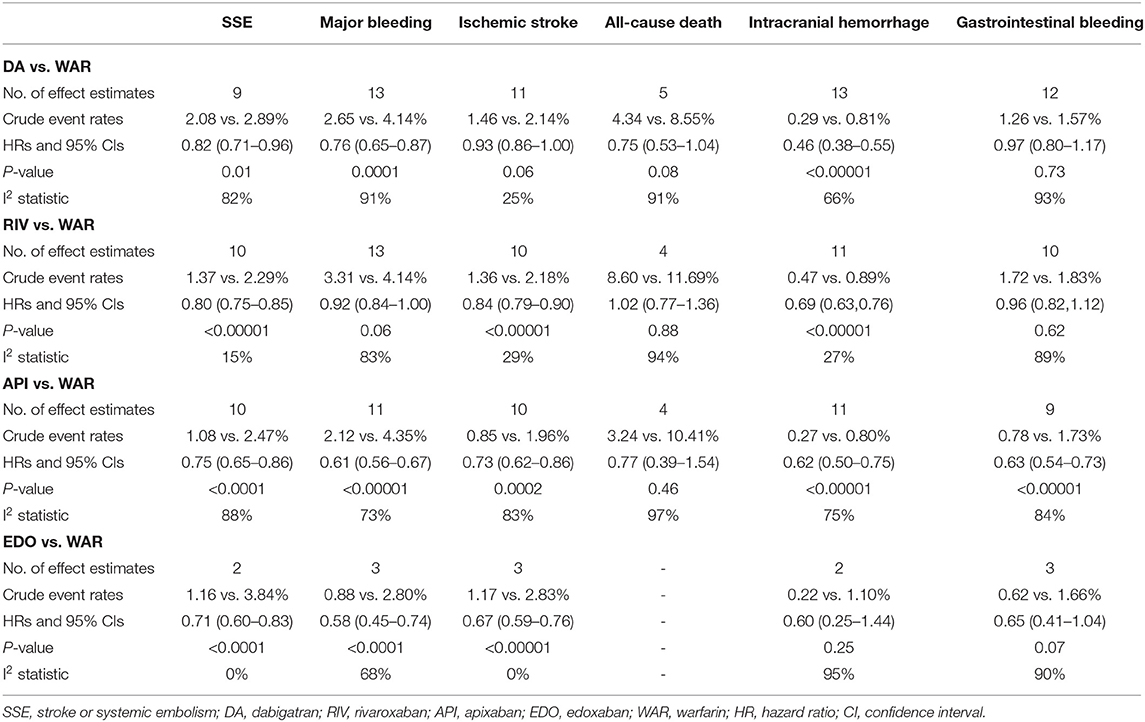
Table 2. Pooled HRs of the effectiveness and safety outcomes between NOACs vs. warfarin in patients with AF.
As presented in Figure 1, compared with warfarin, dabigatran was associated with reduced risks of SSE (2.08 vs. 2.89%; HR, 0.82 [95% CI, 0.71–0.96]) and major bleeding (2.65 vs. 4.14%; HR, 0.76 [95% CI, 0.65–0.87]). The results of rivaroxaban vs. warfarin are shown in Figure 2. Compared with warfarin use, the use of rivaroxaban was markedly associated with a reduced risk of SSE (1.37 vs. 2.29%; HR, 0.80 [95% CI, 0.75–0.85]). Meanwhile, it presented a comparable risk of major bleeding (3.31 vs. 4.14%; HR, 0.92 [95% CI, 0.84–1.00]) between rivaroxaban vs. warfarin. As shown in Figure 3, the use of apixaban vs. warfarin was related to reduced risks of SSE (1.08 vs. 2.47%; HR, 0.75 [95% CI, 0.65–0.86]) and major bleeding (2.12 vs. 4.35%; HR, 0.61 [95% CI, 0.56–0.67]). As shown in Supplementary Figure 2, compared with warfarin use, the use of edoxaban was significantly associated with decreased risks of SSE (1.16 vs. 3.84%; HR, 0.71 [95% CI, 0.60–0.83]) and major bleeding (0.88 vs. 2.80%; HR, 0.58 [95% CI, 0.45–0.74]).
