- 1Division of Cardiology, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 2Institute of Epidemiology and Preventive Medicine College of Public Health, National Taiwan University, Taipei, Taiwan
- 3Cardiovascular Research Center, Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan
- 4Division of Cardiovascular Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
Background: Valve replacement is associated with worse outcomes in individuals who have end-stage renal disease (ESRD) and require a long-term renal replacement therapy. Prosthetic valve selection in patients with ESRD has remained controversial.
Objective: We aimed to investigate long-term outcomes of mechanical and bioprosthetic valve replacement in individuals with ESRD.
Methods: We conducted a population-based retrospective cohort study using data obtained from the Taiwan National Health Insurance Research Database. In total, 10,202 patients, including 912 ESRD and 9,290 non-ESRD patients, were selected after a 1:1 propensity-score matching based on the type of prosthetic valve used. The long-term mortality outcomes were then analyzed.
Results: During a median follow-up period of 59.6 months, the Kaplan–Meier survival analysis revealed that ESRD patients who underwent mechanical valve replacement had higher rates of all-cause mortality and CV deaths than those who underwent bioprosthetic valve replacement (Log-rank test, p = 0.03 and 0.02, respectively). Multivariable regression analyses demonstrated that ESRD patients who underwent bioprosthetic valve replacement had lower rates of all-cause mortality (p < 0.001, hazard ratio: 0.88, 95% confidence interval: 0.82–0.93) and cardiovascular (CV) death (p < 0.001, hazard ratio: 0.83, 95% confidence interval: 0.76–0.90) than those who had mechanical valve replacement.
Conclusion: Bioprosthetic valve replacement is significantly associated with lower rates of all-cause mortality and CV death in the ESRD population.
Introduction
Choosing a prosthetic heart valve can be clinically challenging, and it is commonly based on several factors, such as age, underlying disease requiring the use of anticoagulants, risk of bleeding and thromboembolism, durability of the prosthesis, patients' preferences, and risk of structural deterioration requiring re-interventions (1, 2). Of note, the type of valve prosthesis that should be used in a specific population with comorbidity, including end-stage renal disease (ESRD), has been debated for decades (3–7).
It has long been established that the abnormal calcium and phosphate metabolism due to ESRD is related to calcification and degenerative valvular lesions, which may be explained by an active regulated process associated with an osteoblast-like phenotype (8–11). It results in a major concern regarding structural destruction of the bioprosthetic valves in ESRD. Thus, mechanical valves were previously recommended for ESRD patients (12).
In contrast, patients with ESRD receiving anticoagulants are at higher risk of bleeding, (13, 14) metastatic calcification, and even calciphylaxis (15, 16). In addition, ESRD patients have a short life expectancy. As a result, the increased durability of a mechanical valve may only benefit a small portion of ESRD patients (17–22).
The number of studies investigating the clinical outcome between dialysis patients who had bioprosthetic and mechanical valve replacement is increasing worldwide (23–25). However, owing to the limited sample size, non-uniform characteristics, and emerging advancements in prosthesis and clinical care of dialysis patients, previous studies had conflicting results, and some have shown a similar survival between dialysis patients who had mechanical and bioprosthetic valve replacement (26–30). Thus, the abovementioned findings were not validated in a large-scale nationwide population study. Furthermore, no specific recommendation regarding the selection of prosthetic valves for patients with ESRD was provided in the contemporary guideline (31).
This nationwide population-based study aimed to assess long-term outcomes and associated cardiovascular (CV) events in ESRD patients who underwent bioprosthetic and mechanical valve replacement. We believe that the abovementioned findings could provide insight on decision-making regarding the selection of prosthetic valves among ESRD patients.
Materials and Methods
Study Design and Participants
We conducted a population-based retrospective cohort study, and data were collected from January 1, 2000 to December 31, 2011. The patients who underwent the first valve replacement surgery, without previous or concomitant valve repair, were identified using information from the National Health Insurance Research Database (NHIRD), and they were grouped based on the procedure code of the Specifications of the National Voluntary Consensus Standards for Cardiac Surgery: bioprosthetic valve replacement (procedure code: 35.21, 35.23, 35.25, and 35.27) and mechanical valve replacement (procedure code: 35.22, 35.24, 35.26, and 35.28).
This study was approved in accordance with the Good Clinical Practice Guidelines by the Research Ethics Committee C of the National Taiwan University Hospital.
Databases and Specifications of the Characteristics of the Participants
The Taiwan Collaboration Center of Health Information Application, Ministry of Health and Welfare, provided all the datasets of the NHIRD. The Taiwan's National Health Insurance (NHI) program enrolled 23 million people, which covered 99% of the country's population and included utilization of all NHI resources, including outpatient visits, hospital care, prescribed medications, and the National Death Registry. We obtained permission for the rights from the National Research Institute for the Department of Health and the Health Promotion Administration, Ministry of Health and Welfare. The underlying diseases were identified according to the International Classification of Diseases, 9th Revision—Clinical Modification (ICD 9-CM) codes. The diagnosis must be recorded twice in the outpatient records or at least once in the inpatient records. By linking to the NHIRD, we identified clinical variables, such as age (years), sex, type of valve replacement, number of valve replacements, and presence of chronic kidney disease, congestive heart failure, acute coronary diseases, chronic obstructive pulmonary disease, and thyroid diseases. The selection and grouping of medications were based on the guidelines of the Anatomical Therapeutic Chemical classification system by the World Health Organization.
