
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 November 2021
Sec. Cardiovascular Therapeutics
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.746988
 Duanbin Li1,2†
Duanbin Li1,2† Ya Li1,2†
Ya Li1,2† Maoning Lin1,2
Maoning Lin1,2 Wenjuan Zhang3
Wenjuan Zhang3 Guosheng Fu1,2
Guosheng Fu1,2 Zhaoyang Chen4*
Zhaoyang Chen4* Chongying Jin1,2*
Chongying Jin1,2* Wenbin Zhang1,2*
Wenbin Zhang1,2*Background: Metoprolol is the most used cardiac selective β-blocker and has been recommended as a mainstay drug in the management of acute myocardial infarction (AMI). However, the evidence supporting this regimen in periprocedural myocardial infarction (PMI) is limited.
Methods: This study identified 860 individuals who suffered PMI following percutaneous coronary intervention (PCI) procedure and median followed up for 3.2 years. Subjects were dichotomized according to whether they received chronic oral sustained-release metoprolol succinate following PMI. After inverse probability of treatment weighting (IPTW) adjustment, logistic regression analysis, Kaplan-Meier curve, and Cox regression analysis were performed to estimate the effects of metoprolol on major adverse cardiovascular events (MACEs) which composed of cardiac death, myocardial infarction (MI), stroke, and revascularization. Moreover, an exploratory analysis was performed according to hypertension, cardiac troponin I (cTnI) elevation, and cardiac function. A double robust adjustment was used for sensitivity analysis.
Results: Among enrolled PMI subjects, 456 (53%) patients received metoprolol treatment and 404 (47%) patients received observation. After IPTW adjustment, receiving metoprolol was found to reduce the subsequent 3-year risk of MACEs by nearly 7.1% [15 vs. 22.1%, absolute risk difference (ARD) = 0.07, number needed to treat (NNT) = 14, relative risk (RR) = 0.682]. In IPTW-adjusted Cox regression analyses, receiving metoprolol was related to a reduced risk of MACEs (hazard ratio [HR] = 0.588, 95%CI [0.385–0.898], P = 0.014) and revascularization (HR = 0.538, 95%CI [0.326–0.89], P = 0.016). Additionally, IPTW-adjusted logistic regression analysis showed that receiving metoprolol reduced the risk of MI at the third year (odds ratio [OR] = 0.972, 95% CI [0.948–997], P = 0.029). Exploratory analysis showed that the protective effect of metoprolol was more pronounced in subgroups of hypertension and cTnI elevation ≥1,000%, and was remained in patients without cardiac dysfunction. The benefits above were consistent when double robust adjustments were performed.
Conclusion: In the real-world setting, receiving metoprolol treatment following PCI-related PMI has decreased the subsequent risk of MACEs, particularly the risk of recurrent MI and revascularization.
Coronary artery disease contributes significantly to cardiovascular disease being the leading cause of death around the world (1). Over the past decades, coronary revascularization by the percutaneous coronary intervention (PCI) has been an established therapeutic procedure of coronary artery disease (CAD) (2). However, a silent “killer” still exists. Approximately 3–6% of patients experienced a periprocedural myocardial infarction (PMI) following PCI procedure and up to one-third of patients suffered periprocedural myocardial injury (3, 4). According to the 4th Universal Definition of Myocardial Infarction (UDMI), myocardial infarction (MI) associated with PCI is categorized as type 4a MI, which is primarily determined by the elevation level of cardiac troponin I (cTnI) (5). Numerous studies have demonstrated that PMI is related to the subsequent increased risk of mortality and other adverse cardiovascular events (6). Indeed, even periprocedural myocardial injury has been shown to increase the all-cause mortality following PCI procedure (7). The mechanisms of PCI-related PMI involve acute side branch occlusion, distal embolization, and mechanical process resulting in vulnerable plaque rupture (8). However, the eligible treatment strategy for PCI-related PMI remains in debate. Treatment strategies of acute myocardial infarction (AMI) may benefit PCI-related PMI, but the evidence is limited.
As a competitive and reversible antagonist of beta-1-adrenergic receptors, metoprolol has been the most used β-blocker with over 50 million total prescriptions per year in the U.S. (9). In the Goteborg Metoprolol Trial, metoprolol therapy initiated on admission reduced 3-month mortality and exert a prophylactic effect against ventricular fibrillation in patients with AMI (10, 11). A subsequent study found that long-term administration of 100 mg twice daily of metoprolol reduced the risk of cardiac death and non-fatal reinfarction in patients surviving AMI (12). Besides, early intravenous metoprolol before reperfusion was shown to reduce infarct size and improve left ventricular ejection fraction (LVEF) after ST-segment elevation myocardial infarction (STEMI) (13). The cardiovascular protective effect of metoprolol is established, which is achieved by inhibiting the overactive adrenergic nervous system, reducing oxygen demand, increasing cardiac perfusion, and reducing ventricular remodeling (14). The current clinical guidelines of AMI recommend the β-blockers administration as early as possible and continue thereafter, regardless of STEMI or non-STEMI (15, 16).
Therefore, this real-world multicentric cohort study was conducted to estimate the effects of metoprolol on PMI associated with PCI (type 4a MI) and to optimize clinical decisions.
This was a multicentric retrospective cohort study in the real-world setting. According to the 4th UDMI (5), a total of 1,570 patients diagnosed with PMI following PCI (type 4a MI) were eligible for this study from January 2014 to September 2018. Inclusion to the study required to meet the following criteria: (1) patients diagnosed with unstable angina pectoris (UAP)/stable angina pectoris (SAP)/asymptomatic CAD without an elevated cTnI at baseline; (2) patients who suffered from PCI-related PMI were followed up for 3 years. In contrast, the following patients were excluded: (1) β-blocker administration but not metoprolol, not oral, or not 47.5 mg daily; (2) received PCI again within 90 days of the first PCI; (3) treated with metoprolol due to severe arrhythmia; (4) prolonged PR intervals (>0.24 s), second- or third-degree atrioventricular blocks; (5) active asthma or reactive airway disease; (6) active malignant tumor at baseline; (7) died within 30 days of PCI. The final study population thus included 860 individuals (Figure 1). Ethical approval was granted by the Ethics Committee of Sir Run Run Hospital, College of Medicine, Zhejiang University (20201217-36).
