- 1Division of Nephrology, Department of Medicine, Taipei Veterans General Hospital, Taipei City, Taiwan
- 2Faculty of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 3Institute of Clinical Medicine, National Yang Ming Chiao Tung University, Taipei City, Taiwan
- 4Center for Intelligent Drug Systems and Smart Bio-devices (IDS2B), Hsinchu, Taiwan
- 5Information Management Office, Taipei Veterans General Hospital, Taipei City, Taiwan
- 6Big Data Center, Taipei Veterans General Hospital, Taipei City, Taiwan
- 7Department and Institute of Physiology, National Yang-Ming University, Taipei City, Taiwan
Background: Oral anticoagulants (OAC) plus antiplatelets is recommended for patients with atrial fibrillation (AF) and coronary artery disease (CAD) to reduce thromboembolism. However, there is limited evidence regarding antithrombotic therapy for patients with concomitant chronic kidney disease (CKD), AF, and CAD, especially those not undergoing percutaneous coronary intervention. We aimed to use real-world data assessing the efficacy and safety of antithrombotic regimens in this population.
Methods: We used a single-center database of 142,624 CKD patients to identify those receiving antithrombotic therapy for AF and CAD between 2010 and 2018. Patients taking warfarin or direct OAC (DOAC) alone were grouped in the OAC monotherapy (n = 537), whereas those taking OAC plus antiplatelets were grouped in the combination therapy (n = 2,391). We conducted propensity score matching to balance baseline covariates. The endpoints were all-cause mortality, major adverse cardiovascular events, and major bleedings.
Results: After 1:4 matching, the number of patients in OAC monotherapy and combination therapy were 413 and 1,652, respectively. Between the two groups, combination therapy was associated with higher risks for ischemic stroke (HR 2.37, CI 1.72–3.27), acute myocardial infarction (HR 6.14, CI 2.51–15.0), and hemorrhagic stroke (HR 3.57, CI 1.35–9.81). The results were consistent across CKD stages. In monotherapy, DOAC users were associated with lower risks for all-cause mortality, AMI, and gastrointestinal bleeding than warfarin, but the stroke risk was similar between the two subgroups.
Conclusions: For patients with concomitant CKD, AF and CAD not undergoing PCI, OAC monotherapy may reduce stroke and AMI risks. DOAC showed more favorable outcomes than warfarin.
Introduction
Patients with chronic kidney disease (CKD) have a high risk of cardiovascular (CV) comorbidities including atrial fibrillation (AF) and coronary artery disease (CAD). These three disease entities concomitantly affect each other and share common risk factors such as age, smoking, hypertension, dyslipidemia, and diabetes mellitus (DM). Approximately 5–10% of patients with CKD concomitantly have AF and CAD, and the coexistence creates a vicious cycle (1). With CKD progression, the loss of antioxidant capacity enhances the progression of coronary atherosclerosis and vascular calcification that aggravate myocardial ischemia. The increasing severity of CAD and subsequent cardiac remodeling might predispose individuals to AF. In addition, AF can reduce cardiac output, thus accelerating the deterioration of CKD. Consequently, these patients experience rapid deterioration in renal function and develop adverse CV events.
Combined oral anticoagulants (OAC) and antiplatelets have been suggested for antithrombotic therapy in patients with both AF and CAD. However, such a combination may lead to adverse effects such as hemorrhagic stroke and major bleeding, especially in patients with CKD. Accordingly, previous research aimed to investigate modified antithrombotic regimens with better efficacy and safety for these high-risk patients. Previous randomized controlled trials (RCT) have shown that the combination of warfarin plus clopidogrel exerted an antithrombotic effect equal to that exerted by the conventional triple therapy of warfarin, aspirin, and clopidogrel on patients with AF undergoing percutaneous coronary intervention (2, 3). In comparison to triple therapy, the RE-DUAL trial reported that the use of dabigatran plus clopidogrel leads to comparable CV outcomes and causes less bleeding (4). Furthermore, Yasuda et al. demonstrated that rivaroxaban monotherapy was associated with a lower risk of major bleeding and was non-inferior to rivaroxaban combined with a single antiplatelet agent in terms of efficacy in patients with AF and stable CAD undergoing PCI (5). However, because the number of patients with CKD [defined as those with an estimated glomerular filtration rate (eGFR) of <60 mL/min per 1.73 m2] in these RCTs was low, evidence regarding the safety of antithrombotic therapy in patients with CKD remains limited. Moreover, PCI in the management of CAD among patients with CKD is sometimes limited to the risk of contrast-induced renal failure. Therefore, in this study, we investigated the efficacy and safety of antithrombotic therapy in patients with CKD with concomitant AF and CAD in the Evaluating the Prognosis and Impacts in CKD (EPIC) Research of Taipei Veterans General Hospital (VGH).
Methods
Data Source
This study was based on a single-center, retrospective, observational design. Our main data source was derived from the Big Data Center (BDC) of Taipei VGH. The database contains data regarding demographic characteristics, diagnostic codes, imaging studies, medical procedures, and laboratory findings for outpatient appointments, emergency department visits, and inpatient admissions from January 2010 through December 2018. We used codes from the International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10, respectively) to screen the diagnosis of CKD (ICD-9: 585 and ICD-10: N18), non-valvular AF (ICD-9: 427.31 and ICD-10: I48), and CAD (ICD-9: 410, 411, 412, 413, and 414 and ICD-10: I20, I21, I22, I23, I24, and I25). In addition, we used electronic medical record (EMR) system to collect data that were not included or completely recorded in the Taipei VGH BDC, such as social history, event records, and drug prescriptions. The study protocol was approved by the Institutional Review Board of the Taipei VGH (2017-09-002BC) and fulfilled the ethical guidelines of the Declaration of Helsinki.
