- Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Aim: The connection between revascularization for coronary artery disease (CAD) and the incidence of recurrent events of atrial fibrillation (AF) after ablation is unclear. This study aimed to explore the relationship between coronary revascularization and AF recurrence in patients who underwent radiofrequency catheter ablation (RFCA).
Methods: Four hundred and nineteen patients who underwent performed coronary angiography at the same time as RFCA were enrolled in this study. Obstructive CAD was defined as at least one coronary artery vessel stenosis of ≥75% and percutaneous coronary intervention (PCI) was recommended. Non-obstructive CAD was defined as coronary artery vessel stenosis of <75%. The endpoint was freedom from recurrence from AF after RFCA during the 24-month follow-up.
Results: In total, 102, 95, and 212 patients were undergone coronary angiography and diagnosed as having obstructive CAD, Non-obstructive CAD, and Non-CAD, respectively. During the 24-month follow-up period, patients without obstructive CAD were significantly more likely to achieve freedom from AF than patients with obstructive CAD (hazard ratio [HR]: 1.72; 95% confidence interval [CI]: 1.23–2.41; P = 0.001). The recurrence rate of AF was significantly lower in patients who underwent PCI than in those who did not (HR: 0.45; 95% CI: 0.25–0.80; P = 0.007). The multivariate regression analysis showed that the other predictors of AF recurrence for obstructive CAD were multivessel stenosis (HR: 1.92; 95% CI: 1.04–3.54; P = 0.036) and left atrial diameter (HR: 2.56; 95% CI: 1.31–5.00; P = 0.006).
Conclusions: This study suggests that obstructive CAD is associated with a higher rate of AF recurrence. Additionally, For patients with CAD, coronary revascularization is related to a lower recurrence rate of AF after RFCA.
Introduction
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia, and its incidence increases significantly with age (1). Patients with AF have a significantly higher risk of stroke and mortality (2). Coronary artery disease (CAD) is a common cardiovascular disease and a leading cause of mortality (3). Studies have revealed that the prevalence of CAD in patients with AF ranges from 17 to 46.5% (4–8). Multiple clinical risk factors, including hypertension, diabetes mellitus, increasing age, obesity, and sleep apnea, are shared by both diseases (9). Because the risks for both diseases overlap, CAD is likely to coexist with AF, and patients often undergo percutaneous coronary intervention (PCI) as treatment (10).
Radiofrequency catheter ablation (RFCA) is an important treatment strategy for patients with symptomatic drug-refractory AF (11). A previous meta-analysis proved that RFCA for AF had a higher efficacy rate than antiarrhythmic drug (AAD) therapy while having a lower rate of complications (12). However, the reported success rates of radiofrequency ablation in the treatment of AF are inconsistent. The success rate of atrial fibrillation after a single ablation procedure from different studies varies from 50 to 70% (13–16). Therefore, the success rate of RFCA is not very high. In addition to RFCA therapy, PCI is required for AF and CAD patients with severe coronary artery stenosis. Few studies have investigated the association between revascularization for CAD and recurrence of AF after RFCA. Our study aimed to investigate the relationship between CAD and AF recurrence in patients who underwent RFCA.
Materials and Methods
Study Population
Between January 2017 and January 2019, 2,642 consecutive patients with AF had undergone RFCA in our center. Of these patients, 443 underwent coronary angiography 48 h before the RFCA because of suspected CAD. Of the 443 patients, 18 were excluded due to a previous history of myocardial infarction, and 6 patients could not be followed up. Finally, 419 patients were recruited for the analysis. Obstructive CAD and Non-obstructive CAD were confirmed by coronary angiography and PCI were recommended for patients with obstruction CAD. After RFCA, all patients were followed up and recurrence of AF was investigated in the next 24 months. Study design and flow are presented in Figure 1. This study was approved by the local ethics committee and complied with the Declaration of Helsinki. All patients provided informed consent for the ablation and PCI procedures, which were performed in accordance with the relevant guidelines.