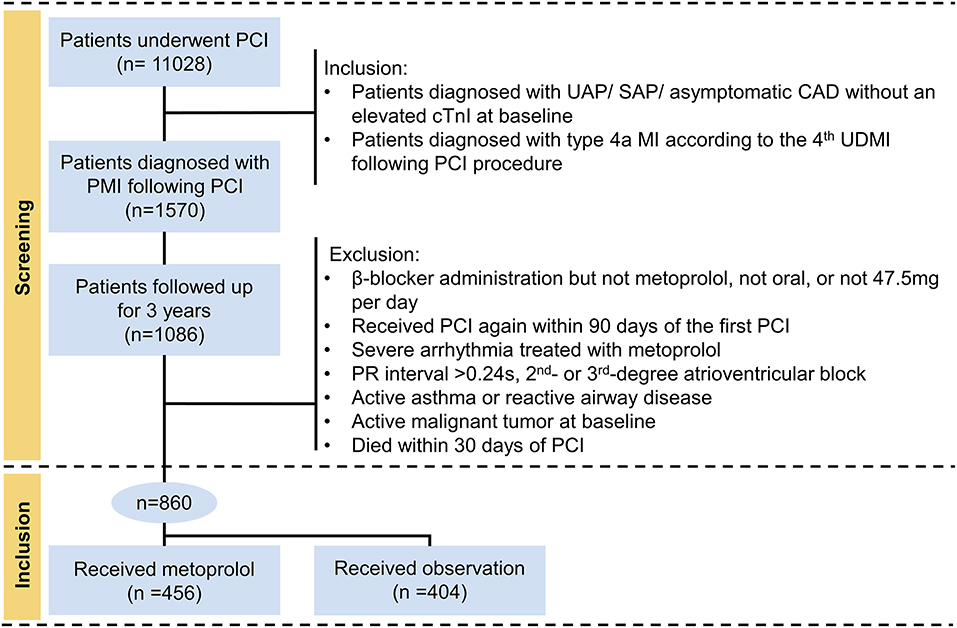
Figure 1. Flowchart describing the selection of subjects. ULN, the upper limit of normal; cTnI, cardiac troponin I; PCI, percutaneous coronary intervention; PMI, periprocedural myocardial infarction; UAP, unstable angina pectoris; SAP, stable angina pectoris; CAD, coronary artery disease.
The decision to perform treatments was made by the physician and the patient in consultation, and the procedure and the placement location of stents were entirely up to the currently recommended guidelines (17). Patients were treated with the optimal strategy of medications, including dual antiplatelet drugs, anticoagulants, and lipid-lowering therapy (18).
The peak value of cTnI was determined by repeated laboratory examination within 48 h following the PCI procedure and was used to diagnose PMI. The upper limit of normal (ULN) of cTnI was determined at 0.011 ng/ml. The criteria of cTnI in PMI was as a post-procedural cTnI ≥5 × ULN with a normal cTnI at baseline or ≥20% cTnI elevation for patients with an elevated cTnI at baseline. Additionally, ischemic symptoms, ECG changes, angiography, or imaging abnormal should be verified according to the 4th UDMI in 2018 (5).
Among the enrolled population, subjects were dichotomized according to treatment strategy. Those who initiated metoprolol treatment at the acute phase of PMI and continued thereafter were categorized into the metoprolol treatment group. Alternatively, the observation group consisted of patients who did not receive metoprolol treatment. Medication data were extracted from the electronic medical record system and verified by telephone interview. Standard metoprolol exposure was defined as oral sustained-release metoprolol succinate 47.5 mg per day.
The study abstracted patient-level variables, including demographic features, laboratory data, PCI-related data, medications in hospitalization, and medications after discharge. Data of PCI treatment was also abstracted, including chronic coronary total occlusions (CTO), lesion location, number of stents, and direct PCI. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) was employed to define diabetes mellitus (DM) (ICD-9-CM 250) and hypertension (ICD-9-CM 362.11, 401–405, and 437.2). Normal NT-proBNP was defined as follows: <50 years old, <450 ng/L; 50–75 years old, <900 ng/L; and >75 years old, <1,800 ng/L.
After discharge, telephone interviews were performed at every 6-month intervals by trained interviewers. The primary analytical endpoint of the study was major adverse cardiovascular events (MACEs), which was consisted of cardiac death, MI, stroke, and revascularization.
First, continuous variables were shown as the mean ± SD and were compared using Mann-Whitney U-tests. Categorical variables were represented as counts (proportions) and were compared using the Chi-square test or Fisher's exact test (if the expected cell value was <5). Missing data were replaced by single imputation with the median value of the cohort. Among these, NT-proBNP had the largest proportion of missing values (4.65%), followed by ejection fraction (EF) (3.49%), C-reactive protein (CRP) (2.91%), direct PCI (2.09%), uric acid (1.63%), the peak value of creatine kinase MB (CK-MB) (1.16%), lipoprotein (a) (1.16%), and very-low-density lipoprotein (VLDL) (1.05%).
Second, to minimize the selection bias, inverse probability of treatment weighting (IPTW) was applied to balance baseline characteristics between cohorts (19). In the IPTW approach, the propensity score (PS) was calculated by a logistic regression model, which predicted the probability of each individual receiving metoprolol. Then each individual was weighted according to PS. The model of PS component variables and their respective weights were shown in Supplementary Table S1. The balance of covariates was assessed by standardized mean difference (SMD) with imbalance defined as SMD >0.1 (20). Besides, IPTW-adjusted PS distribution was depicted in each cohort by kernel density plot.
