- 1National Institute for Health Research (NIHR) Barts Biomedical Research Centre, William Harvey Research Institute, Queen Mary University of London, London, United Kingdom
- 2Barts Heart Centre, St Bartholomew's Hospital, Barts Health National Health Service (NHS) Trust, London, United Kingdom
- 3Department of Health Policy, London School of Economics and Political Science, London, United Kingdom
Aims: To determine financial implications of implementing cardiac magnetic resonance imaging (CMR) in the diagnostic pathway of a population with unexplained acute myocardial injury and normal coronary angiography.
Methods and Results: We performed a focused cost-benefit analysis using a hypothetical population of 2,000 patients with unexplained acute myocardial injury and normal coronary angiography divided into two groups to receive either standard or CMR guided management over a 10-year period. As healthcare practice and costs considerably vary geographically and over time, an algorithm with 15 key variables was developed to permit user-defined calculations of cost-benefit and other analyses. Using current UK costs, routine use of CMR increases healthcare spending by 14% per patient in the first year. After 7 years, CMR guided practice is cost neutral, reducing cost by 3% per patient 10 years following presentation. In addition, CMR -guided therapy results in 7 fewer myocardial infarctions and 14 fewer major bleeding events per 1,000 patients over a 10-year period. The three most sensitive variables were, in decreasing order, the cost of CMR, the cost of ticagrelor and the percentage of the population with MI requiring DAPT.
Conclusion: Routine use of CMR in patients with unexplained acute myocardial injury and normal coronary angiography is associated with cost reductions in the medium to long term. The initial higher cost of CMR is offset over time and delivers a more personalized and higher quality of care.
Introduction
Acute presentations with myocardial injury that occur despite culprit-free coronary angiography were first reported over 70 years ago (1), with a prevalence of 5–9% in patients undergoing coronary angiography for suspected acute coronary syndromes (2–4). Historically, such presentations were often considered to represent myocardial infarction (MI) with non-obstructive coronary artery disease and referred to by the acronym “MINOCA.”
There is now considerable evidence that the majority of “MINOCA” presentations are not due to MI, and that a more accurate diagnosis is readily attainable in routine clinical practice following such presentations. In a meta-analysis of patients provisionally diagnosed with “MINOCA,” the use of cardiac magnetic resonance imaging (CMR) identified 33% had myocarditis, 24% had MI, 20% had other types of heart muscle disease (e.g., Takotsubo cardiomyopathy, dilated cardiomyopathy, or hypertrophic cardiomyopathy), and 26% had no CMR-detectable myocardial abnormalities (2). More recent studies corroborate these findings, emphasizing that MI is not the most frequent cause of this clinical presentation. These data also demonstrate a key role for CMR in the diagnostic pathway for patients with unexplained acute myocardial injury following culprit-free coronary angiography (5–8).
Due to the expectation that MI is the most frequent and/or important etiology of unexplained acute myocardial injury, guidance from the European Society of Cardiology (ESC) and American Heart Association (AHA) have historically focused on therapies for acute coronary syndromes in management following “MINOCA” presentations. In recognition of etiological heterogeneity of this acute presentation, recent guidelines focus on the role of improved diagnostic algorithms as key to improving patient outcomes (9–11). Within these algorithms is a consensus of opinion that CMR has a key role in the detection of the cause of the cardiac injury. However, it must be noted that as coronary artery disease remains the only frequent cause with therapies proven to improve outcomes, the certainty with which MI can be detected or excluded as the cause of acute myocardial injury remains the most important diagnostic aim at this time.
Moreover, while CMR's diagnostic role is supported by single center studies and meta-analyses (Class 1, Level of evidence B), there are no convincing economic evaluations of CMR in this setting (11). We aimed to model the financial impact of implementing CMR in the diagnostic pathway for assessing patients with unexplained acute myocardial injury and culprit-free coronary angiography.
Methods
A hypothetical population of 2,000 patients with unexplained acute myocardial injury and normal coronary angiography was used for this modeling exercise. The population was split evenly into two groups receiving either standard management or CMR guided management. Each population was extrapolated over a 10-year period, with costs determined on an annual basis. Standard management was defined as 66% of the population receiving dual anti-platelet therapy (DAPT) for 1 year followed by aspirin for life, and the remaining 34% of the population receiving aspirin for life (3). CMR guided management ensured that only those with a “true” MI received DAPT for 1 year followed by aspirin for life.