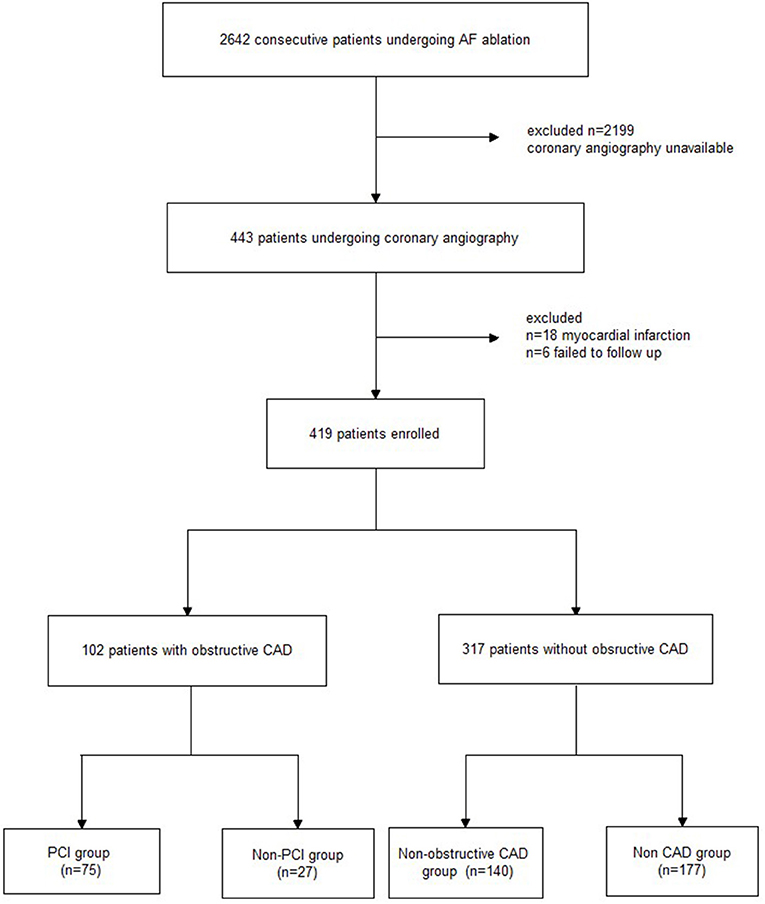
Figure 1. Study flowchart. AF, atrial fibrillation; CAD, coronary artery disease; PCI, percutaneous coronary intervention.
Ablation Procedure
For all enrolled patients, oral anticoagulant drugs (non-vitamin K antagonist oral anticoagulants, NOACs) were administered to the patients, and transoesophageal echocardiography was performed to rule out the possibility of an actual thrombus before the procedure. After the transseptal puncture, unfractionated heparin was administered at 100 U/kg and thereafter at 1,000 U/h to maintain the activated clotting time between 300 and 400 s. Under the guidance of the electroanatomical mapping system (CARTO, Biosense Webster, Diamond Bar, CA, USA), circumferential pulmonary vein isolation (PVI) was performed with the endpoint being the dissociation of all pulmonary vein (PV) potentials and a bidirectional conduction block from the atrium to the PV. Sinus rhythm was restored by cardioversion if AF was sustained after PVI. For patients with persistent AF, linear ablation with the endpoint being a bidirectional block and/or superior vena cava isolation was performed at the operator's discretion. An irrigated catheter was used in the procedure, and the radio frequency power output was usually up to 35–40 W with a maximum temperature of 45°C for each lesion and an irrigation flow of 20–25 mL/min. At the end of the procedure, the PVI and bidirectional block of the lines were evaluated, and the procedure was consolidated if necessary.
Percutaneous Coronary Intervention
PCI was recommended when obstructive CAD (at least one coronary artery vessel had ≥75% stenosis) was identified by coronary angiography (intravascular ultrasound was required if visual critical stenosis was not confirmed). Non-obstructive CAD was confirmed if the coronary artery vessel had <75% stenosis. Before PCI, all patients received dual antiplatelet therapy, including aspirin (100 mg/d) combined with clopidogrel (75 mg/d). Anticoagulant drugs were not discontinued during the perioperative PCI period. Dual antiplatelet therapy combined with anticoagulant drug treatment was prescribed for 3 months. Then, a single antiplatelet drug (clopidogrel) combined with an anticoagulant drug was prescribed. Clopidogrel was continued for at least 12 months after PCI.
Antiarrhythmic Drugs and Blanking Period Management
The first 3 months after RFCA is defined as the blanking period. Oral AADs were administered during the blanking period. Amiodarone was given orally at a dose of 600 mg/day for the first week, 400 mg/day for the second week, and then at a maintenance dose of 200 mg/day. Propafenone were prescribed if the patients could not tolerate amiodarone. Oral AAD treatment was used for 3 months after RFCA according to the physician's decision.