Participants
We enrolled patients who had concurrent CKD, CAD, and AF indicated for OAC therapy such as warfarin or direct OACs (DOACs, referred to as apixaban, dabigatran, rivaroxaban, and edoxaban). Patients who met all the following criteria were considered to be eligible for participation: (1) age >20 years, (2) males with a baseline CHA2DS2-VASc score of >2 and females with a baseline CHA2DS2-VASc score of >3, and (3) use of OACs. Exclusion criteria were as follows: (1) history of PCI before enrollment, (2) use antiplatelets other than aspirin and clopidogrel, (3) use OACs <90 days, (4) no availability of serum creatinine and urine protein measurements at baseline and follow-up. According to the prescriptions, patients receiving warfarin or DOACs alone were grouped into the OAC monotherapy group, whereas patients receiving an OAC plus antiplatelets were grouped into the combination therapy group.
Follow-Up and Endpoints
The index date was defined as the first prescription of OACs. Patients were followed up since the index date until death, loss to follow-up, censoring, or December 31, 2018. We included laboratory tests associated with CV risks such as total and low-density lipoprotein cholesterol, triglyceride, glucose, glycated hemoglobin, and hemoglobin. In addition, the baseline serum creatinine and urine protein-to-creatinine ratio were collected. Data of these measurements closest to the index date within 1 month were defined as the baseline. The eGFR values were calculated for serum creatinine by using the Chronic Kidney Disease Epidemiology Collaboration equation. We identified concomitant medications by using the EMR, and only drug exposure within 90 days before the index date was included. Comorbidity patterns in this study were hypertension, DM, congestive heart failure (CHF), and malignancy. Primary outcomes were all-cause mortality and major adverse cardiac events (MACE) including ischemic stroke, acute myocardial infarction, transient ischemic attack, peripheral artery occlusive disease, and hospitalization for CHF. Secondary endpoints were major bleeding, including hemorrhagic stroke, gastrointestinal (GI) bleeding, and other bleeding events. Renal outcomes included CKD progression characterized by the first occurrence of eGFR declines of >20, >30, >40, and >50%, end-stage renal disease (ESRD), and initiation of dialysis.
Statistical Analysis
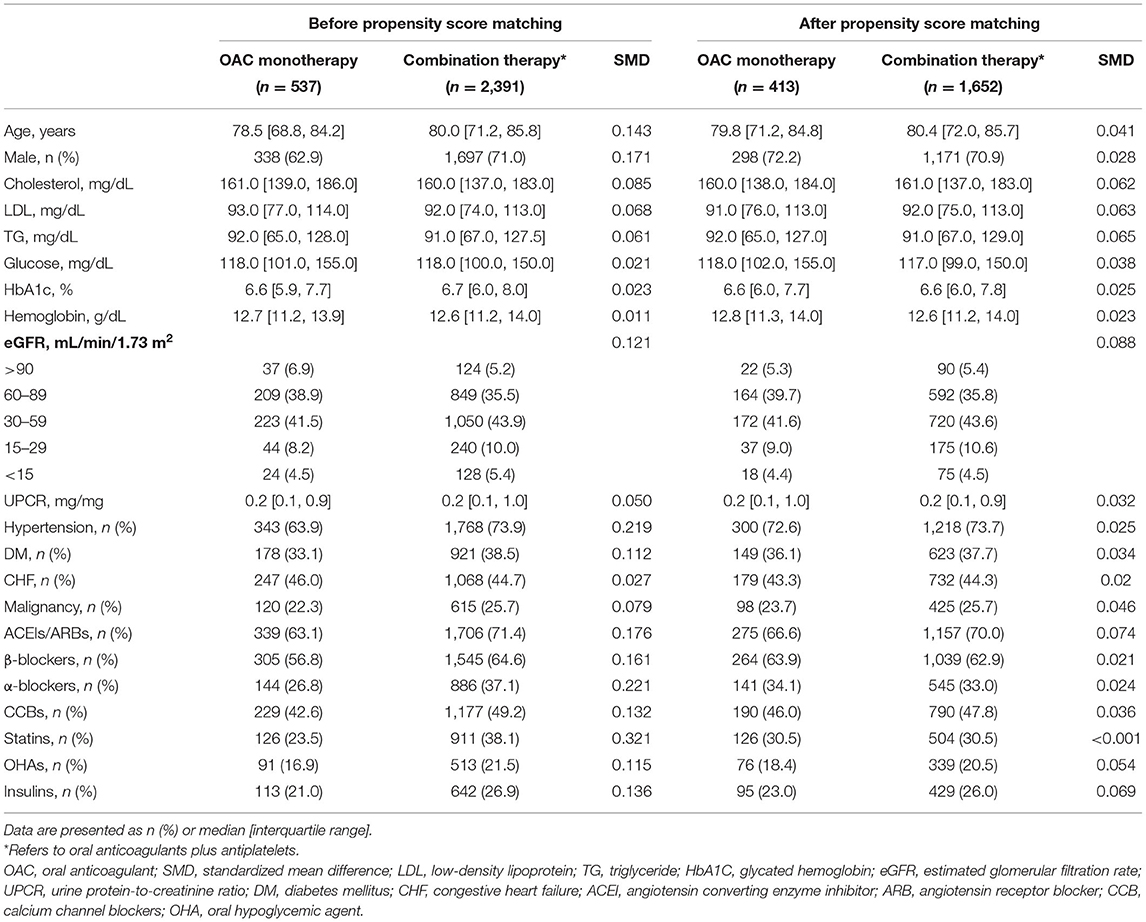
Missing values were imputed using the multiple imputation method by fully conditional specification with five repetitions to establish a complete dataset. The baseline characteristics were compared between the two groups of patients by using the x2 test for categorical variables and independent t-test and Mann–Whitney U-test for parametric and non-parametric continuous variables, respectively. Propensity scores were calculated with all baseline covariates by using a logistic regression model, and propensity score matching was conducted through the nearest neighbor approach with a caliper of 0.01. The standardized difference was calculated to assess the balance between the two groups after matching, and a difference of <0.2 in the score was considered to indicate a negligible imbalance.
