- 1Section of Cardiovascular Medicine, Yale University School of Medicine, New Haven, CT, United States
- 2Ascension Medical Group, Section of Cardiovascular Medicine, Milwaukee, WI, United States
- 3Department of Internal Medicine, Yale New Haven Hospital, New Haven, CT, United States
- 4Section of Infectious Diseases, Yale University School of Medicine, New Haven, CT, United States
Infective endocarditis is a common and treatable condition that carries a high mortality rate. Currently the workup of infective endocarditis relies on the integration of clinical, microbiological and echocardiographic data through the use of the modified Duke criteria (MDC). However, in cases of prosthetic valve endocarditis (PVE) echocardiography can be normal or non-diagnostic in a high proportion of cases leading to decreased sensitivity for the MDC. Evolving multimodality imaging techniques including leukocyte scintigraphy (white blood cell imaging), 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), multidetector computed tomographic angiography (MDCTA), and cardiac magnetic resonance imaging (CMRI) may each augment the standard workup of PVE and increase diagnostic accuracy. While further studies are necessary to clarify the ideal role for each of these imaging techniques, nevertheless, these modalities hold promise in determining the diagnosis, prognosis, and care of PVE. We start by presenting a clinical vignette, then evidence supporting various modality strategies, balanced by limitations, and review of formal guidelines, when available. The article ends with the authors' summary of future directions and case conclusion.
Clinical Vignette
A 61-year-old man is brought to the emergency department with chief concerns of fever and confusion. Medical history is notable for bicuspid aortic stenosis status post bioprosthetic aortic valve replacement 3 months prior to presentation with his postoperative course complicated by sternal wound infection. In the emergency department he is noted to be febrile, tachycardic, and hypotensive. He is treated for septic shock and blood cultures subsequently grew methicillin-susceptible Staphylococcus aureus on day of presentation. Despite treatment with oxacillin, over the next 2 days he continues to be bacteremic with progressive PR prolongation to 240 ms noted on serial electrocardiograms. Given the high clinical concern for prosthetic aortic valve endocarditis with root abscess the patient undergoes transesophageal echocardiogram 2 days after initial positive blood cultures but does not reveal any abscess or vegetation (Supplementary Table 1 and Supplementary Figure 1A). How should this patient be further managed?