Patients diagnosed with ESRD were identified using the order codes (Supplementary Material) of hemodialysis, peritoneal dialysis, and other types of dialysis for at least 3 months (32). In this study, we excluded individuals who were younger than 20 years, underwent both bioprosthetic and mechanical valve replacement, or presented with lethal ventricular arrhythmias before the procedures. In addition, no patient underwent kidney transplantation prior to enrollment.
Study Endpoints During the Long-Term Follow-Up
The primary endpoints were all-cause mortality and CV death (ICD 9-CM codes 390–429) during follow-up. Death was confirmed using data from the Taiwan's National Death Registry. Follow-up was terminated in case of death or if the patients lived beyond December 31, 2016.
Statistical Analysis
The normally distributed continuous variables are presented as mean values ± standard deviation, and non-normally distributed continuous variables are presented as medians with 25 and 75% interquartile ranges (IQRs). Student's T-test was utilized to compare two groups. For testing the distribution of general continuous variable such as age, the normality test was performed before the Student's T-test. Categorical variables were expressed as numbers and percentages and were compared using the chi-square test. The incidence rates of CV events were calculated as the number of cases per 1,000 person-years during follow-up. Propensity-score matching for patients receiving the mechanical valve replacement and the bioprosthetic valve replacement as exposures were performed to minimize the impact of the confounding factors on the clinical outcomes, including age, sex, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, stroke, and total number of valves replaced. A one-to-one matching of pairs was conducted using identical propensity scores with a 0.15 caliper width.