Based on published data, 25% of the modeled population had a true MI (Table 1) (2, 5) and annual rates of a major bleed on DAPT, aspirin or no therapy were estimated as 2, 0.6, and 0.46%, respectively (2, 14–16). The assumed mortality rate in the first year after a diagnosis of unexplained acute myocardial injury and normal coronary angiography was 3.4 and 2.8% for subsequent years (3, 4). Expert consensus based on two seminal DAPT trials was used to determine the additional annual percentage likelihood of MI if not on DAPT and on aspirin only. This value was determined to be 2.6% (Table 1) (16, 17). Further details on the input values and the costs used are available in the Supplementary Material.
However, despite only a fraction of patients having a true MI, the majority will be commenced on DAPT in clinical practice, as demonstrated in the largest registry of such patients where 66% received DAPT (3). Accordingly, the proportion of patients prescribed DAPT (66%) is higher than the assumed prevalence of MI (25%), and the majority of true MI's are likely to receive DAPT, leaving only a small proportion of true MI's at a relatively increased risk of future infarction.
A 10-year time horizon was selected for relevance to policy makers and because extrapolation beyond this would be subject to a high degree of uncertainty from changes in clinical practice and technology. A one-way sensitivity analysis was performed at year 10 to evaluate the variables entered into the model. An additional sensitivity analysis was performed by varying the proportion of patients placed on DAPT from 50 to 66%.
Results
Fifty percent of the study population (1,000 individuals) were treated as per current practice and the remaining 1,000 individuals were treated within a CMR-guided pathway.
Standard Practice
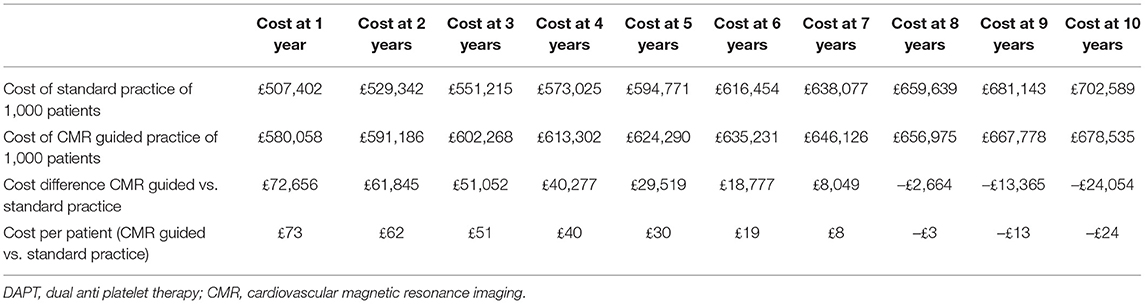
In the first year, the total cost is £507,402 for 1,000 patients, derived from the cost of ticagrelor to 66% of the population (£468,600); 15.24 major bleeding events (£27,158); aspirin for all (£10,000); and 0.75 MI's (£1,644). In the second and subsequent years, costs are significantly lower as ticagrelor and its risks for major bleeding are removed. Without adjusting for inflation, the second-year costs are from aspirin (£9,508), 5.70 major bleeding events (£10,166) and 0.74 MI's (£1,628). Over subsequent years, costs decrease incrementally as the population is censored.
CMR Guided Practice
In the first year, the total cost is £580,058 for 1,000 patients, derived from the cost of 1,000 CMRs to 100% of the population (£358,000), ticagrelor to 25% of the population (£177,500); 8.45 major bleeding events (£15,058); aspirin for 25% (£2,500). In the second and subsequent years, costs are significantly lower as the cost of CMR is removed. Without adjusting for inflation, the second-year costs are from aspirin (£2,365), 4.74 major bleeding events (£8,439). Over subsequent years, costs decrease incrementally as the population is censored.
Comparing Strategies
In the first year the CMR scans themselves account for two-thirds of the cost of the CMR guided practice. However, even in the first year, the costs of CMR (£385,000), are substantially offset by savings on ticagrelor (lower by £291,100), aspirin (£7,500), costs attributable to avoided major bleeds (£12,100), and those attributable to avoided MI (£1,644). When compared to standard practice, the CMR-guided pathway increases costs by 14% (£73) per patient in the first year.