Introduction
Infective endocarditis (IE) is an increasingly common infectious disease with incidence rates in the United States rising from 11 per 100,000 to 15 per 100,000 between 2000 and 2011 (1, 2). The disease also carries a high mortality rate with 5-year mortality of ~40% and in-hospital mortality ranging from 15 to 22% (3–5). Patients with IE require early diagnosis given both the treatable nature of this condition and potential complications of delayed antibiotics and surgery.
In 1994, the original Duke criteria were published by Durack et al. to facilitate the diagnosis of IE (6). However, the extrapolation of these criteria to real-world clinical patients remained challenging. In 2000, citing the need to redefine “possible IE,” and improve the ODC's sensitivity in the detection of Q fever, Li et al. established the modified Duke criteria (MDC; Supplementary Table 2) by proposing several changes to the existing major and minor criteria including further strengthening the role of echocardiography and narrowing the definition of “possible IE” (7). Subsequently, the MDC through the combined use of clinical, echocardiographic, pathologic, and microbiological data have since become one of the most widely used clinical tools for the detection of IE.
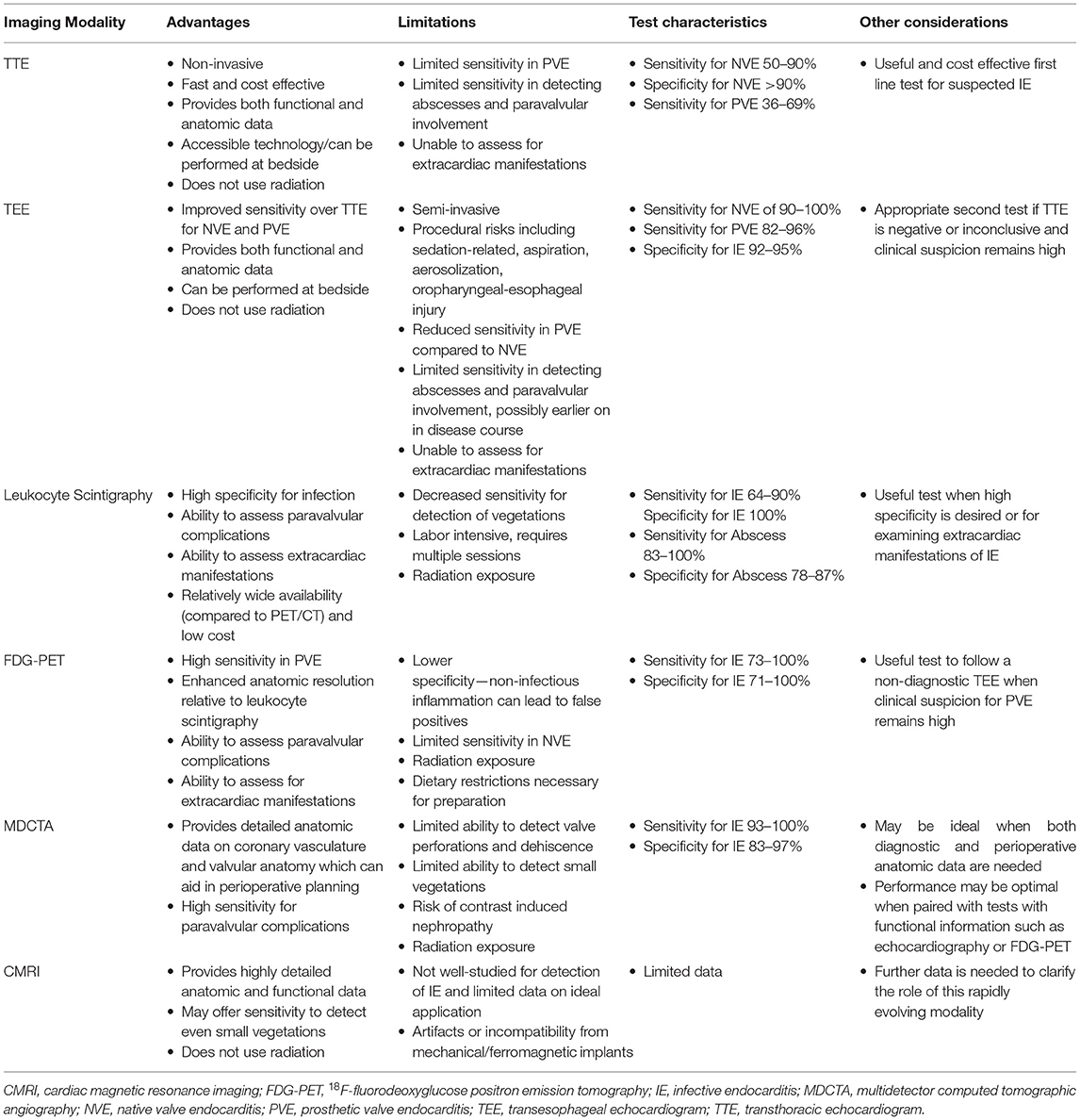
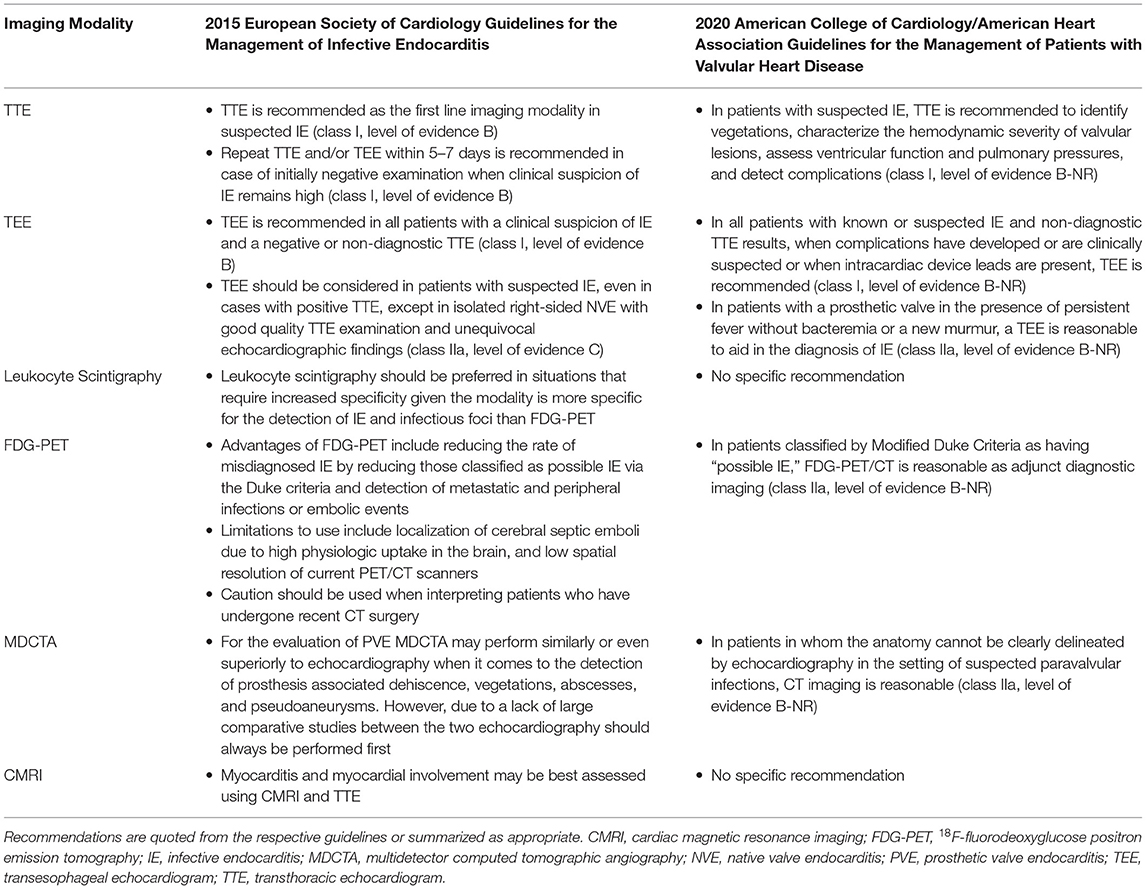
However, in cases of prosthetic valve endocarditis (PVE), echocardiography can be normal or non-diagnostic in around 30% of cases, leading to reduced diagnostic accuracy for the MDC (8, 9). This can be a particularly vexing issue, given the increasing use of intracardiac prosthetic materials, and the relatively high proportion of prosthetic material associated with endocarditis (10–13). Fortunately, newer and novel approaches to cardiac imaging including leukocyte scintigraphy (white blood cell imaging), 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), multidetector computed tomographic angiography (MDCTA), and cardiac magnetic resonance imaging (CMRI) can be considered as potential adjunct tools in the evaluation of suspected PVE (14). The various modalities' advantages, limitations, characteristics, and other considerations are highlighted in Table 1. The various modalities' recommended applications as they pertain to recent clinical guidelines (13, 15) are outlined in Table 2.
