
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 09 February 2022
Sec. Structural Interventional Cardiology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.746774
This article is part of the Research Topic Insights in Structural Interventional Cardiology: 2021 View all 4 articles
 Tarek A. N. Ahmed1,2*
Tarek A. N. Ahmed1,2* You-Jeong Ki1
You-Jeong Ki1 You-Jung Choi1
You-Jung Choi1 Heba M. El-Naggar1,2
Heba M. El-Naggar1,2 Jeehoon Kang1
Jeehoon Kang1 Jung-Kyu Han1
Jung-Kyu Han1 Han-Mo Yang1
Han-Mo Yang1 Kyung Woo Park1
Kyung Woo Park1 Hyun-Jae Kang1
Hyun-Jae Kang1 Bon-Kwon Koo1
Bon-Kwon Koo1 Hyo-Soo Kim1
Hyo-Soo Kim1Background: Systemic inflammatory response syndrome (SIRS) is a systemic insult that has been described with many interventional cardiac procedures. The outcomes of patients undergoing transcatheter aortic valve implantation (TAVI) are thought to be influenced by this syndrome not only on short-term, but also on long-term.
Objective: We assessed the association of SIRS to different clinical, echocardiographic, and computed tomographic (CT) outcomes after TAVI.
Methods: Two hundred and twenty-four consecutive patients undergoing TAVI were enrolled in this study. They were assessed for the occurrence of SIRS within the first 48 h after TAVI. Patients were followed-up for short- and long-term clinical outcomes. Serial echocardiographic follow-ups were conducted at 1-week, 6-months, and 1-year. CT follow-up at 1 year was recorded.
Results: Eighty patients (36%) developed SIRS. Among different parameters, only pre-TAVI total leucocytic count (TLC), pre-TAVI heart rate, and post-TAVI systolic blood pressure independently predicted the occurrence of SIRS. The incidence of HALT was not significantly different between both groups, albeit higher among SIRS patients (p = 0.1) at 1-year CT follow-up. Both groups had similar patterns of LV recovery on serial echocardiography. Long-term follow-up showed that all-cause death, cardiac death, and re-admission for heart failure (HF) or acute coronary syndrome (ACS) were significantly more frequent among SIRS patients. Early safety and clinical efficacy outcomes were more frequently encountered in the SIRS group, while device-related events and time-related valve safety were comparable.
Conclusion: Although SIRS implies an early acute inflammatory status post-TAVI, yet its clinical sequelae seem to extend to long-term clinical outcomes.
The outcome of patients undergoing surgical or interventional therapy is unfavorably influenced by severe systemic inflammation. Systemic inflammatory response syndrome (SIRS) has been reported to frequently occur in the setting of TAVI. Its exact mechanism is still unknown. Periprocedural hypotension and suboptimal organ perfusion with ischemia-reperfusion injury and subsequent cytokine release have been suspected to play a role in the pathogenesis of SIRS post-TAVI (1, 2).
A previous study has accused the inflammatory process post-TAVI to the decline in LV function recovery (3). Likewise, inflammation have been suggested as a contributor to the pathogenesis of acute and chronic heart failure (4).
Hypoattenuation leaflet opacities on computed tomography (CT), or the so called hypoattenuation leaflet thickening (HALT) have been reported after TAVI. It is a hallmark finding of subclinical leaflet thrombosis (5), and have provoked a debate about the necessity of routine anticoagulation post-TAVI, with safety and efficacy concerns. An inflammatory hypothesis has been implicated in the provocation of HALT (6).
Despite SIRS is reported in the early post-TAVI phase, yet its clinical impact has been evidenced to extend to the long-term outcomes with the earlier valve generations (7). Thus, we hypothesize that a sort of a long-standing inflammatory milieu might implicate this finding and is early explicated as SIRS.
In this study, we attempted to elucidate the impact of SIRS on the echocardiographic, CT, and short- and long-term VARC-2-defined individual and composite outcomes, among a real-world population with variable risk scores and utilizing earlier and latest generations transcatheter aortic valves.
We retrospectively included all patients with severe AS (indexed aortic valve area ≤0.6 cm2/m2 or transvalvular mean gradient >40 mm Hg or peak velocity >4 m/s) treated with trans-catheter aortic valve implantation (TAVI) in the period between 2012 till 2019 at Seoul National University Hospital. Patients were divided into 2 groups according to the development of SIRS. All medical records were systematically reviewed and retrieved for patient medical history, vital status, diagnostic tests including lab values, echocardiographic and CT angiographic data as well as procedural details. Patients' clinical data was reported up to the latest follow-up from the hospital electronic medical records. Patients were excluded if they were converted to open heart surgery as the surgical trauma and extracorporeal circulation exacerbate the inflammatory response (8–10), or if they died within <48 h after TAVI, which precludes the feasibility of SIRS data assessment. Part of the study population was also included in the K-TAVI registry previously published (11). The study complied with the declaration of Helsinki and was approved by the local ethics committee.