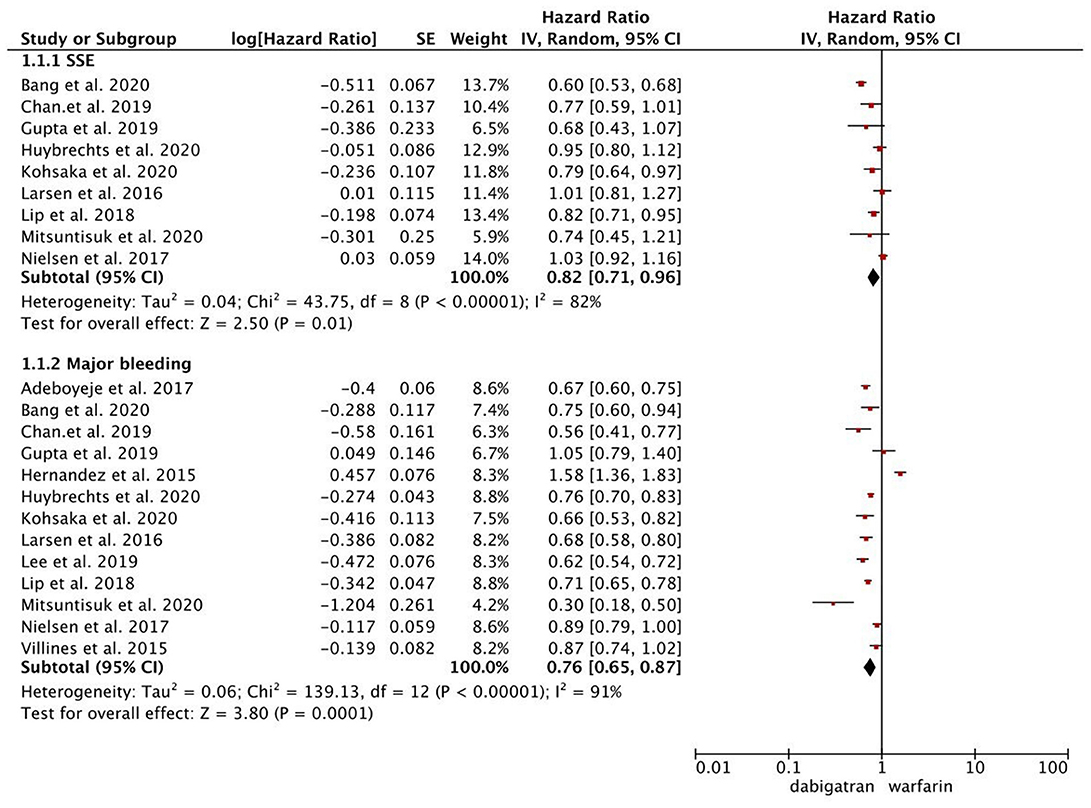
Figure 1. Comparing the primary outcomes including SSE and MB of dabigatran vs. warfarin. SSE, stroke or systemic embolism; MB, major bleeding; HR, hazard ratio; CI, confidence interval; SE, standard error; IV, inverse of the variance.
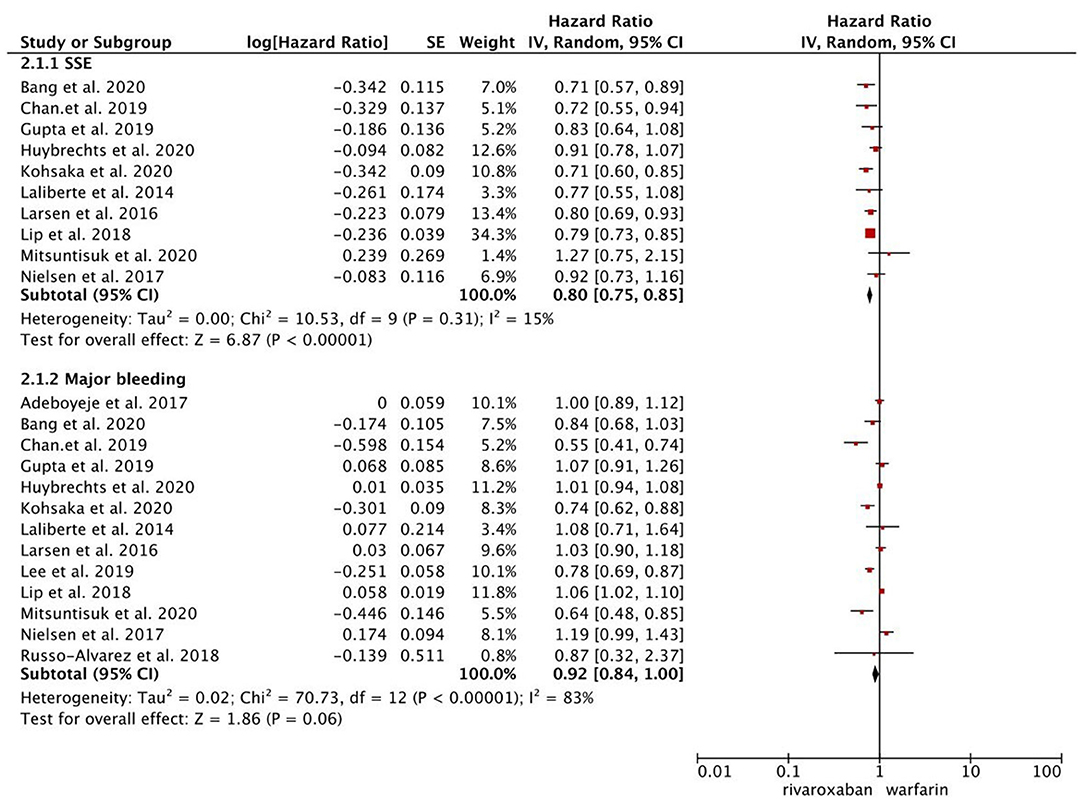
Figure 2. Comparing the primary outcomes including SSE and MB of rivaroxaban vs. warfarin. SSE, stroke