Leukocyte Scintigraphy (White Blood Cell Imaging)
Leukocyte scintigraphy can be highly specific for infection as it allows examination for the pathologic accumulation of radiolabeled granulocytes at involved sites through the use of single photon emission computed tomography (SPECT)/CT. Thus, leukocyte scintigraphy may offer high specificity (100%) for paravalvular infection and abscess detection in patients with suspected PVE (13, 14, 16). Furthermore, this imaging technique allows for evaluation of extracardiac manifestations of PVE, including endovascular infections, ophthalmitis, or intracranial infections, providing a broader picture of both cardiac and non-cardiac sources of infection (17, 18). The modality may yield not as much non-specific radiotracer uptake in the sternum in those who have recently (past 1 month) undergone cardiothoracic surgery, and may avoid confounding inflammation from certain other non-infectious pathologies (e.g., non-calcified atherosclerotic plaque, vasculitis, active thrombus, primary cardiac tumor or non-cardiac tumor that has metastasized to the heart, post-op inflammation, or foreign body reaction) that can mimic the pattern of FDG uptake seen with IE (19). Also, from an availability standpoint there may remain many centers that have SPECT/CT but not yet PET/CT available.
However, leukocyte scintigraphy also has potential limitations. For one, vegetations contain relatively few granulocytes, and therefore leukocyte scintigraphy may offer decreased sensitivity (64%) for the detection of PVE (14, 16). Furthermore, leukocyte scintigraphy offers decreased spatial resolution compared to other imaging modalities and is labor intensive requiring the drawing, preparation, and reinjection of granulocytes over multiple sessions. Currently, there is discordance between the European (13) and American (15) guidelines; white cell scans are recognized in the 2015 European Society of Cardiology (ESC) guideline for the management of IE as being more specific than FDG-PET, and ought to be preferred in clinical situations that would benefit from increased specificity, whereas the 2020 American College Cardiology (ACC)/American Heart Association (AHA) clinical practice valvular heart disease (VHD) guideline does not make a specific recommendation. While the ideal application of leukocyte scintigraphy in the detection of PVE may be currently unknown or debatable, this test may be best implemented when other modalities are inconclusive or when enhanced specificity is needed (13, 14, 16).
18F-fluorodeoxyglucose Positron Emission Tomography
Relative to leukocyte scintigraphy, FDG-PET offers several advantages: it is less labor intensive, has enhanced anatomical resolution, and offers increased sensitivity for the detection of PVE (14, 16, 20). For these reasons FDG-PET is increasingly used in difficult-to-diagnose cases of PVE where initial echocardiography is non-diagnostic. The presence of an abnormal signal in the region of interest in the valve's vicinity by FDG-PET was included as a major criterion for the diagnosis of PVE in the 2015 update (from 2009) ESC IE guideline (13). Adding FDG-PET as a major criterion to the MDC increases the sensitivity of the MDC from 52 to 70% up to 97% without sacrificing specificity, and aids in the early diagnosis of PVE, particularly when echocardiography is equivocal or normal (21, 22). A recent study by Primus et al. showed that FDG-PET improves diagnostic certainty when combined with MDC in both native and PVE (23). A contemporary meta-analysis of PVE or cardiac implantable electronic device (at least 3 months post-placement) endocarditis had a pooled sensitivity of 72–86% and specificity of 83–84% with use of FDG-PET (24). While no FDG uptake excludes the presence of PVE, an elevated ratio of FDG uptake at and around the prosthetic valve relative to background standardized uptake value (SUV) of >4.4 highly suggests PVE (16). Similar to leukocyte scintigraphy, FDG-PET offers the ability to detect distant emboli and foci of infection allowing for the characterization of extracardiac involvement, with the caveat that, due to high physiologic levels of FDG uptake in the brain, it may be limited in the detection of intracerebral infections (13, 20, 21, 25, 26). A related caveat is that cardiac physiologic uptake of glucose must be adequately suppressed with a high-fat, low-carbohydrate diet and prolonged fast prior to FDG-PET to reliably identify pathological uptake in structures adjacent to myocardium (27).
While FDG-PET is highly sensitive, it is less specific than leukocyte scintigraphy because FDG-PET uptake can be increased also in non-infectious sources of inflammation (14, 16). In particular, inflammation from recent cardiac surgery (within a month) has the potential to lead to false positive findings on FDG-PET, and therefore leukocyte scintigraphy with SPECT/CT or other imaging modalities may be preferred in these cases (13, 14, 17). The 2020 ACC/AHA VHD guideline gives FDG-PET a moderate strength (class 2a) recommendation when the MDC is possible IE (15). Due to the several advantages of this modality, including high sensitivity, feasibility, and ability to detect extracardiac involvement, it may be a logical choice to follow a negative or non-diagnostic transesophageal echocardiogram (TEE) when clinical suspicion for PVE remains high (13, 14, 21).
