- 1Department of Radiology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Radiology, University of Washington, Seattle, WA, United States
- 3Internal Medicine Department, University of Massachusetts Memorial Medical Center, Worcester, MA, United States
Background and Aims: Statin therapy is an essential component of cardiovascular preventive care. In recent years, various vessel wall MRI (VW-MRI) techniques have been used to monitor atherosclerosis progression or regression in patients with extracranial or intracranial large-artery atherosclerosis. We aimed to perform a systematic review and meta-analysis on the effects of statin therapy on plaque evolution as assessed by VW-MRI.
Materials and Methods: Prospective studies investigating carotid and intracranial atherosclerotic plaques in patients on statin therapy monitored by serial VW-MRI were systematically identified in the literature. The plaque burden and lipid-rich necrotic core (LRNC) volume of carotid plaque and the imaging features of intracranial plaques were extracted and summarized. For studies investigating carotid artery wall volume and LRNC volume, combined estimates were derived by meta-analysis.
Results: The study identified 21 studies of carotid plaque and two studies of intracranial plaque. While 16 studies investigating carotid plaques that included 780 patients by High-resolution VW-MRI were included in the meta-analysis. There was no significant change in carotid wall volume from baseline to 12 months. A significant change in LRNC volume was observed at > 12 months compared with baseline (Effect = −10.69, 95% CI = −19.11, −2.28, P < 0.01), while no significant change in LRNC volume at 3–6 months or 7–12 months after statin therapy initiation in 6 studies. Increases in fibrous tissue and calcium and reduction in neovascularization density of the plaque were seen in 2/3 studies (including 48/59 patients), 1/3 studies (including 17/54 patients), and 2/2 studies (including 71 patients) after statin therapy, respectively. Two studies with 257 patients in intracranial atherosclerosis showed that statins could effectively decrease wall volume and plaque enhancement volume.
Conclusions: Collective data indicated that statins could potentially stabilize carotid plaques by significantly reducing LRNC with 1 year of therapy as shown on serial carotid VW-MRI. There was no significant decrease in wall volume, which nonetheless indicated that plaque composition changes might be more sensitive to response monitoring than wall volume. It is likely that more sensitive, clinically relevant, and preferably quantitative indicators of therapeutic effects on intracranial vessel plaque morphology will be developed in the future.
Introduction
Patients with extracranial or intracranial large-artery atherosclerosis are treated with aggressive risk factor control to lower their increased risk of ischemic stroke and other vascular events. This typically involves intensive low-density lipoprotein cholesterol (LDL-C) lowering with statin therapy. Meta-analyses of randomized clinical trials on statin therapies have shown that every 1 mmol/L reduction in LDL-C was associated with a 22% reduction in major vascular events (1). Despite LDL being a good marker for the effect of statins, residual stroke risk remains and other markers should be investigated for better pharmacology regime or monitoring the treatment effect (2).
A study in the Chinese population found that compared with the ipsilateral extracranial carotid artery, greater atherosclerotic plaque burden had a closer association with stroke severity (National Institutes of Health Stroke Scale) in the middle cerebral artery, suggesting that intracranial plaques deserve more attention in stroke etiology (3).
Vessel wall MRI (VW-MRI) is a non-Invasive imaging technique with high reproducibility and is used for characterizing atherosclerotic plaques in extracranial carotid or intracranial cerebral arteries (4–6), as well as plaque progression or regression (7–9). Studies of carotid atherosclerosis revealed the plaque characteristics shown on VW-MRI were highly consistent with those shown on histopathology (10, 11), providing the theoretical basis to evaluate the treatment response of atherosclerotic plaques by this imaging technique. Monitoring atherosclerosis with VW-MRI may enable the visual characterization of plaque morphologic and compositional evolution. VW-MRI studies have indicated that lowering LDL-C can remove the cholesterol from plaques, reduce the lipid content, induce plaque regression, even increase the plaque calcification, and therefore stabilize plaques (12–14). Moreover, monitoring atherosclerosis progression or regression in extracranial carotid or intracranial cerebral arteries with VW-MRI may allow for the identification of poor responders to standard-of-care medical therapy, who could be helped with novel lipid-lowering therapies or enrolling in clinical trials evaluating other endovascular treatments.
New studies have been published since the last meta-analysis of using VW-MRI to monitor changes of carotid plaques by Brinjikji et al. (15). The statins therapeutic response assessment in intracranial plaque by VW-MRI has not been systematically studied yet. Studies investigating novel techniques for vessel plaque evaluation are ongoing and show considerable promise. In this systematic review and updated meta-analysis, we aimed to evaluate the effect of statins on plaque components (such as lipid, fibrous tissue, and calcium) and plaque morphology (such as wall volume) as monitored by serial VW-MRI. The effects of statins in carotid plaques assessed by other advanced MRI techniques such as dynamic contrast-enhanced MRI (DCE-MRI), T2 mapping, and MRI targeting macrophages were also reviewed. The selection of parameters has been discussed. Finally, study results investigating intracranial plaques by VW-MRI after statin therapy were summarized.
Materials and Methods
This systematic review was performed with a standardized protocol with reference to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines. Ethical committee approval and patient consent were not required because of their statistical nature.
Search Strategy
A comprehensive search of the PubMed, Embase, Medline, and the Cochrane library databases from January 1st, 2000 through December 31st, 2020 was performed. To identify eligible studies, the following keywords were used in combination with the Boolean operators OR and AND: “intracranial atherosclerosis” or “intracranial plaque” or “intracranial artery stenosis” or “intracranial artery disease” or “cerebral artery atherosclerosis” or “carotid atherosclerosis” or “carotid plaque” or “carotid artery stenosis” or “carotid artery disease” or “lipid-rich necrotic core” and “high-resolution magnetic resonance imaging” or “vessel wall imaging” or “plaque imaging” and “statin” or “lipid-lowering treatment” or “HMG-COA (3-hydroxy-3-methyl glutaryl coenzyme A) reductase inhibitor.” We also hand-searched references from key articles to find any additional studies on the use of serial carotid and intracranial plaque MRI to monitor response to statin therapy. Scoping searches were performed, and an iterative process was used to translate and refine the searches. The list of articles was supplemented with extensive cross-checking of reference lists within the included articles. The search was performed by a senior researcher and reviewed by a second researcher, and discrepancies in judgment were resolved by consensus.