SIRS was defined, according to the existing ACCP/SCCM consensus guidelines, as fulfilling at least two of the following four criteria within the first 48 h after TAVI as assessed by electronic monitoring records in the intensive care unit (ICU): temperature <36.0 or >38.0°C, heart rate >90 beats/min, hyperventilation as respiratory rate >20 breaths/min or PaCO2 <32 mmHg, leucocytic count >12 or <4 (109/L) (12, 13) (Supplementary Table 1). Clinical outcomes were assessed in accordance with the standardized end-point definitions of the Valve Academic Research Consortium 2 (VARC-2) (14).
Trans-thoracic echocardiographic studies were performed for all participants at baseline before TAVI, then follow-up was performed at 1-week, 1-month, and 1-year post-TAVI. Follow-up was available in 78 (97.5%), 143 (99.3%) patients at 1-week, 66 (82.5%), 132 (92%) at 1 month, and 47 (59%), 117 (81%) at 1 year, among the SIRS and no SIRS groups, respectively. Measurements included AV study (aortic valve area (AVA), and mean pressure gradient), left ventricular ejection fraction (LVEF) determined by 2-D biplane Simpson's method, left ventricular mass index (LVMI), left atrial volume index (LAVI), tissue doppler assessment of mitral annulus for estimation of S', E', and E/E' ratio, as well as 2-D LV global longitudinal strain (2-D LV-GLS) by speckle tracking echocardiography. All measurements were standardized according to latest American Society of Echocardiography guidelines (15), and echocardiographic-related endpoints complied to definitions as per VARC-2 consensus document (14).
CT analysis was performed routinely before the TAVI procedure for proper planning of valve size, procedure, and vascular access. Post-TAVI CT was performed routinely at 1-year follow-up, according to rigorous institutional protocols, for the assessment of hypoattenuation leaflet thickening (HALT), that often indicate leaflet thrombosis formation (16), as well as measurements of different aortic dimensions. The protocol adopted was standardized throughout the study period and was largely consistent with the latest consensus document by the Society of Cardiovascular Computed Tomography (SCCT) (17). Follow-up CT studies at 1 year were available for 45 (56.3%), and 109 (75.7%) patients among the SIRS and no SIRS groups, respectively.
Continuous variables were expressed as mean ± standard deviation or median (interquartile range), while categorical variables were presented as frequencies and percentages. Continuous variables were compared by Student's t-test or non-parametric tests according to whether normally distributed or not, respectively. Chi-square or Fisher's exact tests were used to compare categorical variables. Repeated measures ANOVA was used to compare the within-groups and between-groups effect of repeated echocardiographic measurements over time and to assess group-time interaction. Logistic regression modeling was used to assess the independent predictors of SIRS among different covariates. Cox regression analysis was performed to assess the cumulative events over time among the study groups. Two models were created; one for overall mortality and the other for composite clinical efficacy VARC-2 defined endpoints. Variables with p < 0.05 on univariate analyses were included in a multivariate model. Survival analysis according to the occurrence of SIRS was determined for each of overall mortality and clinical efficacy using Kaplan-Meier (K-M) method. The log-rank test was used to express statistical difference in survival analysis. Through-out all tests, statistical significance was assumed when two-sided p < 0.05. Statistical analyses were performed using SPSS statistics version 21 (IBM Corporation, NY, USA).
A total of 224 patients were included in our analysis. Of those 80 patients (36%) developed SIRS, while 144 (64%) had no SIRS. Table 1 shows the baseline clinical characteristics of both study groups; those with SIRS vs. those with no SIRS. Both groups were comparable regarding most clinical parameters. However, those with SIRS had significantly higher STS risk score as well as higher baseline heart rate.
Table 2 shows the baseline laboratory findings of both study groups. Patients with SIRS had significantly lower baseline hemoglobin levels, while they elicited higher total leucocytic count (TLC), Neutrophil lymphocyte ratio (NLR), absolute neutrophilic count (ANC), procalcitonin, and high-sensitive C-reactive protein (HS-CRP) at baseline.
Table 2 shows post-procedural laboratory data, which were quite similar to the baseline findings, with significantly higher indices of inflammation as well as myocardial injury as evidenced by CK-MB and troponin.
There was a diversity among the valve types and generations utilized throughout the study period, which were similarly distributed between the study groups. There was no significant difference in the procedural parameters between both groups with the exception of post-implantation systolic and diastolic blood pressures which were significantly lower among the SIRS group (Table 3).