Multidetector Computed Tomographic Angiography
While TEE, SPECT/CT, and FDG-PET provide functional data, MDCTA has the advantage of providing detailed anatomic images due to its high spatial resolution, with current scanners having the ability to have sub-millimeter (on the order of 0.5-mm) “isotropic” resolution in all three dimensions (28). With a full 3D cardiac dataset scan, post-processing in multiplanar reconstruction allows the valvular and prosthetic structures to be evaluated from any angle to fully interrogate valve and surrounding structures for precise anatomic assessment (29). In particular, MDCTA is adept in the detection of paravalvular abscesses, prosthetic dehiscence, and pseudoaneurysms, and may be better able to distinguish myocardial, pericardial, and coronary sinus involvement relative to TEE, while offering similar ability to detect non-highly mobile and larger vegetations (29–32). Additionally, due to the detailed anatomic information offered, as well as the ability to assess the status of the coronary arteries, MDCTA is particularly well-suited for perioperative evaluation and planning (13, 33). However, when compared with TEE, MDCTA may miss very small valve leaflet perforations and highly mobile vegetations due to the lower temporal resolution compared to echocardiography (30, 31). Therefore, as of the latest guidelines MDCTA may be best utilized as a complementary study to echocardiography, with current ESC guidelines for the management of IE suggesting that echocardiography should typically be performed first (13, 32, 34, 35). Additionally, the 2020 ACC/AHA clinical practice VHD guideline gives MDCTA a class 2a recommendation for suspected paravalvular abscess when echo images are inadequate (15). When added to the standard diagnostic workup including echocardiography, the addition of MDCTA improves sensitivity up to 100% and specificity of 83% (14). However, during the recent COVID-19 pandemic, in cases with risks of aerosolization with TEE, MDCTA has been seen as a reasonable alternative (29). Moreover, another avenue is to complement FDG-PET's functional information with MDCTA's anatomic data in order to maximize sensitivity, specificity, and diagnostic accuracy (22). As a result, while the use of MDCTA is not yet universal as an frontline test in PVE, there is growing data to support its earlier use in the diagnostic assessment in this patient group; a recent meta-analysis published after the 2020 ACC/AHA guideline showed that MDCTA performs better in identifying prosthetic valve infection and showed a trend of improved detection of para-annular complications of abscess and pseudoaneurysm formation compared to TEE (36). Thus, this modality offers promise in scenarios where detailed anatomic or perioperative data is needed to supplement the workup of PVE or as a complementary study to TEE or FDG-PET or even possibly as a frontline study.
Cardiac Magnetic Resonance Imaging
Cardiac MRI is a rapidly evolving imaging modality that can provide both detailed anatomic as well as functional data on valvular regurgitation and the presence of myocardial edema and inflammation (14, 37, 38). When applied to patients with possible infective endocarditis, CMRI can identify valvular vegetations and paravalvular pseudoaneurysms, and detect the paravalvular extension of infection through the presence of delayed contrast enhancement (38–40). This led to the inclusion of CMRI as a new indication along with echocardiography to assess myocardial involvement during infective endocarditis within the subsection regarding complications of infective endocarditis relating to myopericarditis in the 2015 update of ESC guidelines from 2009 (41). Additional strides are being made recently in multimodality comparison between TEE and CMRI for the quantification of paravalvular regurgitation in left-sided (aortic or mitral) prosthetic valves (42). However, to date there are relatively few studies on the use of CMRI in the detection of PVE, and most of the existing data comes from small series or case reports (43, 44). Furthermore, CMRI carries potential limitations including valve-induced susceptibility artifacts with mechanical prosthetic valves, contraindications for patients with certain pacemakers and medical implants, and longer acquisition times (37). As a result, the ideal diagnostic role for CMRI in the workup of IE is still to be determined, and further studies are needed (14).
Discussion
PVE diagnosis remains a challenging and clinically important issue that is expected to be increasingly encountered given the rising use of intracardiac prosthetic materials. In cases of difficult-to-diagnose PVE, multimodality imaging techniques can provide utility beyond the standard diagnostic workup and serve to augment the MDC and echocardiography. Both leukocyte scintigraphy and FDG-PET offer the ability to evaluate for extracardiac involvement while carrying high specificity and sensitivity, respectively. Furthermore, MDCTA can provide key anatomic data, including paravalvular information, and is particularly useful for perioperative planning. While CMRI offers the possibility of detailed anatomic and functional data, currently there is insufficient evidence for the role of this modality in the routine workup of PVE and further studies are needed. The diagnostic approach to PVE is summarized in Figure 1.
