
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 02 December 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.742297
This article is part of the Research Topic Fundamental Enrichment of Ratio-Based Metrics in Cardiology View all 7 articles
The hemodynamic effects of aortic stenosis (AS) consist of increased left ventricular (LV) afterload, reduced myocardial compliance, and increased myocardial workload. The LV in AS patients faces a double load: valvular and arterial loads. As such, the presence of symptoms and occurrence of adverse events in AS should better correlate with calculating the global burden faced by the LV in addition to the transvalvular gradient and aortic valve area (AVA). The valvulo-arterial impedance (Zva) is a useful parameter providing an estimate of the global LV hemodynamic load that results from the summation of the valvular and vascular loads. In addition to calculating the global LV afterload, it is paramount to estimate the stenosis severity accurately. In clinical practice, the management of low-flow low-gradient (LF-LG) severe AS with preserved LV ejection fraction requires careful confirmation of stenosis severity. In addition to the Zva, the dimensionless index (DI) is a very useful parameter to express the size of the effective valvular area as a proportion of the cross-section area of the left ventricular outlet tract velocity-time integral (LVOT-VTI) to that of the aortic valve jet (dimensionless velocity ratio). The DI is calculated by a ratio of the sub-valvular velocity obtained by pulsed-wave Doppler (LVOT-VTI) divided by the maximum velocity obtained by continuous-wave Doppler across the aortic valve (AV-VTI). In contrast to AVA measurement, the DI does not require the calculation of LVOT cross-sectional area, a major cause of erroneous assessment and underestimation of AVA. Hence, among patients with LG severe AS and preserved LV ejection fraction, calculation of DI in routine echocardiographic practice may be useful to identify a subgroup of patients at higher risk of mortality who may derive benefit from aortic valve replacement. This article aims to elucidate the Zva and DI in different clinical situations, correlate with the standard indexes of AS severity, LV geometry, and function, and thus prove to improve risk stratification and clinical decision making in patients with severe AS.
Aortic stenosis (AS) is one of the most common and critical valve diseases in the world. In North America and Europe, aortic valve disease is primarily due to calcification or a congenital bicuspid valve. AS progresses with aging as calcium, thickening, inflammation, or scarring damages the valve, which restricts blood flow. The normal aortic valve area is between 3.0 and 4.0 cm2. The pressure gradient across the aortic valve and aortic transvalvular flow is directly correlated to the aortic valve area. In general, patients with AS will have symptoms of heart failure when the aortic valve area is <1.0 cm2 and/or the mean pressure gradient is over 40 mmHg (Table 1). The pressure gradient utilizing the modified Bernoulli equation indicates a robust correlation with direct pressure measurement.
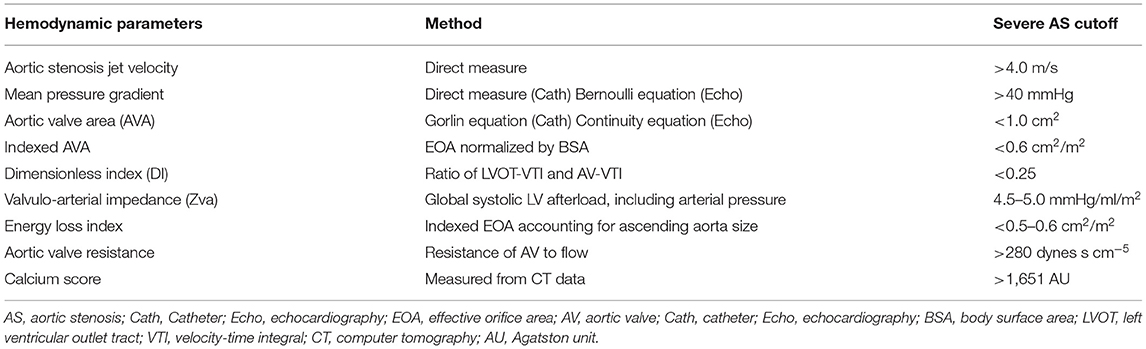
Table 1. Hemodynamic parameters for assessment of aortic stenosis and their cutoff values for severe aortic stenosis.
where ΔP (mmHg) is the maximum pressure gradient between the left ventricle (LV) and the aorta, VAV (m/s) is the maximum stenotic jet velocity. Then, the pressure gradient is applied to estimate the aortic valve area by the continuity principle.
where LVOT-VTI is velocity-time integral in the left ventricular outflow tract, LVOT-CSA is the cross-sectional area of the left ventricular outflow tract, AV-VTI is velocity-time integral across the aortic valve, and AVA is the area of the stenotic aortic valve (Figure 1). Although the severity of AS can be assessed by Doppler echocardiography in almost all patients, it may be underestimated if the range of the interest in Doppler is not well-aligned with the AS jet. Moreover, these conventional methods are limited in low-flow states. As such, the severity of the stenosis may be underestimated in patients with lower left ventricular ejection fraction (LVEF) and cardiac output.
