
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 29 September 2021
Sec. Structural Interventional Cardiology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.747583
This article is part of the Research Topic Transcatheter Aortic Valve Implantation: All transfemoral? Update on vascular access and closure View all 9 articles
Transfemoral access remains the most widely used peripheral vascular approach for transcatheter aortic valve implantation (TAVI). Despite technical improvement and reduction in delivery sheath diameters of all TAVI platforms, 10–20% of patients remain not eligible to transfemoral TAVI due to peripheral artery disease. In this review, we aim at presenting an update of recent data concerning transfemoral access and percutaneous closure devices. Moreover, we will review peripheral non-transfemoral alternative as well as caval-aortic accesses and discuss the important features to assess with pre-procedural imaging modalities before TAVI.
Transcatheter aortic valve implantation (TAVI) has become the new standard of care for patients suffering from symptomatic severe aortic stenosis at high or intermediate surgical risk and is considered as a reasonable alternative to surgery for low risk patients (1–6). Transfemoral access remains the most widely used peripheral vascular approach for TAVI. Current international guidelines recommend transfemoral access as the gold standard for TAVI (7, 8), with American guidelines suggesting even reconsidering surgery for patients in whom anatomy is not suitable for transfemoral access (8). However, concomitant severe peripheral artery disease is frequent in this population and increases the risk of vascular complications. In order to allow direct comparison in the literature, it is recommended to report vascular complications according to the latest Valve Academic Research Consortium (VARC)-3 criteria (9). Accordingly, vascular complications are separated in major or minor complications including closure device failure. Direct impact of major vascular complications on mortality following TAVI has been well reported using the preceding VARC-2 criteria (10, 11). Interestingly, the latest and recent VARC-3 criteria have introduced a separate section defining access-related non-vascular complications referring to surrounding non-vascular structure damage (9).
Despite technical improvement and reduction in delivery sheath diameters of all TAVI platforms, 10–20% of patients remain not eligible to transfemoral TAVI due to peripheral artery disease (12, 13). Accordingly, alternative routes include non-transfemoral peripheral (transsubclavian or transcarotid) or central (caval-aortic, transapical, and direct aortic) vascular approaches. Direct comparison of outcomes between transfemoral and alternative TAVI is difficult since these latter patients present usually more severe comorbidities and are considered at higher procedural risk.
In this review, we aim at presenting an update of recent data concerning transfemoral access and percutaneous closure devices. Moreover, we will review peripheral alternative as well as caval-aortic accesses and discuss the important features to assess with pre-procedural imaging modalities before TAVI. Transapical and direct aortic TAVI will not be addressed in this review since strong evidence has shown worse outcomes in comparison to other alternative accesses and are only rarely considered nowadays (14–16).
Pre-procedural planning is a key step for running a successful TAVI program. Among others, pre-procedural imaging allows both precise aortic root anatomy characterization and peripheral vessel assessment. Routine and systematic use of cardiac multislice computed tomography (MSCT) is currently strongly recommended before TAVI procedures (17). When considering vascular access, MSCT has been reported to predict vascular complications with greater predictive value than traditional peripheral angiography (18). Vascular minimal diameter, tortuosity, and extend and distribution of calcification are major predictors of vascular complications and directly impact feasibility of transfemoral TAVI (19, 20). More detailed specificities of peripheral vascular assessment will be discussed below for each access. Several softwares are nowadays available for MSCT imaging analysis and structure measurements. Among our favorites, FluoroCT is a lightweighted software designed by two interventional cardiologists. It allows operators to perform all the required measurement and analysis of structures before structural heart interventions. Although free, use of FluoroCT needs some learning skills as reconstruction (for example to obtain adequate aortic annulus alignment) should be performed by hand with no automatically reconstruction features. On the other hand, 3mensio (Pie Medical Imaging) offers separate modules addressing specifically each valve or peripheral vasculature with a very intuitive user interface and automatically detection of several structures simplifying procedural planning and measurements. These advanced characteristics are however available at an expensive price imposing costumers to by each module separately.
Recent data from the large Society of Thoracic Surgeons–American College of Cardiology Transcatheter Valve Therapy (STS-ACC TVT) registry (n = 276,316) reported an increasing proportion of patients undergoing transfemoral TAVI from 2013 to 2019, with 95% of all TAVI performed through a femoral access in 2019 (21). Interestingly, proportion of transfemoral procedures dropped from 76% in 2012 to 47% in 2013 as a consequence of alternative access site approval by the Food and Drug Administration (FDA). Since then, a constant increase in transfemoral procedure proportion was observed.
The PARTNER 2 study, who randomized intermediate risk patients to TAVI with a balloon expandable device or surgery, consolidated data regarding benefits of the transfemoral access by reporting superiority of TAVI in patients undergoing transfemoral TAVI in terms of mortality and disabling stroke (hazard ratio 0.79, p = 0.05, TAVI vs. surgery). This superiority was no longer true when considering all vascular approach TAVI procedures compared to surgery.
The national prospective French registry (FRANCE TAVI) recently contributed significantly to the topic by comparing data from 21,611 patients undergoing transfemoral (92.5%) and non-transfemoral peripheral vascular access TAVI (22). After performing a pre-specified propensity score-based matching for comparison of both groups, Beurtheret et al. reported similar procedural mortality (OR of 1.29; 95% CI: 0.87–1.94) and stroke rates (OR of 1.38; 95% CI: 0.88–2.19) between the transfemoral and non-transfemoral TAVI groups of patients, respectively (22). Interestingly, results remained similar when the analysis were performed on patients undergoing TAVI in the more recent half of study period and in intermediate-high/high volume centers (>105 procedures/year).