Follow-Up and Complications
All patients were followed up at 1, 2, 3, and 6 months after the procedure every 3 months in the first year and every 6 months thereafter. At each hospital visit, participants underwent 24-h Holter monitoring and a 12-lead electrocardiogram (ECG). The participants were instructed to obtain an ECG or Holter ECG when they had symptoms indicative of arrhythmia recurrence. Any over-30s episode of atrial tachyarrhythmia (including AF/AT) on ECG was defined as AF recurrence. The study endpoint was freedom from any atrial tachyarrhythmia lasting >30 s after the ablation procedure. A total of four people had gastrointestinal bleeding during the follow-up. Two of them were in the revascularization group. One patient had melena and upper gastrointestinal bleeding in the first month and another patient had gastrointestinal bleeding in the second month after the RFCA. Clopidogrel + NOAC were prescribed instead of triple antithrombotic therapy. The other two patients were in the non-CAD group. After treatment, the patient's bleeding was controlled. No cerebral hemorrhage occurred during the follow-up period.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as counts and percentages. Two-group comparisons of continuous variables were performed using the Student's t-test. Categorical variables in the two groups were compared using the χ2 test. Event-free survival in the two groups was estimated using a Kaplan-Meier analysis with the log-rank test. Arrhythmia recurrence and covariate associations were assessed using multivariate proportional hazard regression models. For each of the selected variables, hazard ratios with corresponding 95% confidence limits and Wald test P-values of the multivariable model were reported. In all analyses, a two-tailed P-value of 0.05 was regarded as statistically significant. All statistical analyses were performed using SPSS (version 17.0; IBM, Armonk, NY, USA).
Results
Patient Characteristics
Out of a total of 419 patients, obstructive CAD was diagnosed in 102 patients (24.3%) according to the extent of coronary stenosis. Patients with obstructive CAD had significantly higher rates of hypertension, dyslipidaemia, diabetes mellitus, and CHA2DS2-VASC scores than those without obstructive CAD. In addition, the obstructive CAD group had a greater left atrial diameter. The differences in the baseline characteristics are summarized in Table 1.
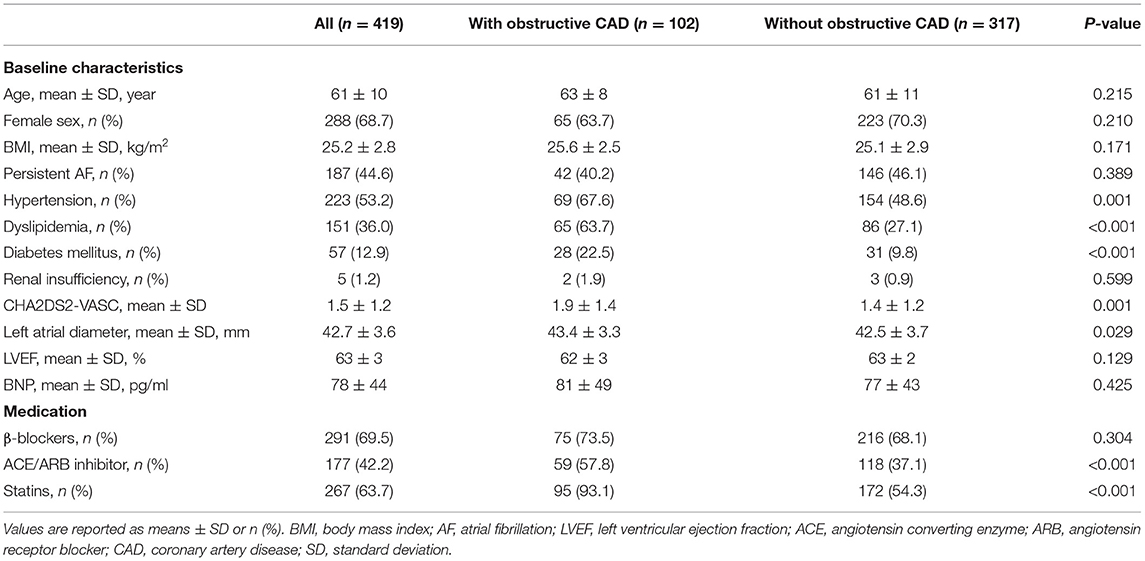
Table 1. Baseline characteristics of the patients with and without obstructive coronary artery disease.