Third, absolute risk difference (ARD), relative risk difference (RRD), number needed to treat (NNT), and relative risk (RR) were estimated in the IPTW-adjusted population. The NNT was the reciprocal of the ARD and indicated how many persons on average need to be exposed to metoprolol treatment to cause benefit in one person who would not otherwise have been benefited (21).
Fourth, IPTW-adjusted logistic regression analyses were conducted at first, second, and third year to assess the effects of metoprolol in population without censored data. After IPTW adjustment, Kaplan-Meier analysis and Cox regression analysis were further performed.
Fifth, an exploratory analysis was conducted to determine the IPTW-adjusted odds ratio (OR) and hazard ratio (HR) of metoprolol treatment according to hypertension (yes or no), the elevation of cTnI (<1,000% or ≥1,000%), and cardiac function (EF ≤ 50% or EF ≥50% with normal NT-proBNP) after rebalancing every covariate in subgroups by the approach depicted above.
Sixth, a sensitivity analysis was conducted applying a double robust approach (IPTW with multivariate regression adjustment) (22). Covariates with SMD >0.05 after IPTW adjustment were further adjusted in multivariate regression models.
Statistical analysis was conducted by SPSS software version 18 (SPSS Inc., Chicago, IL, USA) and R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria). Two-sided statistical significance was defined as P < 0.05.
A total of 860 patients were identified as PMI by the definition of the 4th UDMI and followed up for over 3 years. Among them, 456 (53%) received metoprolol and 404 (47%) received observation, respectively (Table 1). Patients who received metoprolol were younger (67.4 ± 10.7 vs. 70.1 ± 10.3 years, P < 0.001), had higher levels of lipoprotein (a) (27.1 ± 27.4 vs. 22.6 ± 22.2 mg/dl, P = 0.011), higher prevalence of CTO (13.6 vs. 8.2%, P = 0.015), were more likely to receive angiotensin-converting enzyme inhibitor (ACEI) treatment (42.3 vs. 33.7%, P = 0.011), and were less likely to receive calcium channel blocker (CCB) treatment (25.0 vs. 35.1%, P = 0.002). However, there was no significant difference between metoprolol and observation groups in the peak value of cTnI (1.87 ± 1.86 vs. 1.91 ± 1.94 mg/L, P = 0.776), the peak value of CK-MB (28.1 ± 20.9 vs. 25.8 ± 20.5 U/L, P = 0.116), and angiotensin receptor blocker (ARB) treatment (37.9 vs. 41.6%, P = 0.307).
After IPTW adjustment, there was no significant difference between cohorts in demographic features, laboratory data, PCI data, and medications. Baseline characteristics after IPTW adjustment were listed in Table 2. Supplementary Table S1 showed the multivariable logistic regression model that predicted the probability of receiving metoprolol. By using the IPTW method, the SMD of each covariate was below 0.1, indicating that cohorts were comparable thereafter (Figure 2). Besides, distributions of PS between cohorts reached a sufficient balance after IPTW adjustment (Supplementary Figure S1).
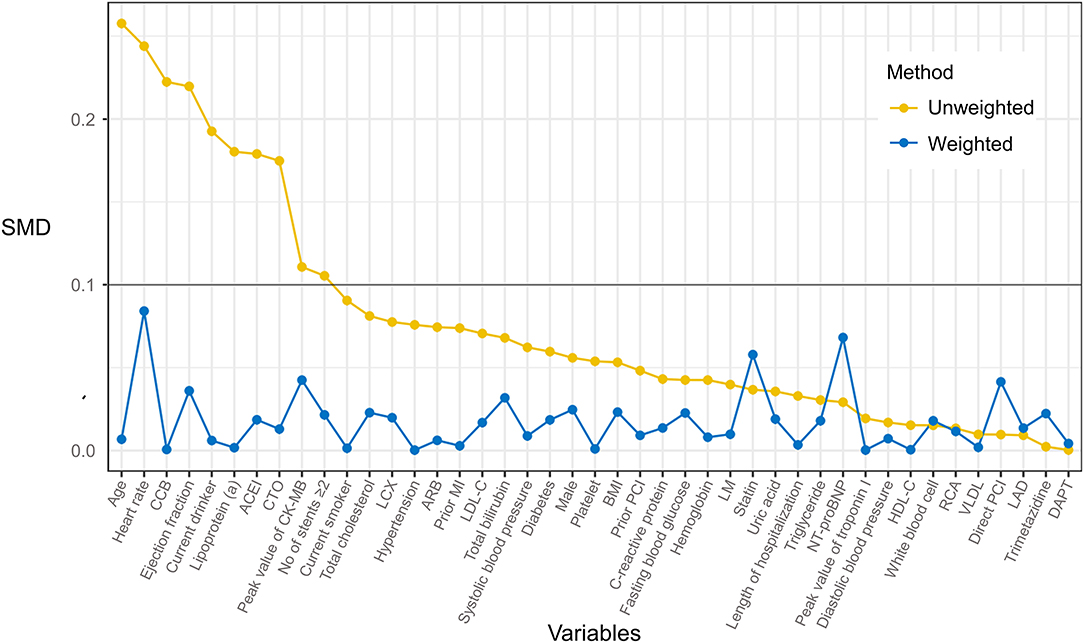
Figure 2. Effect of inverse probability of treatment weighting (IPTW) on baseline characteristics distribution of PMI patients who received metoprolol vs. observation. The imbalance between treatment groups was defined as a standardized mean difference (SMD) >0.1.