Eligibility Criteria and Study Selection
Specific inclusion criteria were: (1) English language articles; (2) all patients were on statin therapy or the results of the statin group were separately reported; (3) patients underwent serial vessel wall MRI to assess the response to statin therapy of intracranial and extracranial carotid atherosclerosis; (4) use of serial carotid or intracranial plaque MRI with dedicated surface coils; (5) minimum field strength of 1.5 T at all time points; (6) total sample size was > 10 patients; (7) follow-up duration was at least 3 months; (8) necessary data were reported to calculate the indicators of plaques. Attempts were made to contact the corresponding author for additional data details when necessary.
Studies must report at least one of the following regarding the plaques: (a) change in wall volume over time; (b) change in lipid-rich necrotic core (LRNC) volume over time; (c) change in calcium volume over time; (d) change in fibrous tissue volume over time.
If multiple studies were thought to contain overlapping patient cohorts and observed similar indicators, only the study with the largest sample size was retained. Review articles, conference abstracts, letters, comments, and case reports were excluded.
Exclusion criteria included retrospective and cross-Sectional studies, studies employing only imaging modalities other than vessel wall MRI, or studies in which the results of the statin arm could not be separated when multiple arms were involved, i.e., statin vs. non-Statin.
This selection strategy was prospectively chosen to provide sufficient and accurate data for a detailed systematic review.
Methodological Quality Assessment
The methodological quality of included studies was independently assessed by two researchers using the “Guidelines for Assessing Quality in Prognostic Studies on the Basis of Framework of Potential Biases” (16). Discrepancies in judgment were resolved by consensus after discussion.
Data Extraction and Analysis
Two reviewers independently extracted data from eligible studies, and disagreements were resolved by consensus. The following study characteristics were extracted: (1) sample size and demographics of study participants; (2) the regimen and dose of statins in all arms; (3) specific MRI protocols employed; (4) the changes in plaque imaging characteristics and in LDL-C levels at all monitoring time points.
We did the meta-analysis of studies investigating variable outcomes of carotid plaques at three time points: (a) 3–6 months, (b) 7–12 months, and (c) > 12 months following statin therapy initiation. The pooled results were expressed as an “Effect” with respective 95% CI. I2 calculated from Q statistic was used to examine the heterogeneity among the included studies, with I values of 0–40, 30–60, 50–90, and 75–100% representing not important, moderate, substantial, and considerable inconsistency, respectively (17). A random-effects model was applicable with an I2 value of over 50%, and a fixed-effects model was applicable with an I2 value of < 50% (18). Meta-analysis was conducted using Stata version 12 (College Station, Texas: StataCorp LP, United States).
Results
Study Selection and Characteristics
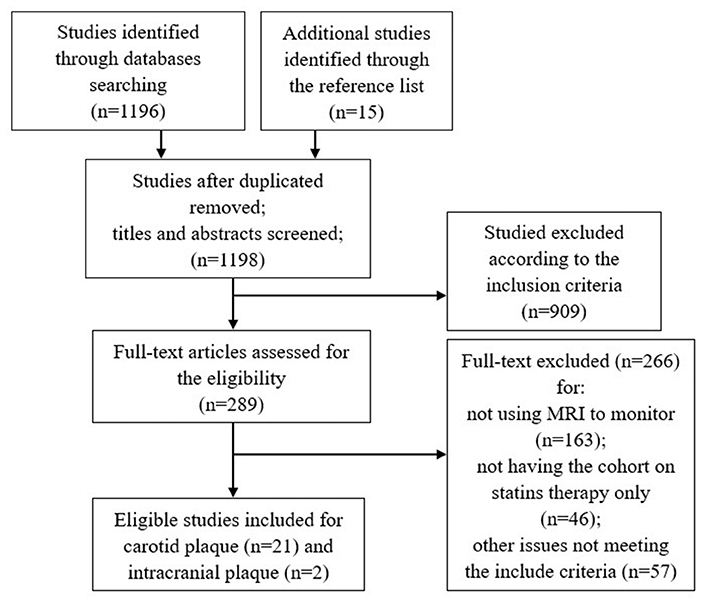
The detailed study selection flow diagram is shown in Figure 1. A total of 1,198 articles were identified after duplicates were removed. From these, 21 articles for carotid plaque and 2 articles for intracranial plaque were identified as eligible. Limited by the number of eligible studies on the intracranial plaque, the meta-analysis included only carotid plaque vessel wall MRI studies.
Table 1 summarizes the 21 included prospective clinical studies that evaluated the changes of carotid plaques on longitudinal VW-MRI in patients on statin therapy published between 2000 and 2020. All studies evaluated the imaging characteristics of the plaques at baseline. There were 12 studies which had imaging follow up at 12 months, 4 studies at 18 months, and 9 studies at 24 months. Six studies did the measurements based on the T1 sequence. Two studies did the measurements based on the T2 sequence. One study did the measurements based on the PD (proton density) sequence. On the other hand, 12 studies did not make the corresponding statement. Eight studies drew the measurements manually, six studies used the automatic custom-designed image analysis tool (CASCADE, IBMarker Company, Seattle, WA, USA) (13, 29–31, 34, 35), and one used the semiautomatic software (Vessel Mass, Leiden University Medical Center, Leiden, Netherlands) (26). Six studies did not make the corresponding statement. All studies recorded the changes in the blood lipid index of the patients, including LDL-C and/or high-density lipoprotein-cholesterol (HDL-C). Nine studies employed plaque burden/volume as the only imaging observation at the experimental endpoint, one study employed LRNC as the only imaging observation, and seven studies used both plaque burden/volume and LRNC as observation indicators at the endpoint. In two studies investigating vascular dynamics, the dynamic changes in the parameters of the plaques were used as the only observation indicators at the endpoint of the experiment. Finally, one study employed plaque inflammation as the indicator (ultrasmall superparamagnetic iron oxide (USPIO) uptake by macrophages) and one employed distensibility as the only observation indicator at the endpoint.
