
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 06 January 2022
Sec. Heart Valve Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.743257
This article is part of the Research Topic Developmental and Acquired Mechanisms of Calcific Aortic Valve Disease View all 13 articles
Background: Permanent pacemaker (PPM) implantation is the main complication of transcatheter aortic valve replacement (TAVR). Few studies have evaluated the requirement for PPM implantation due to ECG changes following TAVR in a Chinese population.
Objective: Our study aimed to evaluate the incidence and predictors of PPM implantation in a cohort of Chinese patients with TAVR.
Methods: We retrospectively evaluated 39 consecutive patients with severe native aortic stenosis referred for TAVR with a self-expandable prosthesis, the Venus A valve (Venus MedTech Inc., Hangzhou, China), from 2019 to 2021 at the Heart Center of Affiliated Zhongshan Hospital of Dalian University. Predictors of PPM implantation were identified using logistic regression.
Results: In our study, the incidence of PPM implantation was 20.5%. PPM implantation occurs with higher risk in patients with negative creatinine clearance (CrCl), dyslipidemia, high Society of Thoracic Surgeons (STS) Morbimortality scores, and lead I T wave elevation. TAVR induced several cardiac electrical changes such as increased R wave and T wave changes in lead V5. The main independent predictors of PPM implantation were new-onset left bundle branch block (LBBB) (coef: 3.211, 95% CI: 0.899–7.467, p = 0.004) and lead I T wave elevation (coef: 11.081, 95% CI: 1.632–28.083, p = 0.016).
Conclusion: New-onset LBBB and lead I T wave elevation were the main independent predictors of PPM implantation in patients undergoing TAVR. Clinical indications such as negative CrCl, dyslipidemia, high STS Morbimortality scores, and an increased T wave elevation before TAVR should be treated with caution to decrease the need for subsequent PPM implantation.
Aortic valve stenosis (AVS) has become the most prevalent acquired heart valve disease pathology (1). Transcatheter aortic valve replacement (TAVR) has been proven to be an efficient treatment for patients with severe AVS. Patients who suffer from this disease are at high-to-intermediate surgical risk (2–4). More recently, new randomized trials have broadened the clinical indications for the procedure, with its efficacy in intermediate and low-risk patients also being demonstrated (2–5). There has been a high demand for TAVR since its introduction in China. These figures are expected to exponentially increase due to the increasing age of the population (6).
This is timeous given that the first TAVR procedure has was performed in China in 2010. To date, only 3,500 patients across approximately 100 hospitals have received TAVR. Currently, self-expandable, mechanically expandable, and balloon-expandable aortic valves are clinically used in TAVR procedures in Western countries. Whereas the majority of TAVR cases in China involve self-expandable valves, investigations into the outcome of these valves in patients with AVS are lacking.
With the development of new-generation valves and operating methods, the occurrence of redo heart valve replacement, paravalvular leakage, and blood vessel complications has significantly decreased. Nevertheless, subsequent permanent pacemaker (PPM) implantation on account of complete atrioventricular block (AVB) is one of the most common complications after TAVR as diagnosed by ECG (7).
Therefore, the purpose of our study was to compare the type and frequency of ECG changes before and after TAVR and at one-month follow-up after discharge. We investigated these ECG changes to determine the main predictors of conduction disorders that lead to PPM implantation after TAVR in Chinese patients with self-expandable valves.
We retrospectively evaluated 47 consecutive patients with severe native aortic stenosis referred for TAVR with the self-expandable Venus A valve (Venus Med Tech Inc., Hangzhou, China) from 2019 to 2021 at the Heart Center of Affiliated Zhongshan Hospital of Dalian University. The exclusion criteria included patients with PPM prior to TAVR (n = 2), intraoperative mortality (n = 1), valve-in-valve procedures (n = 3), and patients where 12-lead ECGs were unavailable (n = 2). Considering this, only 39 patients were included in the study. Surgical indications of patients were assessed and decided upon by a multidisciplinary team of doctors consisting of cardiac surgeons, cardiologists, echocardiologists, and anesthetists. A self-expandable aortic valve was deployed using the transfemoral approach in all patients. The study was approved by the ethics committee of Affiliated Zhongshan Hospital of Dalian University. The study also complied with the Declaration of Helsinki. All patients who participated in the study signed written informed consent forms.