Table 4 shows the baseline and 1-year follow-up CT data for the study groups. Baseline parameters were not significantly different. The follow-up rate was significantly lower among the SIRS group (56.3 vs. 75.7%, p = 0.003). Noticeably, the incidence of HALT, on follow-up CT, was higher among the SIRS group yet not reaching statistical significance (31.1 vs. 20.2%, p = 0.1).
TTE was performed at baseline and at predetermined follow-up points (Table 5). At baseline, no significant difference was encountered among study groups except for higher PASP in the SIRS group, which faded out on follow-up. No significant difference in echocardiographic parameters was encountered upon follow-up at different time intervals. A repeated measure ANOVA was conducted to compare different echocardiographic parameters among the study groups at different time intervals (Figure 1). The within-group difference over time was significant for all echocardiographic parameters; 2D-LVEF, AV mean PG, AVA, LVMI, LAVI, and LV-GLS, while there was no significant difference between both study groups (between-groups effect) for the aforementioned parameters. Regarding the interaction of study groups over time, it was not significant except for LV-GLS which elicited significant interaction with time (p = 0.04).
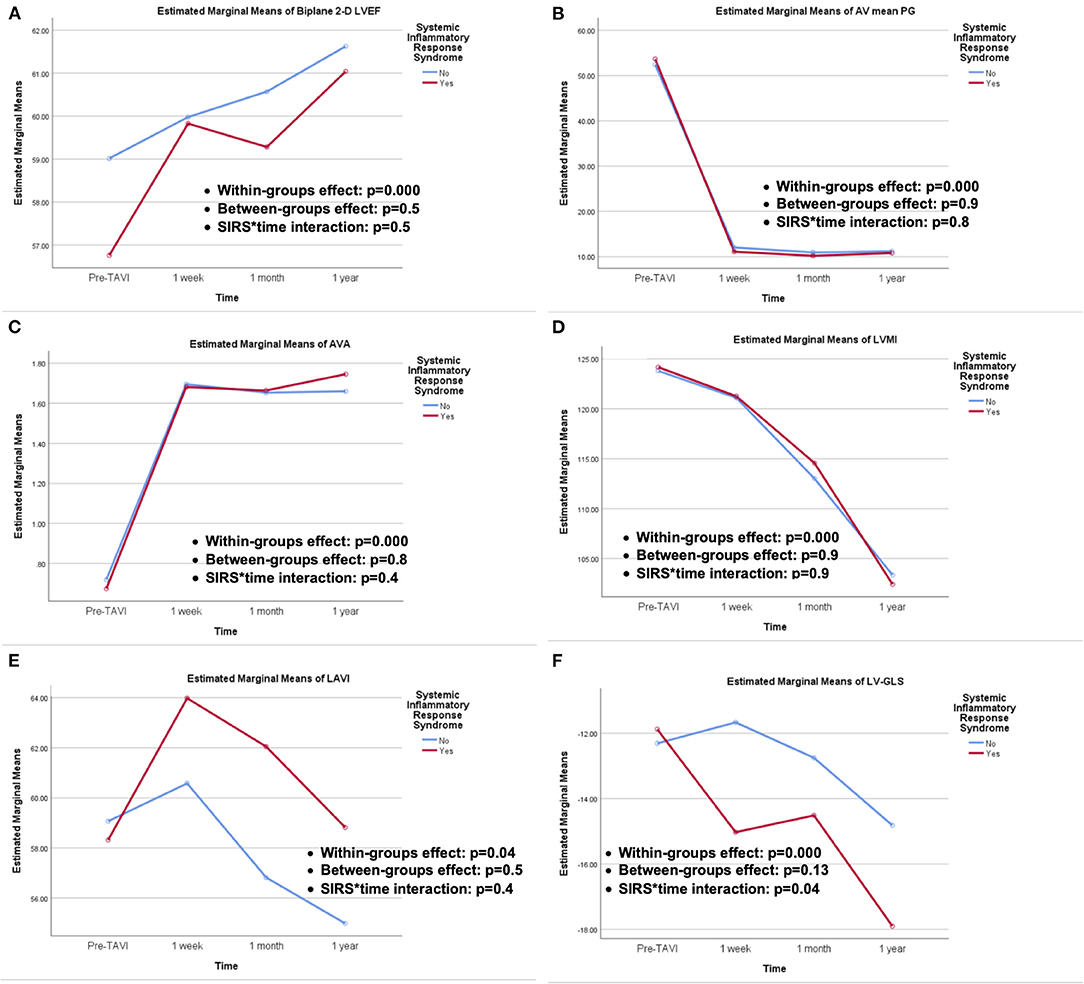
Figure 1. Repeated measures ANOVA for echocardiographic outcomes of; (A) 2-D LVEF, (B) AV mean PG, (C) AVA, (D) LVMI, (E) LAVI, and (F) LV-GLS, showing the within-groups and between-groups effect, as well as SIRS groups and time interactions.