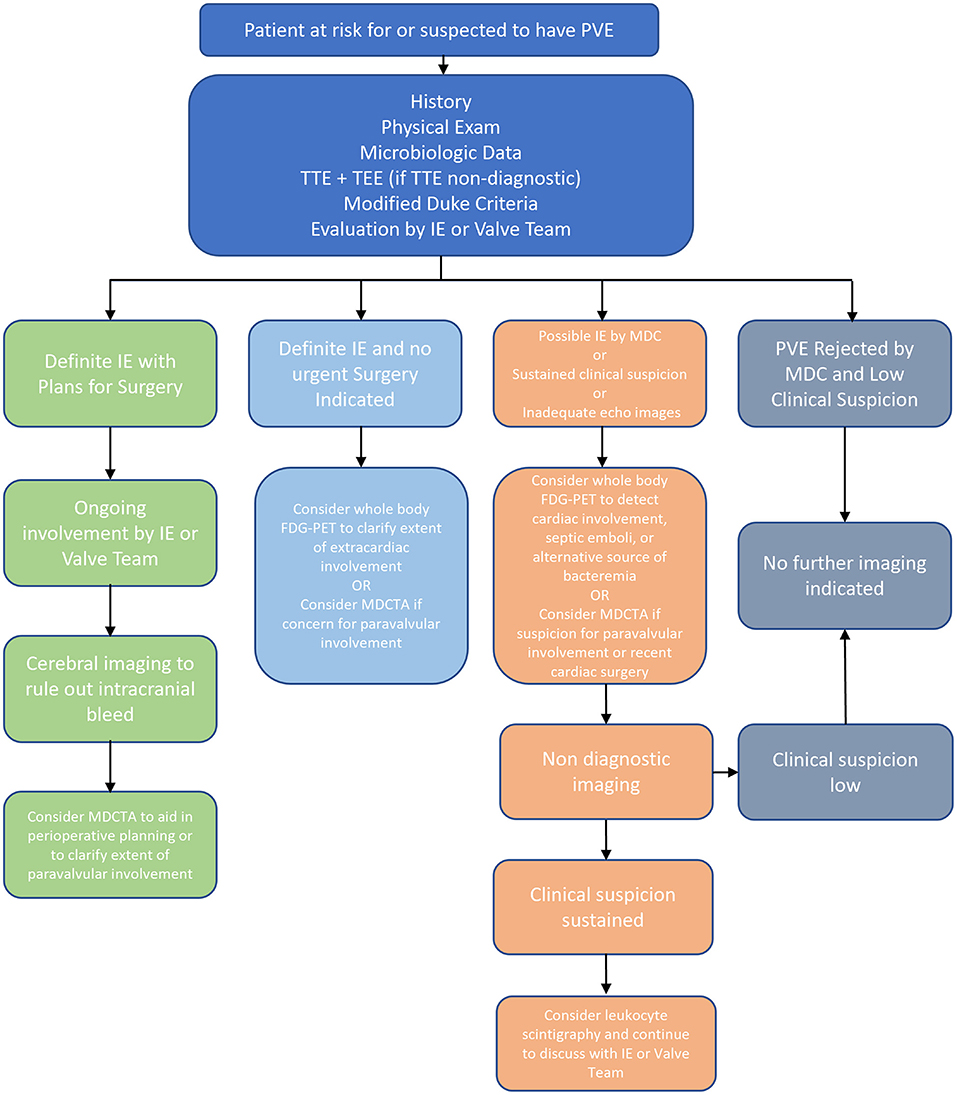
Figure 1. Overview of Diagnosis of PVE. FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; IE, infective endocarditis; MDC, modified duke criteria; MDCTA, multidetector computed tomographic angiography; NVE, native valve endocarditis; PVE, prosthetic valve endocarditis; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.
While an important consideration is the cost effectiveness of each modality, unfortunately contemporary high-quality data in this area is currently lacking. Echocardiography is affordable and widely available although early studies of cost effectiveness in IE found it may be more cost effective to proceed with TEE as an initial diagnostic test in intermediate risk patients than to pursue sequential testing with transthoracic echocardiogram prior to TEE (45). Interestingly, in patients who already have a high pretest probability of IE, the most cost-effective strategy may be to simply pursue empiric treatment without additional echocardiographic imaging (45). Though more limited in availability, the addition of FDG-PET to detect metastatic infections in high-risk patients with Gram-positive bacteremia may be cost effective (46), however it is unclear if this would be true for the evaluation of endocarditis.
Costs and time to diagnosis increase with each sequential diagnostic test, and therefore strategies which reduce test stacking can improve both the cost effectiveness and time effectiveness of diagnosing PVE. Two strategies that are gaining attention are the use of diagnostic flowcharts to streamline the decision-making process, and the use of multimodality endocarditis teams to tailor the diagnostic workup. Contemporary evidence would suggest that diagnostic flow charts may be applicable to real-world clinical practice and may help increase diagnostic yield in PVE (47). However, the full use of complex diagnostic algorithms would require both the necessary imaging technology as well the appropriately specialized team to interpret the data and thus may be limited to certain hospital systems (48). A more individualized approach is the use of highly specialized and multidisciplinary endocarditis teams to guide diagnosis and treatment which may lead to earlier diagnosis and improved outcomes (49, 50). Through the use of these strategies, it may be possible to enhance diagnosis, prognosis, and patient care in PVE.
Future Directions
Despite the utility of multimodality imaging tests, further larger prospective studies are needed to clarify the optimal role and refine the imaging protocol and interpretation of each of these tests in PVE diagnosis, as well as clarify the prognostic value of these tests.
More recently, within radionuclide imaging for PVE, studies have looked at better refining image acquisition time of FDG-PET (~60 min post intravenous injection as opposed to ~150 min) (51) to reduce late false-positive FDG uptake and utilizing SUVs (52). Uniform protocols and standardized metrics would facilitate the comparison of studies from various centers and support the creation of multicenter registries in this area of PVE imaging. The evolution of grading FDG uptake from just positive or negative to more qualitative (absent, mild, moderate, intense) grading, to eventual quantitation with the goal of developing SUV thresholds corresponding to each of the above qualitative levels of FDG uptake would aid to increase diagnostic precision and accuracy and relate these various levels to the probability of PVE in patients.