All patients underwent immediate standardized (10 mm = 1 mV, 25 mm/s) 12-lead ECG before and one month post-operation with subsequent retrospective analysis of the data. Parameters included heart rate, rhythm, axis deviation, PR interval, type of AVB, QRS interval, type of bundle-branch block, QT interval, and corrected QT interval (cQT).
Categorical variables are expressed as numbers and percentages. All continuous data were expressed as means ± standard deviation (SD) or medians ± range as appropriate. Categorical variables were compared using Chi-squared or Fisher exact tests. Continuous variables were compared with the Student's t-test (2-tailed) or Wilcoxon signed-rank tests as appropriate. To identify predictors of patients with PPM dependency after TAVR, logistic regression, with Firth's correction due to the small sample size, was performed. All analyses were conducted using R (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria) or SAS Statistics (version 9.4; North Carolina, America). A p-value < 0.05 was considered statistically significant.
The detailed clinical baseline characteristics of patients in total and with or without PPM implantation are shown in Table 1. The mean age of the 39 consecutive patients was 75.0 ± 8.6 years, and 53.8% of the study population was male. Nearly 70% of the patients with severe AVS were at intermediate or high surgical risk with New York Heart Association (NYHA) classification III/IV. The average EuroScore II and Society of Thoracic Surgeons (STS) Mortality scores were 7.97 ± 8.53 and 3.18 ± 2.81, respectively. No statistical differences were observed between the two groups with the exception of higher dyslipidemia and worse creatinine clearance (CrCl) in the group with PPM implantation. The STS Morbimortality scores at baseline was much higher among the patients with PPM implantation than among those without PPM implantation (with PPM 20.93 ± 8.72 vs. without PPM 13.96 ± 7.20, p = 0.025). Complete AVB (62.5%) was the main indication for patients with PPM implantation after TAVR. In our study, the incidence of PPM implantation was 20.5%. Among patients with PPM implantation within 30 days of undergoing the TAVR procedure, the mean time to PPM implantation after TAVR was 5.8 ± 2.8 days.
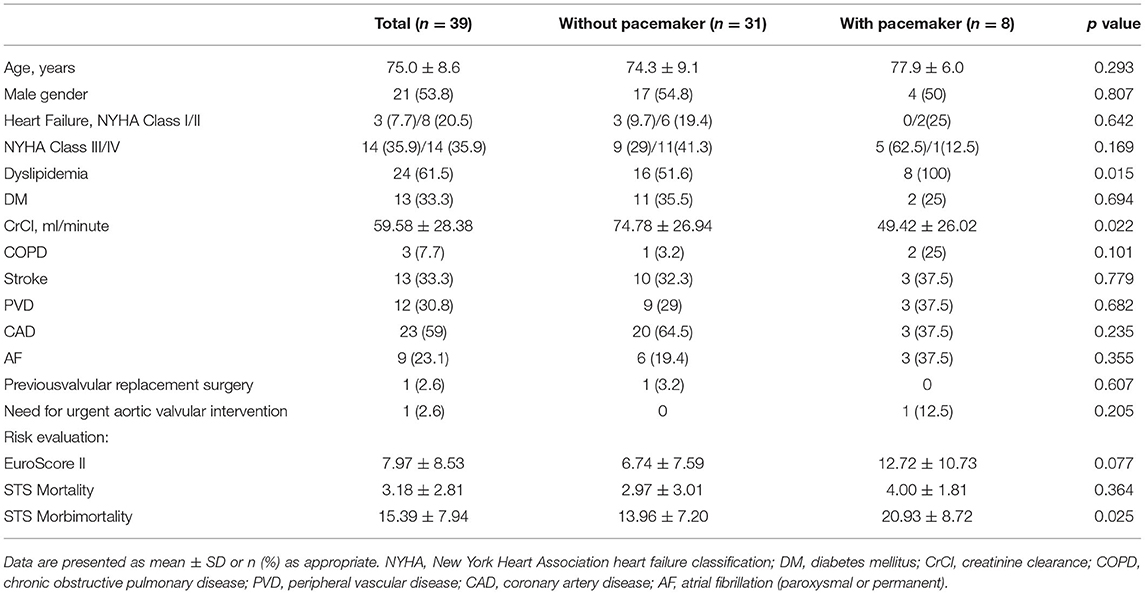
Table 1. Baseline characteristics according to the group of study population: Total, without permanent pacemaker (PPM) and with PPM.