A logistic regression model was created for predicting SIRS including potentially relevant laboratory and hemodynamic covariates that showed significant difference on simple comparisons and avoiding any possible multicollinearity (Table 6). On univariate analysis, the pre-procedural Hgb level, serum creatinine, TLC, pre-TAVI heart rate (HR), post-TAVI systolic and diastolic blood pressures, as well as PASP were predictors of SIRS. However, on multiple regression analysis correcting for significant covariates, only pre-TAVI TLC, pre-TAVI HR, and post-TAVI SBP were independent predictors of SIRS.
In-hospital and over more than 5-years follow-up clinical outcome data were tracked for patient groups through the hospital electronic medical records. In-hospital clinical outcomes showed that patients with SIRS had higher rates of IH death, cardiac tamponade, major, and minor bleeding episodes, as well as stage I AKI (Table 7). Median follow-up was comparable among both study groups (p = 0.4). All-cause death, cardiac death, re-hospitalization for HF, and re-hospitalization for ACS or PCI were significantly more frequent among the SIRS group (Table 7). Looking at the VARC-2 composite endpoints, early safety events (within 30 days), as well as clinical efficacy events (after 30 days), were significantly more frequent among the SIRS group (p < 0.001, p = 0.009, respectively). On the other hand, device-related events and time-related valve safety were not significantly different between both groups (p = 0.6, p = 0.2, respectively) (Table 7).
Cox-regression analyses for time-adjusted events were displayed for the outcomes of all-cause mortality, and composite endpoint of clinical efficacy (Table 8). Multiple analysis showed that for the outcome of all-cause mortality, STS score (HR = 1.1, 95% CI = 1.01–1.18, p = 0.02), Pre-TAVI Hs CRP (HR = 1.3, 95% CI = 1.05–1.51, p = 0.01), and post-TAVI peak CK-MB (HR = 1.0, 95% CI = 1.0–1.09, p = 0.03) independently predicted all-cause mortality. For the outcome of clinical efficacy, STS score was the only independent predictor (HR = 1.06, 95% CI = 1.03–1.09, p < 0.001). Of note, although SIRS showed almost three times the hazard of predicting events for the outcome of clinical efficacy, yet it only showed marginal significance (HR = 2.8, 95% CI = 0.93–8.6, p = 0.06).
Furthermore, elaborating on survival function of both study groups using K-M analysis, showed that SIRS group had more frequent time-related events for all-cause mortality as well as for the composite endpoint of clinical efficacy (log-rank p < 0.001, and =0.01, respectively) (Figure 2).
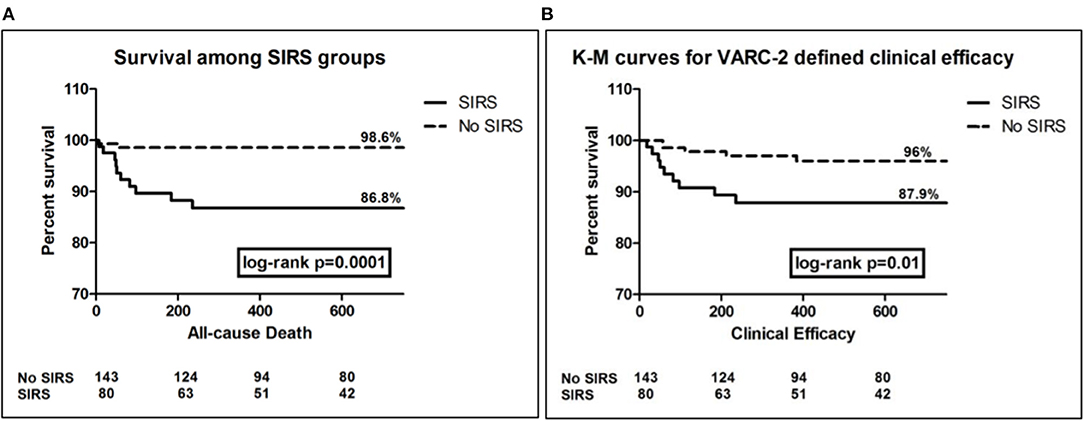
Figure 2. Kaplan Meier survival curves for the outcomes of all-cause death (A), and clinical efficacy composite end-point (B), showing significantly more events among SIRS group, log rank p = 0.0001, and 0.01, respectively.
With the growing application of TAVI in a wide spectrum of aortic stenosis patients ranging from low to high risk, there have been concerns regarding occurrence of SIRS which in turn impacts resource utilization and patient outcomes.