MSCT is the imaging modality of choice for pre-procedural planning in all patients. While a minimal vessel lumen diameter from the left or right common femoral artery to the aortic valve of ≥5.5 mm is recommended with current 18 French delivery systems, the InLine Evolut R and Pro+ 23–29 mm valves (Medtronic) require a minimal diameter of ≥5 mm. Attention should be paid to perform the measures at the location of maximal stenosis and perpendicular to the long axis of the vessel. This recommended minimal lumen diameter cut-off considers a certain degree of vessel distention. Accordingly, calcification extending >270° of the vessel circumference at any level from the common femoral artery to the aorto-iliac bifurcation requires larger minimal lumen diameter in order to allow successful sheaths (Edwards system) or direct delivery system (Medtronic system) insertion. Moreover, calcifications located at the anterior part of the common femoral vessel should be identified as it may prevent percutaneous vessel puncture and percutaneous closure. Finally, significant tortuosity by itself is associated to vascular complications (23). However, in some cases without heavy ilio-femoral calcifications, tortuous vessels may be straighten using a stiff guidewire (24).
Downsizing of current prosthesis delivery catheters has allowed percutaneous transfemoral access to become the standard of care. Percutaneous transfemoral TAVI was widely rapidly used in clinical practice by most of TAVI centers even though no strong data comparing percutaneous to surgical cut-down exist. No randomized clinical trial comparing both techniques has ever been published. The largest and more recent data come from an Asian propensity score matching study comparing outcomes of patients undergoing transfemoral percutaneous or surgical cut-down TAVI using the Edwards Sapien XT valve. As part of the OCEAN TAVI registry, Kawashima et al. (25) reported among 586 patients (305 percutaneous, 281 surgical cut-down) significantly shorter procedural times, lower major vascular complication and bleeding rates, and shorter hospital length of stays in the percutaneous group. In the Brazilian TAVI registry including 402 patients, combined incidence of all-cause mortality, life-threatening bleedings and major vascular complications did not differ at 1 year between patients undergoing transfemoral percutaneous or surgical cut-down TAVI (26).
Traditionally, the common femoral artery is punctured at the level of the center of the femoral head as localized by fluoroscopy. However, the bifurcation height varies in the general population. More recently, Doppler-guided femoral puncture (Figure 1) has been adopted to optimize femoral puncture and secure delivery sheath insertion. Doppler-guided puncture allows precise femoral bifurcation identification and highlights anterior wall calcifications. After identification of the femoral bifurcation in an axial view, the transducer is moved 1–2 centimeters more proximally. The common femoral artery is thereafter punctured by avoiding calcifications either in the axial view by keeping the needle almost vertical or by rotating the transducer 90° counterclockwise in order to obtain a longitudinal view of the artery while inserting the needle at 45° from the skin (Figure 1). While the axial view allows precise anterior arterial wall puncture, the longitudinal view gives the operator a better imaging of the needle entry in the artery. Even though widely used in clinical practice for several years, very few data assessing Doppler-guided femoral puncture outcomes have been reported. Only recently, Vincent et al. published the first propensity score matched comparison between Doppler- and fluoroscopic-guided femoral punctures for transfemoral TAVI (n = 95). Vascular and bleeding complications were largely reduced in the Doppler-guided group in comparison to the fluoroscopic-guided group (vascular complications 16.8 vs. 6.3%, p = 0.023 and life-threatening or major bleedings 22.1 vs. 6%, p = 0.04, respectively) (27). Moreover, micropuncture access set use (Cook Medical, Bloomington, IN) allows to confirm height of the puncture according to both the femoral head and femoral bifurcation (28). In case of inadequate puncture, the four French introducer is removed. Manual compression is performed before the new puncture is performed. Operators should however keep in mind that the 0.018 inch guidewire may easily perforate small arterial branches. We therefore recommend to novel operators to advance the guidewire under fluoroscopic guidance.
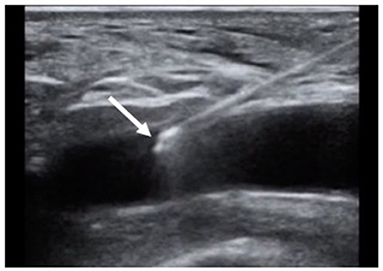
Figure 1. Doppler-guided puncture of the anterior wall of the common femoral artery. Tip of the needle inserted in the lumen of the artery (arrow).
In case of heavy calcified ilio-femoral axis with borderline minimal vessel diameter, intravascular lithotripsy has been reported as feasible and safe for transfemoral TAVI. Indeed, intravascular lithotripsy disrupts intimal and medial calcification and allows large bore sheath insertion after increasing vessel compliance. The only reported experience yet relies on 42 successful TAVI patients with a target lesion diameter of 4.3 ± 1.1 mm, average stenosis of 58.6 ± 17.5% and average maximum calcium arc of 265.5 ± 88.3°. No access site perforation or dissection were reported (29).
Commercially available CE mark vascular closure devices include the Prostar XL and ProGlide (Abbott Cardiovascular, suture-based), the Manta (Teleflex, collagen-based), the PerQseal (Vivasure Medical, patch-based) and the InClosure (InSeal Medical, membrane-based). Currently, the suture-based ProGlide and the collagen-based Manta are the most used vascular closure devices in TAVI and will be discussed in the present review. Both are inserted at the beginning of the TAVI procedure using a pre-closure technique.