We used the as-treated approach to account for switching antithrombotic medications in a real-world setting. The treatment effect for the time to the first event was estimated using Cox proportional-hazards models. The strength of the association between the exposure and outcome is presented as the hazard ratio (HR) with the 95% confidence interval (CI). The cumulative incidences of all-cause mortality, adverse CV events, CKD progression, and major bleeding were compared among patients receiving different antithrombotic therapies by using the modified Kaplan–Meier method and tested using the log-rank statistic. A P < 0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Study Population Characteristics
The patient enrollment process is depicted in Figure 1. A total of 142,624 patients were diagnosed of CKD between 2010 and 2018. After excluding patients who did not meet the inclusion criteria, we identified 2,928 patients with concomitant CAD and AF indicated for OAC therapy stratified by the CHA2DS2-VASc score. Among eligible participants, 537 were included into the OAC monotherapy group, whereas 2,391 were included into the combination therapy group. The baseline clinical characteristics of these patients are listed in Table 1. After 1:4 propensity score matching, the baseline covariates were comparable between the two groups. Furthermore, the distribution balance for the propensity score and the balance plot of absolute standardized effect sizes before and after matching are shown in Supplementary Table 1; Figures 1, 2, respectively.
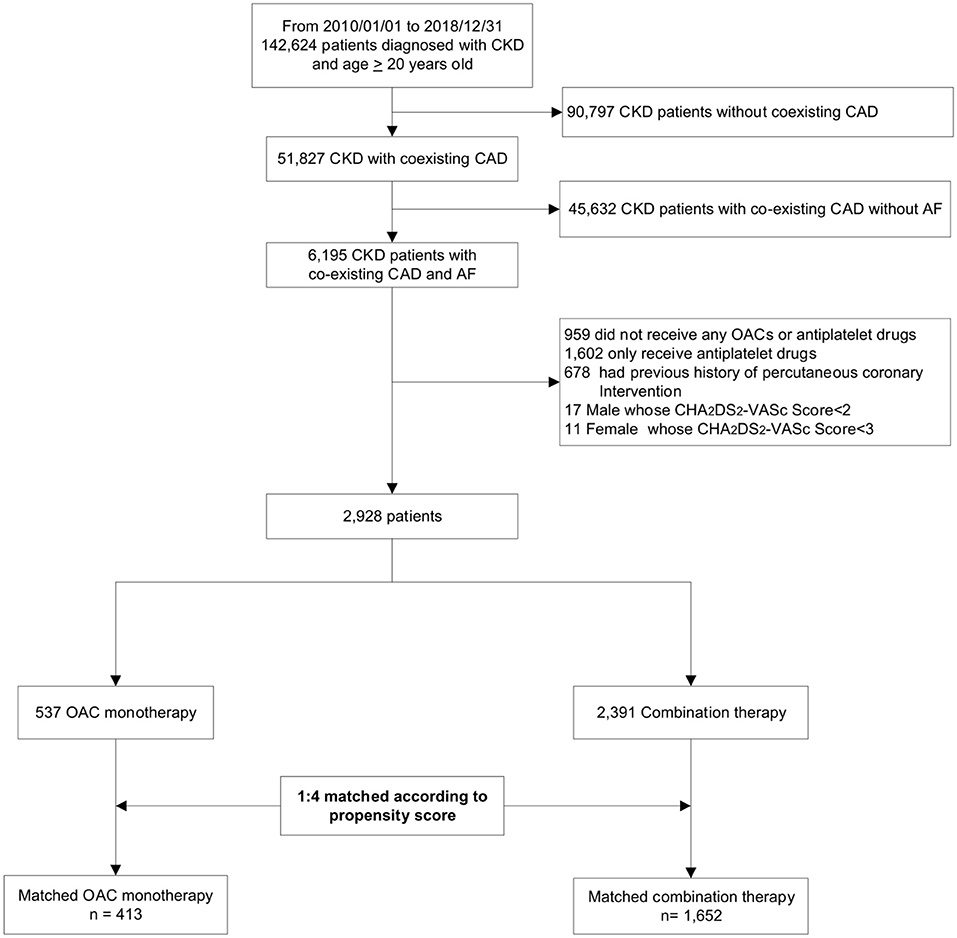
Figure 1. Flowchart of study enrollment. This hospital-based cohort included a total of 142,624 patients with CKD between 2010 and 2018. After excluding ineligible patients, we identified 2,928 patients with concurrent AF and CAD not undergoing percutaneous coronary intervention, and divided them into OAC monotherapy group (n = 537) and combination therapy (OAC plus antiplatelets, n = 2,391) according to their antithrombotic regimens. Finally, we conducted a 1:4 propensity-score matching to balance baseline covariates. CKD, chronic kidney disease; CAD, coronary artery disease; AF, atrial fibrillation; OAC, oral anticoagulant.