The event-free survival curve was plotted using the Kaplan–Meier method with the statistical significance examined using the Log-rank test. The conditional Cox proportional-hazards regression model was utilized to compare the hazard ratios (HRs), with 95% confidence intervals (CIs), of the outcomes. The potential confounders were adjusted using three models (Model 1: age and sex; Model 2: Model 1 plus total number of valves replaced, hypertension, diabetes mellitus, congestive heart failure, coronary artery diseases, and chronic obstructive pulmonary disease; and Model 3: Model 2 plus the use of medications (antiarrhythmic agents of Ia Ib, Ic, III, calcium channel blockers, angiotensin receptor blockers, statins, insulin, and oral hypoglycemic agents). The level of statistical significance was set at a two-tailed alpha level <0.05. The analyses were performed with SAS software (version 9.4, SAS Institute, Cary, NC).
Results
Selection and Characteristics of the Study Population
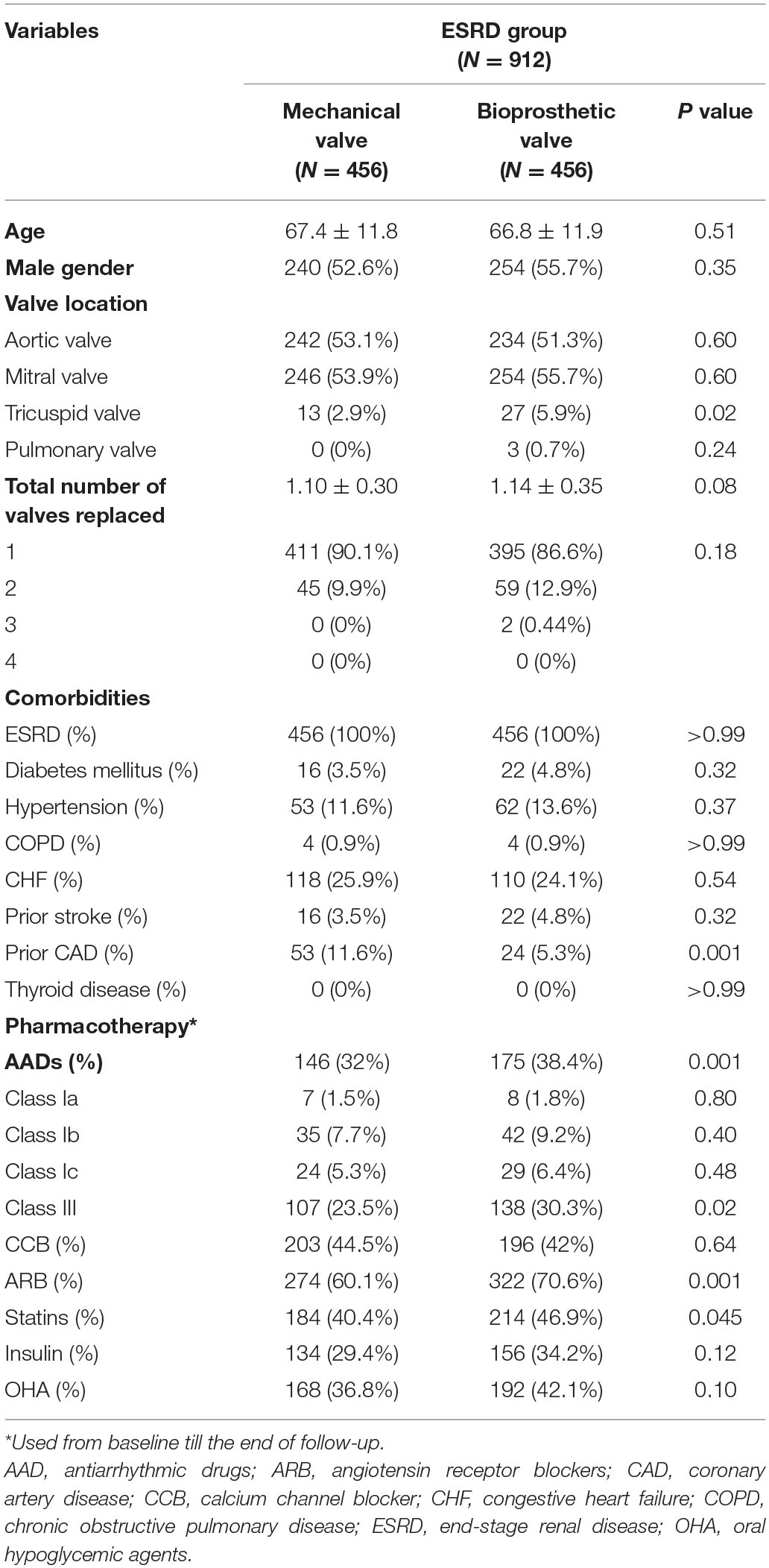
In total, 19,528 patients who had their first valve replacement were identified in the NHIRD. After excluding 883 patients according to the exclusion criteria, 18,645 were included in the original cohort (Supplementary Table 1), and 10,202 patients were selected after propensity-score matching (PSM) (Supplementary Table 2). After PSM, 9,290 and 912 patients were included in the non-ESRD group and ESRD group, respectively. Both groups had equal number of patients who had mechanical and bioprosthetic valve replacement (Figure 1, Table 1, Supplementary Table 3).
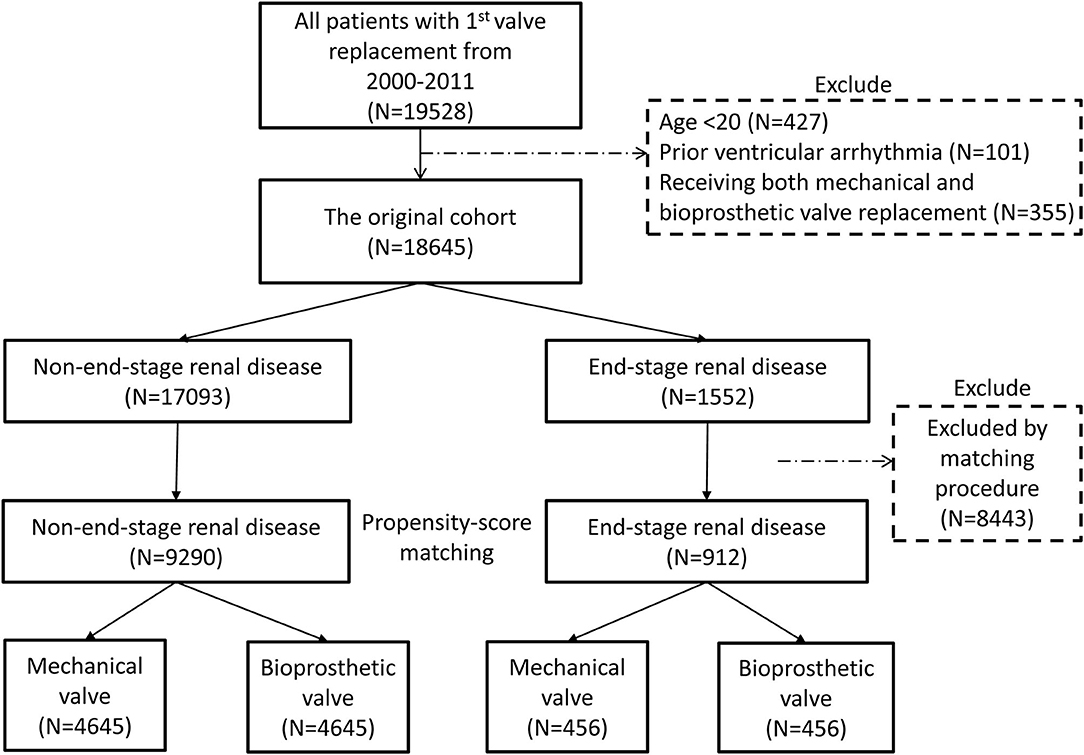
Figure 1. Flow chart of the present study. The process of study population selection and propensity score matching is presented. After PSM, 9,290 and 912 patients were included in the non-ESRD group and ESRD group, respectively. Both groups had equal number of patients who had mechanical and bioprosthetic valve replacement. ESRD, end-stage renal disease; PSM, propensity score matching.