Association Between AF Recurrence and CAD
Of the 419 patients, approximately 61.1% (256 of 419) had no AF recurrence by the end of the 24-month follow-up period. After the ablation, angiotensin receptor blockers (ACEI/ARB) were used more frequently in patients with obstructive CAD than in patients without obstructive CAD (ACEI: 57.8 vs. 37.1%, P < 0.001; ARB: 93.1 vs. 54.3%, P < 0.001). The use of β-blockers was not significantly different between the two groups (Table 1). Patients without obstructive CAD showed a significantly higher proportion of freedom from AF than those in the obstructive CAD group (65 vs. 49%, P = 0.003; Figure 2). The adjusted regression analysis showed a lower rate of recurrence with an estimated hazard ratio of 1.72 [95% confidence interval (CI): 1.23–2.41, P = 0.001], favoring the group without obstructive CAD. The multivariate regression analysis showed that the predictors of AF recurrence after ablation were persistent AF (hazard ratio [HR]: 2.41; 95% CI: 1.72–3.38, P < 0.001) and left atrial diameter (HR: 2.59; 95% CI: 1.79–3.74; P < 0.001) (Table 2).
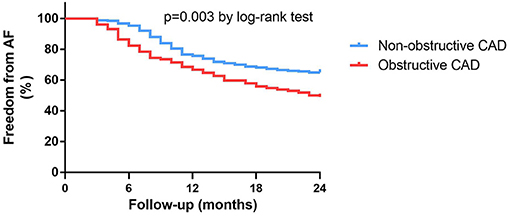
Figure 2. Kaplan-Meier survival curves for freedom from atrial fibrillation recurrence in patients with or without obstructive CAD at 24-month follow-up.
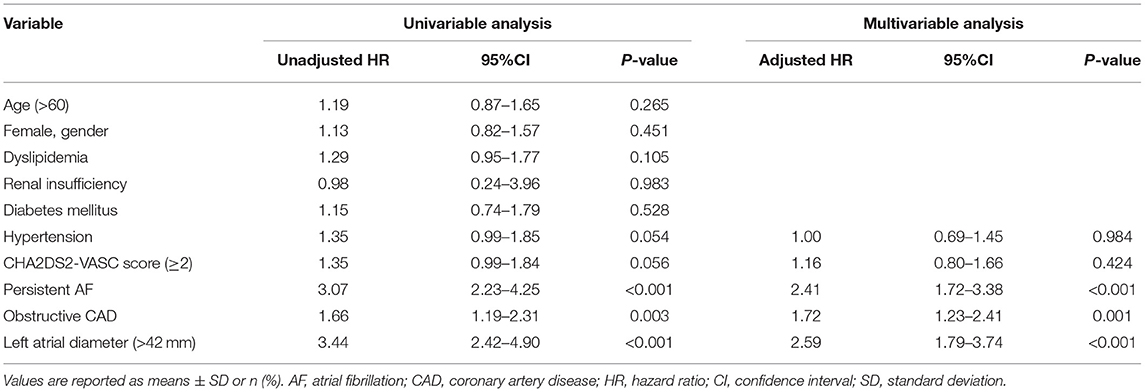
Table 2. Univariable and multivariable regression analyses of the recurrence of atrial fibrillation between the patients with obstructive CAD and without obstructive CAD.
Impact Factors for AF Recurrence in Patients With CAD
In general, 75 of 102 (73.5%) patients with obstructive CAD followed the doctor's recommendation and underwent successful PCI, while the other 27 (26.5%) patients chose to be treated with medications. Coronary angiography showed that 24 of 102 (23.5%) patients had more than one coronary artery stenosis. Patients with multi-vessel stenosis (≥2) had a higher rate of diabetes than patients with one coronary artery stenosis (66.6 vs. 8.9%, P < 0.001). Fourteen of the patients with multi-vessel stenosis received PCI treatment. The multivariable regression analysis showed that patients who chose PCI had a lower recurrence rate of AF than those without PCI (HR: 0.45; 95% CI: 0.25–0.80, P = 0.007, Figure 3). The other predictors of AF recurrence were multivessel stenosis (HR: 1.92; 95% CI: 1.04–3.54; P = 0.036) and left atrial diameter (HR: 2.56; 95% CI: 1.31–5.00; P = 0.006; Table 3).