or systemic embolism; MB, major bleeding; HR, hazard ratio; CI, confidence interval; SE, standard error; IV, inverse of the variance.
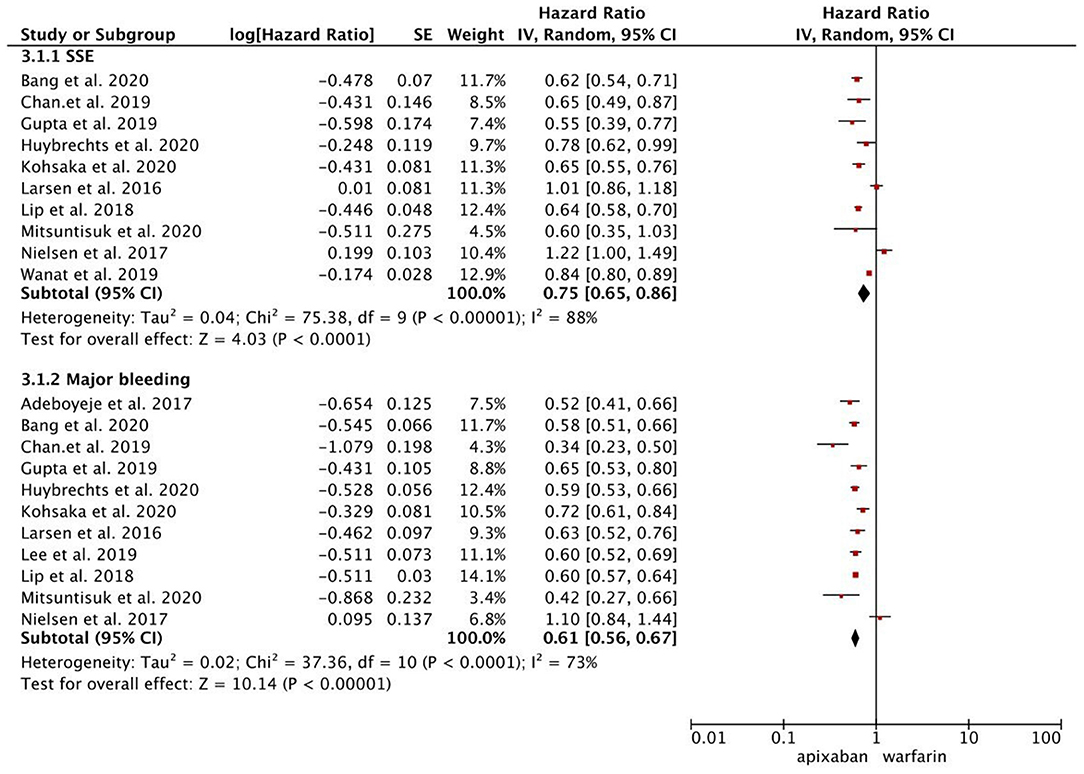
Figure 3. Comparing the primary outcomes including SSE and MB of apixaban vs. warfarin. SSE, stroke or systemic embolism; MB, major bleeding; HR, hazard ratio; CI, confidence interval; SE, standard error; IV, inverse of the variance.