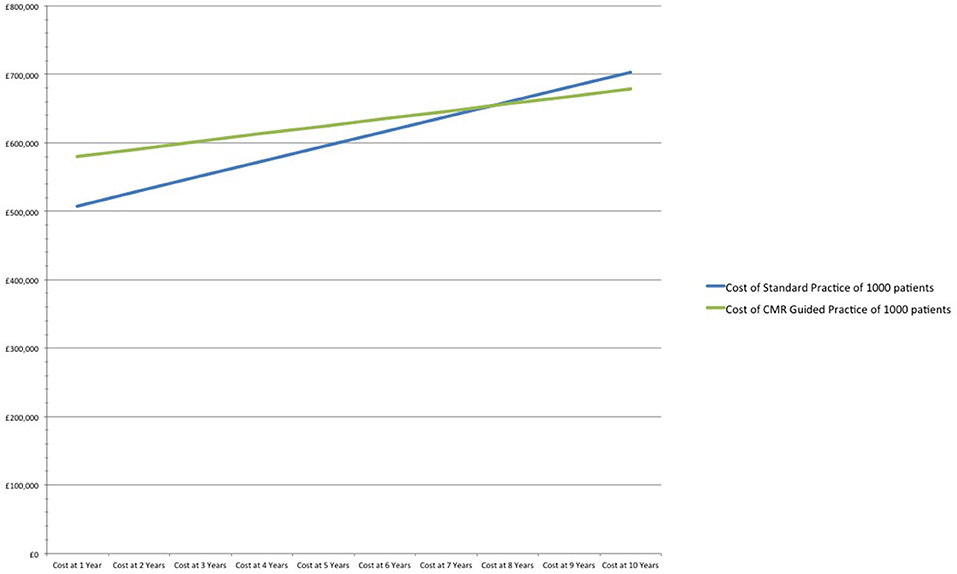
After 7 years CMR guided practice becomes cost neutral, and at 10 years reduces costs by 3% (£23) per patient in comparison to standard practice. The relatively modest cost savings includes the prevention of 7 fewer MIs and 14 fewer major bleeds /1,000 patients over a 10-year period (Table 2 and Figure 1).
Sensitivity Analysis
A one-way sensitivity analysis determined the variables with largest impact on the costs. Each variable was varied by ±20% and the change in cost determined at year 10 (see Figure 2). The three most sensitive variables were, in decreasing order, CMR cost, the cost of ticagrelor and the frequency of true MI.
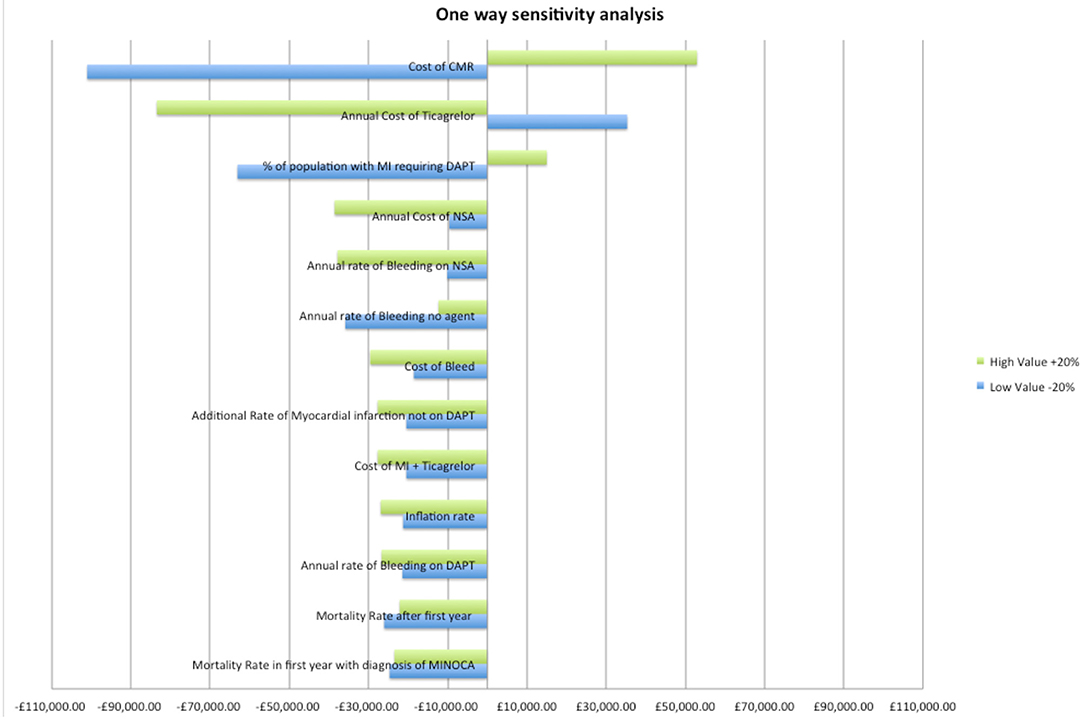
Figure 2. One-way sensitivity analysis of variables. DAPT, dual anti platelet therapy; MI, myocardial infarction; CMR, cardiovascular magnetic resonance imaging; NSA, aspirin.