After PSM, a higher number of patients in the ESRD group underwent bioprosthetic valve replacement for the tricuspid valve (5.9 vs. 2.9%, p = 0.02; Table 1). In the ESRD group, the baseline characteristics were comparable between patients who had mechanical and bioprosthetic valve replacement, except that a high number of patients with mechanical valve replacement had a history of coronary artery disease (11.6 vs. 5.3%, p < 0.001). This could be a potential confounder and was further adjusted by the three models in the conditional Cox regression analysis.
Mortality and CV Events
Crude Incidence Rate
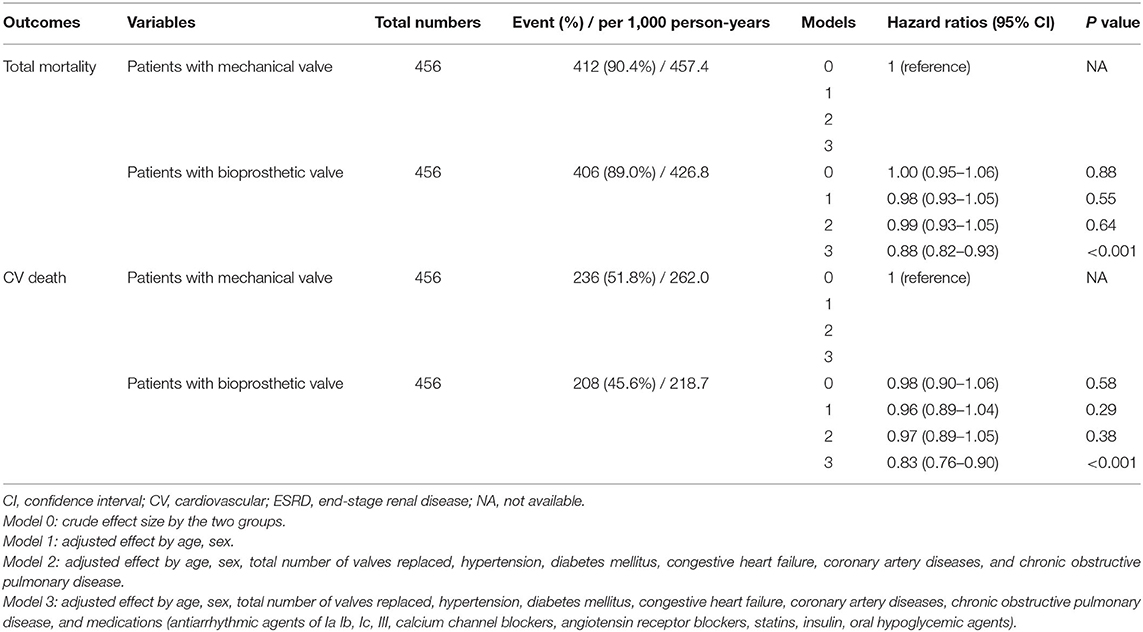
The median follow-up period was 59.6 months (25–75%, IQR: 22.8–108.9) after PSM. In patients without ESRD, the crude incidence rates of all-cause mortality were 80.2 and 80.3/1,000-person-years in patients who had mechanical and bioprosthetic valve replacement, respectively, and the crude incidence values of CV deaths were 43.4 and 41.4/1,000 person-years in patients who had mechanical and bioprosthetic valve replacement, respectively (Supplementary Table 4). In contrast, ESRD patients who underwent mechanical valve replacement had a higher rate of all-cause mortality (457.4/1,000 vs. 426.8/1,000 person-years) and CV death (262.0/1,000 vs. 218.7/1,000 person-years) than those who had bioprosthetic valve replacement (Table 2).
Kaplan–Meier Survival Analysis
The Kaplan–Meier survival analysis revealed that the all-cause mortality and CV deaths were comparable between non-ESRD patients who underwent mechanical and bioprosthetic valve replacement during the 5-year follow-up (Log-rank test, p = 0.88 and 0.58, respectively; Supplementary Figures 1A,B). Meanwhile, ESRD patients who underwent mechanical valve replacement had higher rates of all-cause mortality and CV deaths than those who underwent bioprosthetic valve replacement after the 5-year follow-up (Log-rank test, p = 0.03 and 0.02, respectively; Figures 2A,B).