Compared with warfarin, dabigatran was associated with reduced risks of ischemic stroke (HR, 0.93 [95% CI, 0.86–1.00]) and intracranial hemorrhage (HR, 0.46 [95% CI, 0.38–0.55]), but had similar risks of all-cause death and gastrointestinal bleeding (Supplementary Figure 3). As for rivaroxaban vs. warfarin shown in Supplementary Figure 4, it was associated with reduced risks of ischemic stroke (HR, 0.84 [95% CI, 0.79–0.90]) and intracranial hemorrhage (HR, 0.69 [95% CI, 0.63–0.76]), but had comparable risks of all-cause death and gastrointestinal bleeding. The use of apixaban vs. warfarin was significantly associated with reduced risks of ischemic stroke (HR, 0.73 [95% CI, 0.62–0.86]), intracranial hemorrhage (HR, 0.62 [95% CI, 0.50–0.75]), and gastrointestinal bleeding (HR, 0.63 [95% CI, 0.54–0.73]), but displayed no difference in all-cause death (Supplementary Figure 5). The use of edoxaban vs. warfarin was related to a decreased risk of ischemic stroke (HR, 0.67 [95% CI, 0.59–0.76]), whereas similar risks were observed in intracranial hemorrhage and gastrointestinal bleeding between the two study groups (Supplementary Figure 2).
In the sensitivity analysis, the results of the primary outcomes from the IVhet or QE models (Supplementary Figures 6–9) were similar to those from the primary analysis using the random-effects model. In addition, the results did not change substantially when we re-conducted the analyses using the fixed-effects model (Supplementary Table 4).
As shown in Supplementary Table 4, the subgroup analyses concerning the primary outcomes suggested no significant interactions grouped by the NOAC-dose and follow-up period. For the subgroup analysis based on the regions, Asians showed fewer risks of SSE and major bleeding than non-Asians in the group of dabigatran vs. warfarin. In the group of rivaroxaban vs. warfarin, Asians showed fewer risks of major bleeding compared with non-Asians. In the group of apixaban vs. warfarin, the risk of SSE was significantly lowered in Asians compared with non-Asians. There were not enough studies for the subgroup analyses between edoxaban vs. warfarin.
Comparative effect estimates of NOACs vs. warfarin between observational studies and RCTs are shown in Table 3. For the primary outcomes, dabigatran 150 mg vs. warfarin had a significantly lower risk of major bleeding in the observational studies (HR, 0.72 [95% CI, 0.66–0.78]) than that in the RE-LY trial (HR, 0.93 [95% CI, 0.81–1.07]) (Pinteraction = 0.002). In other comparisons, the pooled effects of the observational studies were consistent with data from the corresponding NOAC trials.
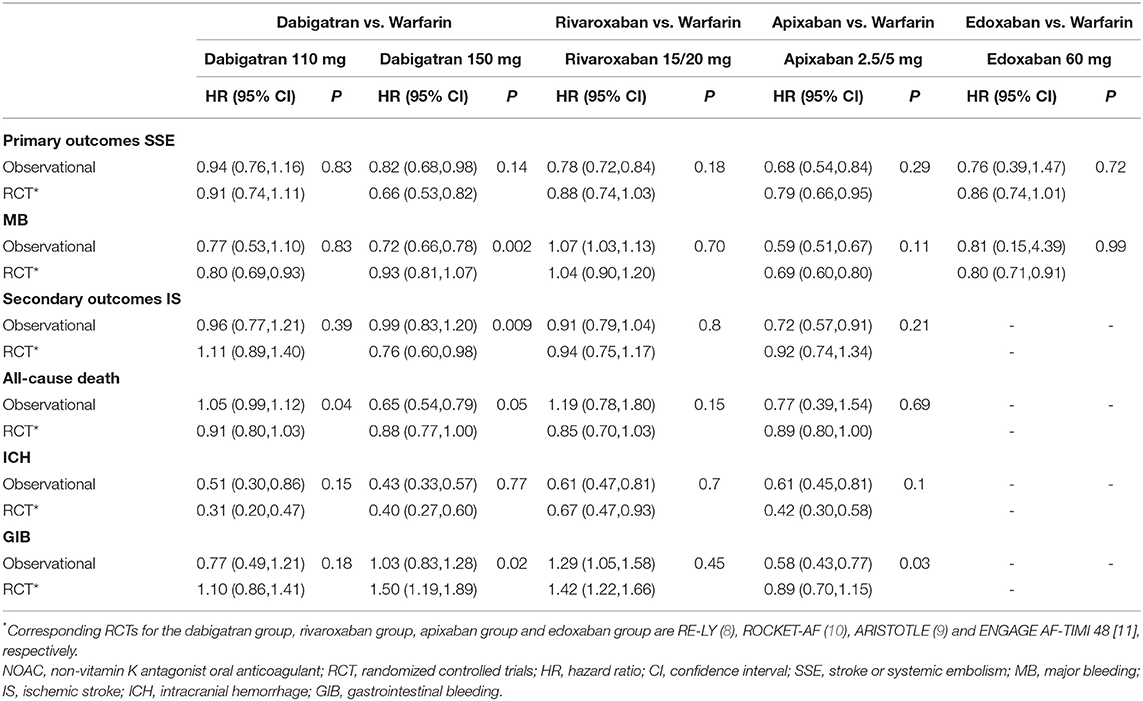
Table 3. Comparing total effect estimates of NOACs vs. warfarin between observational studies and RCTs.