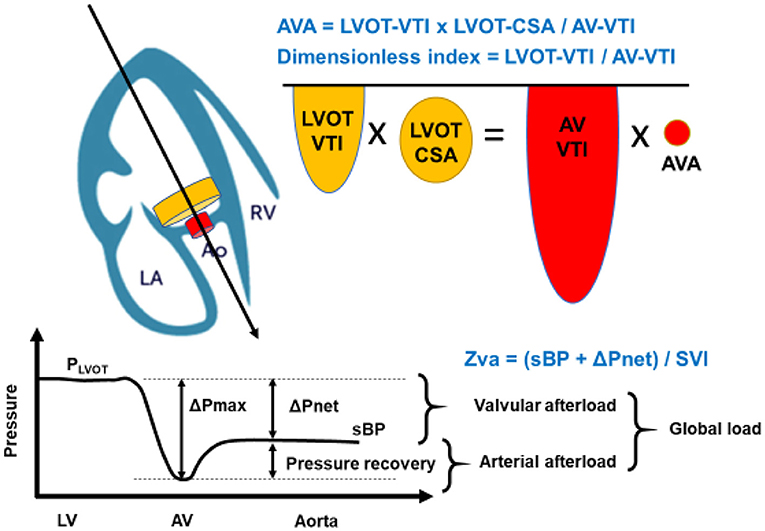
Figure 1. Concept of Aortic Valve Area (AVA) calculation, dimensionless index, and Zva. The calculation of AVA is a standard and must be incorporated into a comprehensive evaluation of aortic stenosis severity. The angle of color Doppler should be accurately aligned. As the LVOT radius is squared to obtain LVOT-CSA in AVA calculation, which may allow inaccuracies and can also contribute substantially to error. The dimensionless index is obtained by LVOT-VTI divided by AV-VTI. The global hemodynamic load imposed on the LV results from the summation of the valvular afterload and the arterial afterload. This global load can be estimated by calculating the valvulo-arterial impedance (Zva). Zva can be calculated with the Doppler mean pressure gradient in place of the ΔPnet: mean pressure gradient. LA, left atrium; Ao, aortic valve; RV, right ventricle; AVA, aortic valve area; LVOT-VTI, left ventricular outlet tract- velocity time integral; LVOT-CSA, cross sectional area; AV-VTI, aortic stenosis- velocity time integral; Zva, valvulo-arterial impedance; sBP, systolic blood pressure; SVI, stroke volume index.
In addition, AS is not just a disease of the valve itself. Indeed, AS is a complex systemic disease with abnormalities of the systemic arterial system such as reduced arterial compliance, LV systolic dysfunction, and reduced transvalvular flow rate, which pose important challenges with regards to diagnostic evaluation and clinical decision making in AS patients (1, 2). Hence, the assessment of AS severity, as well as its therapeutic management, should be conducted with the use of a comprehensive evaluation of the aortic valve, myocardial compliance, and the global LV hemodynamic afterload. The pathophysiology of adverse outcomes in AS is essentially due to an imbalance between the increase in LV hemodynamic afterload due to the valvular obstruction and/or concomitant arterial hypertension, and the capacity of the LV to overcome the “double load.” To reiterate, a double load imposed on the left ventricle by AS, in addition to an increased arterial afterload, the LV hypertrophic process is accelerated due to an aggregated increase in thickness of the LV wall (3). According to the current guidelines, valvular replacement is indicated for severe symptomatic AS and for some groups in asymptomatic individuals with severe AS and preserved LVEF (4–6). In particular, the transvalvular velocity, pressure gradient, and aortic valve area are highlighted in the severity of AS. In this review, we summarize the hemodynamics of severe AS, global LV load, valvulo-arterial impedance (Zva), and accurate assessment of severity with dimensionless index (DI) in different clinical situations, which might aid in improving the prognosis of AS (Figure 1, Table 2).
