
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 15 November 2021
Sec. Cardiovascular Imaging
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.756162
 Chengxi Yan1†
Chengxi Yan1† Ruili Li2†
Ruili Li2† Xiaojuan Guo3
Xiaojuan Guo3 Huan Yu3
Huan Yu3 Wenhuan Li3
Wenhuan Li3 Wenqiao Li2
Wenqiao Li2 Meiji Ren2
Meiji Ren2 Minglei Yang4
Minglei Yang4 Hongjun Li2*
Hongjun Li2*Objectives: To investigate the subclinical imaging changes in terms of myocardial inflammation and fibrosis and to explore the risk factors associated with myocardial fibrosis by cardiac magnetic resonance (CMR) approach in a Chinese HIV/AIDS cohort.
Methods: We evaluated myocardial function (cine), myocardial inflammation (T1, T2), and myocardial fibrosis (through extracellular volume fraction [ECV] and late gadolinium enhancement [LGE]) by a multiparametric CMR scan protocol in a total of 68 participants, including 47 HIV-infected individuals, who were divided into two groups: asymptomatic HIV (HIV+) (n = 30), and acquired immunodeficiency syndrome (AIDS) (n = 17), and 21 healthy controls.
Results: HIV-infected patients had lower left (55.3 ± 6.5 vs. 63.0 ± 7.9%, P < 0.001) and right ventricular systolic function (35.9 ± 15.7 vs. 50.8 ± 9.3%, P < 0.001). Radial systolic strain (30.7 ± 9.3 vs. 39.3 ± 9.4%, P = 0.001), circumferential systolic strain (−17.5 ± 2.6 vs. −19.4 ± 2.7%, P = 0.008), and longitudinal systolic strain (−9.4 ± 5.7 vs. −12.8 ± 3.1%, P = 0.012) were also decreased in HIV. Native T1 relaxation time (1,337.2 ± 70.2 vs. 1,249.5 ± 47.0 ms, P < 0.001), ECV value (33.5 ± 6.2 vs. 28.5 ± 2.9 ms, P = 0.026), and T2 relaxation time (45.2 ± 3.5 vs. 42.0 ± 2.6 ms, P = 0.001) were higher in HIV-infected patients compared with controls. Myocardial fibrosis, predominantly in the mid-inferior wall, was detected in 24.4% of the HIV-infected patients. HIV+ had a significantly lower value of ECV [29.1 (26.1, 31.8) vs. 35.2 (31.8, 41.9) %, P < 0.001] and frequency of LGE [3/25 (8%) vs. 7/16 (43.8%), P = 0.014)] compared with AIDS. AIDS was associated with myocardial fibrosis.
Conclusions: HIV-infected patients were associated with changes in myocardial function and higher rates of subclinical myocardial inflammation and fibrosis, which were more abnormal with greater severity of the disease. AIDS was associated with myocardial fibrosis, where the observations supported earlier initiation of antiretroviral therapy in the Chinese HIV/AIDS cohort.
Cardiac abnormalities were believed to be prevalent in HIV infected patients (1, 2). HIV-related cardiomyopathy was reported significantly higher in postmortem studies than in clinical work due to the lack of highly sensitive and specific diagnostic tools (3). Even though the introduction of antiretroviral therapy (ART) has altered the cardiovascular manifestations, HIV-infected patients are still at an increased risk for cardiovascular disease for the high prevalence of traditional cardiovascular risk factors and concurrent metabolic changes induced by ART (4).
Previous studies (5) demonstrated that a major percentage of HIV infected patients have abnormal ECG findings, and echocardiography studies (6, 7) found that patients with asymptomatic HIV infection noted a relatively low frequency of left ventricular dysfunction and other abnormalities when compared with patients in the later stage of the disease. Other imaging modalities (e.g., PET-CT) could help to detect progressing atherosclerosis on blood vessel walls in HIV-infected patients (8) and demonstrated a chronic vascular inflammation resulting from HIV infection. Nevertheless, only a few autopsy studies had been conducted to address myocardial abnormalities in HIV infection (9).
As a technique to assess myocardial structure, function, and also tissue characterization comprehensively, cardiovascular magnetic resonance (CMR) imaging has been widely used in observing myocardial abnormalities. Previous research (10–13) applied CMR or/and magnetic resonance spectroscopy (MRS) to detect cardiac involvement in asymptomatic HIV subjects and found a high burden of cardiac steatosis, decreased myocardial function, and a high rate of myocardial fibrosis in asymptomatic HIV subjects undergoing ART (14). The late gadolinium enhancement (LGE) pattern, as one of the abnormalities revealed by CMR, represented regional fibrosis which might indicate an irreversible myocardial injury and result in cardiac dysfunction and cardiac death (15, 16). Thus, it is crucial to identify risk factors associated with the presence of LGE in HIV-infected patients. In addition, few CMR studies (17) focus on patients in the late stage of HIV disease despite the cardiac complications that occurred more frequently in those patients compared with patients in the early stage. Furthermore, no CMR study to date has assessed the myocardial involvement among the Chinese HIV/AIDS cohort.
Our study aimed to explore the cardiac involvement in Chinese HIV/AIDS patients by CMR while determining the risk factors associated between the HIV-related clinical parameters and myocardial fibrosis.
The institutional ethics committee approved this prospective study, and all participants gave written informed consent prior to CMR.