In our study, SIRS has been recorded in 36% of the patient population. This was concordant with the prevalence reported by Schwietz et al. (18) (39.1%), and Sinning et al. (7) (40.1%), confirming the wide prevalence of post-TAVI inflammatory response. This incidence was as high as 73% in a study that included patients undergoing aortic valve replacement via TAVR (n = 264) or SAVR (n = 483), using an alternative non-femoral access approach in more than half the cases of TAVR, and trans-femoral cases were performed with a surgical cut-down (19).
In our study, pre- and post-procedural markers of inflammation including HS-CRP were significantly higher among the SIRS group. This is in line with the study by Sinning et al. (7), which showed significant increase in pro-inflammatory cytokines (IL-6 and IL-8), with subsequent elevation in acute phase reactants CRP and PCT.
Among different parameters that were included in a prediction model for the outcome of SIRS, it was concluded that lower post-procedural BP, among other parameters, independently predicted the occurrence of SIRS. It has been previously demonstrated that systemic hypotension results in suboptimal tissue perfusion with subsequent ischemia/reperfusion injury, which in turn triggers a vicious circle of leucocyte and endothelial activation, cytokine release, and finally SIRS (20–22).
It has been postulated that general anesthesia might increase the incidence of SIRS after TAVI (19). However, general anesthesia was employed in the majority of our patient population with no significant difference between both patient groups (p = 0.4). Not to mention that the incidence of SIRS was more or less similar to that reported by Sinning et al. (7), in whom TAVI was performed without general anesthesia (conscious sedation). Nevertheless, in our study population, the duration of anesthesia was significantly longer among the SIRS group, which supports the notion that cardiac hemodynamic challenges, more frequently encountered with longer anesthesia duration, is a major determinant in the pathogenesis of SIRS.
Our study provides the largest patient cohort assessing the impact of various valve types of different generations on the incidence of SIRS. Prior studies have assessed the incidence of SIRS either utilizing only self-expanding valves (Medtronic CoreValve®) (7) or balloon-expandable valves (Edwards SAPIEN®) (19). One might expect that shear stress (23), and transient organ hypoperfusion resulting from rapid pacing with the deployment of balloon-expandable valves (19) or, on the other hand, the hemodynamic compromise with slow deployment or recapturing of self-expandable valves (7), might favor one type of valve or another. However, our data showed that there was no difference between valve types among both groups.
Previous study showed that higher leucocytic count and maximum CRP levels post-TAVI were independent predictors of declined LVEF (3). On the contrary, serial echocardiographic assessments of patients in our study, at different time intervals, did not show difference in LV function recovery between both groups, including LV subclinical dysfunction assessed by speckle-tracking derived LV-GLS. This might be explained by the overwhelming beneficial influence of the valve implantation and relief of the LV overload which supersedes the potential impact of any post-procedural inflammatory status. In fact, an early (1 week), kind of paradoxical, more rapid recovery of LV-GLS was noticed among the SIRS group, with a later catch-up at the subsequent time intervals (1 month, 1 year). A recent study, evaluating circulating inflammatory T-cell phenotypes and its association with adverse LV remodeling post-TAVI, assumed a possible role of IL-10, produced by T-cells, as an anti-inflammatory mediator, which improves cardiac function via activating fibroblasts which has a beneficial effect in the early injury phase, whereas long-term chronic activation of fibroblasts increases myocardial fibrosis resulting in adverse remodeling and functional decline (24). This hypothesis-generating assumption should be elaborated in further dedicated larger scale studies.
For the first time, we assessed the possible relation between post-TAVI SIRS and CT findings on follow-up, particularly the intriguing finding of HALT. Several hypotheses have been postulated in the pathogenesis of HALT (25–28). An inflammatory hypothesis has been implicated in the provocation of HALT (6), which in turn was suspected to trigger inflammatory process with subsequent valve leaflet degeneration (29).
We presumed that subclinical leaflet thrombosis or CT finding of HALT might be partly due to an ongoing inflammatory status that has been early manifested as SIRS. Hypothetically, a sort of chronic activation of systemic inflammatory response might contribute to this delayed finding, and SIRS might be an early trigger. Likewise, inflammatory cascades have been implicated in atherothrombotic coronary disorders and this led to several late therapeutic intervention studies aiming to abort this inflammatory milieu (30, 31). Despite the incidence of HALT in our study was more frequent in the SIRS group of patients (31 vs. 20%), yet this wasn't statistically significant. It is noteworthy that the rate of follow-up CT among SIRS group was less than that of non-SIRS group, thus underestimating the outcome.
Previous studies have elucidated increased 30-days (7, 19), and 1-year mortality (7, 18, 19) among TAVI patients eliciting ≥2 SIRS criteria. We found a higher incidence of in-hospital mortality, major and minor bleeding, and acute kidney injury among the SIRS group. Unlike our results, Schwietz et al. showed no significant difference in early mortality, although a significant increase was observed at 1-year (18). The absence of early difference in mortality in their study might be explained by the fact that they employed apical access in almost 38% of their patient population, which entails higher early in-hospital surgical risk.