As shown in Table 2, evaluation of electrocardiographic parameters and echocardiographic characteristics before TAVR were analyzed. At baseline, there were no differences between the two groups based on mean aortic gradient, aortic valvular area, and valvular aortic area indexed to body surface area. Also, left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension (LVEDD), aortic root diameter (AO), left ventricular posterior wall thickness (PWT), and interventricular septum thickness (IVST) were comparable between the two groups. There was a larger amplitude of the T wave on lead I before TAVR in the patients with PPM implantation than in the patients without PPM implantation (with PPM 0.10 ± 0.17 mv vs. without PPM −0.07 ± 0.19 mv, p = 0.028). No other significant statistical differences were found between the two groups at baseline.
Table 3 shows the pattern of electrocardiographic changes in patients before and after TAVR. Our study did not identify any significant differences in the incidence of electrocardiographic conduction disturbances, such as new-onset atrial fibrillation (AF), new-onset 1st degree AV block, new-onset right bundle branch block (RBBB), or new-onset left anterior fascicular block (LAFB), with the exception of new-onset left bundle branch block (LBBB) (without PPM 22.6% vs. with PPM 75%, p < 0.001), as shown in Table 3.
Figure 1 summarizes the results involving predictors for patients having PPM implantation after TAVR. The main independent predictors of PPM implantation were new-onset LBBB (coef: 3.211, 95% CI: 0.899–7.467, p = 0.004) and T wave magnitude in lead I (coef: 11.081, 95% CI: 1.632–28.083, p = 0.016).
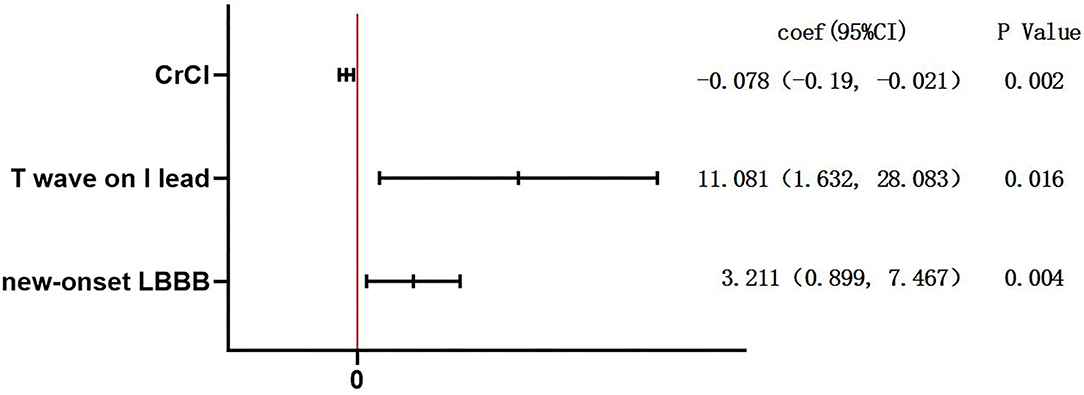
Figure 1. Multiple logistic regression analysis for the predictors of permanent pacemaker (PPM) implantation. To identify predictors of patients with PPM dependency after transcatheter aortic valve replacement (TAVR), logistic regression, with Firth's correction due to the small sample size, was performed. The variables to the right of the red line were predictors of PPM implantation. CrCl, creatinine clearance; LBBB, left bundle branch block.