Human immunodeficiency virus infected patients were consecutively enrolled in this observational study at Beijing Youan Hospital from June 2019 to July 2020. Inclusion criteria were age ≥18 years and a confirmed HIV diagnosis. Exclusion criteria were history of cardiovascular disease, contraindications for CMR, an estimated glomerular filtration rate of < 90 mL/min/1.73 m2, and an impaired liver function (alanine aminotransferase greater than twice the normal upper limit). Clinical histories, physical examinations, and laboratory data were obtained from the enrolled patients, including a detailed review of the HIV disease stage, ART exposure, and cardiovascular disease risk factors. Fasting lipid panels, glucose levels, current plasma CD4+ T-cell counts, CD4+/CD8+ ratios, HIV loads, and hematocrit levels were also acquired. The AIDS stage was defined as the symptomatic stage when the virus becomes highly active and the immune system of the patient weakens, as reported previously (18). The control group consisted of age- and ethnicity-matched (self-defined) subjects (n = 21) with no history of HIV infection or cardiovascular disease. CMR studies and subsequent analyses were performed in a blinded manner, with sequential numbering of subjects. Detailed inclusion and exclusion criteria for participants and healthy control participants are illustrated in Supplementary Figure 1.
Cardiac magnetic resonance was performed for patients with 3.0-T systems (MAGNETOM Trio, Siemens Medical Systems, Erlangen, Germany). For cardiac function assessment, steady-state free precession cine images that were electrocardiogram-gated were also performed (short-axis, four-chamber, and two-chamber views). Native and postcontrast T1 mapping was acquired using an ECG-gated single-shot modified Look-Locker inversion recovery (MOLLI) sequence with protocol 5(3)3 and 4(1)3(1)2, respectively, before and 20 min after contrast administration. Late gadolinium enhancement (LGE) imaging based on segmented inversion-recovery gradient-echo sequences was performed 10–15 min after the administration of a single bolus of a 0.2 mmol/kg bodyweight Gadopentetate dimeglumine (Bayer Healthcare, New Jersey). For ECV calculation, blood hematocrit levels were assessed directly prior to the MRI scan.
Two readers with 2 (Y.C.X.) and 16 years (G.X.J.) of CMR experience analyzed the data and performed the measurements in consensus using a commercially available software CVI42 (Version 5.11.2 Circle Cardiovascular Imaging, Calgary, Canada).
To evaluate cardiac function, the endocardial borders of left and right ventricles were automatically detected by the software and manually adjusted, when necessary, in the short-axis cine-steady-state free precession images. Papillary muscles and trabeculae were excluded. Left ventricular end-diastolic volume (LVEDV), right ventricular end-diastolic volume (RVEDV), left ventricular end-systolic volume (LVESV), right ventricular end-systolic volume (RVESV), left ventricular eject fraction (LVEF), right ventricular eject fraction (RVFE), left ventricular cardiac output (LVCO), and right ventricular cardiac output (RVCO) were determined contemporaneously.
Cardiac magnetic resonance feature tracking (CMR-FT) was performed on short-axis, 4-chamber, and 2-chamber images. Global systolic radial, circumferential, and longitudinal strain values were calculated from peak segmental data. A 16-segment model, according to the American Heart Association model, was applied for segmental strain analysis of the left ventricle (19).
The outline of the endocardium and epicardium were manually contoured on native T1, postcontrast T1, and T2 maps. A 16-segment model was then applied for the acquisition of segmental value. ECV was derived from native T1 and postcontrast T1 of the myocardium as described by the equation in a previous study (20).
Images were evaluated qualitatively for the presence or absence, and the volume fraction of LGE was calculated using a threshold of five SDs (21).
All statistical analyses were performed using SPSS (version 23.0, IBM statistics, Armonk, NY, USA) and GraphPad Prism (Version 8.1, GraphPad Software Inc). Shapiro–Wilk test was used to test the normality of the distribution of the data. Continuous variable with normal distribution or non-normal distribution was expressed as mean ± SD or median (interquartile range). Categorical variables were expressed as counts (percentage). Student's t-test (for normal distribution) and unpaired or Mann–Whitney U test (for non-normal distribution) were performed to compare continuous variables between two groups, and χ2 test was performed to compare categorical variables. Quantitative variables were transformed into categorical variables according to their normal ranges for univariate logistic regression analysis to identify the association of the presence of LGE and HIV-related parameters. P-values of < 0.05 denoted statistically significant.
Among the 49 HIV-1-infected patients recruited, two patients were excluded because of poor image quality. A total of 47 HIV-infected patients (mean age, 37 ± 9 years, range 23–58 years) were divided into two groups: the asymptomatic HIV (HIV+) group (n = 30; 63.83%) and the AIDS group (n = 17; 36.2%). Forty-one patients underwent CMR with Gd-contrast material, and six patients refused Gd injections. Twenty-one healthy adults (mean age, 40 ± 11 years, range 20–60) were also evaluated. No significant differences were shown between the HIV-infected participants and healthy controls in age (P = 0.332), the occurrence of hypertension rate (P = 0.077), heart rate (P = 0.251), weight (P = 0.409), body mass index (P = 0.573), and body surface area (P = 0.157). In keeping with previous reports, the ratio of reported infections was four men to one woman by 2018,1 and there were more men in the subjects with HIV infection than healthy controls (P < 0.001). HIV patients had lower hemoglobin content (135 [126, 143] vs. 164 [136, 163] g/L; P = 0.008) compared with healthy controls (as shown in Supplementary Figure 1; Supplementary Table 1).