According to our study, there was a higher incidence of AKI among the SIRS patients, most of them were AKI stage 1. Looking to the baseline patient data, serum creatinine was significantly higher among the SIRS group. However, baseline serum creatinine did not independently predict the occurrence of SIRS. In the study by Sinning et al. (7), SIRS was strongly associated with the development of AKI. Despite the high prevalence of CRF in their series, yet it was not associated with the occurrence of SIRS after TAVI, in concordance with our finding. Previous studies demonstrated that the inflammatory process resulting from ischemia/reperfusion injury plays a role in the pathophysiology of AKI. In TAVI patients, transient peri-procedural renal and gut hypoperfusion might result in significant cytokine release expressed as SIRS, which plays a crucial role in the pathogenesis of AKI (32, 33).
Noticeably, in our study, the incidence of major and minor bleeding was significantly higher in the SIRS group. One of the proposed contributing factors to the development of SIRS in TAVI patients was transfusion of red blood cell (RBC) units (7). This has been previously elucidated in cardiac surgery patients, and was attributed to co-administration of inflammatory mediators e.g., IL-8 which accumulates in the stored packed RBCs (34, 35). However, in our study, baseline Hgb level did not independently predict the occurrence of SIRS in a multivariable regression model. Likewise, Sinning et al. showed that, in their study cohort, the amount of RBC transfusions as well as the net drop in Hgb level didn't predict SIRS (7). This emphasizes that bleeding and subsequent blood transfusion was only a minor contributor to the occurrence of SIRS.
Over more than 5 years median follow-up, SIRS patients had significantly higher rates of overall mortality, cardiac mortality, readmissions due to HF or due to ACS/PCI. Adopting the VARC-2 composite endpoints, SIRS patients did significantly worse in terms of early safety (<30d) and late clinical efficacy (>30d), yet device success rates and time-related valve safety were not significantly different.
Our study provides the longest follow-up for the impact of SIRS on VARC-2-defined individual and composite clinical outcomes, among a real-world population with variable risk and utilizing earlier and latest generations transcatheter aortic valves. Survival analysis for all-cause mortality and VARC-2 composite endpoint of clinical efficacy showed significantly more events among the SIRS patients. This goes along with previous studies (7, 19), which provided survival analysis for 1-year only. In accordance to Sinning et al. (7), who showed a hazard ratio (95% CI) = 7.4 (3.5–15.6) among SIRS group of patients for the outcome of all-cause mortality, our SIRS group showed a hazard ratio (95% CI) = 10.5 (2.32–47.33). However, adjusted for other covariates, SIRS did not independently predict mortality. Despite SIRS showed almost 3 times the hazard of events for the composite outcome of clinical efficacy, yet it failed to independently predict this outcome as well, with only marginal significance (p = 0.06).
In summary, TAVI might frequently enhance an inflammatory reaction culminating into SIRS. This happens independent of the valve type, and despite moving toward smaller profile devices with better technological refinements, and a more minimalistic approach (conscious sedation, and more transfemoral procedures with no surgical cut-down), yet SIRS remains a devastating problem which needs all efforts to be attenuated.
Recently, there has been an uprising interest in the concept of inflammation in the pathogenesis of cardiovascular disease (36). Several late trials have tested the efficacy of specific anti-inflammatory medications in the context of atherothrombotic coronary artery disease (30, 31, 37). A recent subgroup analysis of the CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) trial showed that IL-1β-targeting therapy may decrease heart failure-related hospitalization and mortality (38). Lately, a proof-of-concept study, showed, for the first time, an association of specific inflammatory phenotypes (immune signatures) with increased mortality after TAVI (39). Thus, suggesting that distinct monocyte and T-cell signatures might stand as novel biomarkers to be considered in risk stratifying patients with severe aortic stenosis undergoing TAVI, and possibly guiding potential anti-inflammatory treatment. This remains to be further elucidated in properly designed studies.
Owing to relatively small sample size and few events, this study ought to be considered hypothesis-generating. The exact pathophysiological mechanism of SIRS post-TAVI remains speculative, and further studies digging deeper into different proposed triggers for such inflammatory response are warranted.
SIRS criteria are partly determined by vital signs like heart rate, respiratory rate and temperature. One might think that this would entail inaccurate recording with individual bias. However, patients undergoing TAVI were subjected to a standardized, rigorous monitoring protocol in the ICU, where vital signs are recorded in the electronic medical record hourly (or even more frequently if necessitated), and this could be easily tracked and retrieved from the electronic medical system. To be more precise, and to avoid the influence of potentially spurious vital signs on determining SIRS criteria, we included abnormal vitals only when having a minimum of 2 consecutive recordings at least 1 h apart.