The ProGlide system (Figure 2A) closes the vessel by delivering a percutaneous suture at the level of the femoral arteriotomy. Typically, large TAVI delivery sheaths require the insertion of 2 devices. Pre-closure is successfully performed by inserting the devices at 10 and 2 o'clock position in the femoral artery. After removing the sheath at the end of the procure, the knocks are pushed and tightened against the vessel wall. Alternatively, some operators prefer to deploy 2 ProGlide in parallel (1 medial and 1 lateral) at 12 o'clock with the addition of a 6 or 8 French Angio-Seal (Abbott Vascular, collagen-based device designed for small accesses) at the end of the procedure.
Despite conflicting results among small reports comparing suture-based closure devices (ProGlide and Prostar XL), 2 recent large propensity score adjusted studies revealed superior efficacity and safety of the ProGlide closure device in terms of vascular and bleeding complications in TAVI patients (30, 31).
More recently, the Manta (Figure 2B), a collagen-based closure system for large percutaneous arteriotomies, has been developed with interesting features. It has been designed in 14 and 18 French allowing vessel closure after sheath removal as large as 25 French. An anchor is inserted in the artery before the bovine collagen plug is pushed against the vessel. Following the initial experience (n = 50) demonstrating safe closure of large arteriotomies (32), the American multicenter SAFE MANTA trial confirmed high technical success (98%) with a single device insertion in 99.6% of patients and low major vascular complications (4.2%) among 341 patients undergoing percutaneous transfemoral TAVI, endovascular abdominal aortic aneurysm repair, or thoracic endovascular aortic aneurysm repair (33). The Manta closure device has shown similar vascular complication rates with lower all-cause mortality and bleeding events when compared to the ProGlide system in a propensity-matched analysis of 111 matched pairs undergoing percutaneous transfemoral TAVI (34). Interestingly, a rapid learning curve of the Manta system use was observed since significant reduction in outcomes was not seen across the different period tertiles of the study.
Atherosclerosis tends to affect less the axillary and subclavian arteries in comparison to the ilio-femoral axis. Accordingly, transsubclavian or transaxillary (TS) approach has been one of the first peripheral alternative vascular access described for TAVI with progressive growing popularity. Data from the large STS-ACC TVT registry showed a progressive increase in TS access use through the years, reaching 2.5% of the TAVI procedures in 2019 (21). A further propensity matched analysis of the STS/ACC TVT registry reported a significant lower 30-day mortality (5.3 vs. 8.4%, p < 0.01) but higher stroke rate (6.3 vs. 3.1%, p < 0.05) with TS TAVI compared to traditional alternative accesses (transapical and transaortic), respectively (35). A slightly higher proportion of TS TAVI (3.2% of the patients) were performed in the FRANCE TAVI registry (n = 21,611). Among these latter patients, major vascular complications were reported in 1.3% of the patients and 4% of all TS approaches needed unplanned vascular repair (22). More recently, Van der Wulp et al. reported a vascular complication rate as high as 18.5% with however a very low major vascular complication rate of 0.5% (1 patient). Unplanned vascular repair was needed in 8.5% of the patients. Overall, procedural success was high (93.5% of the patients) (36). A small propensity-matched comparison between TF (n = 141) and TS (n = 141) access reported similar outcomes at 2 year in terms of procedural success (subclavian 98 vs. femoral 97%, p = ns), major vascular complications (5 vs. 8%, p = ns), life-threatening bleeding (8 vs. 6%, p = ns) and survival (74 vs. 74%, p = ns) (37). Finally, in a recent large meta-analysis including 79,426 patients undergoing TF vs. non-TF peripheral access TAVI (TS or TC), authors reported a trend toward a higher rate of vascular complication in the TS vs. TF group (RR, 1.30; 95% CI, 0.98–1.73), but this difference was no longer true when using more restricted adjusted data (38). Noteworthy, vascular complication definition did however not systematically rely on Valve Academic Research Consortium (VARC) criteria. Similarly, 30-day mortality was higher in the TS vs. TF group only when considering unadjusted data (RR, 1.54; 95% CI, 1.26–1.89). Stroke rates, however, were higher in patients undergoing TS TAVI compared to TF TAVI, using both adjusted and unadjusted data (unadjusted analysis: risk ratio (RR) of 2.28 [95% CI, 1.90–2.72]; adjusted analysis: odds ratio (OR) of 1.53 [95% CI, 1.05–2.22]).
Similarly to TF pre-procedural planning, vessel minimal diameter, tortuosity, and calcification extension and localization are key elements to assess by MSCT. Subclavian artery take-off at the level of the aortic arch needs particular attention since it is a frequent localization of calcification. Significant subclavian artery to aortic arch angulation (>80°), as well as a horizontal aortic root may prevent successful valve delivery (39). A minimal diameter ≥5.5 mm is usually required for TS access in the absence of severe concentric calcifications. Interestingly, more than the minimal diameter by itself, Van der Wulp at al. (36) reported that the ratio of the sheath area to the axillary artery minimal diameter >1.63 is a strong independent predictor of vascular complication following TS TAVI. This ratio remains however experimental and needs further validation. Presence of patent internal mammal arteries in a patient with prior coronary artery bypass grafting is no longer considered as an absolute contraindication to TS approach even though alternative access are usually preferred.