For the secondary outcomes, dabigatran 110 mg vs. warfarin demonstrated a higher risk of all-cause death in observational studies (HR, 1.05 [95% CI, 0.99–1.12]) than that in the RE-LY trial (HR, 0.91 [95% CI, 0.80–1.03]) (Pinteraction = 0.04). Dabigatran 150 mg vs. warfarin showed a lower risk of gastrointestinal bleeding in observational studies (HR, 1.03 [95% CI, 0.83–1.28]) compared with that in the RE-LY trial (HR, 1.50 [95% CI, 1.19–1.89]) (Pinteraction = 0.02). The pooled HR of apixaban 5/2.5 mg vs. warfarin for gastrointestinal bleeding was significantly lower in observational studies (HR, 0.58 [95% CI, 0.43–0.77]) compared to that of the ARISTOTLE trial (HR, 0.89 [95% CI, 0.70–1.15]) (Pinteraction = 0.03). Meanwhile, all the effect estimates of rivaroxaban vs. warfarin were similar between observational studies and the ROCKET AF trial, whereas no enough studies assessed the secondary outcomes of edoxaban vs. warfarin between observational studies and the ENGAGE AF-TIMI 48 trial.
For the observational studies, there were no potential publication biases when inspecting the funnel plots of the primary outcomes (Supplementary Figures 10–13). In addition, the Begg's and Egger's tests also proved no significant publication biases (all P > 0.1; Supplementary Table 5). For the secondary outcomes, the Egger's test showed a potential publication bias in intracranial hemorrhage of the dabigatran vs. warfarin group, and ischemic stroke of the rivaroxaban vs. warfarin group. Nevertheless, the results from the trim-and-fill analysis suggested no trimming performed, and the corresponding pooled results were not changed. For the RCTs, there was no need for publication bias analysis because only four NOAC trials were included.
In the current meta-analysis, we compared the studied outcomes between NOACs and warfarin by only included the PS-based observational studies. Based on the observational studies, the results from different pooled models consistently suggested that compared with warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban were associated with a reduced risk of SSE, whereas dabigatran, apixaban, and edoxaban but not rivaroxaban was associated with a decreased risk of major bleeding. We further tested whether the pooled results of high-quality observational studies were consistent with data from the corresponding RCTs. The risk of major bleeding with dabigatran 150 mg was significantly lower in observational studies than that in the RE-LY trial, whereas the pooled results of observational studies were consistent with data from the corresponding RCTs in other comparisons for both SSE and major bleeding.
Over the past few decades, vitamin K antagonists such as warfarin have been confirmed to be effective for preventing stroke in AF patients (33). However, the shortcomings of warfarin mainly include slow onset time, the significantly varied dose-response relationship among patients, narrow therapeutic window, and frequent interactions with other drugs, potentially limiting its clinical applications (34). Nowadays, there is increasing use of NOACs because they could be more effective, easier to control, and safer than warfarin (7). Previous NOAC trials (RE-LY, ROCKET-AF, ARISTOTLE, and ENGAGE-AF TIMI 48) suggested that NOACs were comparable to warfarin in efficacy, but NOACs significantly reduced the risk of bleeding. Based on data of NOAC trials, current guidelines have recommended NOACs as the first-line drugs for the prevention of thrombogenesis and stroke in patients with nonvalvular AF (5). Although, RCTs have always been hailed as the gold standard for clinical efficacy evaluation, their results may not be well applicable in practice. At this time, observational studies can be a useful complement (35).