A total of 165 (19.2%) MACEs occurred during the 3-year follow-up period. After IPTW adjustment, individuals who received metoprolol reduced the subsequent risk of MACEs by nearly 7.1% (15 vs. 22.1%, ARD = 0.07, RRD = 0.318, NNT = 14, RR = 0.682), recurrent MI by nearly 2.6% (0.9 vs. 3.7%, ARD = 0.028, RRD = 0.766, NNT = 35, RR = 0.234), and revascularization by nearly 5.5% (10.4 vs. 15.9%, ARD = 0.055, RRD = 0.347, NNT = 18, RR = 0.653) (Table 3).
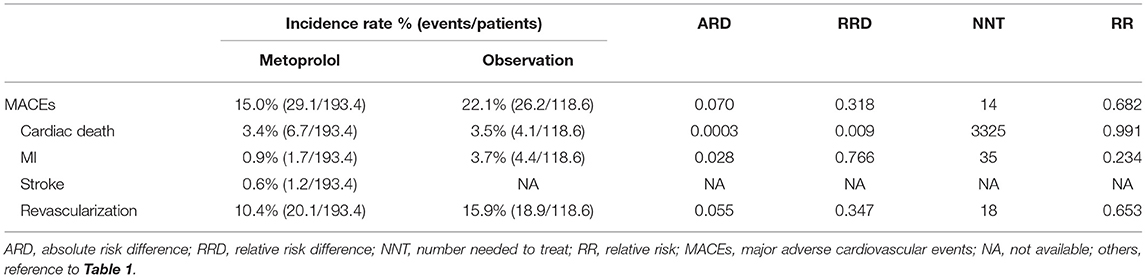
Table 3. Treatment effect of metoprolol vs. observation during the 3 years following the PCI-related PMI in IPTW-adjusted population.
IPTW-adjusted logistic regression (Table 4) showed that receipt of metoprolol significantly reduced the risk of MACEs at the second year (OR = 0.904, 95%CI [0.855–0.955], P < 0.001) and at the third year (OR = 0.925, 95%CI [0.868–0.986], P = 0.017). Specifically, administration of metoprolol significantly reduced the risk of revascularization at 1st year (OR = 0.966, 95%CI [0.937–0.995], P = 0.025), at the second year (OR = 0.931, 95%CI [0.887–0.977], P = 0.004), and at the third year (OR = 0.938, 95%CI [0.888–0.991], P = 0.022). Additionally, metoprolol treatment reduced the risk of recurrent MI at the third year following PMI (OR = 0.972, 95%CI [0.948–0.997], P = 0.029).
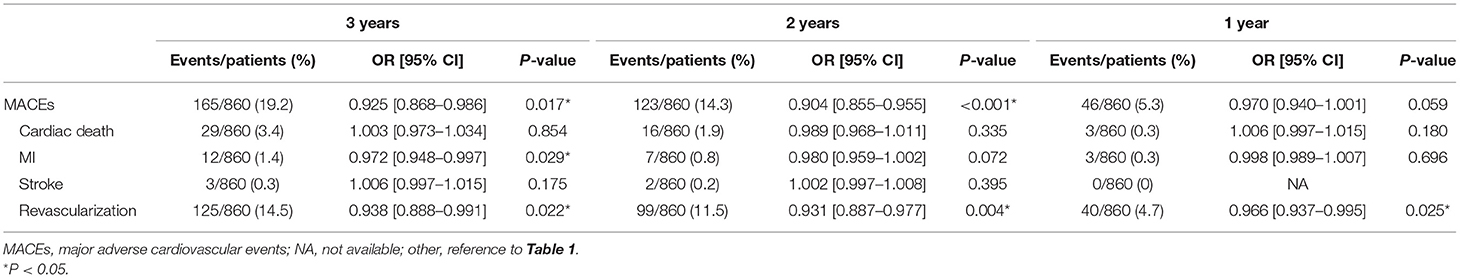
Table 4. Logistic regression analysis of metoprolol treatment on MACEs and its components at 1, 2, and 3 years following PCI-related PMI in IPTW-adjusted population.
For patients who suffered PMI, IPTW-adjusted Kaplan-Meier curves (Figure 3) showed that the metoprolol group achieved a higher survival probability of MACEs (Log-rank P = 0.026) and revascularization (Log-rank P = 0.03) vs. the observation group. IPTW-adjusted Cox regression analyses indicated that receiving metoprolol treatment reduced the 3-year risk of MACEs (HR = 0.588, 95%CI [0.385–0.898], P = 0.014) and revascularization (HR = 0.538, 95%CI [0.326–0.89], P = 0.016; Table 5).