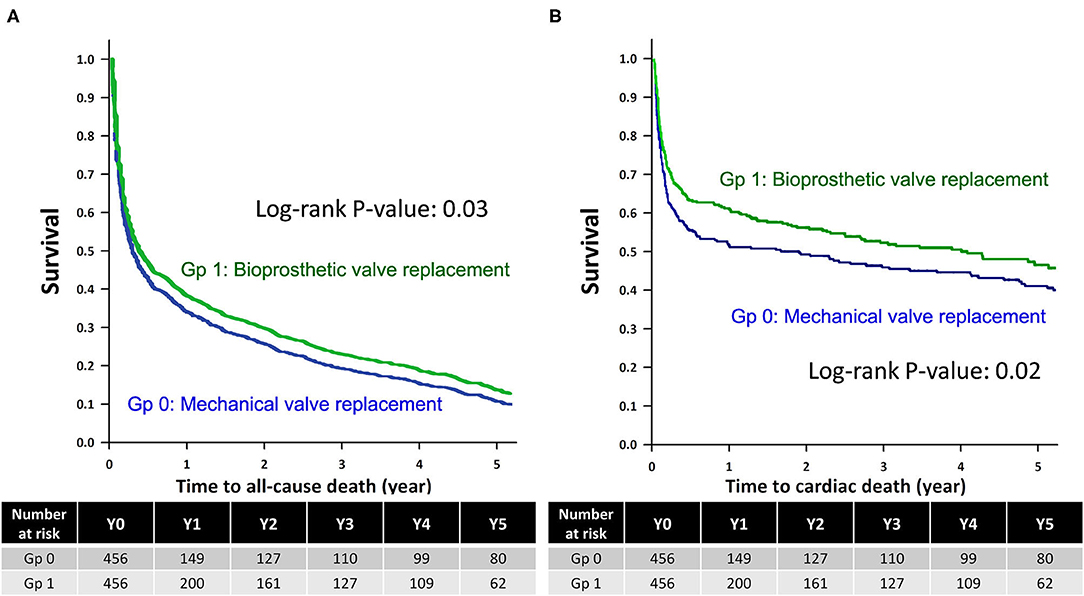
Figure 2. Kaplan–Meier survival analysis of the ESRD group. Kaplan-Meier survival analysis for (A) all-cause mortality and (B) cardiovascular deaths among ESRD patients who underwent mechanical valve replacement (Gp 0) and bioprosthetic valve replacement (Gp 1), with the statistical significance examined using the Log-rank test. ESRD, end-stage renal disease; Gp, group.
Multivariable Regression Analysis
After adjusting for the effects of age, sex, total number of valves replaced, underlying disease, and use of medications via a multivariable regression analysis, results showed a comparable future risk of all-cause mortality in non-ESRD patients who had mechanical and bioprosthetic valve replacement (p = 0.12, HR: 0.89, 95% CI: 0.77–1.03; Supplementary Table 4). However, non-ESRD patients who had bioprosthetic valve replacement had a significant decrease in the rate of CV deaths (p = 0.04, HR: 0.82, 95% CI: 0.67–0.99; Supplementary Table 4). In contrast, in ESRD patients, bioprosthetic valve replacement was significantly associated with a lower rate of all-cause mortality and CV deaths after adjusting for the confounding variables during the 5-year follow-up (p < 0.001, HR: 0.88, 95% CI: 0.82–0.93 and p < 0.001, HR: 0.83, 95% CI: 0.76–0.90, respectively; Table 2).
Short-Term Mortality and the Impact of Total Number of Valves Replaced
Regarding the perioperative and short-term mortality, the mortality rate occurring within 30 days after valve replacement was 19.2%, while the 1-year mortality rate was 63.6% in the present study.
In addition, the increasing total number of valves replaced was significantly associated with a higher rate of all-cause mortality and CV deaths both before and after adjusting for the confounding variables (in all models 0–3) during the 5-year follow-up in patients with ESRD (Supplementary Table 5).
Discussion
Major Findings
The current nationwide population-based study revealed the long-term outcome of ESRD and non-ESRD patients who underwent mechanical and bioprosthetic valve replacement. Firstly, despite the use of different types of valves for replacement, patients with ESRD before valvular surgery had a significantly worse outcome. Secondly, ESRD patients who had bioprosthetic valve replacement had a better long-term outcome in terms of all-cause mortality and CV deaths than those who underwent mechanical valve replacement.
Outcome of Valve Replacement in ESRD Patients
Based on the 2019 Annual Report on Kidney Disease in Taiwan, the overall survival at 1, 3, 5, and 10 years of ESRD patients in Taiwan are 88.7, 69.2, 54.3, and 29.8%, respectively. The present study demonstrated remarkably poorer survival outcomes in ESRD patients with surgical valve replacement. Previous studies have shown that ESRD patients who underwent valve replacement have poor long-term outcomes (17–21). Based on a pooled analysis of eight studies, Altarabsheh et al. (17) have revealed that the overall survival at 2, 4, and 6 years among ESRD patients who had valve replacement was 58, 38, and 29%, respectively (median survival = 2.61 years) (17). Williams et al. (18) have found that only about half of dialysis patients younger than 65 years survived beyond 2 years after valve replacement (18). Moreover, Böning et al. have reported that the median survival time of ESRD patients after aortic valve replacement is 24.7 months (20). All the aforementioned findings were consistent with our results which demonstrated that ESRD patients had a worse prognosis than non-ESRD patients after bioprosthetic and mechanical valve replacement. Nevertheless, the perioperative and short-term mortality rate was higher than most of the previous reports. Firstly, the possible explanation is the remarkably older age of both groups in the present study (mean age 67.4 for the mechanical valve and 66.8 for the bioprosthetic valve group). In addition, unlike previous trials which were mainly based on evidence from single tertiary or referral centers, this nationwide population-based study might have more scientific rigor and external validity to reflect the real-world prognosis of the general population and clinical practice.