It is worth to mention that the different histological structure of subclavian vessel wall makes it more prone to vascular complications with aggressive sheath insertion (mainly dissection or rupture). While the transfemoral artery is more muscular with a thicker and more fibrous adventia, the subclavian artery is characterized by a more thin and elastic wall (40). The left subclavian artery is usually preferred since the vascular path to the aortic root has less tortuosities and mimics TF access (Figure 3A). Until recently, most of TS accesses have been performed by surgical cut-down due to the fear of brachial plexus lesion with percutaneous puncture. A small 6–7 cm incision is performed 1 cm under the clavicle. Muscle fascia are dissected and particular attention should be paid to avoid neural structure injury. Once exposed, the subclavian artery is directly punctured. Subclavian arteriotomy (Figure 3B) is closed by surgical sutures at the end of the procedure. A vascular graft can be anastomosed end-to-side to the subclavian artery to allow safe valve delivery system insertion. In this case, the the graft is directly tied off at the end.
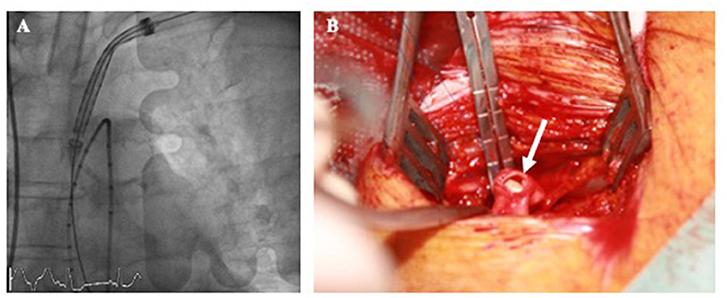
Figure 3. Left transsublavian access with valve delivery system insertion under fluoroscopic guidance at the level of the ascending aorta (A). Subclavian arteriotomy after sheath removal (B, arrow).
Fully percutaneous TS TAVI has been reported to be feasible and safe (41). Performing Doppler-guided punctures, Schafer at al. (42) reported a first German experience of 100 successive patients undergoing percutaneous TS TAVI with a 95% device success. No VARC-2 major vascular complication occurred. Interestingly, closure device failure occurred in 29.2% of the case, all while using the ProStar closure system. No closure device failure was reported when using the ProGlide system. More recently, the Manta system has shown favorable access site closure for TS TAVI (43, 44).
The first successful transcarotid (TC) TAVI has been described in 2010 (45). The most recent and largest data come from the French TAVI registry where 3.4% of patients underwent TC TAVI using the Edwards Sapien 3 prosthesis between 2014 and 2018 (46). Among 314 patients, procedural success was high (97%) with low 30-day mortality (3.2%) and cerebro-vascular ischemic event rate (1.6%). Major vascular complication or bleeding events were reported in 1.6 and 4.1% of the cases, respectively. Interestingly, when comparing TC (n = 911) to TS (n = 702) approach in the French TAVI registry, patients in the TC group had higher major bleeding rates (10 vs. 6.7%, p = 0.002, respectively) but lower major vascular complications rates (0.2 vs. 1.8%, p = 0.02, respectively). Stroke rate was similar between both groups (3.6 vs. 3.0%, p = 0.47) (22). TC TAVI has been compared to TS TAVI after propensity-matched scoring by Debry et al. in French multicenter registry. Interestingly, authors reported similar 30-day and 1-year mortality as well as 30-day stroke/transient ischemic attack (47). Surprisingly, minor bleeding (2.7 vs. 9.3%) and main access hematoma (3.6 vs. 10.3%) were significantly more frequent with the TC access.
MSCT imaging of bilateral supraaortic pre-cerebral arteries is recommended as part of the pre-procedural planning. Ipsilateral calcification extension and localization as well as plaque at high risk of embolization should be assessed. Contralateral significant common carotid artery stenosis or occlusion usually contraindicates TC approach. A minimal lumen diameter of the common carotid artery ≥5.5 mm without >50% stenosis is required. Some centers recommend to evaluate the circle of Willis perfusion in order to assure adequate contralateral blood flow compensation during the TC TAVI procedure. However, no recommendation concerning routine pre-procedural circle of Willis assessment exist yet.
Left common carotid artery is usually preferred for similar aortic root alignment reasons than described in the TS section of this review. However, carotid vascular access should be performed where arterial disease is the worse in order to preserve arterial brain flow on the contralateral side during the TAVI procedure. TC access is systematically performed by surgical exposure of the common carotid artery and avoiding vagal nerve lesion. After anterior wall puncture (Figure 4A) and progressive arteriotomy dilatation, the delivery sheath (Edwards Certitude or Medtronic EnVeo R) is inserted (Figure 4B). A close follow-up of the regional cerebral oxygenation (rSO2) is useful and a >20% relative reduction from baseline (normal range = 55–78%) is a commonly adopted threshold that has been used in major randomized controlled trials of rSO2-guided interventions (44). Surgical carotid repair of the carotid artery is performed at the end of the procedure. Caution should be paid to avoid any air embolism before clamps are removed. Some centers use adjunctive perclose sutures in addition to surgical ligatures to guarantee adequate hemostasis. Interestingly, a French study analyzing data from 174 TC TAVI reported feasibility of a minimally invasive strategy using local anesthesia vs. general anesthesia. While 30-day and 1-year mortality was similar between both groups, patients undergoing general anesthesia suffered from more strokes (8.1 vs. 0%, p < 0.001, respectively) (48).