According to the current American Heart Association/American College of Cardiology and 2021 European Society of Cardiology/European Association of Cardio-thoracic Surgery (ESC/EACTS) guidelines, echocardiographic evaluation of severe AS is defined as: peak transvalvular velocity (Vpeak) ≥ 4.0 m/s, mean pressure gradient (mPG) ≥ 40 mm Hg, aortic valve area (AVA) ≤ 1.0 cm2 and/or AVA indexed for body surface area (BSA) ≤ 0.6 cm2/m2 (4, 6, 7). Indexing AVA for BSA is important in patients whom the valve area may be small but not severely stenotic when adjusted for body size. Discrepancies are frequently encountered between mPG or Vpeak and AVA (8). Moreover, the calculation of the size of the functional aortic orifice by the continuity equation relies on the accurate measurement of the LVOT-CSA (9), frequently underestimated by echocardiography (10) (Table 3). Studies have shown that the LVOT is often elliptical and not circular and thus measuring the LVOT diameter in patients with severe valve calcification is challenging (12, 13). In addition, accurate recording of peak aortic jet velocity requires parallel alignment between the continuous Doppler ultrasound beam and the aortic flow. Non-parallel alignment leads to underestimation of AS severity and thus is operator dependent (2). In clinical practice, these discordant findings may lead to inaccurate assessment of the severity of the AS that could delay therapeutic management and thus produce negative patient outcomes.
Hypertension is found in one-third of patients presenting with symptomatic severe AS. The resultant LV remodeling and hypertrophy are adaptive responses to chronic LV systolic pressure overload and are commonly encountered in patients with hypertension and AS. There are several mechanisms that can explain the important reduction of mean gradient in the presence of hypertension at any AS severity; (1) hypertension causes an increase in systemic vascular resistance, and (2) the decrease in stroke volume induced by increased afterload leads to a reduction in transvalvular gradient and velocity. Thus, the increased “double load,” which includes the arterial and LV afterload, interferes with Doppler echocardiographic evaluation of the severity of AS (Figure 1).
In order to negate the falsely low transvalvular gradient and velocity, the valvulo-arterial impedance index (Zva) can be used (14). Zva is a measure of the global LV afterload. In patients with AS, the hemodynamic index is defined as the ratio of the LV systolic pressure over stroke volume indexed (SVI) to BSA, that is,
where sBP is the systolic blood pressure, and ΔPnet is the mean transvalvular pressure gradient after pressure recovery (14). Thus, this Zva can estimate global LV load and represents the cost in mmHg for each systemic milliliter of blood indexed for BSA pumped by the LV during systole (Figure 1). The index can be calculated via non-invasive means such as the Doppler echocardiogram or invasive means with cardiac catheterization. According to a previous study, a value of Zva ≥ 5.0 mmHg/mL·m2 might represent LV afterload mismatch, and LV systolic dysfunction and Zva ≥ 5.5 mmHg/mL·m2 was associated with a 2.5-fold increase in the risk of overall mortality (15). Moreover, the Zva is particularly useful in patients who do not meet the criteria of severe AS such as with low-flow, low-gradient (LF-LG) severe AS and preserved LVEF. Zva was also the main determinant of LV dysfunction in asymptomatic paradoxical LF-LG severe AS in the SEAS study (16). Similar findings were demonstrated by Hachicha et al. with increased Zva (>3.5 mmHg/mL·m2) as a predictor of poor outcome in asymptomatic severe AS patients due to associated LV systolic and diastolic dysfunction (17). These data suggest that Zva may guide risk stratification and therapeutic management in AS patients who do not fit the criteria for typical severe AS.
The dimensionless index (DI) represents the ratio of the LVOT VTI in relation to that of the aortic valve jet by eliminating the highly erroneous measurement of LVOT cross-sectional area from the continuity equation (18).
The DI is calculated by a ratio of the sub-valvular velocity obtained by pulsed-wave Doppler (LVOT-VTI) divided by the maximum velocity obtained by continuous-wave Doppler across the aortic valve (AV-VTI) (19) (Figure 1). It expresses the size of the valvular effective area as a proportion of the CSA of the LVOT. Alternatively, the ratio of the peak velocity of LVOT to peak AV jet can be used. In the absence of valve stenosis, the velocity ratio approaches 1, with smaller numbers indicating severe stenosis. For instance, severe AS is present when the velocity ratio is 0.25 or less, representing 25% of the valve. Otto et al. reported that DI showed better sensitivity than Doppler pressure gradient to identify severe AS (97 vs. 81%) (20). DI is normalized for body size as it reflects the ratio of the actual valve area compared to the expected valve area.
In cardiovascular research, ratio-based indices are often used, such as LVEF (21), arterial pressure augmentation index (22), coronary fractional flow reserve (23), and pulse pressure (24). When the ratio is used alone, it may provide incomplete information about the content of the two components of a fraction-based index. Indeed, just focusing on the single number of the ratio results in a loss of information. However, the lost information can be regained without additional measurements by applying the Pythagorean mean (25). A polar coordinate description can reveal the companion of the traditional metric. The clinical relevance of both the ratio and the associated companion can often be clarified by demonstrating a statistically significant association with an established physical measure with sound interpretation. In the case of the DI, constitutive metrics are AV-VTI and LVOT-VTI. Companion (the diagonal length of the triangle) is calculated using the Pythagorean theorem (Figure 2A).