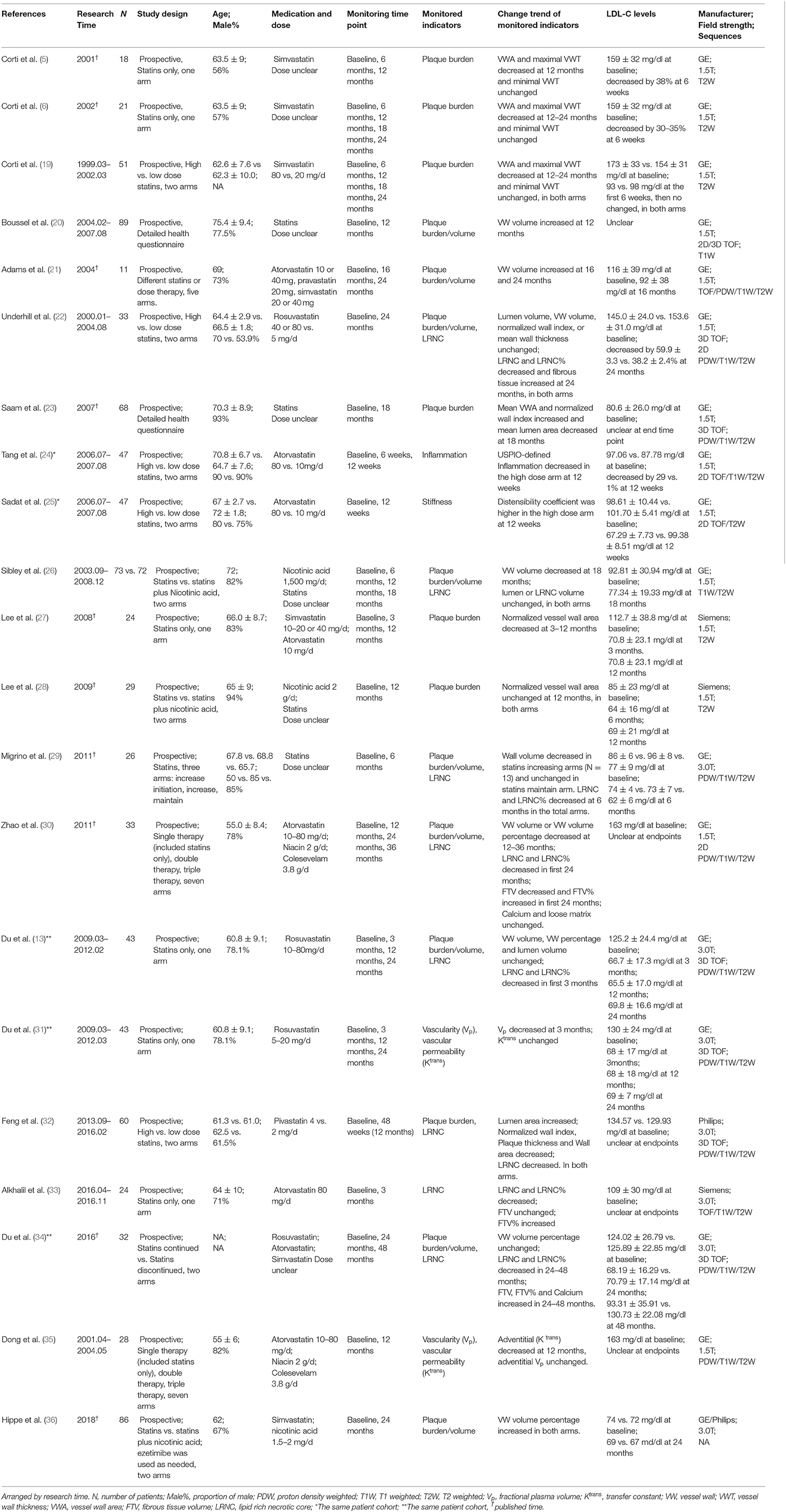
Table 1. Serial vessel wall MRI studies on effects of statin therapy on extracranial carotid atherosclerosis.
Table 2 summarizes the two prospective clinical studies from 2016 to 2020 evaluating treatment response to statins based on imaging findings on longitudinal intracranial VW-MRI studies. These studies monitored plaque burden/volume, intima-media thickness, and plaque number at baseline and after 6 months of statin therapy and recorded the changes of lipid panels, such as LDL-C, HDL-C, etc. The study of Chung et al. made the measurements based on the T2 sequence manually. There was no corresponding statement in another study (4, 37).
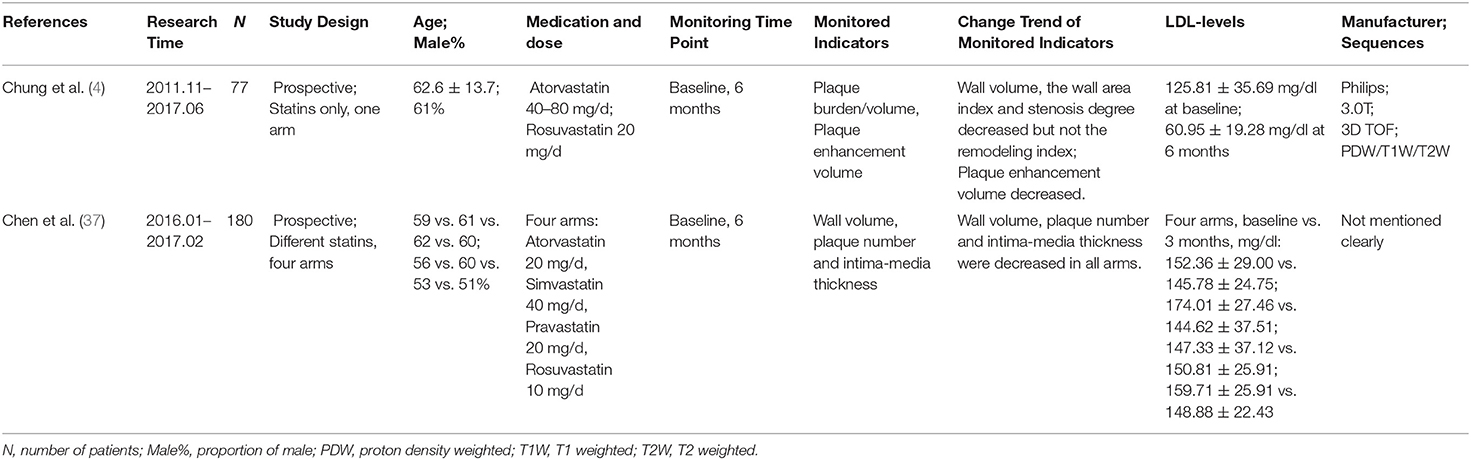
Table 2. Clinical trials of monitoring the efficacy of statins therapy in intracranial atherosclerotic plaques by vessel wall imaging.
Risk of Bias and Quality Assessment
The results of the quality assessment were generally satisfactory. There were 10 studies judged to have a low risk of bias in four assessed domains. Six studies were judged to have a moderate risk of bias in at least one domain. Common sources of moderate risk were study participation and study attrition. Study attrition bias risk came from missing data points, specifically some patients not receiving all follow-up imaging studies and/or lab values at the prescribed time points. Five studies had a high risk of bias in the domain of study participation resulting from a lack of reasonable inclusion and exclusion criteria and/or rationality in variable control. There was no study with a high risk of bias in more than one assessed domain.