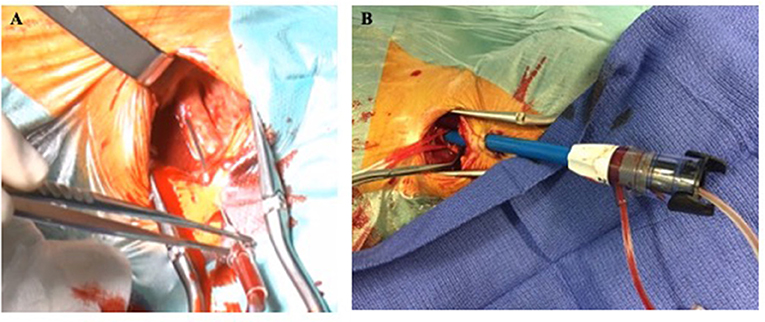
Figure 4. Left transcarotid surgical cut-down with anterior wall arterial puncture (A) and valve delivery sheath insertion (B).
Transcaval or caval-aortic TAVI access has been developed by Halabi at al. (49) and first reported in animals in 2013. Since then, Greenbaum at al. (50) described the first successful experience in humans in 2014. This technique remains however still experimental with limited data. Among 100 consecutive patients non eligible to transfemoral TAVI, percutaneous transcaval access was performed and successful in 99% of the cases. Access closure with a cardiac occluder was successful in all but 1 patient who required a covered stent implantation. Even though 30-day survival was good (92%), VARC-2 life-threatening bleeding and major vascular complication rates were high, respectively 7 and 13% (51). Interestingly, at 12 months, 93% of patients (77 of 83 patients) had CT-proven cavo-aortic fistula occlusion and only 1 patient had persistent asymptomatic patent fistula (52).
MSCT is essential to identify the closest location for crossing from the inferior vena cava to the abdominal aorta. The crossing spot is chosen at the level of the infra-renal aorta by avoiding any significant aortic wall calcification or interfering abdominal structures (bowel loop). Derived fluoroscopic angles and landmarks according to lumber vertebrae are anticipated by MSCT imaging reconstruction.
Simultaneous aortography and venography are performed after positioning a single loop gooseneck snare in the abdominal aorta at the pre-identified crossing spot. A 6-French guiding catheter is inserted in the inferior vena cava and positioned toward the snare loop. The crossing apparatus, consisting of a microcatheter containing a 0.014 Inches stiff wire (for example Confienza Pro 12, Asahi) mounted in a 0.035-inches wire convertor, is advanced in the guiding catheter. Next, crossing from the inferior vena cava to the infra-abdominal aorta is performed using an electrosurgery ablation system connected to the extremity of the 0.014 Inches wire. Once caval-aorta communication is performed, the crossing system is exchanged for a stiff 0.035 Inches wire allowing the insertion of a 22–24-French introducer sheath in the aorta. Aortography assures adequate hemostasis before standard transfemoral TAVI procedure is performed. At the end of the procedure, caval-aorta communication is closed using occluder devices usually approved for patent ductus arteriosus or intracardiac defect closure (Amplatzer Duct Occluder or Amplatzer Muscular VSD occlude St. Jude Medical, St. Paul, Minnesota) (50). Retroperitoneal bleeding is definitively the most feared complication following transcaval access. However, the surrounding retroperitoneal space pressurizes while caval-aorta communication is performed. The highest retroperitoneal pressure in comparison to the venous pressure directs potential aortic bleeding preferentially in the venous system preventing most of the major or life-threatening bleedings.
A second vascular access is required for angiographic guidance during prosthesis deployment. Influenced by data coming from coronary angiogram and percutaneous coronary intervention showing a significant reduction in vascular access-related complication using radial vs. femoral access (53), radial secondary access for TAVI has been progressively used. Encouraging results have been reported by a multicenter registry including 4,949 patients undergoing TAVI using as secondary access either a transfemoral or transradial access (81.1 and 18.9%, respectively) (54). VARC-2 defined secondary access-related complication rate was significantly higher in the transfemoral vs. transradial group with similar results after propensity score matching (4.7 vs. 0.9%, p < 0.001; major vascular complication, 1.8 vs. 0%, p < 0.001). Moreover, the transfemoral group suffered also from a higher 30-day stroke rate (3.1 vs. 1.6%, p = 0.043, respectively) and mortality (4.0 vs. 2.4%, p = 0.047, respectively) (54).
Undiagnosed vascular access-related bleedings may be dramatic since a significant blood loss usually occurs before patients become symptomatic or the bleeding is detected by an imaging modality. Based on tissue impedance change during bleedings, a new monitor has been developed (Early Bird Bleed Monitoring System, Saranas) to identify early subclinical periprocedural bleedings. Briefly, 2 separate electrodes attached on a 6 or 8 French introducer monitor bioimpedance change up to 12 h after the procedure. Visual and audible signals are provided by the system and categorized in three level of bleedings. The first-in-man study reported high level of agreement with MSCT (Cohen's kappa = 0.84) among 60 patients undergoing different endovascular procedure with large-bore vascular access (55). Although encouraging, the system still needs further investigations with larger data.
At the time of femoral vascular closure, femoral crossover using a stiff wire may help prompt bleeding management by balloon occlusion (56). According to the severity of the bleeding or in case of flow limiting dissection, femoral covered self-expanding stent placement is safe and associated with favorable long-term outcomes (57). Crossover was traditionally performed through a contra-lateral femoral access. However, radial secondary access has been recently use to reduce access-related vascular complication rate (54). As a consequence, a modified crossover technique through the secondary radial access has been suggested with encouraging results (58, 59).
More over, heparin reversal has recently been reported to reduce significantly rate of major or life-threatening complications, without increasing the risk of thrombo-embolic events (including stroke or myocardial infarction) (60). However, results should be interpreted cautiously since heparin reversal was performed in presence of vascular complication at the beginning of the study whereas protamine was administered to all patients toward the end of the study.