The cause of the poor prognosis among ESRD patients who had valve replacement are complex and multifactorial. Firstly, the disturbance in mineral metabolism and increased calcium load due to calcium-based phosphorus binders in the ESRD population can result in vascular calcification, which plays a key role in CV death (33). Calcium and phosphorus dysregulation may accelerate the calcification and structural destruction of valve bioprosthesis, which is mediated through a process of osteoblast-like differentiation on these structures (8–11). Moreover, several studies implicate that the host immune response is involved in a major pathogenesis of structural valve degeneration (34, 35). The persistent low-grade inflammation in patients with ESRD, which is associated with increased production and inadequate removal of pro-inflammatory cytokines, may accelerate the degeneration of bioprosthetic valves (36). On the other hand, a higher bleeding and thromboembolic risk has been observed in ESRD patients, (13, 14) particularly in those requiring anticoagulation therapy after mechanical valve replacement. Considering the abovementioned findings, the selection of valve for ESRD patients can be clinically challenging.
Long-Term Outcome of Mechanical and Bioprosthetic Valve Replacement Among ESRD Patients
To the best of our knowledge, this is the first large-scale study that compared the outcome of mechanical and bioprosthetic valve replacement in ESRD patients using data from a nationwide population-based database. Of note, in ESRD patients in this study, bioprosthetic valve replacement was found to be significantly associated with a lower rate of all-cause mortality and CV deaths compared to replacement with mechanical valves. However, this result was not in accordance with that of previous studies showing that ESRD patients who had mechanical vs. bioprosthetic valve replacement had a similar survival time (26–30). The heterogeneous results could be explained by the differences in sample size, follow-up duration, use of medications, presence of comorbidities, advances in the development of prosthetic devices, and improvement in clinical care for dialysis patients between this and the other studies. Notably, several drugs showed a greater distribution in the bioprosthetic valve group, including class III antiarrhythmic drugs, angiotensin receptor blockers, and statins. It suggests the higher prevalence of concealed comorbidities in ESRD patients. It also explains why the benefit of bioprosthetic valves in the ESRD patients could finally be revealed after all the probable bias was minimized in Model 3.
Clinically, the selection of different valves for replacement is based on several factors, and there is no randomized study that compared the long-term outcomes of ESRD patients who underwent different types of valve replacement. Conventionally, ESRD patients were thought to experience early structural deterioration of the bioprosthetic valve due to disturbance in calcium homeostasis (8, 9). However, some studies have reported that this phenomenon is relatively rare due to the limited life expectancy of this population (4, 25). In contrast, some studies have revealed that ESRD patients with mechanical valves more commonly present with bleeding or thromboembolic events than structural deterioration of bioprosthetic valves (17, 24, 27). Patients with ESRD receiving mechanical valve replacement require warfarin for stroke prevention, which is usually associated with a higher risk of bleeding event, metastatic calcification, and catastrophic calciphylaxis (15, 16). The aforementioned complications could increase the periprocedural mortality, as shown in the present findings. However, future prospective studies must be conducted to validate the findings of the current study and to identify ESRD patients who are eligible for bioprosthetic valve replacement.
Limitations
This study had some limitations. Firstly, this study is retrospective in nature, which might have caused inevitable bias. Given the entity of nationwide population-based study, additional information such as laboratory data, post-procedural complication and bleeding rate, causes of non-CV death, or causes of ESRD, cannot be retrieved from National Health Insurance Research Database. In addition, some detailed information was not available in the present study, including the presence of AF, concomitant CABG in valve replacement, specific cause of short-term mortality, or the survival data of ESRD patients without any valve replacement. However, our study can provide insight about clinical decision-making and can be used as a basis in further meta-analysis. Secondly, the baseline characteristics between the mechanical and bioprosthetic valve groups after PSM with potential covariates remained inconsistent. However, these potential confounders were all further adjusted by the three models in the conditional Cox regression analysis. We believe that the probable bias was minimized and assume that it's the reason why the benefit of bioprosthetic valves in the ESRD patients could finally be revealed. Third, diagnostic and procedure coding errors might exist. Nonetheless, the rate of coding error was supposed to be low because all data were double-checked by a professional coding team in each hospital before submission to the NHIRD. Fourth, this is a population-based study enrolling only people in Taiwan. It remains uncertain whether the findings are universal across various racial and ethnic groups in the world. In the end, based on the 2019 Annual Report on Kidney Disease in Taiwan, the majority of patients (around 90%) with ESRD received hemodialysis and <10% of ESRD patients undergoing peritoneal dialysis. Given the unbalanced distribution of hemodialysis vs. peritoneal dialysis and limited case numbers of study population, we didn't perform further analysis of the outcome between the above two groups. The further large cohort will be warranted for this investigation.