Selection of Plaque Measurements
Morphological Parameters
The study found that 16 studies employed wall area, wall thickness, wall area index, wall volume, and lumen volume as the morphological parameters in the clinical trials of carotid atherosclerosis (Table 1). The wall area is defined as wall area = vessel area – lumen area. The wall thickness is the mean vessel wall thickness. The wall area index is defined as the ratio of the wall area of the stenosis site to that of the reference wall. The wall volume and lumen volume are three-dimensional indicators (volume= area × slice thickness × the number of slices) (4–6, 38). The interobserver and interscan intraclass correlation coefficient (ICC) for morphological measurement reported ranged from 0.97 to 0.99. The interobserver and interscan mean coefficient of variation (CV) of the total wall volume measurements were 4.6 and 3.8%, respectively, showing good repeatability (7–9, 20–23, 33).
Compositional Parameters
Eight studies employed LRNC volume, fibrous tissue volume, and Calcium volume as the plaque component variables assessed on carotid VW-MRI (Table 1). The ICCs for component measurement reported in the included studies ranged from 0.88 to 0.96 (9, 22, 33).
Other Novel Parameters
Two studies employed parameters of vascular permeability such as transport constant (Ktrans) and fractional plasma volume (Vp) as the endpoints of clinical medication trials. Two studies employed USPIO-defined inflammation and stiffness as novel parameters.
In atherosclerosis, macrophage accumulation initiates lesion development and triggers clinical events by elaborating various molecules that promote inflammation, plaque disruption, and subsequent thrombus formation (39–42). To identify the plaque inflammation activity, common parameters include USPIO signal intensity in the plaque and compliance coefficient (24, 25). Amax and Amin were defined as the maximum and the minimum vessel cross-sectional areas through the cardiac cycle, respectively. The distensibility was: (Amax–Amin)/Amin* the average brachial pulse pressure. The compliance coefficient was: (Amax–Amin)/the average brachial pulse pressure (25).
Response of Atherosclerosis on Statins
Extracranial Carotid Atherosclerosis
Lipid Rich Necrotic Core Volume
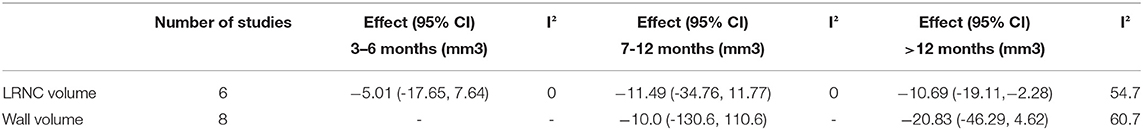
Six studies extracted the change in LRNC volume. The mean LRNC volume was 72.3 ± 35.3 mm3 at baseline and 62.3 ± 34.6 mm3 at the last follow-up. There was a significant decrease in LRNC volume at > 12 months (Effect = −10.69, 95%CI = −19.11, −2.28, P < 0 01). There was no significant decrease in LRNC volume at any time points < 1 year. Moderate heterogeneity was observed for the analysis of LRNC. The data is summarized in Table 3. Representative Forest plots and a detailed line chart are provided in Figures 2A, 3A.
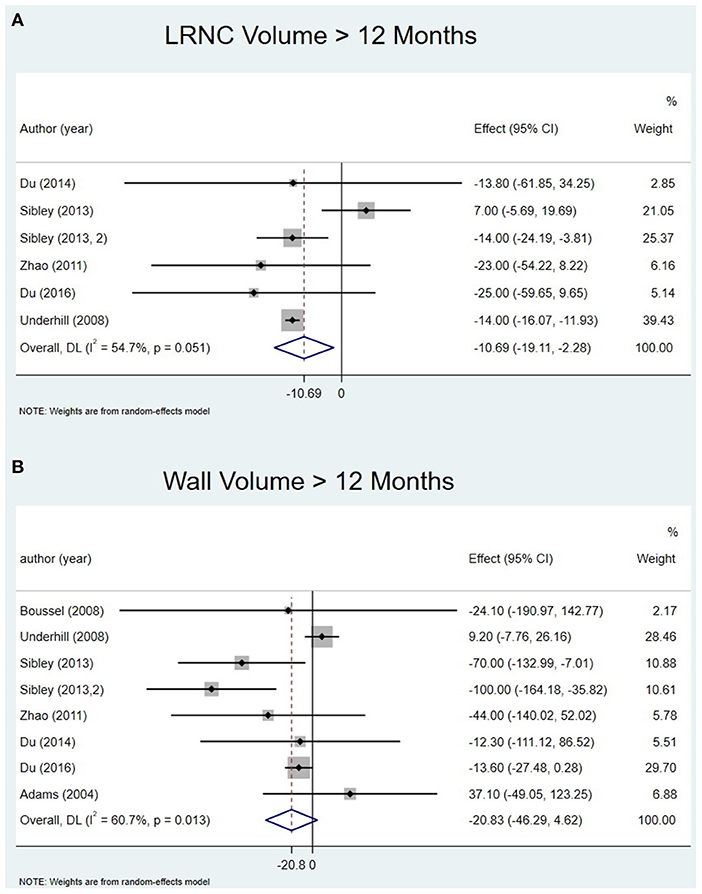
Figure 2. Change in LRNC volume and Wall volume at > 12 months: (A) forest plot of change in LRNC volume at > 12 months; (B) forest plot of change in Wall volume at > 12 months.
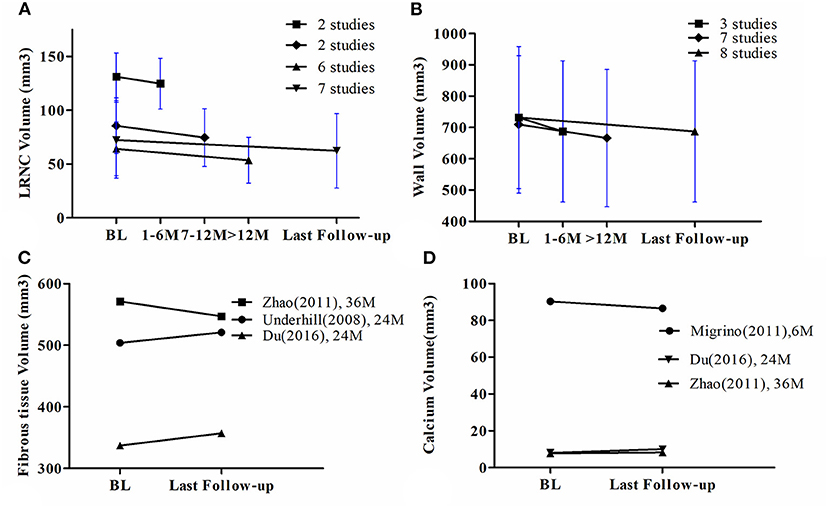
Figure 3. (A) The line chart of LRNC volume, (B) The line chart of wall volume, (C) The line chart of fibrous tissue volume, (D) The line chart of calcium volume. BL = Baseline. The bars mean 95% CI.