In conclusion, percutaneous transfemoral TAVI remains the standard vascular approach. Pre-procedural imaging assessment of peripheral vascular disease plays a major role in the patient's comprehensive evaluation before a TAVI procedure. Figure 5 presents a decision algorithm for vascular access choice according to the authors' preference. Several alternative peripheral accesses have emerged over time with favorable outcomes. In particular, the TS and TC alternative accesses have been associated to similar outcomes to the TF TAVI in non-randomized but adjusted reports. The relative invasiveness of alternative access compared to TF TAVI have prevented the implementation of comparative studies. Moreover, direct comparison of TF and alternative access TAVI will remain limited by different risk profiles of the patients. Both, the TS and TC access are believed to become the new preferred alternative approaches in a near future. Percutaneous TS has been shown feasible and safe but needs further investigations and larger studies. Finally, caval-aortic TAVI is a reasonable approach when all peripheral accesses are precluded but is still experimental.
NP contributed to the design of the review and writing of the manuscript. GB, LL, RI, and TM revised the manuscript. WB contributed to the design of the review and reviewed critically the manuscript. All authors contributed to the article and approved the submitted version.
NP has received research support from the Swiss National Science Foundation (P400PM_194483) and the Geneva University Hospitals.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. (2014) 370:1790–8. doi: 10.1056/NEJMoa1400590
2. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363:1597–607. doi: 10.1056/NEJMoa1008232
3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
4. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. (2020) 382:799–809. doi: 10.1056/NEJMoa1910555
5. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380:1706–15. doi: 10.1056/NEJMoa1816885
6. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2017) 376:1321–31. doi: 10.1056/NEJMoa1700456
7. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1093/eurheartj/ehx391
8. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143:e72–e227. doi: 10.1161/CIR.0000000000000923
9. Varc-3 Writing C, Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. (2021) 42:1825–57. doi: 10.1093/eurheartj/ehaa799
10. Genereux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol. (2012) 60:1043–52. doi: 10.1016/j.jacc.2012.07.003
11. Perrin N, Ellenberger C, Licker M, Hachulla AL, Cikirikcioglu M, Frei A, et al. Management of vascular complications following transcatheter aortic valve implantation. Arch Cardiovasc Dis. (2015) 108:491–501. doi: 10.1016/j.acvd.2015.03.007
12. Auffret V, Lefevre T, Van Belle E, Eltchaninoff H, Iung B, Koning R, et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J Am Coll Cardiol. (2017) 70:42–55. doi: 10.1016/j.jacc.2017.04.053
13. Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. (2017) 69:1215–30. doi: 10.1016/j.jacc.2016.11.033
14. Blackstone EH, Suri RM, Rajeswaran J, Babaliaros V, Douglas PS, Fearon WF, et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation. (2015) 131:1989–2000. doi: 10.1161/CIRCULATIONAHA.114.012525
15. Frohlich GM, Baxter PD, Malkin CJ, Scott DJ, Moat NE, Hildick-Smith D, et al. Comparative survival after transapical, direct aortic, and subclavian transcatheter aortic valve implantation (data from the UK TAVI registry). Am J Cardiol. (2015) 116:1555–9. doi: 10.1016/j.amjcard.2015.08.035
16. O'Hair DP, Bajwa TK, Popma JJ, Watson DR, Yakubov SJ, Adams DH, et al. Direct aortic access for transcatheter aortic valve replacement using a self-expanding device. Ann Thorac Surg. (2018) 105:484–90. doi: 10.1016/j.athoracsur.2017.07.051
17. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc Imaging. (2019) 12:1–24. doi: 10.1016/j.jcmg.2018.12.003
18. Okuyama K, Jilaihawi H, Kashif M, Takahashi N, Chakravarty T, Pokhrel H, et al. Transfemoral access assessment for transcatheter aortic valve replacement: evidence-based application of computed tomography over invasive angiography. Circ Cardiovasc Imaging. (2015) 8:e001995. doi: 10.1161/CIRCIMAGING.114.001995
19. Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. (2010) 55:1080–90. doi: 10.1016/j.jacc.2009.12.014
20. Toggweiler S, Gurvitch R, Leipsic J, Wood DA, Willson AB, Binder RK, et al. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. (2012) 59:113–8. doi: 10.1016/j.jacc.2011.08.069
21. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 76:2492–516. doi: 10.1016/j.jacc.2020.09.595
22. Beurtheret S, Karam N, Resseguier N, Houel R, Modine T, Folliguet T, et al. Femoral versus nonfemoral peripheral access for transcatheter aortic valve replacement. J Am Coll Cardiol. (2019) 74:2728–39. doi: 10.1016/j.jacc.2019.09.054