In the present study of 39 patients with self-expandable valves, we analyzed clinical data, echocardiographic characteristics, and electrocardiographic parameters. Four main findings of our study demonstrated that (1) the total rate of PPM implantation in our consecutive patients was 20.5%; (2) PPM implantation occurs with higher risk in patients with negative CrCl, dyslipidemia, high STS Morbimortality scores, and increased T wave magnitude in lead I; (3) TAVR induced several cardiac electrical changes, such as increased R wave and T wave changes in lead V5; and (4) new-onset LBBB and T wave magnitude in lead I were the main independent predictors of PPM implantation in patients undergoing TAVR.
The most common indication for PPM implantation in those who underwent TAVR was complete AVB in our study, which had also been demonstrated previously (7, 8). Consistent with previous data, a sick sinus syndrome (37.5%), including relevant sinus and bradycardia, was a leading indication in patients who underwent PPM implantation (9).
The anatomic relationship between the aortic valve and the cardiac conduction system is the foundation of the conduction disturbances in patients undergoing TAVR. Anatomically, the proximity of this portion of the atrioventricular node (AVN), the bundle of His, and the membranous part of the interventricular septum makes it more susceptible to conduction disturbances. The left bundle branch is close to the bottom of the interleaflet triangle together with the location of the right coronary leaflets and non coronary aortic valve (10–12). The incidence of complete AVB after TAVR is due to a direct mechanical injury to the AVN and/or the left bundle branch that results in ischemia, edema, and hematoma according to necropsy studies (10). Due to the invention of a self-expandable valve possessing a subannular section, the release of the valve will produce radial force to the left ventricular outflow tract (LVOT). The implantation of a self-expandable valve may lead to conduction block (13). Therefore, it is crucial to improve the surgical manipulations among experienced surgeons in order to decrease the risk of complete AVB after TAVR.
Patients with a higher burden of PPM implantation following TAVR had a higher prevalence of abnormal clinical indications. Some abnormal clinical data before the procedure may be related to injury within the underlying conduction system during TAVR (11). Du et al. (12) demonstrated that patients with prior LBBB had a relatively higher risk of PPM implantation in the Chinese population. Gaede et al. (9) summarized that patients who already had aortic valve replacement prior to TAVR required a PPM implantation less frequently. Our study showed that severe AVS patients who required PPM following TAVR were likely to present with dyslipidemia and negative CrCl. STS Morbimortality also played an important role in evaluating patients with a high frequency of conduction disturbances after TAVR.
Previous studies have reported that renal dysfunction at baseline predicted a higher increase of myocardial injury after the procedure. Additionally, myocardial injury after TAVR could lead to conduction disturbances, which may contribute to the need for PPM implantation (14–16). Thus, lower average CrCl may lead to worse kidney function at baseline, which plays an important role in patients requiring PPM implantation after TAVR. Lindman et al. (17) showed that elevated blood lipid parameters were associated with higher risks of calcific AVS. On account of the thickening and remodeling of the aortic valve leaflets, the formation of calcific AVS will cause severe cardiac outflow tract stenosis. Prosthesis implantation may produce radial force to the cardiac outflow tract, which results in conduction block. It is therefore recommended that doctors focus more attention on reducing the risk factors before TAVR to decrease the need for subsequent PPM implantation.
Few studies have evaluated the relationship between new-onset LBBB and the risk of PPM implantation after TAVR. In our cohort, a new-onset LBBB and T wave magnitude in lead I were the predictors for the patients requiring a PPM implantation after TAVR. However, prior RBBB at baseline was identified as an independent predictor in some previous studies (7, 11, 18, 19). In contrast to our study, smaller studies have shown that prior RBBB plays an important role in cardiac conduction system disorders after TAVR (8, 9, 20, 21).