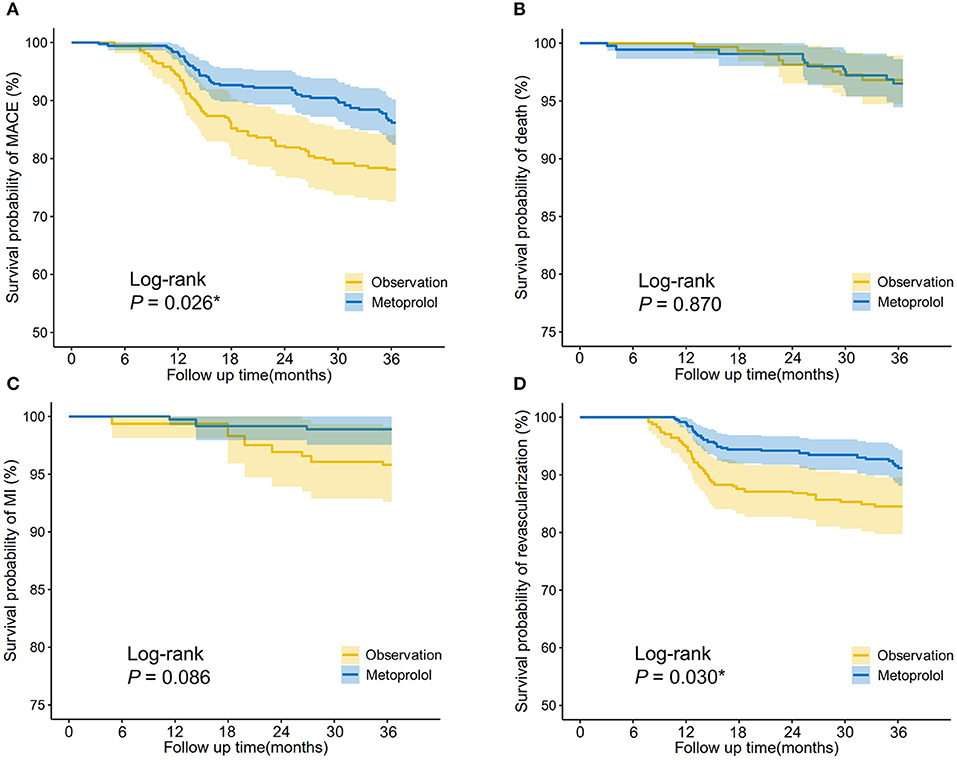
Figure 3. IPTW-adjusted Kaplan-Meier analysis of (A) major adverse cardiovascular events (MACEs), (B) cardiac death, (C) myocardial infarction, and (D) revascularization in patients who received metoprolol vs. observation after PCI-related PMI. The survival curves were indicated by solid lines and 95% CIs by shaded areas. Log-rank P-value was shown. *P < 0.05.
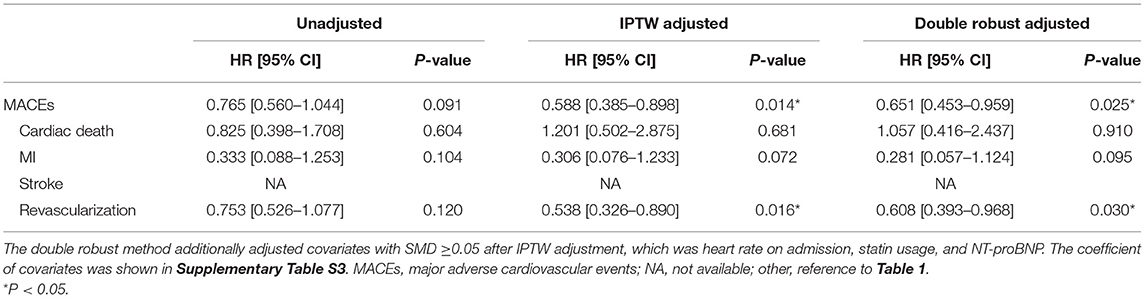
Table 5. Cox regression analysis of metoprolol treatment on MACEs and its components in unadjusted, IPTW adjusted, and double robust adjusted population.
Figure 4 shows the IPTW-adjusted ORs and HRs of receiving metoprolol vs. observation on MACEs according to hypertension, the elevation of cTnI, and cardiac function. Specifically, receipt of metoprolol was associated with a significantly reduced risk of MACEs in patients with hypertension (HR = 0.681, 95%CI [0.466–0.994], P = 0.046), which was independent of the use of other antihypertensive drugs (i.e., ACEI, ARB, and CCB). This protective effect has also been observed in non-hypertensive patients at the second year after PMI (OR = 0.904, 95%CI [0.824–0.992], P = 0.033). In patients with higher cTnI elevation, the protective effect was more pronounced and observed at the third year (OR = 0.918, 95%CI [0.848–0.994], P = 0.035) and the second year after PMI (OR = 0.928, 95%CI [0.864–0.998], P = 0.045). In patients without cardiac dysfunction (EF ≥50% with normal NT-proBNP), the benefit of metoprolol remained with the decreased risk of MACEs at the third year (OR = 0.944, 95% CI [0.891–1], P =0.049) and the second year (OR = 0.948, 95% CI [0.9–0.998], P = 0.042).
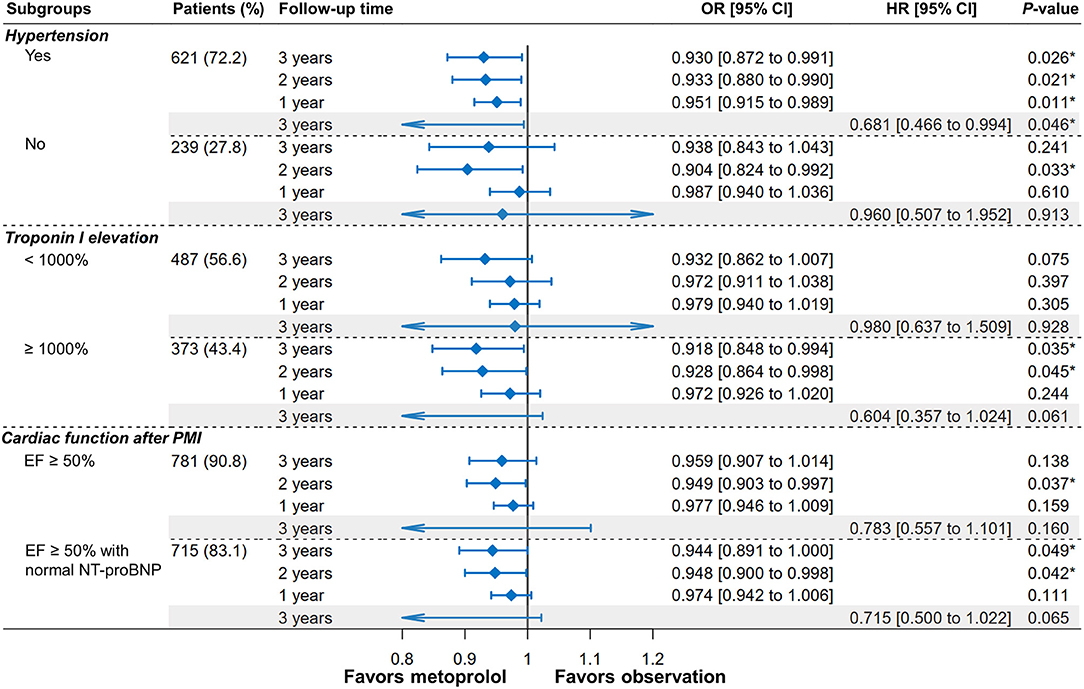
Figure 4. Forest plot of MACEs depicting the IPTW-adjusted odds ratios (ORs) and hazard ratios (HRs) of metoprolol administration vs. observation after PMI according to hypertension, troponin I elevation, and cardiac function. *P < 0.05.