Nowadays, clinical practice-based data are increasingly used to evaluate the effectiveness and safety profiles of NOACs compared to warfarin. Xue et al. (34) compared the overall effectiveness and safety outcomes of three NOACs (dabigatran, rivaroxaban, and apixaban) with warfarin in Asians with AF. Based on the real-world studies, the authors demonstrated that in Asians with AF, the use of NOACs could have potential advantages in all the effectiveness and safety profiles when compared to warfarin irrespective of the type and drug doses. Nevertheless, the heterogeneous real-world studies without proper methods to balance the covariate distribution could be influenced by the potential confounders (36), thus potentially influencing the reliability of results. The PS methods including PSM and IPTW are the most frequently used methods to deal with this issue. The PS methods comprehensively consider all measured characteristic variables, especially confounding factors, making the matched sample more similar to the population of an RCT. PSM can match the treatment and non-treatment group based on the PS from low to high, and thus it can control multiple confounders at the same time by only using the matching of PS (37). IPTW is capable of eliminating confounders by conforming to the distribution of PS in each group (37). However, PSM and IPTW are often failed to be properly conducted (36). Therefore, to further improve the reliability of the study outcomes and reduce the influence of confounding factors, PS diagnostics such as standardized differences, C-statistic, and eye-balling could be conducted after PSM or IPTW. Standardized differences are an attribute of the sample, independent of the sample size. It is easy to compute and understand and is the most commonly used diagnostic method to measure the balance of covariate distribution between treatment groups (36, 38). In our current analysis, all of the 20 observational studies applied PSM or IPTW to balance the covariates between NOACs and warfarin regimen group. For the PS diagnostics, 14 studies used standardized differences, and 6 studies failed to report any further diagnostic use.
Reaching an agreement between RCTs and observational studies can greatly improve the accuracy of the results and offer more confidence in the reference of clinical routine practice. It is still known that whether the findings of observational studies were consistent with data from the NOAC trials. Siontis et al. (35) compared the consistency between RCTs and observational studies of the profiles of NOACS and warfarin. The authors found that the effect of NOACs and warfarin were consistent between RCTs and observational studies for most outcomes. However, some exceptions appeared in the dabigatran vs. warfarin group. The RE-LY trial found an increased risk of myocardial infarction in patients treated with dabigatran 150 mg compared with patients using warfarin, whereas the reverse outcomes were found in observational studies. Also, significantly higher risks of major and gastrointestinal bleeding were found in observational studies when compared to the RE-LY trial in the dabigatran group. Conversely, the data of the RE-LY trial demonstrated a lower rate of SSE compared with that of the observational studies. However, Siontis et al. did not describe the baseline characteristics of the treated and non-treated groups in detail, nor did they clarify the statistical methods used in the included studies. Lacking rigorous study design and statistical analysis could make the results easily affected by confounding bias, and thus reduced its reliability. Given these issues, we decided to conduct a more comprehensive meta-analysis by only included the PS-based observational studies. In our analysis, the results of the effectiveness and safety profiles are largely in agreement with some discrepancies that mainly happened in the dabigatran vs. warfarin group. The results of the consistency between the observational studies and RCTs of Siontis et al. are quite similar to our study.
There were still several limitations in this meta-analysis. First, most of the observational studies included were retrospective, and therefore, the association between the drug and the event outcomes rather than their causal relationships were evaluated. Second, despite the detailed information extracted from the included studies, there were still some articles that lack major data (e.g., drug dosage, follow-up period of NOAC treatment) which may provide potential uncertainties to the results. Third, several important cardiovascular events including myocardial infarction were not included in our analysis due to a lack of data. Fourth, in this meta-analysis, we did not include observational studies that only focused on the special populations with AF. Nevertheless, we have previously discussed the effect of NOACs in the special AF populations (e.g., chronic kidney disease, hypertrophic cardiomyopathy, peripheral artery disease, prior stroke) (39–42). Finally, although we included comparisons of outcomes between edoxaban and warfarin, we still failed to assess the results for some outcomes due to insufficient data.