Although the study comprises a very small number of patients undergoing TAVI via trans-apical approach, yet the present results might only apply to patients undergoing TAVI using the transfemoral approach.
Finally, despite adjusting for multiple covariates, yet our findings might have been influenced by unmeasured confounders.
Regardless of valve type, SIRS is frequently encountered after TAVI. It was associated with more frequent in-hospital events, admissions for HF or ACS, as well as long-term mortality. The notion that SIRS might influence the occurrence of HALT is intriguing but couldn't be established. This warrants further research and would impact treatment plans post-TAVI.
The datasets presented in this article are not readily available because the dataset belongs to Seoul National University Hospital any access to the dataset needs permission from Seoul National University Hospital. Requests to access the datasets should be directed to Hyo-Soo Kim, aHlvc29vQHNudS5hYy5rcg==.
The studies involving human participants were reviewed and approved by Seoul National University Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
TA, Y-JK, HE-N, and JK contributed to conception and design of the study. TA, Y-JK, Y-JC, and HE-N organized the database. TA and Y-JK performed the statistical analysis. TA wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
TA received a fully funded post-doctoral scholarship from the Egyptian Ministry of Higher Education and Assiut University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.746774/full#supplementary-material
1. Patel MR, Mahaffey KW, Armstrong PW, Weaver WD, Tasissa G, Hochman JS, et al. Prognostic usefulness of white blood cell count and temperature in acute myocardial infarction (from the CARDINAL Trial). Am J Cardiol. (2005) 95:614–8. doi: 10.1016/j.amjcard.2004.11.008
2. Théroux P, Armstrong PW, Mahaffey KW, Hochman JS, Malloy KJ, Rollins S, et al. Prognostic significance of blood markers of inflammation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty and effects of pexelizumab, a C5 inhibitor: a substudy of the COMMA trial. Eur Heart J. (2005) 26:1964–70. doi: 10.1093/eurheartj/ehi292
3. Vavuranakis M, Kariori M, Voudris V, Thomopoulou S, Vrachatis D, Aznaouridis K, et al. Impact of inflammatory process on left ventricular recovery after Transcatheter Aortic Valve Implantation. Int J Cardiol. (2013) 168:e118–20. doi: 10.1016/j.ijcard.2013.08.040
4. Backes JM, Howard PA, Moriarty PM. Role of C-Reactive protein in cardiovascular disease. Ann Pharmacother. (2004) 38:110–8. doi: 10.1345/aph.1D203
5. Jilaihawi H, Asch FM, Manasse E, Ruiz CE, Jelnin V, Kashif M, et al. Systematic CT methodology for the evaluation of subclinical leaflet thrombosis. JACC Cardiovasc Imaging. (2017) 10:461–70. doi: 10.1016/j.jcmg.2017.02.005
6. Karády J, Apor A, Nagy AI, Kolossváry M, Bartykowszki A, Szilveszter B, et al. Quantification of hypo-attenuated leaflet thickening after transcatheter aortic valve implantation: clinical relevance of hypo-attenuated leaflet thickening volume. Eur Heart J Cardiovasc Imaging. (2020) 21:1395–404. doi: 10.1093/ehjci/jeaa184
7. Sinning JM, Scheer AC, Adenauer V, Ghanem A, Hammerstingl C, Schueler R, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. (2012) 33:1459–68. doi: 10.1093/eurheartj/ehs002
8. Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: Pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. (1997) 85:766–82. doi: 10.1213/00000539-199710000-00011
9. Franke A, Lante W, Fackeldey V, Becker HP, Kurig E, Zöller LG, et al. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: is what we see what we know? Eur J Cardiothorac Surg. (2005) 28:569–75. doi: 10.1016/j.ejcts.2005.07.007
10. Fransen E, Maessen J, Dentener M, Senden N, Geskes G, Buurman W. Systemic inflammation present in patients undergoing CABG without extracorporeal circulation. Chest. (1998) 113:1290–5. doi: 10.1378/chest.113.5.1290
11. Yu CW, Kim WJ, Ahn JM, Kook H, Kang SH, Han JK, et al. Trends and outcomes of transcatheter aortic valve implantation (TAVI) in Korea: the results of the first cohort of Korean TAVI registry. Korean Circ J. (2018) 48:382–94. doi: 10.4070/kcj.2018.0117
12. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. In Chest, 1644–55. doi: 10.1378/chest.101.6.1644
13. Rangel Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the Systemic Inflammatory Response Syndrome (SIRS): a prospective study. JAMA. (1995) 273:117–23. doi: 10.1001/jama.1995.03520260039030
14. Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research Consortium-2 consensus document. Eur Heart J. (2012) 33:2403–18. doi: 10.1093/eurheartj/ehs255
15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–71. doi: 10.1093/ehjci/jev014
16. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. (2015) 373:2015–24. doi: 10.1056/NEJMoa1509233
17. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): an expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc Imaging. (2019) 12:1–24. doi: 10.1016/j.jcmg.2018.12.003
18. Schwietz T, Behjati S, Gafoor S, Seeger F, Doss M, Sievert H, et al. Occurrence and prognostic impact of systemic inflammatory response syndrome in transfemoral and transapical aortic valve implantation with balloon- and self-expandable valves. EuroIntervention. (2015) 10:1468–73. doi: 10.4244/EIJY14M06_05
19. Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart. (2015) 101:537–45. doi: 10.1136/heartjnl-2014-307057
20. Asimakopoulos G. Mechanisms of the systemic inflammatory response. Perfusion. (1999) 14:269–77. doi: 10.1177/026765919901400406
21. Kohsaka S, Menon V, Lowe AM, Lange M, Dzavik V, Sleeper LA, et al. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med. (2005) 165:1643–50. doi: 10.1001/archinte.165.14.1643
22. Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. (2003) 107:2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2
23. Stühle S, Wendt D, Hou G, Wendt H, Schlamann M, Thielmann M, et al. In-vitro investigation of the hemodynamics of the Edwards SapienTM transcatheter heart valve. J Heart Valve Dis. (2011) 20:53–63.
24. Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. (2009) 104:e9–18. doi: 10.1161/CIRCRESAHA.108.188243
25. Pache G, Schoechlin S, Blanke P, Dorfs S, Jander N, Arepalli CD, et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J. (2016) 37:2263–71. doi: 10.1093/eurheartj/ehv526
26. Yanagisawa R, Hayashida K, Yamada Y, Tanaka M, Yashima F, Inohara T, et al. Incidence, predictors, and mid-term outcomes of possible leaflet thrombosis after TAVR. JACC Cardiovasc Imaging. (2017) 10:1–11. doi: 10.1016/j.jcmg.2016.11.005
27. Hansson NC, Grove EL, Andersen HR, Leipsic J, Mathiassen ON, Jensen JM, et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol. (2016) 68:2059–69. doi: 10.1016/j.jacc.2016.08.010
28. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. (2017) 389:2383–92. doi: 10.1016/S0140-6736(17)30757-2
29. Rodriguez-Gabella T, Voisine P, Puri R, Pibarot P, Rodés-Cabau J. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J Am Coll Cardiol. (2017) 70:1013–28. doi: 10.1016/j.jacc.2017.07.715
30. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
31. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
32. Simmons EM, Himmelfarb J, Tugrul Sezer M, Chertow GM, Mehta RL, Paganini EP, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. (2004) 65:1357–65. doi: 10.1111/j.1523-1755.2004.00512.x
33. Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, et al. Serum Interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. (2009) 13:R104. doi: 10.1186/cc7940
34. Izbicki G, Rudensky B, Na'amad M, Hershko C, Huerta M, Hersch M. Transfusion-related leukocytosis in critically ill patients. Crit Care Med. (2004) 32:439–42. doi: 10.1097/01.CCM.0000104951.94820.A9
35. Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. (2007) 116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977
36. Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res. (2019) 124:437–50. doi: 10.1161/CIRCRESAHA.118.313129
37. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. (2019) 380:752–62. doi: 10.1056/NEJMoa1809798
38. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. (2019) 139:1289–99. doi: 10.1161/CIRCULATIONAHA.118.038010
Keywords: systemic inflammatory response syndrome, hypoattenuation leaflet thickening, transcatheter aortic valve implantation, subclinical leaflet thrombosis, inflammatory markers
Citation: Ahmed TAN, Ki Y-J, Choi Y-J, El-Naggar HM, Kang J, Han J-K, Yang H-M, Park KW, Kang H-J, Koo B-K and Kim H-S (2022) Impact of Systemic Inflammatory Response Syndrome on Clinical, Echocardiographic, and Computed Tomographic Outcomes Among Patients Undergoing Transcatheter Aortic Valve Implantation. Front. Cardiovasc. Med. 8:746774. doi: 10.3389/fcvm.2021.746774
Received: 24 July 2021; Accepted: 18 November 2021;
Published: 09 February 2022.
Edited by:
Micha Tobias Maeder, Kantonsspital St. Gallen, SwitzerlandReviewed by:
Henrique Barbosa Ribeiro, Universidade de São Paulo, BrazilCopyright © 2022 Ahmed, Ki, Choi, El-Naggar, Kang, Han, Yang, Park, Kang, Koo and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tarek A. N. Ahmed, dGFyZWsuYS5uLmFobWVkQG1lZC5hdW4uZWR1LmVn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.