Conclusion
Preoperative ESRD was associated with a significantly worse outcome in patients who had valve replacement. Patients with ESRD who underwent bioprosthetic valve replacement had significantly better long-term outcomes, including a lower rate of all-cause mortality and CV deaths. However, to shed light on clinical decision-making in this specific population, future prospective cohort studies based on independent databases are warranted to validate the present findings.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of the National Taiwan University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Center for Dynamical Biomarkers and Translational Medicine, Ministry of Science and Technology (Grant Numbers 107-2314-B-010-061-MY2, MOST 106-2314-B-075-006-MY3, MOST 106-2314-B-010-046-MY3, and MOST 106-2314-B-075-073-MY3), Research Foundation of Cardiovascular Medicine, Szu-Yuan Research Foundation of Internal Medicine (Grant Number 107-02-036), and Taipei Veterans General Hospital (Grant Numbers V108C-032, V108C-107, V109C-113, V109D48-001-MY2-1, C17-095, V106C-158, V106C-104, V107B-014, V107C-060, and V107C-054).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.745370/full#supplementary-material
Abbreviations
CI, confidence interval; CV, cardiovascular; ESRD, end-stage renal disease; HR, hazard ratio; IQR, interquartile range; NHIRD, National Health Insurance Research Database; PSM, propensity-score matching.
References
1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2017) 135:e1159–95. doi: 10.1161/CIR.0000000000000503
2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Rev Esp Cardiol. (2018) 71:110. doi: 10.1016/j.rec.2017.12.013
3. Lucke JC, Samy RN, Atkins BZ, Silvestry SC, Douglas JM Jr, Schwab SJ, et al. Results of valve replacement with mechanical and biological prostheses in chronic renal dialysis patients. Ann Thorac Surg. (1997) 64:129–32; discussion 32–3. doi: 10.1016/S0003-497500342-1
4. Kaplon RJ, Cosgrove DM 3rd, Gillinov AM, Lytle BW, Blackstone EH, Smedira NG. Cardiac valve replacement in patients on dialysis: influence of prosthesis on survival. Ann Thorac Surg. (2000) 70:438–41. doi: 10.1016/S0003-497501544-7
5. Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation. (2002) 105:1336–41. doi: 10.1161/hc1102.100075
6. Brinkman WT, Williams WH, Guyton RA, Jones EL, Craver JM. Valve replacement in patients on chronic renal dialysis: implications for valve prosthesis selection. Ann Thorac Surg. (2002) 74:37–42; discussion 42. doi: 10.1016/s0003-4975(02)03692-5
7. Chan V, Jamieson WR, Fleisher AG, Denmark D, Chan F, Germann E. Valve replacement surgery in end-stage renal failure: mechanical prostheses versus bioprostheses. Ann Thorac Surg. (2006) 81:857–62. doi: 10.1016/j.athoracsur.2005.09.009
8. Lamberti JJ, Wainer BH, Fisher KA, Karunaratne HB, Al-Sadir J. Calcific stenosis of the porcine heterograft. Ann Thorac Surg. (1979) 28:28–32. doi: 10.1016/S0003-497563387-5
9. Mao M, Madhavan M, Blauwet L, Schaff HV, Qian Q. Bioprosthetic tricuspid valve stenosis in end-stage renal failure. Am J Med Sci. (2012) 343:252–4. doi: 10.1097/MAJ.0b013e318238e92d
10. Bohbot Y, Candellier A, Diouf M, Rusinaru D, Altes A, Pasquet A, et al. Severe aortic stenosis and chronic kidney disease: outcomes and impact of aortic valve replacement. J Am Heart Assoc. (2020) 9:e017190. doi: 10.1161/JAHA.120.017190
11. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. (2003) 107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69
12. Bonow RO, Carabello B, de Leon AC Jr, Edmunds LH Jr, Fedderly BJ, Freed MD, et al. Guidelines for the management of patients with valvular heart disease: executive summary a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on management of patients with valvular heart disease). Circulation. (1998) 98:1949–84. doi: 10.1161/01.CIR.98.18.1949
13. Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. (2007) 50:433–40. doi: 10.1053/j.ajkd.2007.06.017
14. Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. (2008) 3:105–10. doi: 10.2215/CJN.01810407
15. Yu WY, Bhutani T, Kornik R, Pincus LB, Mauro T, Rosenblum MD, et al. Warfarin-associated nonuremic calciphylaxis. JAMA Dermatol. (2017) 153:309–14. doi: 10.1001/jamadermatol.2016.4821
16. Lehman JS, Chen TY, Lohse CM, El-Azhary RA. Evaluating the validity of subclassifying warfarin-associated nonuremic calciphylaxis: a retrospective cohort study. Int J Dermatol. (2018) 57:572–4. doi: 10.1111/ijd.13884
17. Altarabsheh SE, Deo SV, Dunlay SM, Obeidat YM, Erwin PJ, Rababa'h A, et al. Tissue valves are preferable for patients with end-stage renal disease: an aggregate meta-analysis. J Card Surg. (2016) 31:507–14. doi: 10.1111/jocs.12805
18. Williams ML, Bavaria JE, Acker MA, Desai ND, Vallabhajosyula P, Hargrove WC, et al. Valve selection in end-stage renal disease: should it always be biological? Ann Thorac Surg. (2016) 102:1531–5. doi: 10.1016/j.athoracsur.2016.04.092
19. Okada N, Tajima K, Takami Y, Kato W, Fujii K, Hibino M, et al. Valve selection for the aortic position in dialysis patients. Ann Thorac Surg. (2015) 99:1524–31. doi: 10.1016/j.athoracsur.2014.11.055
20. Böning A, Boedeker RH, Rosendahl UP, Niemann B, Haberer S, Roth P, et al. Long-term results of mechanical and biological heart valves in dialysis and non-dialysis patients. Thorac Cardiovasc Surg. (2011) 59:454–9. doi: 10.1055/s-0030-1271028
21. D'Alessandro DA, Skripochnik E, Neragi-Miandoab S. The significance of prosthesis type on survival following valve replacement in dialysis patients. J Heart Valve Dis. (2013) 22:743–50.
22. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. (2012) 33:2451–96. doi: 10.1093/eurheartj/ehs109
23. Zhibing Q, Xin C, Ming X, Lele L, YingShuo J, LiMing W. Should bioprostheses be considered the valve of choice for dialysis-dependent patients? J Cardiothorac Surg. (2013) 8:42. doi: 10.1186/1749-8090-8-42
24. Umezu K, Saito S, Yamazaki K, Kawai A, Kurosawa H. Cardiac valvular surgery in dialysis patients: comparison of surgical outcome for mechanical versus bioprosthetic valves. Gen Thorac Cardiovasc Surg. (2009) 57:197–202. doi: 10.1007/s11748-008-0365-1
25. Pai VB, Tai CK, Bhakri K, Kolvekar S. Should we use mechanical valves in patients with end-stage renal disease? Interact Cardiovasc Thorac Surg. (2012) 15:240–3. doi: 10.1093/icvts/ivs115
26. Takemura H. Selection of artificial valve for the patients on hemodialysis. Gen Thorac Cardiovasc Surg. (2013) 61:314–9. doi: 10.1007/s11748-012-0173-5
27. Bianchi G, Solinas M, Bevilacqua S, Glauber M. Are bioprostheses associated with better outcome than mechanical valves in patients with chronic kidney disease requiring dialysis who undergo valve surgery? Interact Cardiovasc Thorac Surg. (2012) 15:473–83. doi: 10.1093/icvts/ivs236
28. Chan V, Chen L, Mesana L, Mesana TG, Ruel M. Heart valve prosthesis selection in patients with end-stage renal disease requiring dialysis: a systematic review and meta-analysis. Heart. (2011) 97:2033–7. doi: 10.1136/heartjnl-2011-300727
29. Thourani VH, Sarin EL, Keeling WB, Kilgo PD, Guyton RA, Dara AB, et al. Long-term survival for patients with preoperative renal failure undergoing bioprosthetic or mechanical valve replacement. Ann Thorac Surg. (2011) 91:1127–34. doi: 10.1016/j.athoracsur.2010.12.056
30. Phan K, Zhao DF, Zhou JJ, Karagaratnam A, Phan S, Yan TD. Bioprosthetic versus mechanical prostheses for valve replacement in end-stage renal disease patients: systematic review and meta-analysis. J Thorac Dis. (2016) 8:769–77. doi: 10.21037/jtd.2016.02.74
31. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
32. National Health Research Institutes. Annual Report of Kidney Disease in Taiwan. Taipei: National Health Research Institutes (2016).
33. Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. (2014) 307:F891–900. doi: 10.1152/ajprenal.00163.2014
34. Kostyunin AE, Yuzhalin AE, Rezvova MA, Ovcharenko EA, Glushkova TV, Kutikhin AG. Degeneration of bioprosthetic heart valves: update 2020. J Am Heart Assoc. (2020) 9:e018506. doi: 10.1161/JAHA.120.018506
35. Gong G, Seifter E, Lyman WD, Factor SM, Blau S, Frater RW. Bioprosthetic cardiac valve degeneration: role of inflammatory and immune reactions. J Heart Valve Dis. (1993) 2:684–93.
Keywords: bioprosthetic valve, cardiovascular event, end-stage renal disease, valve replacement, mechanical valve, mortality
Citation: Li G-Y, Chen Y-Y, Chung F-P, Chien K-L, Hsu C-P and Lin Y-J (2021) Long-Term Outcomes of Bioprosthetic or Mechanical Valve Replacement in End-Stage Renal Disease: A Nationwide Population-Based Retrospective Study. Front. Cardiovasc. Med. 8:745370. doi: 10.3389/fcvm.2021.745370
Received: 21 July 2021; Accepted: 29 November 2021;
Published: 17 December 2021.
Edited by:
Peter Zilla, University of Cape Town, South AfricaReviewed by:
Simon Kraler, University of Zurich, SwitzerlandPaul Human, University of Cape Town, South Africa
Copyright © 2021 Li, Chen, Chung, Chien, Hsu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fa-Po Chung, bWFyeHRhaWppQGdtYWlsLmNvbQ==; Chiao-Po Hsu, aHN1Y3BAdmdodHBlLmdvdi50dw==
†These authors have contributed equally to this work and share first authorship
 Guan-Yi Li
Guan-Yi Li Yun-Yu Chen
Yun-Yu Chen Fa-Po Chung
Fa-Po Chung Kuo-Liong Chien
Kuo-Liong Chien Chiao-Po Hsu
Chiao-Po Hsu Yenn-Jiang Lin
Yenn-Jiang Lin