This meta-analysis suggested that the use of NOACs for stroke prevention in AF was non-inferior or even superior to warfarin based on data from PS-based observational studies. The consistency between the observational studies and corresponding RCTs further confirmed this view.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This study was funded by National Natural Science Foundation of China (8210020907), China National Postdoctoral Program for Innovative Talents (BX20200400), and China Postdoctoral Science Foundation (2020M673016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.757188/full#supplementary-material
1. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. (2015) 313:1950–62. doi: 10.1001/jama.2015.4369
2. Jeong HK, Lee KH, Park HW, Yoon NS, Kim MC, Lee N, et al. Real world comparison of rivaroxaban and warfarin in korean patients with atrial fibrillation: propensity matching cohort analysis. Chonnam Med J. (2019) 55:54–61. doi: 10.4068/cmj.2019.55.1.54
3. Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. (2018) 49:2933–44. doi: 10.1161/STROKEAHA.118.020232
4. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JJ, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of Cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000665
5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
6. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of Non-Vitamin k antagonist oral anticoagulants in patients with atrial fibrillation. Europace. (2021). doi: 10.1093/europace/euab157. [Epub ahead of print].
7. Bradley M, Welch EC, Eworuke E, Graham DJ, Zhang R, Huang TY. Risk of stroke and bleeding in atrial fibrillation treated with apixaban compared with warfarin. J Gen Intern Med. (2020) 35:3597–604. doi: 10.1007/s11606-020-06180-8
8. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
9. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 365:981–92. doi: 10.1056/NEJMoa1107039
10. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
11. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
12. Collins R, Bowman L, Landray M, Peto R. The magic of randomization versus the myth of real-world evidence. N Engl J Med. (2020) 382:674–8. doi: 10.1056/NEJMsb1901642
13. Gershon AS, Jafarzadeh SR, Wilson KC, Walkey AJ. Clinical knowledge from observational studies. Everything you wanted to know but were afraid to ask. Am J Respir Crit Care Med. (2018) 198:859–67. doi: 10.1164/rccm.201801-0118PP
14. Dahabreh IJ, Sheldrick RC, Paulus JK, Chung M, Varvarigou V, Jafri H, et al. Do observational studies using propensity score methods agree with randomized trials? A systematic comparison of studies on acute coronary syndromes. Eur Heart J. (2012) 33:1893–901. doi: 10.1093/eurheartj/ehs114
15. Mitsuntisuk P, Nathisuwan S, Junpanichjaroen A, Wongcharoen W, Phrommintikul A, Wattanaruengchai P, et al. Real-World comparative effectiveness and safety of non-vitamin k antagonist oral anticoagulants vs. Warfarin in a developing country. Clin Pharmacol Ther. (2021) 109:1282–92. doi: 10.1002/cpt.2090
16. Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. (2017) 356:j510. doi: 10.1136/bmj.j510
17. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. (2016) 353:i3189. doi: 10.1136/bmj.i3189
18. Kohsaka S, Katada J, Saito K, Jenkins A, Li B, Mardekian J, et al. Safety and effectiveness of non-vitamin K oral anticoagulants versus warfarin in real-world patients with non-valvular atrial fibrillation: a retrospective analysis of contemporary Japanese administrative claims data. Open Heart. (2020) 7:e1232. doi: 10.1136/openhrt-2019-001232
19. Lee SR, Choi EK, Kwon S, Han KD, Jung JH, Cha MJ, et al. Effectiveness and safety of contemporary oral anticoagulants among Asians with non-valvular atrial fibrillation. Stroke. (2019) 50:2245–49. doi: 10.1161/STROKEAHA.119.025536
20. Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S, et al. Effectiveness and safety of Non-Vitamin k antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. (2017) 48:3040–8. doi: 10.1161/STROKEAHA.117.018773
21. Bang OY, On YK, Lee MY, Jang SW, Han S, Han S, et al. The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: results from a real-world data analysis. PLoS ONE. (2020) 15:e242922. doi: 10.1371/journal.pone.0242922
22. Chan YH, Lee HF, See LC, Tu HT, Chao TF, Yeh YH, et al. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest. (2019) 156:529–543. doi: 10.1016/j.chest.2019.04.108
23. Laliberté F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. (2014) 30:1317–25. doi: 10.1185/03007995.2014.907140