The predictors of PPM implantation in patients receiving TAVR have been examined in seven studies (9, 12, 22–26). All studies were cohort studies. RBBB prior to TAVR was considered to be a predictor in most of the selected studies (9, 12, 22, 24, 26) in Table 4. However, the association of new-onset LBBB with PPM implantation after TAVR has not been fully characterized. A study by Chorianopoulos et al. (22), in which 46 patients with PPM implantation and 83 patients without PPM implantation were enrolled, showed that prior RBBB was the only predictor. A retrospective cohort study by Muillet et al. (23) showed that post-TAVR QRS duration and depth of implantation were the predictors of PPM implantation in patients receiving TAVR. In a cohort study by Luise Gaede et al. (9), in which 176 patients with PPM implantation and 849 patients without PPM implantation were enrolled, not only prior RBBB but also higher MPG and post-dilatation of the prosthesis were observed to be the predictors. A study focusing on 38 Chinese patients with PPM implantation and 218 Chinese patients without PPM implantation showed that prior RBBB, tricuspid aortic valve (TAV), and implantation depth at the non-coronary sinus side were the predictors, especially in Chinese population (12). The study of Kiani S, et al. (24) showed the history of syncope, prior RBBB, QRS duration ≥138 ms, and valve oversizing >15.6% were the predictors. Ferreira T et al. (25) demonstrated that increased H-V interval, which showed in post-TAVR electrophysiological study, was the best predictor of PPM implantation undergoing TAVR. A previous study by Johny Nicolas et al. (26) showed that RBBB before TAVR was the only predictor.
A positive correlation between PPM implantation after TAVR and new-onset LBBB was demonstrated in our study. However, no correlation was found between PPM implantation and prior RBBB or 1° AVB. We attribute the finding to mechanical injury that mainly affected the left bundle branch. Preexisting damage to the right bundle branch may lead to complete AVB when exposed to TAVR. The mechanism of the relationship between T wave magnitude in lead I and PPM implantation is unknown. Meanwhile, other predictors, including the type of the valve and the depth of prosthesis implantation, were not confirmed in our cohort (27–30). To date, no detailed guidelines for the management of new-onset LBBB following TAVR are available. Consequently, any recommendation about the management of new-onset LBBB after TAVR should be treated with caution and further investigation is warranted.
The limitations of our study are as follows. First, our study had a small sample size and a short follow-up time. The patients undergoing TAVR were also from a single center. The numbers of patients with PPM implantation and without PPM implantation were low, which limits the statistics of the study. However, the data were revised with Firth's correction due to the small sample size. All analyses were conducted using R (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria) or SAS Statistics (version 9.4; North Carolina, America). Thus, we strongly believe that our findings are reliable. Second, following discharge, we observed the patients only after a one-month follow-up. The interpretation may differ based on six-month or one-year follow-up periods. Finally, other variables, such as the position of the valve and valve type, may have generated a significant variation to the risk of conduction abnormalities after TAVR. Although our study cohort was small, only involved a single center, and was limited to a short follow-up time, we strongly believe that our findings are representative and may assist in providing valuable insight toward future identification of high-risk cases requiring PPM. Hence, our findings about PPM implantation after TAVR contributes to not only patients but also doctors for improving the postoperative quality of life of patients.
The overall incidence of PPM implantation in our consecutive patients was 20.5%. PPM implantation occurs with higher risk in patients with negative CrCl, dyslipidemia, high STS Morbimortality score, and increased T wave magnitude in lead I. TAVR induced several cardiac electrical changes, such as increased R wave and T wave changes in lead V5. New-onset LBBB and T wave magnitude in lead I were the main independent predictors of PPM implantation in patients undergoing TAVR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Affiliated Zhongshan Hospital of Dalian University. The patients/participants provided their written informed consent to participate in this study.