In comparison with HIV+ group, patients in the AIDS group were significantly older (35 [29, 38] vs. 41 [36, 46] years; P = 0.010) and had more comorbidity of diabetes mellitus (P = 0.035). AIDS group had a longer duration of HIV diagnosis than HIV+ group (6.0 [5.0, 11.5] vs. 3.4 [1.4, 7.3] years, P = 0.008). Laboratory results showed a significantly higher current CD4+ (717.2 ± 209.8 vs. 188.2 ± 192.0 cells/mm3; P < 0.001), current CD4+/CD8+ ratio (0.68 [0.53, 0.98] vs. 0.14 [0.57, 0.36]; P < 0.001), HCT (45.3 [42.8, 47.9] vs. 40.2 [29.9, 43.7] %; P < 0.001), and lower glucose (5.1 [4.5, 5.3] vs. 5.6 [4.9, 6.2] mmol/L; P = 0.025) in HIV+ group compared with AIDS group. Lower hemoglobin content was shown in the AIDS group compared with the HIV+ group (157 [147, 165] vs. 136 [110, 156] g/dL, P = 0.003), (as shown in Supplementary Table 2).
Compared with healthy controls, HIV-infected patients had a significantly lower LVEF (55.3 ± 6.5 vs. 63.0 ± 7.9%; P < 0.001), lower RVEF (35.9 ± 15.7 vs. 50.8 ± 9.3%; P < 0.001), lower RVCO (3.5 ± 1.4 vs. 5.0 ± 1.6 l/min; P < 0.001), higher RVESV (83.4 [69.5, 108.2] vs. 61.8 [49.2, 73.3] mL; P < 0.001), and higher RVESVi (83.4 [69.5, 108.2] vs. 61.8 [49.2, 73.3]; P = 0.012) (as shown in Table 1).
CMR-FT analysis showed that HIV patients had a lower GRS (30.7 ± 9.3 vs. 39.3 ± 9.4%; P < 0.001), lower GCS (–17.5 ± 2.6 vs. –19.4 ± 2.7%; P = 0.008), lower GLS (–9.4 ± 5.7 vs. –12.8 ± 3.1%; P = 0.012) when compared with healthy controls (as shown in Table 1).
Myocardial T1 relaxation times (1,337.2 ± 70.2 vs. 1,249.5 ± 47.0 ms; P < 0.001) and ECV (33.5 ± 6.2 vs. 28.5 ± 2.9%; P < 0.001) as well as T2 relaxation times (45.2 ± 3.5 vs. 42.0 ± 2.6 ms; P < 0.001) were elevated in participants with HIV patients. The visible sign of myocardial fibrosis was present in a total of 10 of 41 (21.95%) HIV patients, significantly higher than subjects in the control group (P = 0.013) (Table 1; Figure 1). Around 7.4% (49 of 656) segments showed LGE and were mainly distributed in the midinferior wall (Figure 2).
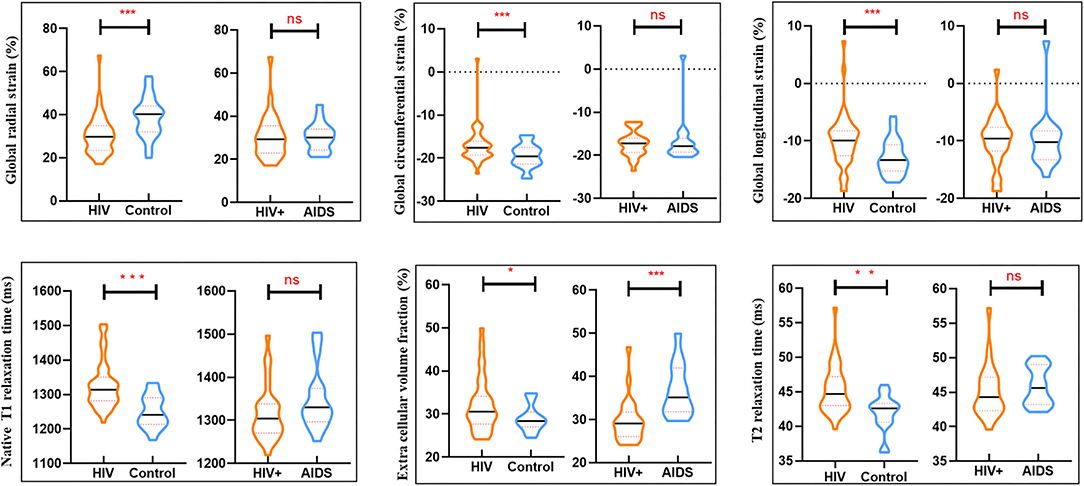
Figure 1. Box plots showed differences in cardiac parameters between groups (HIV group and controls) and within clinically subclassified HIV subgroup (HIV+ and AIDS group). *P < 0.05, **P < 0.01, ***P < 0.001; ns no significant difference.