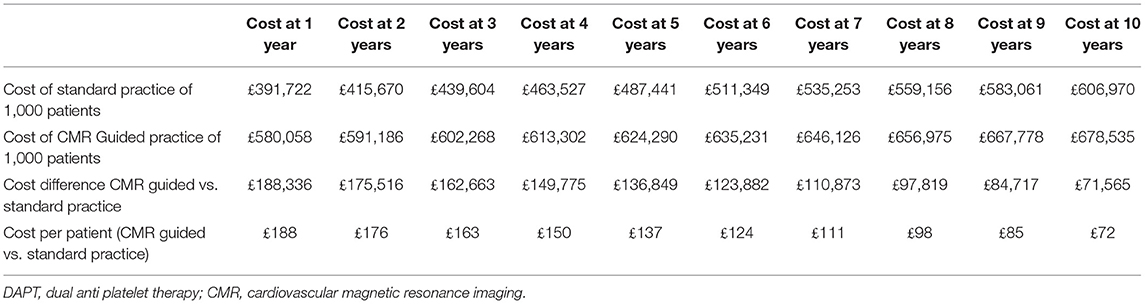
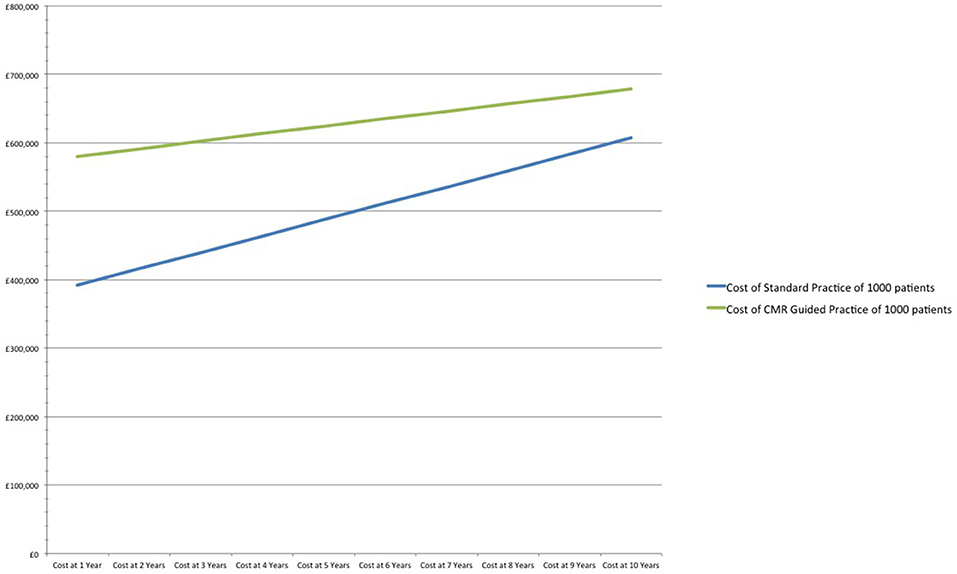
If prescription rate of DAPT decreases from 66 to 50% in the standard practice arm, CMR's relative cost increases by 48% (£188/patient) in the first year when compared to standard practice. After 10-years, the CMR strategy remains 12% more expensive (£72/patient) in comparison to standard practice. However, CMR prevents 16 MIs and 12 major bleeds in the 10-year period (Table 3 and Figure 3).