JZ, CC, and ST: conceptualization and methodology. JZ and CC: investigation and writing—original draft. JZ: writing—review and English editing. ST, SZ, and JL: supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.743257/full#supplementary-material
1. Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. (2020) 40:885–900. doi: 10.1161/ATVBAHA.119.313067
2. Arora S, Vavalle JP. Transcatheter aortic valve replacement in intermediate and low risk patients-clinical evidence. Ann Cardiothorac Surg. (2017) 6:493–7. doi: 10.21037/acs.2017.07.01
3. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364:2187–98. doi: 10.1056/NEJMoa1103510
4. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. (2010) 363:1597–607. doi: 10.1056/NEJMoa1008232
5. Chamandi C, Barbanti M, Munoz-Garcia A, Latib A, Nombela-Franco L, Gutiérrez-Ibanez E, et al. Long-term outcomes in patients with new-onset persistent left bundle branch block following TAVR. JACC Cardiovasc Interv. (2019) 12:1175–84. doi: 10.1016/j.jcin.2019.03.025
6. Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. (2014) 56:565–71. doi: 10.1016/j.pcad.2014.02.006
7. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. (2015) 8:60–9. doi: 10.1016/j.jcin.2014.07.022
8. Erkapic D, De Rosa S, Kelava A, Lehmann R, Fichtlscherer S, Hohnloser SH. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol. (2012) 23:391–7. doi: 10.1111/j.1540-8167.2011.02211.x
9. Gaede L, Kim WK, Liebetrau C, Dörr O, Sperzel J, Blumenstein J, et al. Pacemaker implantation after TAVI: predictors of AV block persistence. Clin Res Cardiol. (2018) 107:60–9. doi: 10.1007/s00392-017-1158-2
10. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. (2017) 136:1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352
11. Chen S, Chau KH, Nazif TM. The incidence and impact of cardiac conduction disturbances after transcatheter aortic valve replacement. Ann Cardiothorac Surg. (2020) 9:452–67. doi: 10.21037/acs-2020-av-23
12. Du F, Zhu Q, Jiang J, Chen H, Liu X, Wang J. Incidence and predictors of permanent pacemaker implantation in patients who underwent transcatheter aortic valve replacement: observation of a chinese population. Cardiology. (2020) 145:27–34. doi: 10.1159/000502792
13. Tzamtzis S, Viquerat J, Yap J, Mullen MJ, Burriesci G. Numerical analysis of the radial force produced by the Medtronic-CoreValve and Edwards-SAPIEN after transcatheter aortic valve implantation (TAVI). Med Eng Phys. (2013) 35:125–30. doi: 10.1016/j.medengphy.2012.04.009
14. De Marzo V, Crimi G, Vercellino M, Benenati S, Pescetelli F, Della Bona R, et al. Impact of bioprosthetic valve type on peri-procedural myocardial injury and mortality after transcatheter aortic valve replacement. Heart Vessels. (2021). doi: 10.2459/JCM.0000000000001178
15. Stundl A, Schulte R, Lucht H, Weber M, Sedaghat A, Shamekhi J, et al. Periprocedural myocardial injury depends on transcatheter heart valve type but does not predict mortality in patients after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2017) 10:1550–60. doi: 10.1016/j.jcin.2017.05.029
16. Nara Y, Watanabe Y, Kataoka A, Nakashima M, Hioki H, Nagura F, et al. Incidence, predictors, and midterm clinical outcomes of myocardial injury after transcatheter aortic-valve implantation. Int Heart J. (2018) 59:1296–302. doi: 10.1536/ihj.17-645
17. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM. et al. Calcific aortic stenosis. Nat Rev Dis Primers. (2016) 2:16006. doi: 10.1038/nrdp.2016.6
18. Mauri V, Reimann A, Stern D, Scherner M, Kuhn E, Rudolph V, et al. Predictors of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement With the SAPIEN 3. JACC Cardiovasc Interv. (2016) 9:2200–9. doi: 10.1016/j.jcin.2016.08.034