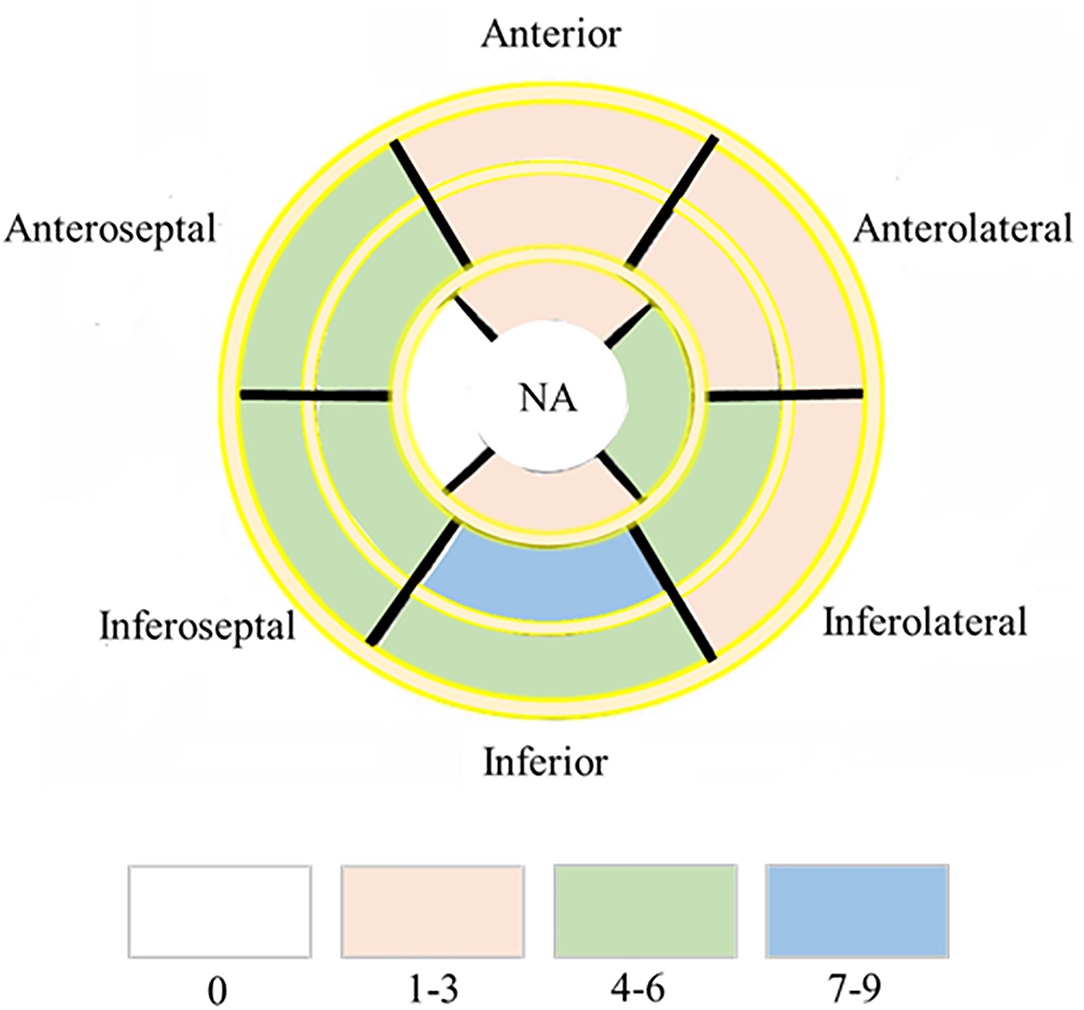
Figure 2. Dominant location and distribution of myocardial fibrosis segments in the AHA 16 segments' model. Number of LGE distributed in AHA 16 segments' model in 41/47 HIV infected patients. NA, not applicable.
There were no significant differences in LVEF, LVEDV, LVESV, LVCO, LVEDVi, LVESVi, RVEF, RVEDV, RVESV, RVCO, RVEDVi, RVESVi between HIV+ and AIDS subjects, nor were there significant differences in GRS, GCS, and GLS. T1-derived ECV measures showed a significantly elevated ECV value in AIDS group compared with HIV+ group (35.2 [31.8, 41.9] vs. 29.1 [26.1, 31.8]%; P < 0.001). No differences showed between subgroups in T2 and native T1 relaxation time. Three of the 25 (8%) patients in the HIV+ group showed the presence of LGE, which was significantly lower than those in the AIDS group (seven of 16, 43.8%; P = 0.014). Although AIDS patients had a higher volume fraction of LGE compared with HIV+ patients (5.3 [2.1, 10.5] vs. 4.5 [2.8, 7.2]%; P = 0.129), the difference was not significant. There was no difference in the proportion of pericardial effusion between HIV subgroups (P = 0.337) (Table 2; Figure 3).