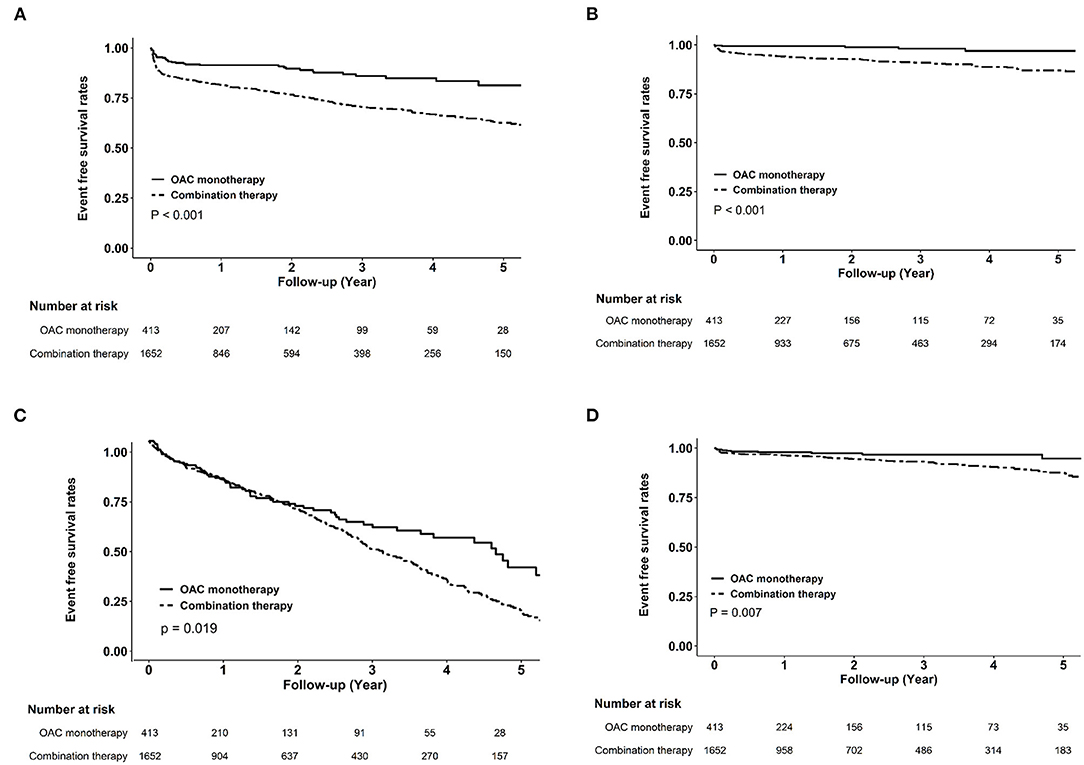
Figure 2. Kaplan–Meier curves of clinical endpoints between oral anticoagulant monotherapy and combination therapy in the matched cohort. Compared with combination therapy, oral anticoagulant monotherapy was associated with higher cumulative event-free probabilities for ischemic stroke (A), myocardial infarction (B), all-cause mortality (C), and hemorrhagic stroke (D).
MACE and Bleeding Risks Associated Wih OAC in Combination With Antiplatelets
Figure 2 shows the cumulative event-free probability curve of all-cause mortality, AMI, ischemic stroke, and hemorrhagic stroke between the two groups. During the follow-up period of 88.5 ± 66.4 months, patients receiving OAC monotherapy were found to have more favorable outcomes (all P < 0.05; log-rank test). As shown in Table 2, compared with the OAC monotherapy group, the combination therapy group had increased risks of all-cause mortality (HR 1.31, 95% CI 1.01–1.71, P = 0.044), ischemic stroke (HR 2.37, 95% CI 1.72–3.27, P < 0.001), and AMI (HR 6.14, 95% CI 2.51–15.0, P < 0.001). Moreover, the combination therapy group had a higher risk of hemorrhagic stroke (HR 3.57, 95% CI 1.3–9.81, P = 0.014). The risks of decline in eGFR and ESRD were similar between the two groups.
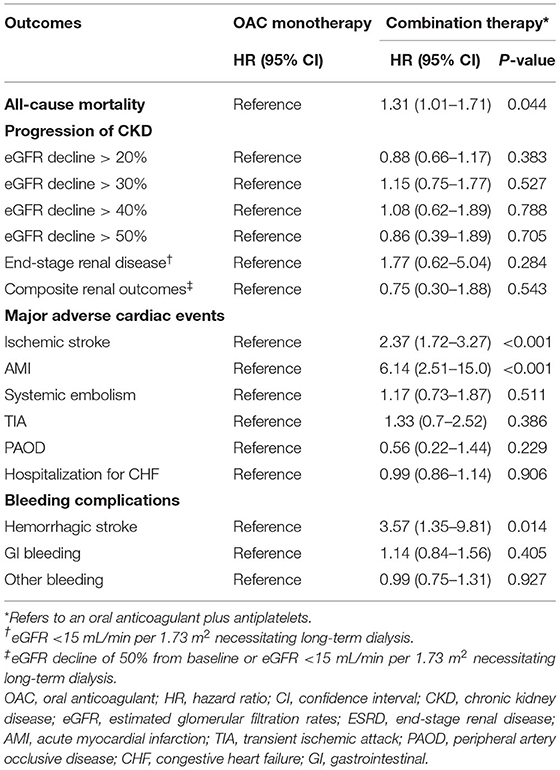
Table 2. Risks of all-cause mortality, progression of chronic kidney disease, adverse cardiovascular events and bleeding complications between oral anticoagulant monotherapy or combination therapy in patients with chronic kidney disease with atrial fibrillation and coronary artery disease.