Currently, the diagnosis of PVE should continue to rely on clinical judgment, microbiological data, and echocardiographic studies with multimodality imaging augmenting this workup when additional sensitivity, specificity, functional data, or anatomic clarification is necessary. Analogous to how echocardiography is used in the modified Duke paradigm, the precise quantification of valvular FDG uptake SUVs could be how more major and minor criteria of PVE using nuclear imaging become delineated, perhaps in combination with the analysis of the pattern and location of FDG uptake in the valve and paravalvular regions of interest.
In the area of multidetector cross-sectional imaging with computed tomography, dual-energy scans, which can reduce beam hardening and partial volume averaging artifacts to improve tissue characterization quality via distinguishing between high- and low-photon energies, have shown some early promise, but thus far have been limited in clinical use (29). Spectral computed tomography may further delineate different tissue densities by utilizing multiple energy levels, and these modalities may aid in earlier vegetation and abscess identification while continued improvements in stent resolution and minimizations in artifacts with ultra-high-resolution scans (detector rows as low as 0.25 mm in width) may improve evaluation of the prostheses themselves.
Conclusion
Following a non-diagnostic TEE, the patient in the clinical vignette underwent FDG-PET 4 days later, which revealed increased FDG uptake at the aortic valve prosthesis. Subsequent repeat TEE 3 days later and 1 week since original TEE revealed vegetations and an aortic root abscess (Supplementary Figures 1B-E and Supplementary Table 3). The patient was taken for redo aortic valve replacement with ascending aortic graft and root replacement. In summary, while PVE may provide a diagnostic challenge to the conventional workup of IE, multimodality imaging techniques such as leukocyte scintigraphy, FDG-PET, MDCTA, and CMRI can provide additional diagnostic value and aid in the correct diagnosis of PVE.
Author Contributions
ME, LS, and DH devised the manuscript concept. ME collected articles and wrote the manuscript in collaboration with KU, JP, MR, and DH. JP, MR, MM, BY, LS, and DH critically reviewed and revised the manuscript. All authors approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.750573/full#supplementary-material
Abbreviations
ACC, American College of Cardiology; AHA, American Heart Association; CMRI, cardiac magnetic resonance imaging; ESC, European Society of Cardiology; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; IE, infective endocarditis; MDC, modified Duke criteria; MDCTA, multidetector computed tomographic angiography; PVE, prosthetic valve endocarditis; SPECT/CT, single photon emission computed tomography; SUV, standardized uptake value; TEE, transesophageal echocardiogram; VHD, valvular heart disease.
References
1. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. (2015) 65:2070–6. doi: 10.1016/j.jacc.2015.03.518
2. Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York State, 1998-2013. JAMA. (2017) 317:1652–60. doi: 10.1001/jama.2017.4287
3. Hoen B, Duval X. Infective endocarditis. N Engl J Med. (2013) 368:1425–33. doi: 10.1056/NEJMcp1206782
4. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. (2016) 387:882–93. doi: 10.1016/S0140-6736(15)00067-7
5. Bannay A, Hoen B, Duval X, Obadia J-F, Selton-Suty C, Le Moing V, et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Eur Heart J. (2011) 32:2003–15. doi: 10.1093/eurheartj/ehp008
6. Durack DT, Lukes AS, Bright DK, Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. (1994) 96:200–9. doi: 10.1016/0002-9343(94)90143-0
7. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. (2000) 30:633–8. doi: 10.1086/313753
8. Hill EE, Herijgers P, Claus P, Vanderschueren S, Peetermans WE, Herregods M-C. Abscess in infective endocarditis: the value of transesophageal echocardiography and outcome: a 5-year study. Am Heart J. (2007) 154:923–8. doi: 10.1016/j.ahj.2007.06.028
9. Vieira MLC, Grinberg M, Pomerantzeff PMA, Andrade JL, Mansur AJ. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart. (2004) 90:1020–4. doi: 10.1136/hrt.2003.025585
10. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. (2011) 58:1001–6. doi: 10.1016/j.jacc.2011.04.033
11. Kim DH, Tate J, Dresen WF, Papa FC Jr, Bloch KC, Kalams SA, et al. Cardiac implanted electronic device-related infective endocarditis: clinical features, management, and outcomes of 80 consecutive patients: cardiac device-related endocarditis. Pacing Clin Electrophysiol. (2014) 37:978–85. doi: 10.1111/pace.12452
12. Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Paré C, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. (2007) 297:1354–61. doi: 10.1001/jama.297.12.1354
13. Habib G, Lancellotti P, Antunes MJ. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology. Eur Heart J. (2015) 36:21. doi: 10.1093/eurheartj/ehv319
14. Gomes A, Glaudemans AWJM, Touw DJ, van Melle JP, Willems TP, Maass AH, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis. (2017) 17:e1–14. doi: 10.1016/S1473-3099(16)30141-4
15. Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77:e25–197. doi: 10.1016/j.jacc.2020.11.018
16. Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, et al. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. (2014) 55:1980–5. doi: 10.2967/jnumed.114.141895
17. Erba PA, Conti U, Lazzeri E, Sollini M, Doria R, De Tommasi SM, et al. Added value of 99mTc-HMPAO–labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med. (2012) 53:1235–43. doi: 10.2967/jnumed.111.099424
18. Hyafil F, Rouzet F, Lepage L, Benali K, Raffoul R, Duval X, et al. Role of radiolabelled leucocyte scintigraphy in patients with a suspicion of prosthetic valve endocarditis and inconclusive echocardiography. Eur Heart J Cardiovasc Imaging. (2013) 14:586–94. doi: 10.1093/ehjci/jet029
19. Lee JC, Wee YS, Horvath RL. Nuclear imaging in the diagnosis of infective endocarditis. J Nucl Cardiol. (2020) 27:1049. doi: 10.1007/s12350-019-01819-4
20. Kestler M, Munoz P, Rodriguez-Creixems M, Rotger A, Jimenez-Requena F, Mari A, et al. Role of 18F-FDG PET in patients with infectious endocarditis. J Nucl Med. (2014) 55:1093–8. doi: 10.2967/jnumed.113.134981
21. Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. (2013) 61:2374–82. doi: 10.1016/j.jacc.2013.01.092
22. Pizzi MN, Roque A, Fernández-Hidalgo N, Cuéllar-Calabria H, Ferreira-González I, Gonzàlez-Alujas MT, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. (2015) 132:1113–26. doi: 10.1161/CIRCULATIONAHA.115.015316
23. Primus CP, Clay TA, McCue MS, Wong K, Uppal R, Ambekar S, et al. 18F-FDG PET/CT improves diagnostic certainty in native and prosthetic valve Infective Endocarditis over the modified Duke Criteria. J Nucl Cardiol. (2021). doi: 10.1007/s12350-021-02689-5. [Epub ahead of print].
24. Wang TKM, Sánchez-Nadales A, Igbinomwanhia E, Cremer P, Griffin B, Xu B. Diagnosis of Infective endocarditis by subtype using 18F-fluorodeoxyglucose positron emission tomography/computed tomography: a contemporary meta-analysis. Circ Cardiovasc Imaging. (2020) 13:e010600. doi: 10.1161/CIRCIMAGING.120.010600
25. Orvin K, Goldberg E, Bernstine H, Groshar D, Sagie A, Kornowski R, et al. The role of FDG-PET/CT imaging in early detection of extra-cardiac complications of infective endocarditis. Clin Microbiol Infect. (2015) 21:69–76. doi: 10.1016/j.cmi.2014.08.012
26. Asmar A, Ozcan C, Diederichsen ACP, Thomassen A, Gill S. Clinical impact of 18F-FDG-PET/CT in the extra cardiac work-up of patients with infective endocarditis. Eur Heart J Cardiovasc Imag. (2014) 15:1013–9. doi: 10.1093/ehjci/jeu054
27. Martineau P, Grégoire J, Harel F, Pelletier-Galarneau M. Assessing cardiovascular infection and inflammation with FDG-PET. Am J Nucl Med Mol Imaging. (2021) 11:46–58.
28. Choi JW, van Rosendael AR, Bax AM, van den Hoogen IJ, Gianni U, Baskaran L, et al. The journal of cardiovascular computed tomography year in review - 2019. J Cardiovasc Comput Tomogr. (2020) 14:107–17. doi: 10.1016/j.jcct.2020.01.003
29. Khalique OK, Veillet-Chowdhury M, Choi AD, Feuchtner G, Lopez-Mattei J. Cardiac computed tomography in the contemporary evaluation of infective endocarditis. J Cardiovasc Comput Tomogr. (2021) 15:304–12. doi: 10.1016/j.jcct.2021.02.001
30. Feuchtner GM, Stolzmann P, Dichtl W, Schertler T, Bonatti J, Scheffel H, et al. Multislice computed tomography in infective endocarditis. J Am Coll Cardiol. (2009) 53:436–44. doi: 10.1016/j.jacc.2008.01.077
31. Fagman E, Perrotta S, Bech-Hanssen O, Flinck A, Lamm C, Olaison L, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol. (2012) 22:2407–14. doi: 10.1007/s00330-012-2491-5
32. Suchá D, Symersky P, Tanis W, Mali WPTM, Leiner T, van Herwerden LA, et al. Multimodality imaging assessment of prosthetic heart valves. Circ Cardiovasc Imaging. (2015) 8:e003703. doi: 10.1161/CIRCIMAGING.115.003703
33. Gahide G, Bommart S, Demaria R, Sportouch C, Dambia H, Albat B, et al. Preoperative evaluation in aortic endocarditis: findings on cardiac CT. AJR Am J Roentgenol. (2010) 194:574–8. doi: 10.2214/AJR.08.2120
34. Habets J, Budde RP, Symersky P, van den Brink RB, de Mol BA, Mali WP, et al. Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol. (2011) 8:466–78. doi: 10.1038/nrcardio.2011.71
35. Rajiah P, Moore A, Saboo S, Goerne H, Ranganath P, MacNamara J, et al. Multimodality imaging of complications of cardiac valve surgeries. Radiographics. (2019) 39:932–56. doi: 10.1148/rg.2019180177