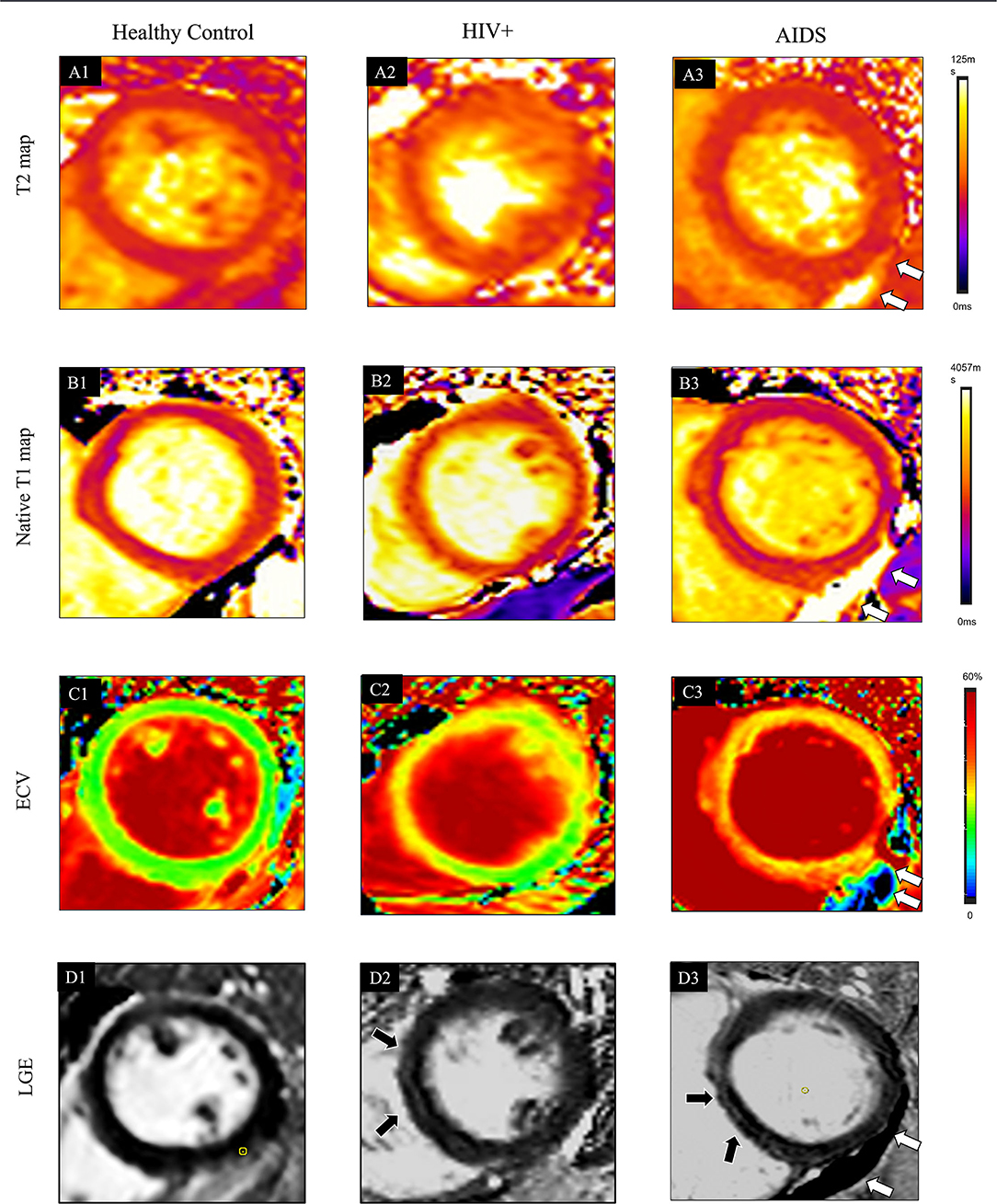
Figure 3. Typical cases showed comprehensive CMR parameters in a healthy control participant (38-year-old man, A1–D1), HIV+ patients (47-year-old man, A2–D2), and AIDS participant (38-year-old man with the complication of pneumocystis carinii pneumonia; A3–D3). Quantitative maps include cardiac T2 relaxation time (T2 map, A1), native T1 relaxation time (T1 native map, B1), and ECV map (C1). Also, LGE images (D1) are provided. In the healthy control case, all the quantitative CMR parameters are normal. In HIV+ patients, increased native T1 (1277 ± 209 ms) and ECV (31 ± 6%) are shown in the ventricular septum region of the myocardium on the T1 (B2) and ECV maps (C2), which also located in the corresponding region of LGE (D2, black arrows). T2 (A2, 39 ± 6 ms) values are normal. Global T2 (A3, 47 ± 8 ms), T1-native (B3, 1314 ± 150 ms), and ECV (C3, 33 ± 8%) are further elevated in AIDS patients. LGE (D3) pattern is only shown at the ventricular septum area (black arrow). The white arrow indicates pericardial effusion.
The results of univariate logistic regression analyses related to cardiac inflammation and fibrosis were shown in Table 3. The clinical factor of AIDS was the risk factor for the presence of LGE (odds ratio 6.300, 95% CI 1.358, 29.235; p = 0·019).
To the best of our knowledge, it was the first study to provide an extensive assessment of the cardiac structure, function, and myocardial tissue characterization using a comprehensive CMR approach in the Chinese HIV/AIDS cohort. The novel key finding of this observational, prospective study was the high burden of cardiac disease with signs of focal and diffuse myocardial edema and fibrosis, as well as a high incidence of pericardial effusion, indicating the subclinical myocardial inflammation in HIV-infected patients. ECV value and LGE presence were higher depending on the stage of HIV disease, revealing a link between the clinical grade of HIV disease and cardiac fibrosis. Besides, AIDS was a risk factor of myocardial fibrosis, further supporting the proposal that all patients with HIV should be commenced on ART to control the development of the disease.
T2 mapping was widely used to detect edema and inflammation in myocardial disease (22); however, it was poorly investigated in the context of HIV-related cardiomyopathy to date. Our findings of elevated T2 relaxation time in HIV-infected patients, when compared with healthy controls, indicated the presence of edema which was also evidence of inflammation (23). A slight increase in T2 relaxation time was shown in the AIDS group compared with HIV+, but the difference was not significant. Gutberlet et al. (24) pointed out that T2-weighted imaging had lower sensitivities as well as specificities in patients with suspected chronic myocarditis when compared with acute myocarditis. Abdel-Aty et al. (25) also found that T2-weighted CMR was sensitive not only to edema-related signal changes but also to a variety of pathological phenomena that occurred during infarct healing so that it could not differentiate acute from chronic myocardial infarction. T2 relaxation times are negatively correlated to collagen content in muscle tissue (26). A higher ratio of fibrosis tissue in chronic myocardial injury may result in the apparent “normalization” of T2 value (27) as observed in our study.