Subgroup Analyses of MACE and Bleeding Risks Associated With OAC in Combination With Antiplatelets
Supplementary Figures 3–6 compare MACE and bleeding risks among different subgroups of patients receiving OAC monotherapy vs. those receiving combination therapy. Similar to the aforementioned findings, the use of combination therapy was associated with significantly higher risks of ischemic stroke, AMI, all-cause mortality, and hemorrhagic stroke than OAC monotherapy in all subgroups except for age; however, patients aged ≥65 years had higher risks of all-cause mortality (P for interaction = 0.029) and hemorrhagic stroke (P for interaction = 0.011).
MACE and Bleeding Risks Associated With Warfarin Alone or in Combination With Antiplatelets
As shown in Table 3, we divided the matched cohort into four groups in terms with OACs: DOAC monotherapy, DOAC plus antiplatelets, warfarin monotherapy, and warfarin plus antiplatelets. We found that warfarin was associated with higher risks of all-cause mortality (warfarin monotherapy: HR 1.90, 95% CI 1.12–3.22, P = 0.018; warfarin plus antiplatelets: HR 2.33, 95% CI 1.47–3.69, P < 0.001) compared with DOAC monotherapy. Furthermore, we found that the combination of warfarin with antiplatelets was associated with increased risks of ischemic stroke (HR 2.31, 95% CI 1.47–3.62, P < 0.001), AMI (HR 9.4, 95% CI 2.31–38.32, P = 0.002), and GI bleeding (HR 2.35, 95% CI 1.38–4.00, P = 0.002) among the four subgroups. The risk of CKD progression did not differ between those receiving DOACs and those receiving warfarin, irrespective of whether they were used in combination with antiplatelets.
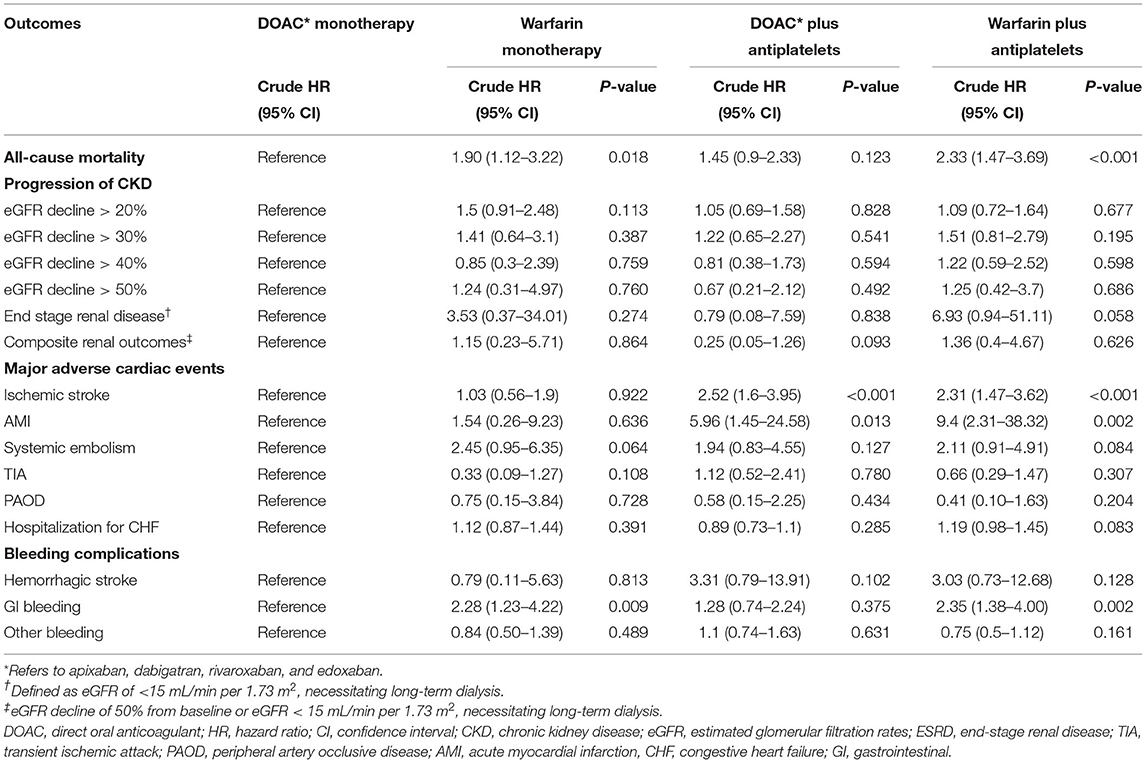
Table 3. Risks of all-cause mortality, progression of chronic kidney disease, adverse cardiovascular events and bleeding complications between the four groups of antithrombotic treatments in patients with chronic kidney disease with atrial fibrillation and coronary artery disease.
Risks of Thromboembolism and Bleeding Between Warfarin and DOACs
Figure 3 illustrates the risks of thromboembolism and major bleeding associated with warfarin compared with DOACs. In the matched CKD cohort, the risks of all-cause mortality (HR 0.60, 95% CI 0.49–0.74, P < 0.001), AMI (HR 0.62, 95% CI 0.43–0.89, P = 0.013), and GI bleeding (HR 0.52, 95% CI 0.41–0.68, P < 0.001) were significantly lower in patients treated with DOACs compared with those treated with warfarin. Nevertheless, the risks of ischemic and hemorrhage stroke were not different between the two groups. These findings were consistent before and after propensity score matching.