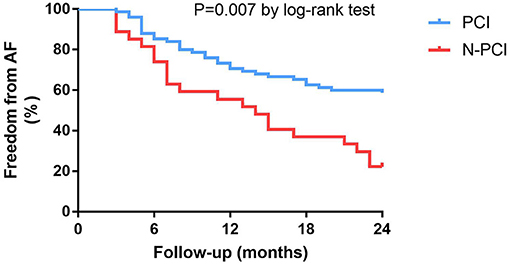
Figure 3. Kaplan-Meier survival curves for freedom from atrial fibrillation recurrence in patients with obstructive CAD at 24-month follow-up.
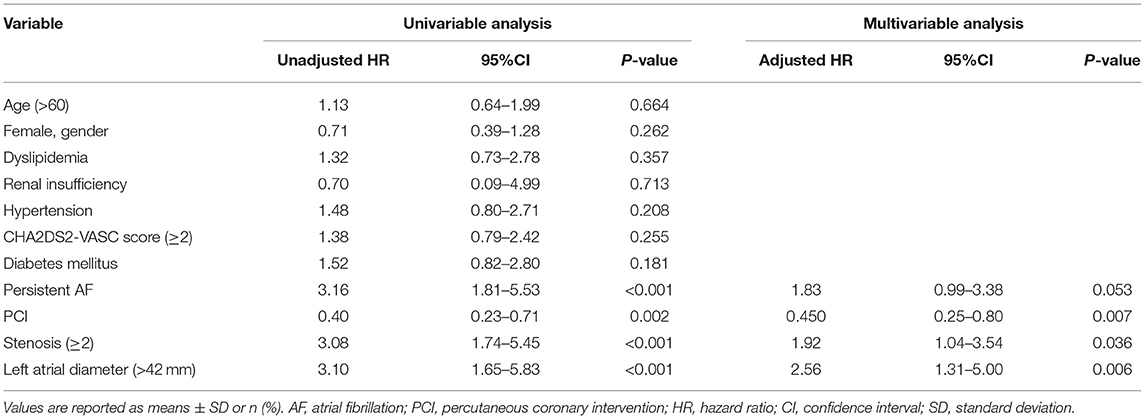
Table 3. Univariable and multivariable regression analyses of the recurrence of atrial fibrillation in the patients with obstructive CAD.
Subgroup Analysis of Recurrence in Patients With Non-obstructive CAD and Patients Without CAD
Of those who underwent coronary angiography, 140 (33.4%) were diagnosed as Non-obstructive CAD and 177 (42.2%) patients were diagnosed as without CAD. Non-obstructive CAD group had a similar recurrence of AF compared to Non-CAD group (40.7 vs. 30.5%, P = 0.075). The univariable proportional hazards analyses showed that there was no significant difference in AF recurrence between the Non-obstructive CAD group and Non-CAD group (HR: 1.33; 95% CI: 0.92–1.93; P = 0.128). The predictors of AF recurrence were persistent AF (HR: 2.41; 95% CI: 1.59–3.66; P < 0.001) and left atrial diameter (HR: 2.68; 95% CI: 1.72–4.17; P < 0.001) (Table 4).
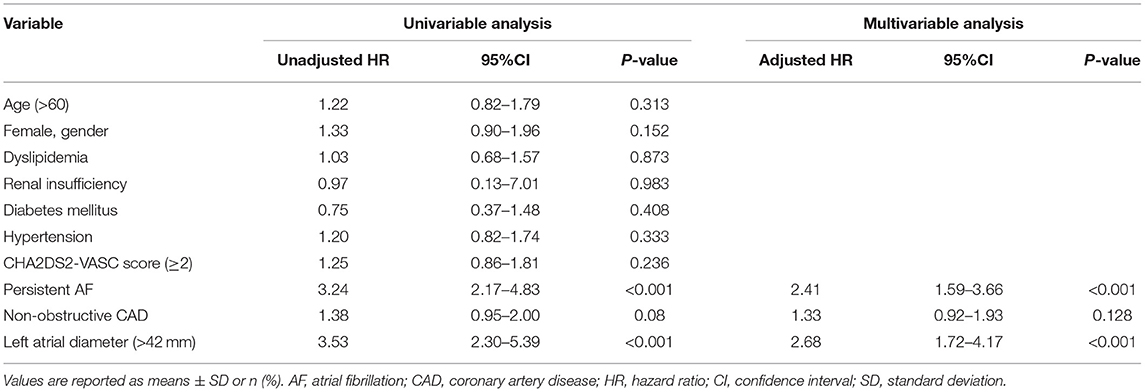
Table 4. Univariable and multivariable regression analyses of the recurrence of atrial fibrillation in the patients without obstructive CAD.