The association of metoprolol treatment with MACEs and its components remained in the sensitivity analysis using the double robust adjustment (IPTW with multivariable regression). Multivariable regression additionally adjusted covariates with SMD ≥0.05, which was heart rate on admission, statin usage, and NT-proBNP. The protective effect of metoprolol on MACEs (HR = 0.651, 95%CI [0.453–0.959], P = 0.025) and revascularization (HR = 0.608, 95%CI [0.393–0.968], P = 0.03) remained (Table 4). Consistently, metoprolol reduced the risk of MI at the third year (OR = 0.972, 95%CI [0.945–0.998], P = 0.037) after double robust adjustment (Supplementary Table S2).
This real-world multicentric study demonstrated the protective effects of metoprolol treatment for PCI-related PMI. Chronic receiving oral sustained-release metoprolol succinate reduces the subsequent 3-year risk of MACEs by nearly 7.1% than their counterparts who received observation, particularly the risk of recurrent MI and revascularization. The exploratory analysis showed that the protective effect of metoprolol was more pronounced in subgroups of hypertension and cTnI elevation ≥1,000%. Besides, the benefits of receiving metoprolol were observed to be consistent in patients without cardiac dysfunction.
Metoprolol is the most frequently used β-receptor blocker (9). In patients with AMI, the protective effect of metoprolol has been confirmed by numerous clinical studies, whether it is taken orally or intravenously (10–13). The clinical practice guidelines for AMI strongly recommend the use of β-blockers as soon as possible and continue to use thereafter, regardless of STEMI and non-STEMI (15, 16). The current study employed 47.5 mg daily oral sustained-release metoprolol succinate as exposure, which is <200 mg daily oral dose commonly used in previous RCTs (10–12). However, even a lower dose of metoprolol has been shown to reduce the risk of subsequent MACEs in the current study, which is consistent with the results of previous RCTs in AMI patients (10–13). In the current study, decreased risk of MACEs (nearly 7.1%) mainly came from the reduced risk of recurrent MI and revascularization, but not mortality. In contrast, for patients surviving AMI, chronic administration of β-blockers certainly leads to a reduction in subsequent mortality (23). This discrepancy may be because PMI is a minor myocardial infarction with a lower mortality rate, which masks the benefit of receiving β-blockers therapy.
The latest ESC clinical practice guidelines on AMI prefer to recommend (class IA) β-blockers administration when LVEF is ≤ 40%, regardless of STEMI or non-STEMI (15, 16). On the contrary, when LVEF is >40%, the evidence of benefits from β-blockers was limited. For this, several large clinical trials are underway to evaluate the effects of β-blockers on AMI patients without LVEF reduction (24). By propensity score matching, Choo et al. (25) confirmed a reduction in all-cause and cardiac mortality with β-blocker therapy at 3 years in AMI patients with EF ≥50%. Therefore, the current study additionally observed the effectiveness of receiving metoprolol treatment in subgroups of EF ≥50% and EF ≥50% with normal NT-proBNP. The results showed that the benefits of receiving metoprolol in reducing the risk of MACEs have remained and consistent with the main finding, which supports the chronic administration of metoprolol in PMI patients with preserved cardiac function.
Metoprolol is often used to lower blood pressure and has been shown to reduce subsequent mortality in the primary prevention of hypertensive patients (26). In the current study, the benefit of metoprolol for PMI patients seemed to be more pronounced in the subgroup of hypertension, which suggested that the benefit may partly come from the antihypertensive effect. However, the antihypertensive effect might not be the only mechanism. On the one hand, the benefits of metoprolol had also been observed in non-hypertensive patients despite relatively few subjects. On the other hand, the benefit of metoprolol was found in the overall population analysis after balancing the covariates between groups by IPTW adjustment (including hypertension, ACEI, ARB, and CCB). Therefore, the protective mechanism of metoprolol might partly come from the antihypertensive effect, but not all.
Periprocedural myocardial infarction is considered a minor MI. The previous study had shown that higher levels of cTnI were associated with a poorer prognosis (27). In this study, the protective effect of metoprolol appeared to be more pronounced in patients with a cTnI increase of ≥1,000%. This might be due to a greater cTnI elevation leading to a more significant prognostic difference, which in turn made the protective effect of metoprolol more prominent.
According to the fourth UDMI, MI was categorized into type 1 to type 5 considering the difference in pathology, clinical features, and prognosis, and therapeutic strategy (5). PCI-related PMI was termed type 4a MI, in which many complicated mechanisms intertwined including acute side branch occlusion, distal embolization, and mechanical process resulting in vulnerable plaque rupture (8, 28). For PCI-related PMI, the benefits from metoprolol may come from the following underlying mechanisms.