Another CMR parameter for quantification of myocardial inflammatory is native T1 relaxation time (28, 29). Higher native T1 values, as shown in our study, probably represented a combination of patchy myocardial edema, fibrosis, and inflammation. LGE existed in 10 of 41 (24.2%) HIV patients, and participants in the AIDS stage (43.8%) had a higher occurrence rate of LGE than HIV+ (8%). LGE, as a part of the healing response to subclinical myocarditis, might represent irreversible myocardial injury as previously described for the non-infected population (15). The presence of LGE predicted a worse outcome than did the absence of LGE (30). In accordance with the LGE pattern, a significant difference of ECV values which were known to represent an indirect measure of diffuse interstitial myocardial fibrosis (31) was shown between patients and controls and also HIV subgroups. Myocarditis was reported frequently on biopsy in the majority of HIV patients during the pre-ART era (32), whereas in the ART era, subclinical myocarditis has not been reported extensively. Even though most of the HIV participants in our study (37 of 47, 78.7%) received ART, myocardial inflammation still seems to prevail. We postulated immune restoration that is mediated by ART was incomplete even in long-term virologically suppressed patients, and the immune dysfunction could translate to a higher risk for developing chronic diseases (33). Besides, ART side effects should also be recognized as a possible confounding factor of systemic inflammation (34). Effusion was also shown in our study with the occurrence rate of 19.1%, and the ratio was even higher in AIDS group (29.4%) compared with HIV+ group (13.3%). The higher sensitivity of CMR has enabled the demonstration of small pericardial effusions which likely support the presence of a higher degree inflammatory state in the AIDS group.
A striking tendency was found toward a reduced ejection fraction of left ventricular in HIV-positive subjects and it further decreased in the AIDS group; besides, GRS, GCS, and GLS values were all decreased in HIV patients, which were in line with previous echocardiography and CMR studies (10, 11, 13, 35). HIV-associated cardiomyopathy often occurred as reduced LVEF or dilated LV, and studies with a higher proportion of AIDS reported a higher prevalence of left ventricular dysfunction (36). The mechanisms of cardiac dysfunction remain unclear. There are several pathogens: HIV-infected patients are more prone to have subclinical coronary atherosclerosis due to HIV infection and antiretroviral therapy (37). As reported by a previous study that long-term exposure to antiretroviral medications was prone to substantial metabolic risk, with about 50% of patients having dyslipidemias and 1/3 had accompanying impaired glucose tolerance (38). We also found a higher glucose content in AIDS patients compared with HIV+ patients (5.6 vs. 5.1 mmol/L), which may further lead to cardiovascular risk (38). Subclinical myocardial inflammation as revealed by CMR further predisposed to left myocardial dysfunction. Besides, HIV therapy may further compound cardiovascular function. Nucleoside analog reverse-transcriptase inhibitors, such as zidovudine and stavudine, may have marked negative effects on myocardial structure through mitochondrial toxicity inhibition of the mtDNA replicate and DNA pol-γ (39).
Cine-MRI data also confirmed RV anatomic alterations and also RV function impairment in HIV subjects. Casalino et al. (40) demonstrated that when using echocardiography, AIDS patients had a significantly increased RVESV and deceased RVEF compared with healthy controls. The thinner-walled RV tends to manifest myocardial abnormalities and structural and functional modifications (41). Pulmonary hemodynamic disturbances as well as fluid loss caused by diarrhea, which was common during HIV infection and was believed to explain RV modifications in AIDS (42). The diagram shows the proposed pathogenic mechanism of cardiac involvement in patients with HIV disease (Figure 4).
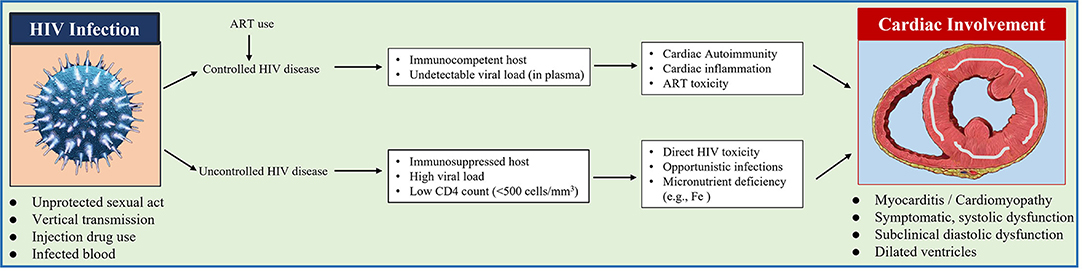
Figure 4. Diagram shows the proposed pathogenic mechanism of cardiac involvement in patients with HIV disease.
There were still several limitations because of the observational and explorative study design. The exact causality or pathogenesis for our findings cannot be determined since there is no endomyocardial biopsy. Yet, several studies have validated these applied CMR parameters against histologic analysis in the detection of myocardial fibrosis and inflammation (43). Second, in this Chinese HIV cohort, 45 of 47 (95.74%) patients were men; therefore, these results are likely not generalizable to women. However, this may reflect the gender distribution of the AIDS epidemic in China (44). Further study with a larger sample size of women was needed to confirm these findings before the study results may be generalized. Third, patients in the AIDS stage were limited in numbers. Therefore, research with a larger sample size of AIDS would be recommended in the future. Finally, ART use might be confounders. However, in accordance with previous studies, cardiac involvement in HIV patients was independent of the virological suppression and was observed in participants on ART as well as in those naïve to ART (11).