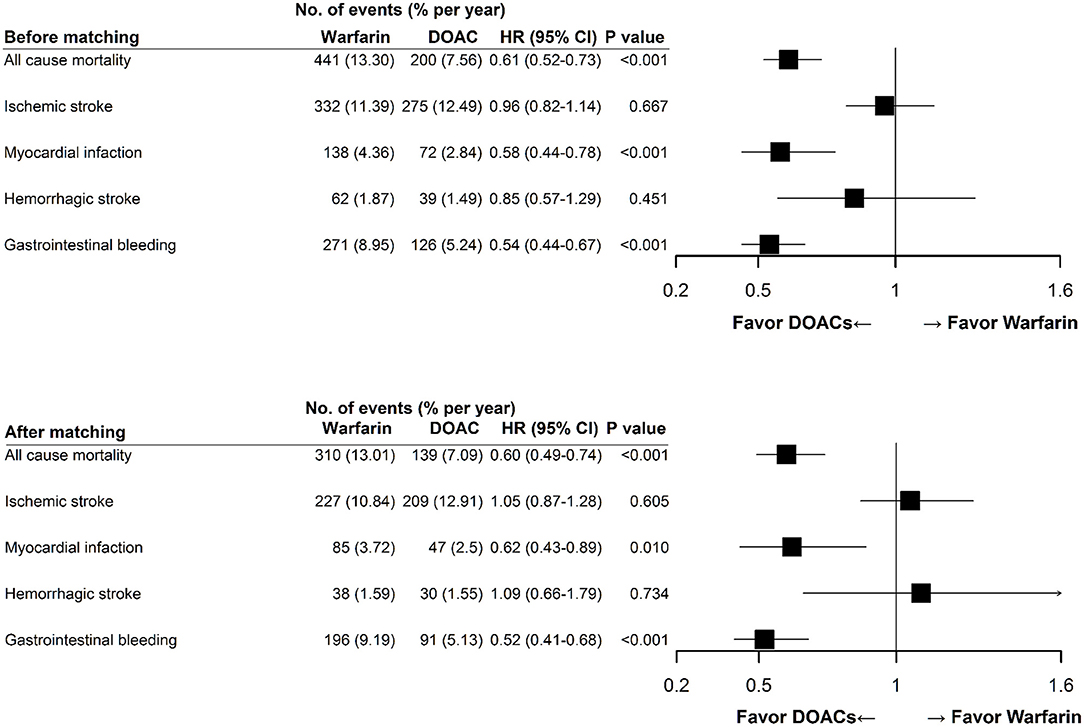
Figure 3. Clinical endpoints of direct oral anticoagulant use compared with warfarin use. Multivariable Cox proportional hazards model showed that DOAC use was associated with lower rates of all-cause mortality, myocardial infarction, and gastrointestinal bleeding, and a similar rate of ischemic and hemorrhagic stroke compared with warfarin use. These findings were consistent before and after propensity score matching. DOAC, direct oral anticoagulant; HR, hazard ratio; CI, confidence interval.
Discussion
This large-scale retrospective cohort study showed that OAC monotherapy appears to a preferable antithrombotic therapy in patients with CKD with concomitant AF and CAD who had not undergone PCI. The findings of propensity score-matched analysis revealed that the additional use of antiplatelets along with OACs did not exert a stronger protective effect on ischemic stroke and AMI but significantly increased the risk of hemorrhagic stroke. The results were consistent across subgroups categorized by sex, history of hypertension or DM, and baseline eGFR. We also found that the use of DOACs in patients with CKD was associated with a lower risk of all-cause mortality, AMI, and GI bleeding than the use of warfarin. Our study indicated that DOAC monotherapy might be feasible for the management of concurrent AF and CAD in high-risk CKD patients.
To the best of our knowledge, this is the first study to show that OAC alone significantly reduced the CV risks and resulted in lower bleeding and mortality risks compared with combination therapy among patients with CKD and concomitant AF and CAD. Antiplatelets have been considered the drug of choice on primary and secondary preventions in patients with CAD and CKD (6), whereas OAC therapy is crucial for lowering the risk for stroke and thromboembolism in patients with concomitant AF and CKD (7). Our finding has clinical relevance since some physicians prefer prescribing antiplatelets instead of OACs for CKD patients at high bleeding risk. However, irrespective of the antiplatelet regimen used, the deletion of OAC from treatment is inadequate for the prevention of thromboembolism in patients with CKD with a CHA2DS2-VASc score of 2 or higher (8). On the other hand, we observed that the combined use of antiplatelets with OACs might enhance the risk of AMI among patients with CKD compared to use OACs alone. Although the use of antiplatelets can reduce the risk of ischemic stroke and acute coronary syndrome in patients with normal kidney function, some studies have reported the phenomenon of “antiplatelet resistance” characterized by a poor response to aspirin or clopidogrel in patients with CKD (9, 10). Besides, Jeong et al. have reported that the suboptimal response to antiplatelets is nearly 70% in some of the Asian communities due to genetic polymorphism, and suggested a different therapeutic window of platelet reactivity in East Asians (11, 12). The failure to suppress platelet activity leads to the increased thrombogenicity and may explain the higher risks of ischemic stroke and AMI despite using combination therapy among our participants. Therefore, we suggested that an appropriate OAC use is critical to overcoming the low effectiveness of antiplatelets in patients with CKD with concomitant AF and CAD, especially in those for whom PCI for coronary revascularization is not suitable.
Previous studies and a recent meta-analysis have reached the same conclusion to use OAC monotherapy in patients with AF with stable CAD for 1 year or more after PCI (13, 14). Nevertheless, patients with CKD were often excluded from such clinical trials. By contrast, we included patients with CKD with concomitant CAD who had not yet received PCI. Large-scale prospective CKD trials evaluating the relevance of the proposed treatment strategy are lacking. Our study is the first to illustrate the suitability of OAC prescription in patients with CKD with concomitant AF and CAD.