Discussion
In the present study, we analyzed the relationship between coronary revascularization and AF recurrence in patients who underwent initial RFCA. The main findings are summarized as follows. First, we found that patients with obstructive CAD were less likely to achieve freedom from AF compared to patients without obstructive CAD. Second, for patients with CAD, coronary revascularization was associated with a lower recurrence rate after the initial RFCA. The multivariable regression analysis confirmed that coronary revascularization, multivessel stenosis, and left atrial diameter were important predictive factors for the recurrence of AF in patients with obstructive CAD after RFCA. Finally, there were no significant differences in AF recurrence between patients with Non-obstructive CAD (coronary stenosis <75%) and those without CAD.
Coronary Artery Disease and Recurrence of AF
Since there are common risk factors in both diseases such as hypertension, diabetes mellitus, obesity, and sleep apnea, patients with AF often have coexisting CAD. The relationship between the AF substrate and chronic atrial ischemia has been demonstrated in previous animal experimental studies (17, 18). Right coronary artery occlusion promotes AF triggers and substrate formation, which facilitates the initiation and maintenance of AF (18). In addition, a substrate is formed to maintain AF, while acute atrial ischemia occurs after several hours (19). Acute atrial ischemia can lead to atrial effective refractory period shortening, increased conduction heterogeneity, and decreased conduction velocity (17, 20). Stable re-entrant sources at the border zone of an atrial myocardial infarction are associated with previous myocardial infarction fibrosis, which is an important reason for stabilizing re-entrant rotors. The myocardial fibers in the infarcted area give rise to conducting tracts that can set up a re-entry circuit. As described in an atrial myocardial infarction, fibrosis in the ventricle is also a factor in stabilizing re-entrant rotors (21), which can lead to fibrillatory conduction to maintain AF (18, 22). Another study from McLellan et al. (23) showed that ventricular fibrosis predicted the success of atrial fibrillation ablation.
The relationship between CAD and the outcome of AF after RFCA treatment has been analyzed in limited clinical studies. The Leipzig study, which enrolled 1,310 consecutive patients (12% patients with CAD), analyzed the impact of the presence and extent of CAD on recurrences after AF catheter ablation (24). The results demonstrated that there was no significant difference in arrhythmia recurrence rates in patients with and without CAD. In addition, the origin or extent of coronary stenosis was not associated with the recurrence ratio after catheter ablation in patients with or without CAD. Den Uijl's study recruited 125 AF patients and investigated the association of coronary atherosclerosis with the efficacy of RFCA during 12 month follow up (25). They found that the presence of coronary atherosclerosis is not associated with a higher risk for AF recurrence after RFCA. Another clinical study by Hiraya et al. revealed that there was a significantly higher rate of AF recurrence in patients with CAD than in patients without CAD (26). These results are inconsistent, highlighting the need for further research.
In the present study, persistent AF and left atrial diameter were predictors of AF recurrence after catheter ablation. We also found that patients with Non-obstructive CAD were significantly more likely to achieve freedom from AF than patients with obstructive CAD. The multivariate regression analysis showed that CAD was an independent risk factor for AF recurrence. CAD is a disease that could cause myocardial ischemia, which results in oxidative stress and atrial remodeling (27). Furthermore, myocardial ischemia can impair the left ventricle and increase the pressure in the left atrium, which causes changes in the tissue. These factors facilitate the initiation and recurrence of AF and reduce the success rate of RFCA. In the subgroup analysis, we compared AF recurrence after catheter ablation in patients with Non-obstructive CAD and those with normal coronary vessels. Interestingly, the results revealed that the recurrence of AF in patients with Non-obstructive CAD was similar to that of patients with normal coronary vessels, showing that patients with Non-obstructive CAD (vessel obstruction <75%) were not significantly different from those without coronary stenosis. It seems that the presence of coronary atherosclerosis does not impact the efficacy of RFCA because coronary atherosclerosis does not contribute to significant myocardial ischemia. Therefore, coronary atherosclerosis, which has no severe stenosis, has a limited impact on the recurrence of AF after RFCA, and other factors, such as persistent AF and left atrial diameter, limit the efficacy of RFCA.