36. Jain V, Wang TKM, Bansal A, Farwati M, Gad M, Montane B, Kaur S, Bolen MA, Grimm R, Griffin B, et al. Diagnostic performance of cardiac computed tomography versus transesophageal echocardiography in infective endocarditis: a contemporary comparative meta-analysis. J Cardiovasc Comput Tomogr. (2021) 15:313–21. doi: 10.1016/j.jcct.2020.11.008
37. Pham N, Zaitoun H, Mohammed TL, DeLaPena-Almaguer E, Martinez F, Novaro GM, et al. Complications of aortic valve surgery: manifestations at CT and MR imaging. Radiographics. (2012) 32:1873–92. doi: 10.1148/rg.327115735
38. Dursun M, Yilmaz S, Yilmaz E, Yilmaz R, Onur I, Oflaz H, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol. (2015) 21:28–33. doi: 10.5152/dir.2014.14239
39. Akins EW, Slone RM, Wiechmann BN, Browning M, Martin TD, Mayfield WR. Perivalvular pseudoaneurysm complicating bacterial endocarditis: MR detection in five cases. AJR Am J Roentgenol. (1991) 156:1155–8. doi: 10.2214/ajr.156.6.2028858
40. Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol. (2016) 118:1410–8. doi: 10.1016/j.amjcard.2016.07.053
41. von Knobelsdorff-Brenkenhoff F, Schulz-Menger J. Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J Cardiovasc Magn Reson. (2016) 18:6. doi: 10.1186/1532-429X-18-S1-Q57
42. Haberka M, Malczewska M, Pysz P, Kozłowski M, Wojakowski W, Smolka G. Cardiovascular magnetic resonance and transesophageal echocardiography in patients with prosthetic valve paravalvular leaks: towards an accurate quantification and stratification. J Cardiovasc Magn Reson. (2021) 23:31. doi: 10.1186/s12968-021-00722-7
43. Sievers B, Brandts B, Franken U, Trappe H-J. Cardiovascular magnetic resonance imaging demonstrates mitral valve endocarditis. Am J Med. (2003) 115:681–2. doi: 10.1016/S0002-9343(03)00441-8
44. Dursun M, Yilmaz S, Ali Sayin O, Olgar S, Dursun F, Yekeler E, et al. Rare cause of delayed contrast enhancement on cardiac magnetic resonance imaging: infective endocarditis. J Comput Assist Tomogr. (2005) 29:709–11. doi: 10.1097/01.rct.0000177520.02175.28
45. Heidenreich PA, Masoudi FA, Maini B, Chou TM, Foster E, Schiller NB, et al. Echocardiography in patients with suspected endocarditis: a cost-effectiveness analysis. Am J Med. (1999) 107:198–208. doi: 10.1016/S0002-9343(99)00216-8
46. Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EMM, Oyen WJG. Cost-effectiveness of routine 18F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med. (2011) 52:1673–8. doi: 10.2967/jnumed.111.089714
47. Gomes A, van Geel PP, Santing M, Prakken NHJ, Ruis ML, van Assen S, et al. Imaging infective endocarditis: adherence to a diagnostic flowchart and direct comparison of imaging techniques. J Nucl Cardiol. (2020) 27:592–608. doi: 10.1007/s12350-018-1383-8
48. Pizzi MN, Fernández-Hidalgo N. Optimizing the diagnostic workup of infective endocarditis: an urgent need for studies focused on defining the decision-making process. J Nucl Cardiol. (2020) 27:609–11. doi: 10.1007/s12350-018-1434-1
49. Erba PA, Pizzi MN, Roque A, Salaun E, Lancellotti P, Tornos P, et al. Multimodality imaging in infective endocarditis: an imaging team within the endocarditis team. Circulation. (2019) 140:1753–65. doi: 10.1161/CIRCULATIONAHA.119.040228
50. Kaura A, Byrne J, Fife A, Deshpande R, Baghai M, Gunning M, et al. Inception of the “endocarditis team” is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. (2017) 4:e000699. doi: 10.1136/openhrt-2017-000699
51. Scholtens AM, Swart LE, Verberne HJ, Budde RPJ, Lam MGEH. Dual-time-point FDG PET/CT imaging in prosthetic heart valve endocarditis. J Nucl Cardiol. (2018) 25:1960–7. doi: 10.1007/s12350-017-0902-3
Keywords: prosthetic, valve, endocarditis (infectious), multimodality, imaging, endocarditis team
Citation: Eder MD, Upadhyaya K, Park J, Ringer M, Malinis M, Young BD, Sugeng L and Hur DJ (2021) Multimodality Imaging in the Diagnosis of Prosthetic Valve Endocarditis: A Brief Review. Front. Cardiovasc. Med. 8:750573. doi: 10.3389/fcvm.2021.750573
Received: 30 July 2021; Accepted: 29 November 2021;
Published: 20 December 2021.
Edited by:
Grigorios Korosoglou, GRN Klinik Weinheim, GermanyReviewed by:
Ilias Ninios, Interbalkan Medical Center, GreeceAnastasios Panagopoulos, University of Nebraska Medical Center, United States
Copyright © 2021 Eder, Upadhyaya, Park, Ringer, Malinis, Young, Sugeng and Hur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David J. Hur, ZGF2aWQuaHVyQHlhbGUuZWR1
 Maxwell D. Eder
Maxwell D. Eder Krishna Upadhyaya
Krishna Upadhyaya Jakob Park
Jakob Park Matthew Ringer
Matthew Ringer Maricar Malinis
Maricar Malinis Bryan D. Young
Bryan D. Young Lissa Sugeng
Lissa Sugeng David J. Hur
David J. Hur