Although warfarin is the standard treatment for patients with CKD stage 4–5D, we found that DOACs can be a preferred option for the prevention of thromboembolism irrespective of the levels of eGFR. Besides, some observational studies have shown that the renal function of warfarin users tends to decline faster than that of DOACs (15, 16). To explain this phenomenon, Brodsky et al. reported that the nephrotoxicity of warfarin were positively correlated with excessive anticoagulation with an international normalized ratio (INR) of >3.0, which may cause clinically relevant bleeding or glomerular hemorrhage termed “anticoagulant-related nephropathy” (17). In this study, we did not find that DOACs were superior to warfarin in terms of renal outcomes; however, DOACs can be an appropriate and safe antithrombotic treatment for patients with CKD, even in the pre-dialysis stages. This finding was parallel to recent studies that suggested DOACs had comparable efficacy and were safer than warfarin in patients with an eGFR of <15 mL/min per 1.73 m2 and those on hemodialysis (HD) (18–21). Recently the Kidney Disease: Improving Global Outcomes (KDIGO) conference approved the consideration regarding the use of a lower dose of apixaban or rivaroxaban in patients with advanced CKD (22). Accordingly, DOACs potentially have equal effectiveness as warfarin and better safety outcomes in patients with AF with advanced CKD.
A substantial proportion of CKD patients inevitably develop ESRD, leading to high CV risk attributed to anemia, endothelial dysfunction, vascular calcification, and oxidative stress (23). Dialysis therapy per se significantly impacts coagulation and thrombosis, and warfarin has also been associated with vascular calcification in HD patients (24–26). Randhawa et al. conducted a meta-analysis with 15 observational studies reporting the outcomes of 47,480 patients with AF and ESRD. They found the use of warfarin had no benefits on lowering risks for ischemic stroke, major bleeding, and mortality, but with a significantly higher risk of hemorrhagic stroke in ESRD patients (27). A recent network meta-analysis also showed no evidence of reducing thromboembolic events with warfarin or DOACs in patients with AF and ESRD (28). Although an increasingly popular approach is to use apixaban for stroke prevention among patients with AF and ESRD, the outcomes derived from previous studies were inconstant (18, 20, 29, 30). Therefore, the lack of robust evidence of an appropriate OAC therapeutic approach for dialysis patients highlights the urgent need for additional research in this population.
This study has some limitations. First, the study population was composed of Taiwanese patients from a single center; thus, the results may not be applicable to other ethnic groups. Second, because of the lack of specific details regarding the time in the therapeutic range (6) of warfarin, we could not assess the adequacy of OAC therapy in patients treated with warfarin. In consideration of bleeding tendency attributed to uremic toxins, anemia, and platelet dysfunction in patients with advanced CKD and that undergoing dialysis, the INR target of warfarin therapy in patients with ESRD was apparently conservative in clinical practice. We estimated a low TTR in warfarin users, which reflects poor anticoagulation control and might have affected outcomes in this study. Third, selection bias may exist in a retrospective cohort design. However, we adjusted for potential confounders by using propensity score matching. Moreover, we have to acknowledge that the distributions of CKD among our participants were mainly in stages 3 and 4, whereas the number of ESRD patients taking OACs is small; thereby, our findings might not apply to dialysis-dependent CKD patients. Finally, our study is observational in nature; thus, it cannot prove causality. Nevertheless, given the limited evidence from RCTs on antithrombotic therapy exclusively for patients with CKD, our results indicate the effectiveness and safety of using DOACs alone for AF and CAD among patients with CKD in routine clinical practice.
Conclusions
In patients with CKD with concomitant AF and CAD who had not undergone PCI, OAC in combination with antiplatelets might not provide additional benefits for the prevention of MACE and further be associated with a higher risk of bleeding events. DOACs can be the preferred OAC therapy than warfarin, with a lower risk of all-cause mortality as well as AMI and GI bleeding. Additional prospective clinical studies are needed to reinforce our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Taipei Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Y-PL and D-CT were involved in planning and supervised the work. K-HL and S-MO processed the experimental data, performed the analysis, drafted the manuscript, and designed the figures. Y-CC designed the model and the computational framework. M-TT aided in interpreting the results and worked on the manuscript. All authors discussed the results and commented on the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology, ROC (109-2314-B-010-056-MY3 and 109-2314-B-075-066) and Taipei Veterans General Hospital (V108D42-004-MY3-2, V109D50-001-MY3-1, V108C-103, V109C-114, and V109B-016). This work was also financially supported by the Center for Intelligent Drug Systems and Smart Bio-devices (IDS2B) from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, and Foundation for Poison Control (FPC-110-003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Members of Evaluating the Prognosis and Impacts in CKD (EPIC) Taiwan Research Group in Taipei Veterans General Hospital include D-CT, S-MO, Y-CC, Y-PL, K-HL, M-TT, Chih-Yu Yang, Wei-Cheng Tseng, and Jia-Sin Liu.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.751359/full#supplementary-material