23. Vavuranakis M, Kariori M, Voudris V, Kalogeras K, Vrachatis D, Aznaouridis C, et al. Predictive factors of vascular complications after transcatheter aortic valve implantation in patients treated with a default percutaneous strategy. Cardiovasc Ther. (2013) 31:e46–54. doi: 10.1111/1755-5922.12023
24. Noble S, Roffi M. Overcoming the challenges of the transfemoral approach in transcatheter aortic valve implantation. Interv Cardiol. (2013) 8:131–4. doi: 10.15420/icr.2013.8.2.131
25. Kawashima H, Watanabe Y, Kozuma K, Nara Y, Hioki H, Kataoka A, et al. Propensity-matched comparison of percutaneous and surgical cut-down approaches in transfemoral transcatheter aortic valve implantation using a balloon-expandable valve. EuroIntervention. (2017) 12:1954–61. doi: 10.4244/EIJ-D-16-00408
26. Bernardi FL, Gomes WF, de Brito FS Jr, Mangione JA, Sarmento-Leite R, Siqueira D, et al. Surgical cutdown versus percutaneous access in transfemoral transcatheter aortic valve implantation: Insights from the Brazilian TAVI registry. Catheter Cardiovasc Interv. (2015) 86:501–5. doi: 10.1002/ccd.25820
27. Vincent F, Spillemaeker H, Kyheng M, Belin-Vincent C, Delhaye C, Pierache A, et al. Ultrasound guidance to reduce vascular and bleeding complications of percutaneous transfemoral transcatheter aortic valve replacement: a propensity score-matched comparison. J Am Heart Assoc. (2020) 9:e014916. doi: 10.1161/JAHA.119.014916
28. Scarsini R, De Maria GL, Joseph J, Fan L, Cahill TJ, Kotronias RA, et al. Impact of complications during transfemoral transcatheter aortic valve replacement: how can they be avoided and managed? J Am Heart Assoc. (2019) 8:e013801. doi: 10.1161/JAHA.119.013801
29. Di Mario C, Goodwin M, Ristalli F, Ravani M, Meucci F, Stolcova M, et al. A Prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2019) 12:502–4. doi: 10.1016/j.jcin.2019.01.211
30. Barbash IM, Barbanti M, Webb J, Molina-Martin De Nicolas J, Abramowitz Y, Latib A, et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J. (2015) 36:3370–9. doi: 10.1093/eurheartj/ehv417
31. Berti S, Bedogni F, Giordano A, Petronio AS, Iadanza A, Bartorelli AL, et al. Efficacy and safety of proglide versus prostar XL vascular closure devices in transcatheter aortic valve replacement: the RISPEVA Registry. J Am Heart Assoc. (2020) 9:e018042. doi: 10.1161/JAHA.120.018042
32. Van Mieghem NM, Latib A, van der Heyden J, van Gils L, Daemen J, Sorzano T, et al. Percutaneous plug-based arteriotomy closure device for large-bore access: a Multicenter Prospective Study. JACC Cardiovasc Interv. (2017) 10:613–9. doi: 10.1016/j.jcin.2016.12.277
33. Wood DA, Krajcer Z, Sathananthan J, Strickman N, Metzger C, Fearon W, et al. Pivotal clinical study to evaluate the safety and effectiveness of the MANTA percutaneous vascular closure device. Circ Cardiovasc Interv. (2019) 12:e007258. doi: 10.1161/CIRCINTERVENTIONS.119.007258
34. Moriyama N, Lindstrom L, Laine M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve replacement using MANTA versus ProGlide. Eurointervention. (2019) 14:e1558–e65. doi: 10.4244/EIJ-D-18-00769
35. Dahle TG, Kaneko T, McCabe JM. Outcomes following subclavian and axillary artery access for transcatheter aortic valve replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc Interv. (2019) 12:662–9. doi: 10.1016/j.jcin.2019.01.219
36. van der Wulp K, Thijs I, van Wely M, Loverbos A, Gehlmann H, Verkroost M, et al. Incidence and predictors of vascular complications in transaxillary TAVI. Eurointervention. (2020) 15:e1325–e31. doi: 10.4244/EIJ-D-19-00588
37. Petronio AS, De Carlo M, Bedogni F, Maisano F, Ettori F, Klugmann S, et al. 2-year results of CoreValve implantation through the subclavian access: a propensity-matched comparison with the femoral access. J Am Coll Cardiol. (2012) 60:502–7. doi: 10.1016/j.jacc.2012.04.014
38. Faroux L, Junquera L, Mohammadi S, Del Val D, Muntane-Carol G, Alperi A, et al. Femoral versus nonfemoral subclavian/carotid arterial access route for transcatheter aortic valve replacement: a systematic review and meta-analysis. J Am Heart Assoc. (2020) 9:e017460. doi: 10.1161/JAHA.120.017460
39. Bapat V, Tang GHL. Axillary/subclavian transcatheter aortic valve replacement: the default alternative access? JACC Cardiovasc Interv. (2019) 12:670–2. doi: 10.1016/j.jcin.2019.02.017
40. Biasco L, Ferrari E, Pedrazzini G, Faletra F, Moccetti T, Petracca F, et al. Access sites for TAVI: patient selection criteria, technical aspects, and outcomes. Front Cardiovasc Med. (2018) 5:88. doi: 10.3389/fcvm.2018.00088
41. Schafer U, Ho Y, Frerker C, Schewel D, Sanchez-Quintana D, Schofer J, et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: “the Hamburg Sankt Georg approach”. JACC Cardiovasc Interv. (2012) 5:477–86. doi: 10.1016/j.jcin.2011.11.014
42. Schafer U, Deuschl F, Schofer N, Frerker C, Schmidt T, Kuck KH, et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int J Cardiol. (2017) 232:247–54. doi: 10.1016/j.ijcard.2017.01.010
43. Ruck A, Eriksson D, Verouhis D, Saleh N, Linder R, Corbascio M, et al. Percutaneous access and closure using the MANTA vascular closure device in transaxillary transcatheter aortic valve implantation. Eurointervention. (2020) 16:266–8. doi: 10.4244/EIJ-D-19-00809