If the prescription rate of DAPT was to increase to 100% in the standard practice arm, CMR is more cost effective with savings of £175,582 in the first year, and increased savings of £254,960 at year 10. Furthermore, using CMR prevents 19 major bleeds in the 10-year period.
An example equation used to calculate the costs associated with standard practice in Year 1 is available in the Supplementary File.
Wider Perspectives
An estimation of the impact of nationwide adoption of CMR in the diagnostic pathway was based on 80,000 admissions with a diagnosis of presumed myocardial infarction in 2019/2020 (18) and with a 7% incidence of unexplained acute myocardial injury despite normal coronary angiography (~5,600 patients/year) (2–4). Routine use of CMR in the diagnostic pathway, when compared to standard practice, increases costs by £406,874 for year one. However, CMR use is associated with modest savings after 10 years (£138,226) and results in 42 fewer myocardial infarctions and 80 fewer major bleeding events.
Discussion
Recent International guidelines have placed importance on the use of CMR in patients with unexplained acute myocardial injury and normal coronary angiography; however, the costs and benefits of adapting this approach remain poorly explored (10, 11).
Our analysis focuses on the benefits of identifying the fraction of such patients that have had a “true” MI: this group may benefit from secondary prevention, whilst those with alternative causes of myocardial injury are spared the costs of secondary prevention and its unwanted side effects (Figure 4). This relatively simple analysis demonstrates that CMR-guided management is relatively inexpensive in the short-term, may result in modest cost-savings in the medium to long term, and may reduce morbidity and mortality from major bleeding and recurrent MI.
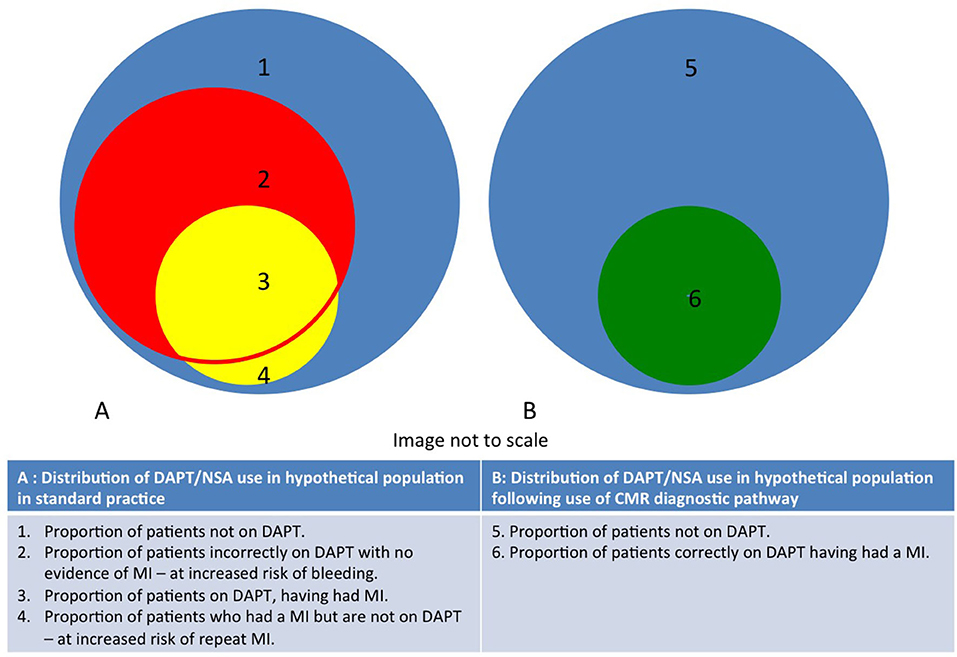
Figure 4. Diagram demonstrating distribution of DAPT/NSA use in populations with unexplained acute myocardial injury and normal coronary angiography in both CMR and standard practice. The routine use of CMR reduces the proportion of patients in areas 2 and 4.