References
1. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. (2009) 30:1038–45. doi: 10.1093/eurheartj/ehn579
2. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. (2013) 381:1107–15. doi: 10.1016/S0140-6736(12)62177-1
3. Lamberts M, Gislason GH, Olesen JB, Kristensen SL, Schjerning Olsen AM, Mikkelsen A, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. (2013) 62:981–9. doi: 10.1016/j.Jacc.2013.05.029
4. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. (2017) 377:1513–24. doi: 10.1056/NEJMoa1708454
5. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. (2019) 381:1103–13. doi: 10.1056/NEJMoa1904143
6. Agrawal H, Aggarwal K, Littrell R, Velagapudi P, Turagam MK, Mittal M, et al. Pharmacological and non pharmacological strategies in the management of coronary artery disease and chronic kidney disease. Curr Cardiol Rev. (2015) 11:261–9. doi: 10.2174/1573403x1103150514155757
7. Turagam MK, Addepally NS, Velagapudi P. Novel anticoagulants for stroke prevention in atrial fibrillation and chronic kidney disease. Expert Rev Cardiovasc Ther. (2013) 11:1297–9. doi: 10.1586/14779072.2013.839188
8. Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) Endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. (2014) 35:3155–79. doi: 10.1093/Eurheartj/Ehu298
9. Morel O, Muller C, Jesel L, Moulin B, Hannedouche T. Impaired platelet P2Y12 inhibition by thienopyridines in chronic kidney disease: mechanisms, clinical relevance and pharmacological options. Nephrol Dial Transplant. (2013) 28:1994–2002. doi: 10.1093/ndt/Gft027
10. Polzin A, Dannenberg L, Sansone R, Levkau B, Kelm M, Hohlfeld T, et al. Antiplatelet effects of aspirin in chronic kidney disease patients. J Thromb Haemost. (2016) 14:375–80. doi: 10.1111/jth.13211
11. Jeong YH. “East Asian Paradox”: Challenge for the current antiplatelet strategy of “one-Guideline-Fits-All Races” in acute coronary syndrome. Curr Cardiol Rep. (2014) 16:485. doi: 10.1007/S11886-014-0485-4
12. Kim HK, Tantry US, Smith SC Jr., Jeong MH, Park SJ, Kim MH, et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. (2021) 121:422–32. doi: 10.1055/s-0040-1718729
13. Lee SR, Rhee TM, Kang DY, Choi EK, Oh S, Lip GYH. Meta-analysis of oral anticoagulant monotherapy as an antithrombotic strategy in patients with stable coronary artery disease and nonvalvular atrial fibrillation. Am J Cardiol. (2019) 124:879–85. doi: 10.1016/j.Amjcard.2019.05.072
14. Fischer Q, Georges JL, Le Feuvre C, Sharma A, Hammoudi N, Berman E, et al. Optimal long-term antithrombotic treatment of patients with stable coronary artery disease and atrial fibrillation: “OLTAT registry”. Int J Cardiol. (2018) 264:64–9. doi: 10.1016/j.Ijcard.2018.03.018
15. Chan YH, Yeh YH, Hsieh MY, Chang CY, Tu HT, Chang SH, et al. The risk of acute kidney injury in asians treated with apixaban, rivaroxaban, dabigatran, or warfarin for non-valvular atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. (2018) 265:83–9. doi: 10.1016/j.Ijcard.2018.02.075
16. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. (2017) 69:2779–90. doi: 10.1016/j.Jacc.2017.03.600
17. Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. (2018) 29:2787–93. doi: 10.1681/ASN.2018070741
18. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. (2018) 138:1519–29. doi: 10.1161/circulationaha.118.035418
19. Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. (2019) 132:1078–83. doi: 10.1016/j.Amjmed.2019.04.013
20. Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. (2018) 2:291–98. doi: 10.1002/Rth2.12083
21. Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and efficacy of apixaban versus warfarin in patients with advanced chronic kidney disease. Ann Pharmacother. (2018) 52:1078–84. doi: 10.1177/1060028018781853
22. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J. (2018) 39:2314–25. doi: 10.1093/Eurheartj/Ehy060
23. Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. (2008) 2008:S4–9. doi: 10.1038/ki.2008.516
24. Lee KH Li SY, Liu JS, Huang CT, Chen YY, Lin YP, et al. Association of warfarin with congestive heart failure and peripheral artery occlusive disease in hemodialysis patients with atrial fibrillation. J Chin Med Assoc. (2017) 80:277–82. doi: 10.1016/j.Jcma.2016.10.012
25. Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol. (2008) 3:1504–10. doi: 10.2215/CJN.00770208
26. Tsai MT, Chen YY, Chang WJ, Li SY. Warfarin accelerated vascular calcification and worsened cardiac dysfunction in remnant kidney mice. J Chin Med Assoc. (2018) 81:324–30. doi: 10.1016/j.Jcma.2017.08.021
27. Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G, et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. (2020) 3:e202175. doi: 10.1001/Jamanetworkopen.2020.2175
28. Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N, et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol. (2020) 75:273–85. doi: 10.1016/j.Jacc.2019.10.059
29. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. (2020) 15:1146–54. doi: 10.2215/CJN.11650919
Keywords: anticoagulation, acute myocardial infarction, atrial fibrillation, stroke, thromboembolism
Citation: Lee K-H, Ou S-M, Chu Y-C, Lin Y-P, Tsai M-T and Tarng D-C (2021) Antithrombotic Therapy for Chronic Kidney Disease Patients With Concomitant Atrial Fibrillation and Coronary Artery Disease. Front. Cardiovasc. Med. 8:751359. doi: 10.3389/fcvm.2021.751359
Received: 01 August 2021; Accepted: 15 September 2021;
Published: 08 October 2021.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Mohit Turagam, Mount Sinai Hospital, United StatesJing-Song Ou, The First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2021 Lee, Ou, Chu, Lin, Tsai and Tarng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Der-Cherng Tarng, dctarng@vghtpe.gov.tw
†These authors have contributed equally to this work and share first authorship
 Kuo-Hua Lee
Kuo-Hua Lee