44. De Palma R, Ruck A, Settergren M, Saleh N. Percutaneous axillary arteriotomy closure during transcatheter aortic valve replacement using the MANTA device. Catheter Cardiovasc Interv. (2018) 92:998–1001. doi: 10.1002/ccd.27383
45. Modine T, Lemesle G, Azzaoui R, Sudre A. Aortic valve implantation with the CoreValve ReValving System via left carotid artery access: first case report. J Thorac Cardiovasc Surg. (2010) 140:928–9. doi: 10.1016/j.jtcvs.2010.03.001
46. Overtchouk P, Folliguet T, Pinaud F, Fouquet O, Pernot M, Bonnet G, et al. Transcarotid approach for transcatheter aortic valve replacement with the sapien 3 prosthesis: a multicenter French Registry. JACC Cardiovasc Interv. (2019) 12:413–9. doi: 10.1016/j.jcin.2018.11.014
47. Debry N, Trimech TR, Gandet T, Vincent F, Hysi I, Delhaye C, et al. Transaxillary compared with transcarotid access for TAVR: a propensity-matched comparison from a French multicentre registry. Eurointervention. (2020) 16:842–9. doi: 10.4244/EIJ-D-20-00117
48. Debry N, Delhaye C, Azmoun A, Ramadan R, Fradi S, Brenot P, et al. Transcarotid transcatheter aortic valve replacement: general or local anesthesia. JACC Cardiovasc Interv. (2016) 9:2113–20. doi: 10.1016/j.jcin.2016.08.013
49. Halabi M, Ratnayaka K, Faranesh AZ, Chen MY, Schenke WH, Lederman RJ. Aortic access from the vena cava for large caliber transcatheter cardiovascular interventions: pre-clinical validation. J Am Coll Cardiol. (2013) 61:1745–6. doi: 10.1016/j.jacc.2013.01.057
50. Greenbaum AB, O'Neill WW, Paone G, Guerrero ME, Wyman JF, Cooper RL, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol. (2014) 63:2795–804. doi: 10.1016/j.jacc.2014.04.015
51. Greenbaum AB, Babaliaros VC, Chen MY, Stine AM, Rogers T, O'Neill WW, et al. Transcaval access and closure for transcatheter aortic valve replacement: a prospective investigation. J Am Coll Cardiol. (2017) 69:511–21. doi: 10.1016/j.jacc.2016.10.024
52. Lederman RJ, Babaliaros VC, Rogers T, Stine AM, Chen MY, Muhammad KI, et al. The fate of transcaval access tracts: 12-month results of the prospective NHLBI transcaval transcatheter aortic valve replacement study. JACC Cardiovasc Interv. (2019) 12:448–56. doi: 10.1016/j.jcin.2018.11.035
53. Ferrante G, Rao SV, Juni P, Da Costa BR, Reimers B, Condorelli G, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv. (2016) 9:1419–34. doi: 10.1016/j.jcin.2016.04.014
54. Junquera L, Urena M, Latib A, Munoz-Garcia A, Nombela-Franco L, Faurie B, et al. Comparison of transfemoral versus transradial secondary access in transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2020) 13:e008609. doi: 10.1161/CIRCINTERVENTIONS.119.008609
55. Genereux P, Nazif TM, George JK, Barker CM, Klodell CT, Slater JP, et al. First-in-human study of the saranas early bird bleed monitoring system for the detection of endovascular procedure-related bleeding events. J Invasive Cardiol. (2020) 32:255–61.
56. Genereux P, Kodali S, Leon MB, Smith CR, Ben-Gal Y, Kirtane AJ, et al. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc Interv. (2011) 4:861–7. doi: 10.1016/j.jcin.2011.05.019
57. Sedaghat A, Neumann N, Schahab N, Sinning J-M, Hammerstingl C, Pingel S, et al. Routine endovascular treatment with a stent graft for access-site and access-related vascular injury in transfemoral transcatheter aortic valve implantation. Circ Cardiovasc Interv. (2016) 9:e003834. doi: 10.1161/CIRCINTERVENTIONS.116.003834
58. Buchanan GL, Chieffo A, Montorfano M, Maccagni D, Maisano F, Latib A, et al. A “modified crossover technique” for vascular access management in high-risk patients undergoing transfemoral transcatheter aortic valve implantation. Cathet Cardiovasc Intervent. (2013) 81:579–83. doi: 10.1002/ccd.24380
59. Curran H, Chieffo A, Buchanan GL, Bernelli C, Montorfano M, Maisano F, et al. A comparison of the femoral and radial crossover techniques for vascular access management in transcatheter aortic valve implantation: The milan experience. Cathet Cardiovasc Intervent. 83:156–61. doi: 10.1002/ccd.24913
Keywords: transcatheter aortic valve implantation, vascular access, transfemoral, alternative access, vascular complication
Citation: Perrin N, Bonnet G, Leroux L, Ibrahim R, Modine T and Ben Ali W (2021) Transcatheter Aortic Valve Implantation: All Transfemoral? Update on Peripheral Vascular Access and Closure. Front. Cardiovasc. Med. 8:747583. doi: 10.3389/fcvm.2021.747583
Received: 26 July 2021; Accepted: 30 August 2021;
Published: 29 September 2021.
Edited by:
Hendrik Ruge, Technical University Munich, GermanyReviewed by:
Alexander Sedaghat, University Hospital Bonn, GermanyCopyright © 2021 Perrin, Bonnet, Leroux, Ibrahim, Modine and Ben Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Modine, dGhvbWFzbW9kaW5lQGdtYWlsLmNvbQ==; Walid Ben Ali, ZHIud2FsaWRiZW5hbGlAZ21haWwuY29t
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.