19. Keßler M, Gonska B, Seeger J, Rottbauer W, Wöhrle J. Predictors of permanent pacemaker implantation after transfemoral aortic valve implantation with the Lotus valve. Am Heart J. (2017) 192:57–63. doi: 10.1016/j.ahj.2017.07.011
20. Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R, et al. Incidence, predictors, and outcome of conduction disorders after transcatheter self-expandable aortic valve implantation. Am J Cardiol. (2011) 107:747–54. doi: 10.1016/j.amjcard.2010.10.054
21. Boerlage-Van Dijk K, Kooiman KM, Yong ZY, Wiegerinck EM, Damman P, Bouma BJ, et al. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. (2014) 37:1520–9. doi: 10.1111/pace.12460
22. Chorianopoulos E, Krumsdorf U, Pleger ST, Katus HA, Bekeredjian R. Incidence of late occurring bradyarrhythmias after TAVI with the self-expanding CoreValve(®) aortic bioprosthesis. Clin Res Cardiol. (2012) 101:349–55. doi: 10.1007/s00392-011-0398-9
23. Mouillet G, Lellouche N, Lim P, Meguro K, Yamamoto M, Deux J, et al. Patients without prolonged QRS after TAVI with CoreValve device do not experience high-degree atrio-ventricular block. Catheter Cardiovasc Interv. (2013) 81:882–7. doi: 10.1002/ccd.24657
24. Kiani S, Kamioka N, Black GB, Lu MLR, Lisko JC, Rao B, et al. Development of a risk score to predict new pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2019) 12:2133–42. doi: 10.1016/j.jcin.2019.07.015
25. Ferreira T, Da Costa A, Cerisier A, Vidal N, Guichard JB, Romeyer C, et al. Predictors of high-degree conduction disturbances and pacemaker implantation after transcatheter aortic valve replacement: Prognostic role of the electrophysiological study. Pacing Clin Electrophysiol. (2021) 44:843–55. doi: 10.1111/pace.14225
26. Nicolas J, Guedeney P, Claessen BE, Mehilli J, Petronio AS, Sartori S, et al. Incidence, predictors and clinical impact of permanent pacemaker insertion in women following transcatheter aortic valve implantation: Insights from a prospective multinational registry. Catheter Cardiovasc Interv. (2021) 98:E908–17. doi: 10.1002/ccd.29807
27. Schymik G, Tzamalis P, Bramlage P, Heimeshoff M, Würth A, Wondraschek R, et al. Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin Res Cardiol. (2015) 104:351–62. doi: 10.1007/s00392-014-0791-2
28. Husser O, Pellegrini C, Kessler T, Burgdorf C, Thaller H, Mayr NP, et al. Predictors of permanent pacemaker implantations and new-onset conduction abnormalities with the SAPIEN 3 balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv. (2016) 9:244–54. doi: 10.1016/j.jcin.2015.09.036
29. Rodríguez-Olivares R, van Gils L, El Faquir N, Rahhab Z, Di Martino LF, van Weenen S, et al. Importance of the left ventricular outflow tract in the need for pacemaker implantation after transcatheter aortic valve replacement. Int J Cardiol. (2016) 216:9–15. doi: 10.1016/j.ijcard.2016.04.023
Keywords: aortic stenosis, transcatheter aortic valve replacement, complete atrioventricular block, permanent pacemaker, predictors
Citation: Zhang J, Chi C, Tian S, Zhang S and Liu J (2022) Predictors of Permanent Pacemaker Implantation in Patients After Transcatheter Aortic Valve Replacement in a Chinese Population. Front. Cardiovasc. Med. 8:743257. doi: 10.3389/fcvm.2021.743257
Received: 18 July 2021; Accepted: 06 December 2021;
Published: 06 January 2022.
Edited by:
Lakshmi Prasad Dasi, Georgia Institute of Technology, United StatesReviewed by:
Paul Human, University of Cape Town, South AfricaCopyright © 2022 Zhang, Chi, Tian, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jihong Liu, ODY0MzA4OUBxcS5jb20=; Shulong Zhang, emhhbmdzaHVsb25nbWRAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.