First, metoprolol may reduce the ischemia-reperfusion injury and infarct size of PMI, although it is a minor MI. In a porcine ischemia/reperfusion model, Ibanez et al. (29) proved that receiving metoprolol intravenously can reduce the size of MI. The subsequent METOCARD-CNIC trial demonstrated the protective effect of metoprolol administration before reperfusion in reducing infarct size and promoting prognosis in STEMI patients (13, 30). In the current study, patients had initiated to receive metoprolol at the acute phase of MI, which potentially reduce myocardial injury and thus improve the prognosis. Second, receiving metoprolol treatment in the acute phase of PMI may bring about more myocardial perfusion. In the acute phase of MI, β-blocker attenuates excessive sympathetic nervous system activity through a variety of mechanisms, including lowering heart rate to prolong diastolic periods, reducing cardiac contractility to reduce oxygen consumption, and dilating epicardial coronary arteries to increase coronary blood flow (14). Third, metoprolol may attenuate ventricular remodeling in patients surviving PMI and thus achieve long-term benefits (31). Unlike transmural necrosis in STEMI, subendocardial necrosis often appears in minor MI, which is prone to reverse remodeling in the setting of contractile reserve and revascularization (32). Through IPTW-adjustment, the current study balanced the potential inhibitor of cardiac remodeling (i.e., ACEI and ARB) between groups, which further supports the benefit of inhibiting remodeling from metoprolol.
In general, compared with AMI, PCI-related PMI can be deemed as a minor MI, which occurs mostly in patients with stable CAD who underwent index PCI. The current study found that PCI-related PMI patients can also benefit from the chronic administration of β-blockers, which was initially recommended for AMI patients by international guidelines.
First, this study was bound by inherent biases as a retrospective study. Second, 200 mg oral metoprolol was often used as the exposure dose in previous RCTs of AMI. However, due to the real-world setting, the current study identified 47.5 mg daily oral sustained-release metoprolol succinate as the exposure, thus its results may not be applicable to explain the effects of other doses of metoprolol on PMI. Third, revascularization was observed as a secondary endpoint, but target lesion revascularization or target vessel revascularization was not further analyzed. Fourth, the definition of PMI was adopted from the fourth UDMI based on cTnI, which cannot avoid the potential limitations of the definition of PMI.
In the real-world setting, receiving metoprolol treatment following PCI-related PMI has decreased the subsequent risk of MACEs, particularly the risk of recurrent MI and revascularization.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University (20201217-36). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
WenbZ, CJ, and ZC conceived and designed the study. DL organized these data and drafted the manuscript with the help of ML. DL, YL, and WenjZ analyzed the data. YL drew the pictures. WenbZ, CJ, ZC, and GF detected any errors in the whole process. All authors have read and approved the manuscript for submission.
This work was supported by grants from the National Natural Science Foundation of China (82070408 and 81800212), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2021RC014), the Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2021ZB172), and the Joint Funds for the innovation of science and Technology, Fujian province (2018Y9094).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.746988/full#supplementary-material
CAD, coronary artery disease; PCI, percutaneous coronary intervention; PMI, periprocedural myocardial infarction; UDMI, universal definition of myocardial infarction; MI, myocardial infarction; AMI, acute myocardial infarction; cTnI, cardiac troponin I; β-blocker, β-adrenoceptor antagonist; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction; ULN, the upper limit of normal; CTO, chronic total occlusion; ICD-9-CM, International Classification of Diseases, 9th Revision Clinical Modification; DM, diabetes mellitus; BMI, body mass index; NT-proBNP, N-terminal pro-brain natriuretic peptide; MACEs, major adverse cardiovascular events; EF, ejection fraction; CRP, C-reactive protein; CK-MB, creatine kinase MB; VLDL, very low-density lipoprotein; IPTW, inverse probability of treatment weighting; PS, propensity score; SMD, standardized mean difference; ARD, absolute risk difference; RRD, relative risk difference; NNT, number needed to treat; RR, relative risk; HR, hazard ratio; OR, odds ratio; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; RCT, randomized controlled trial.
1. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
2. Chacko L, Howard JP, Rajkumar C, Nowbar AN, Kane C, Mahdi D, et al. Effects of percutaneous coronary intervention on death and myocardial infarction stratified by stable and unstable coronary artery disease: a meta-analysis of randomized controlled trials. Circ Cardiovasc Qual Outcomes. (2020) 13:e006363. doi: 10.1161/CIRCOUTCOMES.119.006363
3. Cho MS, Ahn J-M, Lee C-H, Kang D-Y, Lee J-B, Lee PH, et al. Differential rates and clinical significance of periprocedural myocardial infarction after stenting or bypass surgery for multivessel coronary disease according to various definitions. JACC Cardiovasc Intervent. (2017) 10:1498–507. doi: 10.1016/j.jcin.2017.05.051
4. Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. (2005) 111:1027–32. doi: 10.1161/01.CIR.0000156328.28485.AD
5. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
6. Lee DW, Cavender MA. Periprocedural myocardial infarction in contemporary practice. Interv Cardiol Clin. (2019) 8:209–23. doi: 10.1016/j.iccl.2018.12.001
7. Li Y, Pei H, Bulluck H, Zhou C, Hausenloy DJ. Periprocedural elevated myocardial biomarkers and clinical outcomes following elective percutaneous coronary intervention: a comprehensive dose-response meta-analysis of 44,972 patients from 24 prospective studies. EuroIntervention. (2020) 15:1444–50. doi: 10.4244/EIJ-D-19-00737
8. Park D-W, Kim Y-H, Yun S-C, Ahn J-M, Lee J-Y, Kim W-J, et al. Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur Heart J. (2013) 34:1662–9. doi: 10.1093/eurheartj/eht048
9. ClinCalc LLC ClinCalc DrugStats Database. Available online at: https://clincalc.com/Drugstats/ (accessed July 8, 2021).