Coronary Revascularization and Recurrence of AF
Few studies have examined the impact of revascularization on AF recurrence in patients with CAD. Hiraya et al. investigated the role of revascularization in patients with CAD after initial PVI (26). The patients with CAD who had undergone PCI before PVI had a lower recurrence rate than those who did not undergo PCI. Our study demonstrated that coronary revascularization for CAD reduces the recurrence of AF after catheter ablation, and this finding concurs with that of Hiraya's study. Furthermore, we found that patients with multivessel stenosis (≥2) had a higher recurrence rate than patients with single-vessel stenosis. In addition to left atrial diameter and coronary revascularization, the multivariable regression analysis also showed that multi-vessel stenosis (≥2) was an independent predictive factor for AF recurrence in patients with CAD. Multi-vessel stenosis, which causes a higher degree of ischemia, is an important reason for the increased pressure in the left atrium, as discussed above. Coronary revascularization may decrease atrial ischemia and left atrial pressure in patients with CAD and AF. Therefore, revascularization for patients with CAD was associated with a lower recurrence rate of AF after RFCA.
Limitations
This study had some inherent limitations. First of all, this study was a non-randomized study. There was a bias when selecting patients for coronary angiography. In many cases, it was difficult to distinguish chest symptoms between the discomfort caused by atrial fibrillation and angina due to CAD. Therefore, patients with atypical angina might not receive coronary angiography, On the other hand, it might cause a lower positive rate for patients undergoing coronary angiography. Secondly, when the coronary angiography results showed critical stenosis, although we used intravascular ultrasound to estimate the degree of obstruction, it still had the selection bias of CAD patients for revascularization. Thirdly, although we encouraged the patient to contact the physician in the case of suspected recurrence symptoms, data were limited by reliance on occasional and periodic ECG/Holter recording and clinical symptoms. Because asymptomatic arrhythmia episodes were difficult to be diagnosed, recurrence rates have been possibly underestimated.
Conclusion
This study suggests that obstructive CAD is associated with a higher rate of AF recurrence. Additionally, for patients with CAD, coronary revascularization is related to a lower recurrence rate of AF after RFCA.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The present study was performed by all authors. HT and XC contributed to the conception, design, and supervision of the study. XC, KZ, and JZ collected clinical information and performed data analysis. FQ and HL performed the clinical follow-up. XC wrote the manuscript. All authors discussed the results and contributed to the approval of the manuscript.
Funding
This work was supported by the Joint Project of Medical Science and Technology Research of Henan (grant no. LHGJ20190099).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the patients of this study for spending time as well as for their continued participation in our follow-up. We would like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.756552/full#supplementary-material
References
1. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. (2014). 64:e1–76. doi: 10.1016/j.jacc.2014.03.022
2. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. (1998) 82:2–9. doi: 10.1016/S0002-9149(98)00583-9
3. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. (2014) 35:2950–9. doi: 10.1093/eurheartj/ehu299
4. Crijns HJ, Van Gelder IC, Van Gilst WH, Hillege H, Gosselink AM, Lie KI. Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter. Am J Cardiol. (1991) 68:335–41. doi: 10.1016/0002-9149(91)90828-9
5. Baseline characteristics of patients with atrial fibrillation: the AFFIRM Study. Am Heart J. (2002) 143:991–1001. doi: 10.1067/mhj.2002.122875
6. Lip GY, Beevers DG. ABC of atrial fibrillation. History, epidemiology, and importance of atrial fibrillation. BMJ. (1995) 311:1361–3. doi: 10.1136/bmj.311.7016.1361
7. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. (1995) 98:476–84. doi: 10.1016/S0002-9343(99)80348-9
8. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. (2009) 360:668–78. doi: 10.1056/NEJMoa0803778
9. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