In conclusion, the comprehensive CMR approach showed a high burden of myocardial structural and functional abnormalities as well as chronic inflammation, which intensify with the severity of HIV disease; besides, AIDS was associated with myocardial fibrosis. Our findings prompt the need for closer cardiologic check-ups (e.g., for cardiac medication adjustment or antiinflammatory therapy), especially in patients of late-stage HIV disease or if imaging-based inflammation markers are particularly high.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Youan approved this prospective study. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CY: conceptualization, formal analysis, investigation, data curation, writing—original draft, and writing—review and editing. RL: formal analysis, investigation, data curation, writing—original draft, and writing—review and editing. XG: formal analysis, investigation, and resource. HY and WenhL: review and editing. WenqL and MR: data curation. MY: software and writing—review and editing. HL: conceptualization, writing—review and editing, resource, and supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China [Grant No. 81771806 and 61936013]; Beijing Natural Science Foundation [7212051]; Beijing Excellent Talent Plan [Grant No. 2018000021469G290].
Author MY was employed by Neusoft Research of Intelligent Healthcare Technology, Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all the patients and participants who were involved in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.756162/full#supplementary-material
1. Moskowitz L, Hensley GT, Chan JC, Adams K. Immediate causes of death in acquired immunodeficiency syndrome. Arch Pathol Lab Med. (1985) 109:735–8.
2. De Castro S, Migliau G, Silvestri A, D'Amati G, Giannantoni P, Cartoni D, et al. Heart involvement in AIDS: a prospective study during various stages of the disease. Eur Heart J. (1992) 13:1452–9. doi: 10.1093/oxfordjournals.eurheartj.a060085
3. Magula NP, Mayosi BM. Cardiac involvement in HIV-infected people living in Africa: a review. Cardiovasc J S Afr. (2003) 14:231–7.
4. Duprez DA, Jacqueline N, Kuller LH, Russell T, Waldo B, Stephane DW, et al. Inflammation, coagulation and cardiovascular disease in HIV-Infected. Individuals. (2012) 7:e44454. doi: 10.1371/journal.pone.0044454
5. Raffanti SP, Chiaramida AJ, Sen P, Wright P, Middleton JR, Chiaramida S. Assessment of cardiac function in patients with the acquired immunodeficiency syndrome. Chest. (1988) 93:592–4. doi: 10.1378/chest.93.3.592
6. Herskowitz A, Wu TC, Willoughby SB, Vlahov D, Ansari AA, Beschorner WE, et al. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol. (1994) 24:1025–32. doi: 10.1016/0735-1097(94)90865-6
7. Himelman RB, Chung WS, Chernoff DN, Schiller NB, Hollander H. Cardiac manifestations of human immunodeficiency virus infection: a two-dimensional echocardiographic study. J Am Coll Cardiol. (1989) 13:1030–6. doi: 10.1016/0735-1097(89)90256-8
8. Mikail N, Sinigaglia M, Hyafil F. Could FDG-PET imaging play a role in the detection of progressing atherosclerosis in HIV-infected patients? J Nucl Cardiol. (2019) 26:1266–8. doi: 10.1007/s12350-018-1247-2
9. Hofman P, Michiels JF, Saint Paul MC, Bernard E, Dellamonica P, Loubiere R. [Cardiac lesions in acquired immunodeficiency syndrome (AIDS). Apropos of an autopsy series of 25 cases]. Ann Pathol. (1990) 10:247–57.
10. Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. (2013) 128:814–22. doi: 10.1161/CIRCULATIONAHA.113.001719
11. Ntusi N, O'Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. (2016) 9:e004430. doi: 10.1161/CIRCIMAGING.115.004430
12. Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. (2015) 212:1544–51. doi: 10.1093/infdis/jiv274
13. Luetkens JA, Doerner J, Schwarze-Zander C, Wasmuth JC, Boesecke C, Sprinkart AM, et al. cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-Infected patients. Circ Cardiovasc Imaging. (2016) 9:e004091. doi: 10.1161/CIRCIMAGING.115.004091
14. Neilan TG, Nguyen KL, Zaha VG, Chew KW, Morrison L, Ntusi NAB, et al. Myocardial steatosis among antiretroviral therapy-treated people with human immunodeficiency virus participating in the REPRIEVE Trial. J Infect Dis. (2020) 222:S63–s69. doi: 10.1093/infdis/jiaa245
15. Zagrosek A, Abdel-Aty H, Boyé P, Wassmuth R, Messroghli D, Utz W, et al. cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging. (2009) 2:131–8. doi: 10.1016/j.jcmg.2008.09.014
16. de Leuw P, Arendt CT, Haberl AE, Froadinadl D, Kann G, Wolf T, et al. Myocardial fibrosis and inflammation by CMR predict cardiovascular outcome in people living with HIV. JACC Cardiovasc Imaging. (2021) 14:1548–57. doi: 10.1016/j.jcmg.2021.01.042
17. Piñeirua-Menéndez A, Flores-Miranda R, Sánchez-Nava D, Ortega-Pérez R, Belaunzaran-Zamudio PF, Pérez-Patrigeon S, et al. Myocardial inflammatory changes before and after antiretroviral therapy initiation in people with advanced human immunodeficiency virus disease. Open Forum Infect Dis. (2020) 7: 297. doi: 10.1093/ofid/ofaa297
18. HIV/AIDS. Mayo Clinic. Available online at: https://wwwmayoclinicorg/diseases-conditions/hiv-aids/basics/symptoms/con-20013732.