A CMR guided strategy will also contribute to the identification of causes of cardiac injury other than MI (5, 11). It is important to note that healthcare and financial consequences of these other diagnoses are not included in the current analysis. Similarly, effect on quality of life, insurance premiums, critical illness status and several other ramifications following a diagnosis of MI have not been explored here. Although a more accurate diagnosis achieved with CMR may be expected to offer significant advantages in several of these domains, determining the attributable costs and benefits is uncertain; for example, the relevant financial implications of a diagnosis of myocarditis are undefined. Recognizing these challenges, our cost-benefit analysis necessarily focused on CMR's ability to accurately identify those patients who have had an MI in order to stratify accurately for antiplatelet therapy.
We only address treatment with DAPT and aspirin as other pharmaceutical agents commonly prescribed following MI (such as angiotensin converter enzyme inhibitors, angiotensin receptor blockers, beta-blockers and statins) are relatively inexpensive, have a low risk profiles, and include several agents commonly used for other cardiac conditions that present with acute cardiac injury. For the majority of these other causes of acute myocardial injury, the dearth of robust therapeutic data for the underlying diagnosis also limits our ability to estimate the effects of these agents on outcome (3, 19).
Within the constraints of a study limited to the benefits attributable to the identification of “true” MI, we identify the variables most sensitive to adjustment. These include the costs of CMR and ticagrelor, the percentage of the population with true MI, and the proportion of patients that would be prescribed DAPT in the absence of CMR stratification. In order to facilitate different healthcare practices, and account for cross-country variation in key determinants, our algorithm is available for download (Supplementary Material). This tool includes 15 adjustable variables to enable analyses to be better tailored to local factors and to accommodate changes in costs and in guidelines.
Limitations
This analysis is limited by using a single study for determination of the proportion of missed myocardial infarctions and a single study for the determination of standard practice of DAPT usage (3, 8). In addition, the assumption of 100% sensitivity and specificity of CMR to diagnose infarction and the impact of false negatives, and false positives have not been included in the calculations and is an inherent limitation. This study is also restricted as it does not encompass the plethora of benefits that CMR has in this population, namely from a diagnostic, prognostic and management perspective. It is likely that the cost savings associated with CMR are underestimated as the timeline is only 10 years, and the population with unexplained acute myocardial injury with normal coronary angiography would likely be on aspirin for life, resulting in a greater number of bleeding events than reported in this study. Furthermore, the costs associated with MI are likely underestimated as this study did not account for the lifelong downstream costs, for example heart failure admissions and cardiac rehabilitation, again underestimating cost savings associated with CMR utilization. Although we have focused on invasive coronary angiography as the test that defines culprit-free coronary arteries, cardiac CT (and other techniques) may also determine culprit-free coronary status in patients presenting on ACS pathways. It is predicted that the use of CT will not alter this study's fundamental findings, except perhaps by identifying larger numbers of patients with cardiac injury where coronary disease is unlikely to be the cause and for whom CMR may help make important decisions for long-term management.
Conclusion
This study, limited to an assessment of the utility of CMR to detect MI, demonstrates that the use of CMR in patients presenting with unexplained acute myocardial injury and normal coronary angiography includes modest short-term costs, is cost-reducing in the medium to long term, prevents treatment-related major bleeds, and reduces risks of subsequent MI.
This data advocates for the wider adoption of CMR, as its incorporation into acute cardiac pathways will help deliver a cost-effective, more personalized, and higher quality of care to patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
TM, DJ, IU, SM, and SP contributed to conception and design of the study. TM created the calculator and wrote the first draft of the manuscript. TM, DJ, RF, and IU wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This project was supported by the European Association of Cardiovascular Imaging (EACVI)—Task Force for Value Based Healthcare, and the British Heart Foundation accelerator award AA/18/5/34222.