Fibrous Tissue Volume
Only three studies had corresponding data and therefore meta-analysis was not performed. Two studies found an increase in fibrous tissue volume at the last follow-up. However, one study found a decreased volume at the 24-month time point after initiation of statin therapy and at the last follow-up. The detailed line chart is provided in Figure 3C.
Calcium Volume
Only three studies had corresponding data and therefore meta-analysis was not performed. One study found an increase in calcium volume at the last follow-up. However, two studies found a decrease and no change in calcium volume at the last follow-up, respectively. The detailed line chart is shown in Figure 3D.
Wall Volume
All eight studies evaluated the change in wall volume. The mean wall volume at baseline was 731.8 ± 227.1 mm3 and at the last follow-up was 687.2 ± 225.6 mm3. There was no significant decrease at > 12 months (Effect = −20.83, 95%CI = −46.29, 4.62, P >0.05). I-squared value was 60.7% at > 12 months indicating substantial heterogeneity. There was no significant decrease at any time point except the last follow-up. The data is summarized in Table 3. Representative Forest plots and a detailed line chart are provided in Figures 2B, 3B.
Neovascularization
Two studies with 71 total patients found a decrease in Vp or Ktrans of plaque at 3 and 12 months after statin therapy initiation, which indicated a reduction in plaque neovascularization density.
The Relationship Between LRNC Volumes and LDL Levels
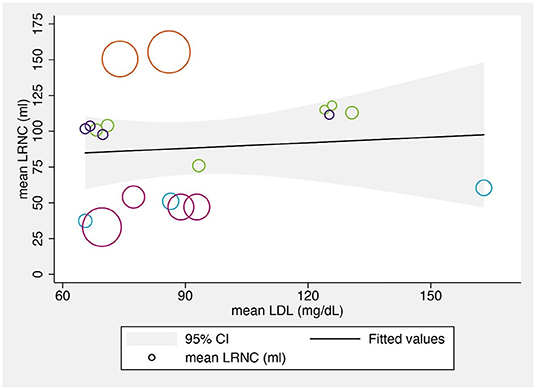
We also explored the relationship between the mean LRNC volumes and the mean LDL-C levels at various time points. Although several previous studies found that changes in the LRNC were significantly correlated with LDL-C levels (22, 32, 36), the reported LRNC volume changes varied across the reviewed studies, possibly due to the different study populations, designs, and statin regimens. The bubble chart showing the results across included studies is shown in Figure 4.
Intracranial Atherosclerosis
In the evaluation of 77 patients with symptomatic acute ischemic stroke treated with high-dose statins (Atorvastatin 40–80 mg or rosuvastatin 20 mg), the study of Chung et al. found that high-dose statins could effectively decrease plaque enhancement volume, wall area index, and degree of stenosis. Longer duration (more than 1 month) of statin treatment and higher reduction of LDL-C were associated with decreased plaque enhancement volume (4, 38). Another study by Chen et al. compared the efficacy and safety of different statins in the treatment of intracranial atherosclerotic plaques involving middle cerebral arteries and found that different statins could significantly reduce blood lipid levels, intima-media thickness, plaque number, and wall volume, but rosuvastatin and atorvastatin had more effective anti-inflammatory and plaque stabilizing effects than simvastatin and pravastatin (37).
Discussion
This updated meta-analysis of patients who received serial carotid plaque imaging while on statin therapy demonstrated a significant reduction in LRNC volume only with > 1 year of therapy, whereas wall volume did not change significantly at any time points. It suggested that VW-MRI could visualize the changes of plaque components and morphology in vivo after statin therapies, and the former could be a more sensitive marker of therapeutic response. Increasing LRNC size was associated with the development of new ulceration, fibrous cap rupture, increasing plaque burden, as well as ischemic stroke events (43–46). Therefore, LRNC size was a good target for the evaluation of therapeutic response. A review of studies that monitored changes of intracranial atherosclerotic plaques by VW-MRI suggested that changes in multiple parameters such as decreased plaque number, wall volume, and plaque enhancement volume could be readily detected.
Compared with the meta-analysis by Brinjikji et al. (15) which also explored the change of plaque characteristics after statin therapies, our analysis provided additional information listed below. (1) One new prospective study with both vessel wall and LRNC data were included (34); (2) we summarized the change in plaque components such as fibrous tissue and calcium in addition to LRNC volume and wall volume; (3) the relationship between mean LRNC volumes and mean LDL-C levels were plotted; (4) given the technical developments in plaque imaging, a review of advanced MRI techniques including T2 mapping and DCE was performed and the established indicators for therapy monitoring of atherosclerotic plaques were summarized; (5) the results derived from studies of intracranial atherosclerosis were also summarized.
Carotid Atherosclerosis
Two of three studies showed a significant increase in fibrous tissue volume of carotid plaques after 2 years of statin treatment. The timing of the change suggested that the decrease in the LRNC volume probably preceded the increase in fibrous tissue volume, which could be consistent with the proposed mechanism that the LRNC component might be replaced by fibrous tissue after statin treatment (15).
Calcium volume did not change significantly at any time points including for follow-up duration as long as 3 years (30). Sun et al. (44) reported that high plaque calcification content was not associated with poor prognosis in AIM-HIGH. Calcium might not be the crucial component to evaluate in carotid plaques when a therapeutic response of statins is being assessed by VW-MRI.
The study of Hellings et al. (47) found that carotid plaque features including IPH and intraplaque vessel formation identified in endarterectomy specimens were associated with future cardiovascular events in 818 patients who underwent carotid endarterectomy. Currently, advanced MRI techniques such as DCE MRI and USPIO enhanced MRI show unique advantages in characterizing plaque micro-vascularization and inflammation. These parameters could be further explored in future prospective studies to monitor the effects of therapy on plaque morphology (48–51).
Intracranial Atherosclerosis
Because of the unique structure and environment, intracranial arteries receive nutrient supply directly from cerebrospinal fluid and have fewer vasa vasorum than carotid arteries (52). Fewer vasa vasorum, however, makes intracranial arteries more vulnerable to hypoxic injury. The hypoxic environment caused by pathological wall thickening in atherosclerosis can stimulate angiogenic factors (e.g., vascular endothelial growth factor) that consequently promote neovascularization (53–55). Statins can reduce the release of vascular endothelial growth factors through anti-inflammatory effects, thereby reducing neovascularization and plaque enhancement volume on MRI seen in carotid plaque (35, 56). These observations suggest that statins might have different mechanisms to stabilize intracranial plaques than extracranial ones, and the assessment of intracranial plaque changes requires further investigation, which is indeed necessary given the crucial role that intracranial atherosclerosis plays in stroke development. Notably, a recent study on intracranial atherosclerosis indicated that 35% of patients had a poor response to statin therapy; post-Treatment VW-MRI showed no change or increased plaque enhancement volume and increased degree of stenosis. The study suggested that these patients might need to choose alternative therapeutic options such as PCSK9 inhibitors or a combination with ezetimibe to lower lipid levels (4).