19. Fattori R, Biagini E, Lorenzini M, Buttazzi K, Lovato L, Rapezzi C. Significance of magnetic resonance imaging in apical hypertrophic cardiomyopathy. Am J Cardiol. (2010) 105:1592–6. doi: 10.1016/j.amjcard.2010.01.020
20. Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 mapping. JACC: Cardiovascul Imaging. (2015) 8:526–36. doi: 10.1016/j.jcmg.2014.12.025
21. Sun Z, Zhang Q, Zhao H, Yan C, Yang H-J, Li D, et al. Retrospective assessment of at-risk myocardium in reperfused acute myocardial infarction patients using contrast-enhanced balanced steady-state free-precession cardiovascular magnetic resonance at 3T with SPECT validation. J Cardiovascul Magn Resonance. (2021) 23:25. doi: 10.1186/s12968-021-00730-7
22. Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging. (2016) 17:154–61. doi: 10.1093/ehjci/jev246
23. Yilmaz A, Ferreira V, Klingel K, Kandolf R, Neubauer S, Sechtem U. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev. (2013) 18:747–60. doi: 10.1007/s10741-012-9356-5
24. Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, et al. Suspected chronic myocarditis at cardiac mr: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. (2008) 246:401–9. doi: 10.1148/radiol.2461062179
25. Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, et al. Delayed Enhancement and T2-Weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. (2004) 109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6
26. Nieminen M, Töyräs J, Hakumäki J, Närväinen J, Hyttinen M, Helminen H, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. (2001) 46:487–93. doi: 10.1002/mrm.1218
27. Scholz TD, Fleagle SR, Burns TL, Skorton DJ. Tissue determinants of nuclear magnetic resonance relaxation times. effect of water and collagen content in muscle and tendon. Invest Radiol. (1989) 24:893–8. doi: 10.1097/00004424-198911000-00010
28. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. (2004) 52:141–6. doi: 10.1002/mrm.20110
29. Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, et al. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. (2007) 58:34–40. doi: 10.1002/mrm.21272
30. O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. (2010) 56:867–74. doi: 10.1016/j.jacc.2010.05.010
31. Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovascul Magn Resonance. (2016) 18:89. doi: 10.1186/s12968-016-0308-4
32. Karvounis HI, Papadopoulos CE, Zaglavara TA, Nouskas IG, Gemitzis KD, Parharidis GE, et al. Evidence of left ventricular dysfunction in asymptomatic elderly patients with non-insulin-dependent diabetes mellitus. Angiology. (2004) 55:549–55. doi: 10.1177/000331970405500511
33. Rönsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PLoS ONE. (2013) 8:e65182. doi: 10.1371/journal.pone.0065182
34. De Pablo-Bernal RS, Ruiz-Mateos E, Rosado I, Dominguez-Molina B, Alvarez-Ríos AI, Carrillo-Vico A, et al. TNF-α levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother. (2014) 69:3041–6. doi: 10.1093/jac/dku263
35. Erqou S, Lodebo BT, Masri A, Altibi AM, Echouffo-Tcheugui JB, Dzudie A, et al. cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. JACC: Heart Failure. (2019) 7:98–108. doi: 10.1016/j.jchf.2018.10.006
36. Cerrato E, D'Ascenzo F, Biondi-Zoccai G, Calcagno A, Frea S, Grosso Marra W, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. (2013) 34:1432–6. doi: 10.1093/eurheartj/ehs471
37. Post WS, Budoff M, Kingsley L, Palella FJ Jr, Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. (2014) 160:458–67. doi: 10.7326/M13-1754
38. Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. (2003) 349:1993–2003. doi: 10.1056/NEJMoa030218
39. Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. (1995) 1:417–22. doi: 10.1038/nm0595-417
40. Casalino E, Laissy J-P, Soyer P, Bouvet E, Vachon F. Assessment of right ventricle function and pulmonary artery circulation by cine-mri in patients with AIDS. Chest. (1996) 110:1243–7. doi: 10.1378/chest.110.5.1243
41. Marmor AT, Mijiritsky Y, Plich M, Frenkel A, Front D. Improved radionuclide method for assessment of pulmonary artery pressure in COPD. Chest. (1986) 89:64–9. doi: 10.1378/chest.89.1.64
42. Saito H, Dambara T, Aiba M, Suzuki T, Kira S. Evaluation of cor pulmonale on a modified short-axis section of the heart by magnetic resonance imaging. Am Rev Respir Dis. (1992) 146:1576–81. doi: 10.1164/ajrccm/146.6.1576
43. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. (2009) 53:1475–87. doi: 10.1016/j.jacc.2009.02.007
44. UGHAsfs. (2019). Available online at: https://share-netinternational.org/unaids-global-hiv-aids-statistics-2019-fact-sheet/.
Keywords: HIV, cardiovascular magnetic resonance, cardiac involvement, myocardial inflammation, myocardial fibrosis
Citation: Yan C, Li R, Guo X, Yu H, Li W, Li W, Ren M, Yang M and Li H (2021) Cardiac Involvement in Human Immunodeficiency Virus Infected Patients: An Observational Cardiac Magnetic Resonance Study. Front. Cardiovasc. Med. 8:756162. doi: 10.3389/fcvm.2021.756162
Received: 10 August 2021; Accepted: 13 October 2021;
Published: 15 November 2021.
Edited by:
Nay Aung, Queen Mary University of London, United KingdomReviewed by:
Yeon Hyeon Choe, Sungkyunkwan University, South KoreaCopyright © 2021 Yan, Li, Guo, Yu, Li, Li, Ren, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjun Li, bGlob25nanVuMDAxMTNAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.