Conflict of Interest
TM acknowledges support from the Irish Cardiac Society. SP acknowledges support from the National Institute for Health Research (NIHR) Biomedical Research Centre at Barts. He provides consultancy to and is a shareholder of Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada. RF acknowledges support from AstraZeneca and Circle Cardiovascular Imaging Inc. These organizations were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.749668/full#supplementary-material
References
1. Miller R. Burchell H, Edwards J. Myocardial infarction with and without acute coronary occlusion. AMA Arch Intern Med. (1951) 88:597–607. doi: 10.1001/archinte.1951.03810110049005
2. Pasupathy S, Air T, Dreyer R, Tavella R, Beltrame J. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. (2015) 131:861–70. doi: 10.1161/CIRCULATIONAHA.114.011201
3. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjöld A, Gard A, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. (2017) 135:1481–9. doi: 10.1161/CIRCULATIONAHA.116.026336
4. Pasupathy S, Lindahl B, Litwin P, Tavella R, Williams M, Air T, et al. 2389 Survival after myocardial infarction with non-obstructive coronary arteries (MINOCA) - a comprehensive systematic review and meta-analysis. Eur Heart J. (2019) 40(Suppl. 1):ehz748.0147. doi: 10.1093/eurheartj/ehz748.0147
5. Vágó H, Szabó L, Dohy Z, Czimbalmos C, Tóth A, Suhai F, et al. Early cardiac magnetic resonance imaging in troponin-positive acute chest pain and non-obstructed coronary arteries. Heart. (2020) 106:992–1000. doi: 10.1136/heartjnl-2019-316295
6. Dastidar A, Baritussio A, De Garate E, Drobni Z, Biglino G, Singhal P, et al. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging. (2019) 12:1973–82. doi: 10.1016/j.jcmg.2018.12.023
7. Gerbaud E, Harcaut E, Coste P, Erickson M, Lederlin M, Labèque J, et al. Cardiac magnetic resonance imaging for the diagnosis of patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Int J Cardiovasc Imaging. (2011) 28:783–94. doi: 10.1007/s10554-011-9879-1
8. Dastidar A, Rodrigues J, Johnson T, De Garate E, Singhal P, Baritussio A, et al. Myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging. (2017) 10:1204–6. doi: 10.1016/j.jcmg.2016.11.010
9. Agewall S, Beltrame J, Reynolds H, Niessner A, Rosano G, Caforio A, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. (2016) 38:143–53. doi: 10.1093/eurheartj/ehw149
10. Tamis-Holland J, Jneid H, Reynolds H, Agewall S, Brilakis E, Brown T, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. (2019) 139:e891–908. doi: 10.1161/CIR.0000000000000670
11. Collet J, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt D, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020 42:1289–367. doi: 10.1093/eurheartj/ehaa909
12. England N. NHS England National Cost Collection for the NHS. (2020). Available online at: https://www.england.nhs.uk/national-cost-collection (accessed December 10, 2020).
13. NHSBSA. Drug Tariff. (2020). Available online at: https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed December 10, 2020).
14. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg P, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. (2018) 392:940–9. doi: 10.1016/S0140-6736(18)31858-0
15. The ASCEND. Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. (2018) 379:1529–39. doi: 10.1056/NEJMoa1804988
16. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. (2001) 345:1506. doi: 10.1056/NEJM200111153452023
17. Wallentin L, Becker R, Budaj A, Cannon C, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
18. NHS Digital. Hospital Admitted Patient Care Activity 2019-20. (2020). Available online at: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2019-20 (accessed December 10, 2020).
Keywords: cost-benefit analysis, cardiac magnetic resonance (CMR) imaging, myocardial infarction with non-obstructive coronary atherosclerosis, healthcare planning and management, financial modeling/forecasting
Citation: Murphy T, Jones DA, Friebel R, Uchegbu I, Mohiddin SA and Petersen SE (2021) A Cost Analysis of Cardiac Magnetic Resonance Imaging in the Diagnostic Pathway of Patients Presenting With Unexplained Acute Myocardial Injury and Culprit-Free Coronary Angiography. Front. Cardiovasc. Med. 8:749668. doi: 10.3389/fcvm.2021.749668
Received: 29 July 2021; Accepted: 27 September 2021;
Published: 20 October 2021.
Edited by:
Ali Yilmaz, University Hospital Münster, GermanyReviewed by:
Grigorios Korosoglou, GRN Klinik Weinheim, GermanyMoritz Montenbruck, Katholisches Marienkrankenhaus GmbH, Germany
Copyright © 2021 Murphy, Jones, Friebel, Uchegbu, Mohiddin and Petersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steffen E. Petersen, cy5lLnBldGVyc2VuQHFtdWwuYWMudWs=
†These authors share first authorship
‡These authors share senior authorship
 Theodore Murphy
Theodore Murphy Daniel A. Jones1,2†
Daniel A. Jones1,2† Rocco Friebel
Rocco Friebel Saidi A. Mohiddin
Saidi A. Mohiddin Steffen E. Petersen
Steffen E. Petersen