Regarding the selection of measurements on imaging, several clinical studies provide a theoretical basis and report practical experience for the assessment of responses to statin therapy in extracranial atherosclerosis, however, the clinical studies investigating intracranial atherosclerotic plaques are relatively few, with a limited assessment of VW-MRI characteristics. The consensus of experts of the American Society of Neuroradiology on intracranial vessel wall imaging along with a recent meta-analysis indicates that intracranial plaque enhancement, positive remodeling, and plaque irregularity are associated with increased risk of stroke (57, 58). These features are therefore highly relevant when assessing and validating response to therapy. Furthermore, evaluation of plaque compositional characteristics in addition to morphological features on VW-MRI might be clinically relevant as suggested by the imaging features of carotid plaques. Given the unique role of neovascularization in the pathogenesis of vessel plaques, other novel techniques to assess neovascularization should be developed.
Imaging Techniques
The standard multi-contrast black blood MRI (fast-spin-echo based) for carotid vessel wall imaging has been widely used for the evaluation of plaque morphology, and the sequences are widely available on the major commercial scanners at both 1.5 and 3T. All included studies used specific neck surface coils to improve the image signal-to-noise ratio/resolution. However, the neck surface coils are not widely available in clinical radiology departments and a separate purchase is required. Without using neck surface coils, the clinical head-neck or neurovascular coils produce noisier images which limit the image resolution and therefore limit the measurement accuracy of plaque morphology. This is a major barrier for clinical translation and multi-center trials.
In the past decade, 3D fast-spin-echo sequences with variable flip angle train (product sequence in all major vendors, termed SPACE in Siemens, CUBE in GE, and VISTA in Philips) have been widely used for carotid vessel wall imaging due to its high scan efficiency, isotropic resolution, and intrinsic blood suppression (59, 60). Such 3D techniques allow high-resolution imaging using clinical neurovascular coils (61), which significantly improve their feasibility in a clinical setting.
While the evaluation of plaque volume and LRNC volume using standard multi-contrast black blood MRI is the most popular and feasible approach, more advanced but less popular methods including T2 mapping, DCE and USPIO-MRI have their unique advantages. The T2 mapping method provides quantitative analysis of the plaque component and is more reproducible across scanners and centers. The DCE method provides unique information on the vasa vasorum, which is an important factor in plaque vulnerability. USPIO-MRI provides unique information of the vessel function and inflammation (macrophage activity). However, their disadvantages are also apparent. For the T2 mapping and DCE techniques, specific sequences are required, and specific post-Processing is required, too. Thus, only a few research centers performed such analysis. The USPIO contrast is off-label for medical imaging usage. Thus, despite that its safety profile is comparable to gadolinium, it is not widely available for imaging, and only a few centers performed USPIO MRI (62). In addition, to visualize inflammation, two imaging sessions (1–3 days apart) are required to enable macrophages to uptake the contrast agent. There is still a big gap to fill before these novel techniques can be used more widely. Better sequences and easier post-Processing pipelines will improve the availability of these techniques in the future.
Conclusion
Collective data indicated that statin therapy could stabilize carotid plaques by significantly reducing LRNC of therapy as shown on serial carotid VW-MRI. Future studies and clinical practice could focus on changes in plaque characteristics (such as components) rather than morphological features. It is expected that more sensitive, clinically relevant, and preferably quantitative indicators to monitor therapeutic effects on intracranial plaques will be developed. VW-MRI may play an important role in assessing and guiding clinical statin therapies in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
PZ, YW, and CZ contributed to conception and design of the study. PZ organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. YW, CZ, JS, MM-B, and YY wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the Research Project of Healthcare for Cadres of Sichuan province, Grant No. 2021-229. CZ is supported by United States National Institute of Health (NIH) grant R00HL136883.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.742935/full#supplementary-material
References
1. Cholesterol Treatment Trialists Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. (2010) 376:1670–81. doi: 10.1016/S0140-6736(10)61350-5
2. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
3. Cao Y, Sun Y, Zhou B, Zhao HL, Zhu Y, Xu JR, et al. Atherosclerotic plaque burden of middle cerebral artery and extracranial carotid artery characterized by MRI in patients with acute ischemic stroke in China: association and clinical relevance. Neurol Res. (2017) 39:344–50. doi: 10.1080/01616412.2017.1281196
4. Chung JW, Cha J, Lee MJ, Yu IW, Park MS, Seo WK, et al. Intensive statin treatment in acute ischaemic stroke patients with intracranial atherosclerosis: a high-resolution magnetic resonance imaging study (STAMINA-MRI study). J Neurol Neurosurg Psychiatry. (2020) 91:204–11. doi: 10.1136/jnnp-2019-320893
5. Corti R, Fayad ZA, Fuster V, Worthley SG, Helft G, Chesebro J, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. (2001) 104:249–52. doi: 10.1161/01.CIR.104.3.249
6. Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Smith D, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. (2002) 106:2884–7. doi: 10.1161/01.CIR.0000041255.88750.F0
7. El Aidi H, Mani V, Weinshelbaum K, Aguiar S, Taniguchi H, Postley J, et al. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med. (2009) 6:219–28. doi: 10.1038/ncpcardio1444
8. Zhang X, Zhu C, Peng W, Tian B, Chen L, Teng Z, et al. Scan-rescan reproducibility of high resolution magnetic resonance imaging of atherosclerotic plaque in the middle cerebral artery. PLoS ONE. (2015) 10:e0134913. doi: 10.1371/journal.pone.0134913
9. Sun J, Zhao XQ, Balu N, Hippe DS, Hatsukami TS, Isquith DA, et al. Carotid magnetic resonance imaging for monitoring atherosclerotic plaque progression: a multicenter reproducibility study. Int J Cardiovasc Imaging. (2015) 31:95–103. doi: 10.1007/s10554-014-0532-7
10. Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. (2005) 112:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174
11. Takaya N, Cai J, Ferguson M, Yarnykh V, Chu B, Saam T, et al. Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J Magn Reson Imaging. (2006) 24:203–10. doi: 10.1002/jmri.20599
12. Alkhalil M, Chai JT, Choudhury RP. Plaque imaging to refine indications for emerging lipid-lowering drugs. Eur Heart J Cardiovasc Pharmacother. (2017) 3:58–67. doi: 10.1093/ehjcvp/pvw034
13. Du R, Cai J, Zhao XQ, Wang QJ, Liu DQ, Leng WX, et al. Early decrease in carotid plaque lipid content as assessed by magnetic resonance imaging during treatment of rosuvastatin. BMC Cardiovasc Disord. (2014) 14:83. doi: 10.1186/1471-2261-14-83
14. Mujaj B, Bos D, Selwaness M, Leening M, Kavousi M, Wentzel J, et al. Statin use is associated with carotid plaque composition: the rotterdam study. Int J Cardiol. (2018) 260:213–8. doi: 10.1016/j.ijcard.2018.02.111
15. Brinjikji W, Lehman VT, Kallmes DF, Rabinstein AA, Lanzino G, Murad MH, et al. The effects of statin therapy on carotid plaque composition and volume: a systematic review and meta-analysis. J Neuroradiol. (2017) 44:234–40. doi: 10.1016/j.neurad.2016.12.004
16. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. (2006) 144:427–37. doi: 10.7326/0003-4819-144-6-200603210-00010
17. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Availabli online at: https://handbook-5-1.Cochrane.Org (updated March 2011).
18. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Chaplin WF, et al. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J Am Coll Cardiol. (2005) 46:106–12. doi: 10.1016/j.jacc.2005.03.054
20. Boussel L, Arora S, Rapp J, Rutt B, Huston J, Parker D, et al. Atherosclerotic plaque progression in carotid arteries: monitoring with high-spatial-resolution MR imaging–multicenter trial. Radiology. (2009) 252:789–96. doi: 10.1148/radiol.2523081798
21. Adams G, Greene J, Vick G, Harrist R, Kimball K, Karmonik C, et al. Tracking regression and progression of atherosclerosis in human carotid arteries using high-resolution magnetic resonance imaging. Magn Reson Imaging. (2004) 22:1249–58. doi: 10.1016/j.mri.2004.08.020
22. Underhill HR, Yuan C, Zhao XQ, Kraiss LW, Parker DL, Saam T, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J. (2008) 155:584 e1–8. doi: 10.1016/j.ahj.2007.11.018
23. Saam T, Yuan C, Chu B, Takaya N, Underhill H, Cai J, et al. Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis. (2007) 194:e34–42. doi: 10.1016/j.atherosclerosis.2006.08.016
24. Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ JM UK-I, et al. The ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) study. evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. (2009) 53:2039–50. doi: 10.1016/j.jacc.2009.03.018
25. Sadat U, Howarth SP, Usman A, Taviani V, Tang TY, Graves MJ, et al. Effect of low-and high-dose atorvastatin on carotid artery distensibility using carotid magnetic resonance imaging -a post-hoc sub group analysis of ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) study. J Atheroscler Thromb. (2013) 20:46–56. doi: 10.5551/jat.12633
26. Sibley CT, Vavere AL, Gottlieb I, Cox C, Matheson M, Spooner A, et al. MRI-measured regression of carotid atherosclerosis induced by statins with and without niacin in a randomised controlled trial: the NIA plaque study. Heart. (2013) 99:1675–80. doi: 10.1136/heartjnl-2013-303926
27. Lee JM, Wiesmann F, Shirodaria C, Leeson P, Petersen SE, Francis JM, et al. Early changes in arterial structure and function following statin initiation: quantification by magnetic resonance imaging. Atherosclerosis. (2008) 197:951–8. doi: 10.1016/j.atherosclerosis.2007.09.001
28. Lee JM, Robson MD, Yu LM, Shirodaria CC, Cunnington C, Kylintireas I, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. (2009) 54:1787–94
29. Migrino RQ, Bowers M, Harmann L, Prost R, LaDisa JF. Carotid plaque regression following 6-month statin therapy assessed by 3T cardiovascular magnetic resonance: comparison with ultrasound intima media thickness. J Cardiovasc Magn Reson. (2011) 13:37. doi: 10.1186/1532-429X-13-37
30. Zhao XQ, Dong L, Hatsukami T, Phan BA, Chu B, Moore A, et al. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging. (2011) 4:977–86. doi: 10.1016/j.jcmg.2011.06.013
31. Du R, Cai J, Cui B, Wu H, Zhao X, Ye P. Rapid improvement in carotid adventitial angiogenesis and plaque neovascularization after rosuvastatin therapy in statin treatment-naïve subjects. J Clin Lipidol. (2019) 13:847–53. doi: 10.1016/j.jacl.2019.07.008
32. Feng T, Huang X, Liang Q, Liang Y, Yuan Y, Feng L, et al. Effects of pitavastatin on lipid-rich carotid plaques studied using high-resolution magnetic resonance imaging. Clin Ther. (2017) 39:620–9. doi: 10.1016/j.clinthera.2017.01.013
33. Alkhalil M, Biasiolli L, Akbar N, Galassi F, Chai JT, Robson MD, et al. T2 mapping MRI technique quantifies carotid plaque lipid, and its depletion after statin initiation, following acute myocardial infarction. Atherosclerosis. (2018) 279:100–6. doi: 10.1016/j.atherosclerosis.2018.08.033
34. Du R, Zhao XQ, Cai J, Cui B, Wu HM, Ye P. Changes in carotid plaque tissue composition in subjects who continued and discontinued statin therapy. J Clin Lipidol. (2016) 10:587–93. doi: 10.1016/j.jacl.2016.01.004
35. Dong L, Kerwin WS, Chen H, Chu B, Underhill HR, Neradilek MB, et al. Carotid artery atherosclerosis: effect of intensive lipid therapy on the vasa vasorum–evaluation by using dynamic contrast-enhanced MR imaging. Radiology. (2011) 260:224–31. doi: 10.1148/radiol.11101264
36. Hippe DS, Phan BAP, Sun J, Isquith DA, O'Brien KD, Crouse JR, et al. Lp(a) (lipoprotein(a)) levels predict progression of carotid atherosclerosis in subjects with atherosclerotic cardiovascular disease on intensive lipid therapy: an analysis of the aim-high (atherothrombosis intervention in metabolic syndrome with low hdl/high triglycerides: impact on global health outcomes) carotid magnetic resonance imaging substudy-brief report. Arterioscler Thromb Vasc Biol. (2018) 38:673–8. doi: 10.1161/ATVBAHA.117.310368
37. Chen X, Wang S, Lin L, Li Y, Zhang H. Drug effect of atorvastatin on middle cerebral atherosclerotic stenosis and high resolution NMR diagnosis. Pak J Pharm Sci. (2018) 31:1169–73.