24. Wanat MA, Wang X, Paranjpe R, Chen H, Johnson ML, Fleming ML, et al. Warfarin vs. Apixaban in nonvalvular atrial fibrillation, and analysis by concomitant antiarrhythmic medication use: a national retrospective study. Res Pract Thromb Haemost. (2019) 3:674–83. doi: 10.1002/rth2.12221
25. Gupta K, Trocio J, Keshishian A, Zhang Q, Dina O, Mardekian J, et al. Effectiveness and safety of direct oral anticoagulants compared to warfarin in treatment naïve non-valvular atrial fibrillation patients in the US Department of defense population. BMC Cardiovasc Disord. (2019) 19:142. doi: 10.1186/s12872-019-1116-1
26. Villines TC, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, et al. A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. (2015) 114:1290–298. doi: 10.1160/TH15-06-0453
27. Russo-Alvarez G, Martinez KA, Valente M, Bena J, Hu B, Luxenburg J, et al. Thromboembolic and major bleeding events with rivaroxaban versus warfarin use in a real-world setting. Ann Pharmacother. (2018) 52:19–25. doi: 10.1177/1060028017727290
28. Adeboyeje G, Sylwestrzak G, Barron JJ, White J, Rosenberg A, Abarca J, et al. Major bleeding risk during anticoagulation with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. (2017) 23:968–78. doi: 10.18553/jmcp.2017.23.9.968
29. Chang HY, Zhou M, Tang W, Alexander GC, Singh S. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. (2015) 350:h1585. doi: 10.1136/bmj.h1585
30. Hernandez I, Baik SH, Piñera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. Jama Intern Med. (2015) 175:18–24. doi: 10.1001/jamainternmed.2014.5398
31. Huybrechts KF, Gopalakrishnan C, Bartels DB, Zint K, Gurusamy VK, Landon J, et al. Safety and effectiveness of dabigatran and other direct oral anticoagulants compared with warfarin in patients with atrial fibrillation. Clin Pharmacol Ther. (2020) 107:1405–19. doi: 10.1002/cpt.1753
32. Go AS, Singer DE, Toh S, Cheetham TC, Reichman ME, Graham DJ, et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice: a retrospective cohort study. Ann Intern Med. (2017) 167:845–54. doi: 10.7326/M16-1157
33. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
34. Xue Z, Zhang H. Non-Vitamin k antagonist oral anticoagulants versus warfarin in asians with atrial fibrillation: meta-analysis of randomized trials and real-world studies. Stroke. (2019) 50:2819–28. doi: 10.1161/STROKEAHA.119.026054
35. Siontis KC, Checkole S, Yao X, Gersh BJ, Noseworthy PA. Do observational studies agree with randomized trials?: evaluation of oral anticoagulants in atrial fibrillation. J Am Coll Cardiol. (2020) 75:562–3. doi: 10.1016/j.jacc.2019.12.007
36. Zhang Z, Kim HJ, Lonjon G, Zhu Y. Balance diagnostics after propensity score matching. Ann Transl Med. (2019) 7:16. doi: 10.21037/atm.2018.12.10
37. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
38. Granger E, Watkins T, Sergeant JC, Lunt M. A review of the use of propensity score diagnostics in papers published in high-ranking medical journals. BMC Med Res Methodol. (2020) 20:132. doi: 10.1186/s12874-020-00994-0
39. Chen C, Cao Y, Zheng Y, Dong Y, Ma J, Zhu W, et al. Effect of rivaroxaban or apixaban in atrial fibrillation patients with stage 4–5 chronic kidney disease or on dialysis. Cardiovasc Drug Ther. (2021) 35:273–81. doi: 10.1007/s10557-021-07144-8
40. Zhou Y, He W, Zhou Y, Zhu W. Non-vitamin K antagonist oral anticoagulants in patients with hypertrophic cardiomyopathy and atrial fibrillation: a systematic review and meta-analysis. J Thromb Thrombolys. (2020) 50:311–7. doi: 10.1007/s11239-019-02008-3
41. Liao X, Fu Y, Ma J, Zhu W, Yuan P. Non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and peripheral artery disease: a systematic review and meta-analysis. Cardiovasc Drug Ther. (2020) 34:391–9. doi: 10.1007/s10557-020-06962-6
Keywords: anticoagulants, atrial fibrillation, propensity score, outcomes, meta-analysis
Citation: Liu F, Yang Y, Cheng W, Ma J and Zhu W (2021) Reappraisal of Non-vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:757188. doi: 10.3389/fcvm.2021.757188
Received: 11 August 2021; Accepted: 21 September 2021;
Published: 15 October 2021.
Edited by:
Roberto Pola, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Håkan Wallen, Karolinska Institutet (KI), SwedenCopyright © 2021 Liu, Yang, Cheng, Ma and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuwei Liu, Z3psaXVmdXdlaUAxNjMuY29t; Wengen Zhu, emh1d2c2QG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.