10. Ryden L, Ariniego R, Arnman K, Herlitz J, Hjalmarson A, Holmberg S, et al. A double-blind trial of metoprolol in acute myocardial infarction. Effects on ventricular tachyarrhythmias. New Engl J Med. (1983) 308:614–8. doi: 10.1056/NEJM198303173081102
11. Hjalmarson A, Herlitz J, Holmberg S, Ryden L, Swedberg K, Vedin A, et al. The Goteborg metoprolol trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation. (1983) 67(6 Pt 2):I26–32.
12. Olsson G, Rehnqvist N, Sjogren A, Erhardt L, Lundman T. Long-term treatment with metoprolol after myocardial infarction: effect on 3 year mortality and morbidity. J Am Coll Cardiol. (1985) 5:1428–37. doi: 10.1016/S0735-1097(85)80360-0
13. Ibanez B, Macaya C, Sánchez-Brunete V, Pizarro G, Fernández-Friera L, Mateos A, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. (2013) 128:1495–503. doi: 10.1161/CIRCULATIONAHA.113.003653
14. Hong J, Barry AR. Long-term beta-blocker therapy after myocardial infarction in the reperfusion era: a systematic review. Pharmacotherapy. (2018) 38:546–54. doi: 10.1002/phar.2110
15. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320
16. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
17. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. (2012) 79:453–95. doi: 10.1002/ccd.23438
18. Lyubarova R, Schulman-Marcus J, Boden WE. Contemporary management of patients with stable ischemic heart disease. Cardiovasc Innovat Appl. (2019) 3:269–78. doi: 10.15212/CVIA.2017.0071
19. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. (2015) 34:3661–79. doi: 10.1002/sim.6607
20. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
21. Guyot P, Cheng W, Tremblay G, Copher R, Burnett H, Li X, et al. Number needed to treat in indirect treatment comparison. J Comp Eff Res. (2018) 7:259–69. doi: 10.2217/cer-2017-0023
22. Khalaf K, Johnell K, Austin PC, Tyden P, Midlov P, Perez-Vicente R, et al. Low adherence to statin treatment during the 1st year after an acute myocardial infarction is associated with increased 2nd-year mortality risk-an inverse probability of treatment weighted study on 54 872 patients. Eur Heart J Cardiovasc Pharmacother. (2021) 7:141–7. doi: 10.1093/ehjcvp/pvaa010
23. Dezsi CA, Szentes V. The real role of beta-blockers in daily cardiovascular therapy. Am J Cardiovasc Drugs. (2017) 17:361–73. doi: 10.1007/s40256-017-0221-8
24. Zeitouni M, Kerneis M, Lattuca B, Guedeney P, Cayla G, Collet JP, et al. Do patients need lifelong beta-blockers after an uncomplicated myocardial infarction? Am J Cardiovasc Drugs. (2019) 19:431–8. doi: 10.1007/s40256-019-00338-4
25. Choo EH, Chang K, Ahn Y, Jeon DS, Lee JM, Kim DB, et al. Benefit of beta-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart. (2014) 100:492–9. doi: 10.1136/heartjnl-2013-305137
26. Wikstrand J, Warnold I, Olsson G, Tuomilehto J, Elmfeldt D, Berglund G. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA. (1988) 259:1976–82. doi: 10.1001/jama.1988.03720130040027
27. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. (2013) 61:1240–9. doi: 10.1016/j.jacc.2012.12.026
28. Park D-W, Kim Y-H, Yun S-C, Ahn J-M, Lee J-Y, Kim W-J, et al. Impact of the angiographic mechanisms underlying periprocedural myocardial infarction after drug-eluting stent implantation. Am J Cardiol. (2014) 113:1105–10. doi: 10.1016/j.amjcard.2013.12.016
29. Ibanez B, Cimmino G, Prat-Gonzalez S, Vilahur G, Hutter R, Garcia MJ, et al. The cardioprotection granted by metoprolol is restricted to its administration prior to coronary reperfusion. Int J Cardiol. (2011) 147:428–32. doi: 10.1016/j.ijcard.2009.09.551
30. Podlesnikar T, Pizarro G, Fernandez-Jimenez R, Montero-Cabezas JM, Sanchez-Gonzalez J, Bucciarelli-Ducci C, et al. Effect of early metoprolol during ST-segment elevation myocardial infarction on left ventricular strain. Feature-tracking cardiovascular magnetic resonance substudy from the METOCARD-CNIC trial. JACC Cardiovasc Imaging. (2019) 12(7 Pt 1):1188–98. doi: 10.1016/j.jcmg.2018.07.019
31. Duncker DJ, Boontje NM, Merkus D, Versteilen A, Krysiak J, Mearini G, et al. Prevention of myofilament dysfunction by beta-blocker therapy in postinfarct remodeling. Circ Heart Fail. (2009) 2:233–42. doi: 10.1161/CIRCHEARTFAILURE.108.806125
Keywords: coronary artery disease, percutaneous coronary intervention, periprocedural myocardial infarction, β-blocker, metoprolol
Citation: Li D, Li Y, Lin M, Zhang W, Fu G, Chen Z, Jin C and Zhang W (2021) Effects of Metoprolol on Periprocedural Myocardial Infarction After Percutaneous Coronary Intervention (Type 4a MI): An Inverse Probability of Treatment Weighting Analysis. Front. Cardiovasc. Med. 8:746988. doi: 10.3389/fcvm.2021.746988
Received: 25 July 2021; Accepted: 25 October 2021;
Published: 23 November 2021.
Edited by:
Krishnaraj Sinhji Rathod, Queen Mary University of London, United KingdomReviewed by:
Guangzhi Cong, General Hospital of Ningxia Medical University, ChinaCopyright © 2021 Li, Li, Lin, Zhang, Fu, Chen, Jin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Zhang, MzMxMzAxMUB6anUuZWR1LmNu; Chongying Jin, amluY3lfbHZAMTYzLmNvbQ==; Zhaoyang Chen, Y2hlbnpoYW95YW5nODg4QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.