38. Chung JW, Hwang J, Lee MJ, Cha J, Bang OY. Previous statin use and high-resolution magnetic resonance imaging characteristics of intracranial atherosclerotic plaque: the intensive statin treatment in acute ischemic stroke patients with intracranial atherosclerosis study. Stroke. (2016) 47:1789–96. doi: 10.1161/STROKEAHA.116.013495
39. Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. (2003) 41(4 Suppl S):15S–22. doi: 10.1016/S0735-1097(02)02834-6
40. Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. (2004) 13:125–38. doi: 10.1016/S1054-8807(04)00004-3
41. Virmani R, Burke A, Farb A, Kolodgie F. Pathology of the vulnerable plaque. J Am Coll Cardiol. (2006) 47:C13–8. doi: 10.1016/j.jacc.2005.10.065
42. Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. (2002) 8:1257–62. doi: 10.1038/nm1102-1257
43. Porambo ME, DeMarco JK MR imaging of vulnerable carotid plaque. Cardiovasc Diagn Ther. (2020) 10:1019–31. doi: 10.21037/cdt.2020.03.12
44. Sun J, Zhao XQ, Balu N, Neradilek MB, Isquith DA, Yamada K, et al. Carotid plaque lipid content and fibrous cap status predict systemic CV outcomes: the MRI substudy in AIM-HIGH. JACC Cardiovasc Imaging. (2017) 10:241–9. doi: 10.1016/j.jcmg.2016.06.017
45. Beddhu S, Boucher RE, Sun J, Balu N, Chonchol M, Navaneethan S, et al. Chronic kidney disease, atherosclerotic plaque characteristics on carotid magnetic resonance imaging, and cardiovascular outcomes. BMC Nephrol. (2021) 22:69. doi: 10.1186/s12882-021-02260-x
46. Brunner G, Virani S, Sun W, Liu L, Dodge R, Nambi V, et al. Associations between carotid artery plaque burden, plaque characteristics, and cardiovascular events: The ARIC carotid magnetic resonance imaging study. JAMA cardiology. (2021) 6:79–86. doi: 10.1001/jamacardio.2020.5573
47. Hellings W, Peeters W, Moll F, Piers S, van Setten J, Van der Spek P, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. (2010) 121:1941–50. doi: 10.1161/CIRCULATIONAHA.109.887497
48. Gkagkanasiou M, Ploussi A, Gazouli M, Efstathopoulos EP. USPIO-enhanced MRI neuroimaging: a review. J Neuroimaging. (2016) 26:161–8. doi: 10.1111/jon.12318
49. Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. (2006) 241:459–68. doi: 10.1148/radiol.2412051336
50. Kerwin W, Hooker A, Spilker M, Vicini P, Ferguson M, Hatsukami T, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. (2003) 107:851–6. doi: 10.1161/01.CIR.0000048145.52309.31
51. Ruehm S, Corot C, Vogt P, Kolb S, Debatin J. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. (2001) 103:415–22. doi: 10.1161/01.CIR.103.3.415
52. Zervas N, Liszczak T, Mayberg M, Black P. Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg. (1982) 56:475–81. doi: 10.3171/jns.1982.56.4.0475
53. Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. (2006) 113:2245–52. doi: 10.1161/CIRCULATIONAHA.105.578955
54. Langheinrich AC, Kampschulte M, Buch T, Bohle RM. Vasa vasorum and atherosclerosis - quid novi? Thromb Haemost. (2007) 97:873–9. doi: 10.1160/TH06-12-0742
55. Moulton KS. Plaque angiogenesis and atherosclerosis. Curr Atheroscler Rep. (2001) 3:225–33. doi: 10.1007/s11883-001-0065-0
56. O'Brien K, Hippe D, Chen H, Neradilek M, Probstfield J, Peck S, et al. Longer duration of statin therapy is associated with decreased carotid plaque vascularity by magnetic resonance imaging. Atherosclerosis. (2016) 245:74–81. doi: 10.1016/j.atherosclerosis.2015.11.032
57. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American society of neuroradiology. AJNR Am J Neuroradiol. (2017) 38:218–29. doi: 10.3174/ajnr.A4893
58. Lee HN, Ryu CW, Yun SJ. Vessel-wall magnetic resonance imaging of intracranial atherosclerotic plaque and ischemic stroke: a systematic review and meta-analysis. Front Neurol. (2018) 9:1032. doi: 10.3389/fneur.2018.01032
59. Zhu C, Sadat U, Patterson AJ, Teng Z, Gillard JH, Graves MJ. 3D high-resolution contrast enhanced MRI of carotid atheroma–a technical update. Magn Reson Imaging. (2014) 32:594–7. doi: 10.1016/j.mri.2014.01.019
60. Saba L, Yuan C, Hatsukami TS, Balu N, Qiao Y, DeMarco JK, et al. Carotid artery wall imaging: perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American society of neuroradiology. AJNR Am J Neuroradiol. (2018) 39:E9–E31. doi: 10.3174/ajnr.A5488
61. Brinjikji W, DeMarco JK, Shih R, Lanzino G, Rabinstein AA, Hilditch CA, et al. Diagnostic accuracy of a clinical carotid plaque MR protocol using a neurovascular coil compared to a surface coil protocol. J Magn Reson Imaging. (2018) 48:1264–72. doi: 10.1002/jmri.25984
Keywords: vessel wall imaging, intracranial, carotid, atherosclerosis, plaque, statin
Citation: Zhou P, Wang Y, Sun J, Yu Y, Mossa-Basha M and Zhu C (2021) Assessment of Therapeutic Response to Statin Therapy in Patients With Intracranial or Extracranial Carotid Atherosclerosis by Vessel Wall MRI: A Systematic Review and Updated Meta-Analysis. Front. Cardiovasc. Med. 8:742935. doi: 10.3389/fcvm.2021.742935
Received: 17 July 2021; Accepted: 28 September 2021;
Published: 27 October 2021.
Edited by:
Venkatesh Mani, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Hao Li, University of Cambridge, United KingdomYisen Zhang, Capital Medical University, China
Copyright © 2021 Zhou, Wang, Sun, Yu, Mossa-Basha and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuting Wang, d2FuZ3l1dGluZ18zMzBAMTYzLmNvbQ==
 Pengyu Zhou
Pengyu Zhou Yuting Wang
Yuting Wang Jie Sun2
Jie Sun2 Yannan Yu
Yannan Yu Mahmud Mossa-Basha
Mahmud Mossa-Basha Chengcheng Zhu
Chengcheng Zhu