10. Sutton NR, Seth M, Ruwende C, Gurm HS. Outcomes of patients with atrial fibrillation undergoing percutaneous coronary intervention. J Am Coll Cardiol. (2016) 68:895–904. doi: 10.1016/j.jacc.2016.05.085
11. Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2007) 4:816–61. doi: 10.1016/j.hrthm.2007.04.005
12. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythmia Electrophysiol. (2009) 2:349–61. doi: 10.1161/CIRCEP.108.824789
13. Nilsson B, Chen X, Pehrson S, Køber L, Hilden J, Svendsen JH. Recurrence of pulmonary vein conduction and atrial fibrillation after pulmonary vein isolation for atrial fibrillation: a randomized trial of the ostial versus the extraostial ablation strategy. Am Heart J. (2006) 152:537. doi: 10.1016/j.ahj.2006.05.029
14. Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythmia Electrophysiol. (2010) 3:237–42. doi: 10.1161/CIRCEP.109.923771
15. Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. (2003) 42:185–97. doi: 10.1016/S0735-1097(03)00577-1
16. Bertaglia E, Tondo C, De Simone A, Zoppo F, Mantica M, Turco P, et al. Does catheter ablation cure atrial fibrillation? Single-procedure outcome of drug-refractory atrial fibrillation ablation: a 6-year multicentre experience. Europace. (2010) 12:181–7. doi: 10.1093/europace/eup349
17. Jayachandran JV, Zipes DP, Weksler J, Olgin JE. Role of the Na(+)/H(+) exchanger in short-term atrial electrophysiological remodeling. Circulation. (2000) 101:1861–6. doi: 10.1161/01.CIR.101.15.1861
18. Nishida K, Qi XY, Wakili R, Comtois P, Chartier D, Harada M, et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. (2011) 123:137–46. doi: 10.1161/CIRCULATIONAHA.110.972778
19. Rivard L, Sinno H, Shiroshita-Takeshita A, Schram G, Leung TK, Nattel S. The pharmacological response of ischemia-related atrial fibrillation in dogs: evidence for substrate-specific efficacy. Cardiovasc Res. (2007) 74:104–13. doi: 10.1016/j.cardiores.2007.01.018
20. Lammers WJ, Kirchhof C, Bonke FI, Allessie MA. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Am J Physiol. (1992) 262(1 Pt 2):H47–55. doi: 10.1152/ajpheart.1992.262.1.H47
21. de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, et al. Slow conduction in the infarcted human heart. ‘Zigzag' course of activation. Circulation. (1993). 88:915–26. doi: 10.1161/01.CIR.88.3.915
22. Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, et al. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. (2006) 113:626–33. doi: 10.1161/CIRCULATIONAHA.105.575340
23. McLellan AJ, Ling LH, Azzopardi S, Ellims AH, Iles LM, Sellenger MA, et al. Diffuse ventricular fibrosis measured by T1 mapping on cardiac MRI predicts success of catheter ablation for atrial fibrillation. Circ Arrhythmia Electrophysiol. (2014) 7:834–40. doi: 10.1161/CIRCEP.114.001479
24. Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Rolf S, et al. Presence and extent of coronary artery disease as predictor for AF recurrences after catheter ablation: the Leipzig Heart Center AF Ablation Registry. Int J Cardiol. (2015) 181:188–92. doi: 10.1016/j.ijcard.2014.12.039
25. den Uijl DW, Boogers MJ, Compier M, Trines SA, Scholte AJ, Zeppenfeld K, et al. Impact of coronary atherosclerosis on the efficacy of radiofrequency catheter ablation for atrial fibrillation. Eur. Heart J Cardiovasc Imaging. (2013) 14:247–52. doi: 10.1093/ehjci/jes144
26. Hiraya D, Sato A, Hoshi T, Watabe H, Yoshida K, Komatsu Y, et al. Impact of coronary artery disease and revascularization on recurrence of atrial fibrillation after catheter ablation: Importance of ischemia in managing atrial fibrillation. J Cardiovasc Electrophysiol. (2019) 30:1491–8. doi: 10.1111/jce.14029
Keywords: atrial fibrillation, radiofrequency catheter ablation, coronary artery disease, percutaneous coronary intervention, recurrence
Citation: Chen X, Zhao J, Zhu K, Qin F, Liu H and Tao H (2021) The Association Between Recurrence of Atrial Fibrillation and Revascularization in Patients With Coronary Artery Disease After Catheter Ablation. Front. Cardiovasc. Med. 8:756552. doi: 10.3389/fcvm.2021.756552
Received: 10 August 2021; Accepted: 27 October 2021;
Published: 19 November 2021.
Edited by:
Peter Larsen, University of Otago, Wellington, New ZealandReviewed by:
Martin Stiles, The University of Auckland, New ZealandMinglong Chen, The First Affiliated Hospital of Nanjing Medical University, China
Copyright © 2021 Chen, Zhao, Zhu, Qin, Liu and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailong Tao, dGFvaGxkckAxMjYuY29t
 Xiaowei Chen
Xiaowei Chen Jiangtao Zhao
Jiangtao Zhao