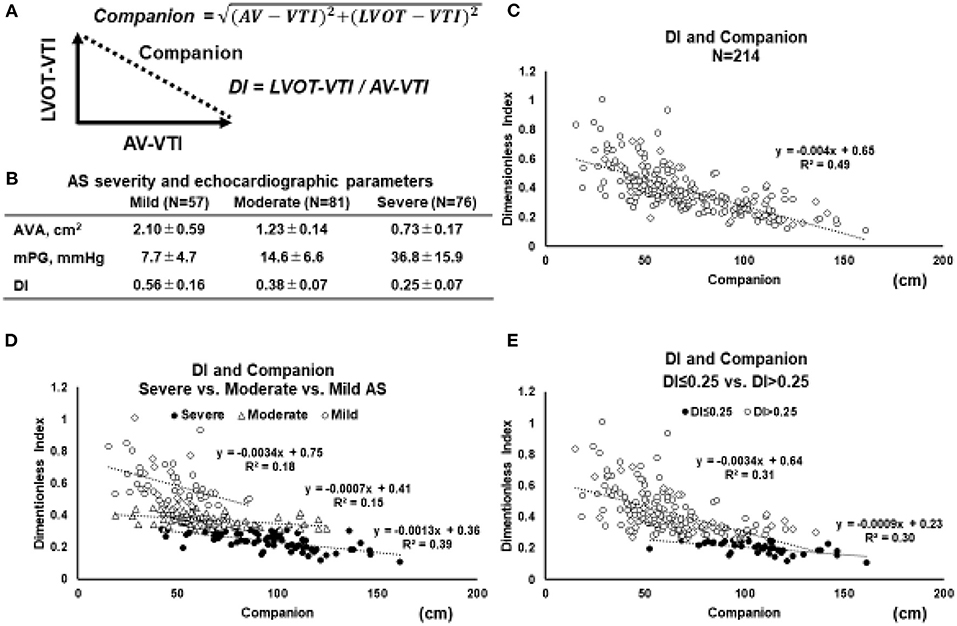
Figure 2. Relation between DI and its companion. (A) Geometric relationship between the two parameters and the companion. Based on Pythagorean theorem, the companion (diagonal length of the triangle) is obtained by the following equation: Companion = √(AV − VTI)2 + (LVOT − VTI)2. (B) In order to elucidate the behavior of the relation between DI and companion, the echocardiographic parameters in 214 patients with AS are retrospectively reviewed. (C) The relations between the DI and companion in whole AS patients (N = 214). (D) The relation between the DI and companion in separated by the severity of AS (mild vs. moderate vs. severe). (E) The relation between the DI and companion in AS patients with DI ≤ 0.25 vs. DI > 0.25 (E). Abbreviations are as same as in Figure 1.
To elucidate the behavior of the relation between DI and its companion, echocardiographic parameters in 214 patients with AS are retrospectively reviewed (Figure 2B). The relation between the DI and companion in whole AS patients (N = 214) (Figure 2C), separated by the severity of AS (mild vs. moderate vs. severe) (Figure 2D), and in patients with DI ≤ 0.25 vs. DI > 0.25 (Figure 2E). According to the 2021 ESC/ EACTS guidelines, DI value of < 0.25 suggests severe AS, especially when other clinical and echocardiographic parameters are equivocal (6). When stratified by AS severity, it was found that each group could be separated relatively clearly (Figure 2D). These figures indicated that the smaller the DI, the more the slope asymptotes to zero, regardless of the companion. Especially in the AS patients with DI < 0.25, the slope became almost parallel to the x-axis, and the triangle formed by AV-VTI and LVOT-VTI changed with a constant similarity ratio. Taken together, the DI can separate AS severity with clarity and enables clinicians to assess the severity of AS without worrying about the magnitude of AV-VTI and LVOT-VTI if it is <0.25.
Asymptomatic severe AS is classified into the two groups: (1) asymptomatic severe AS with normal LVEF (Stage C1) and (2) asymptomatic severe AS with low LVEF (Stage C2) (5) (Table 3). Surgical intervention is indicated for patients with asymptomatic severe AS (Stage C2). In patients with asymptomatic severe AS, the determinant factors resulting in poor prognosis are higher peak velocity (≥5 m/s) (26), rapid progression of stenosis (incremental peak velocity > 0.3 m/s/year) (27, 28), elevated BNP (29), and pulmonary hypertension (tricuspid valve regurgitation velocity ≥3.5 m/s or systolic pulmonary arterial pressure ≥60 mmHg) (30, 31). Current guidelines recommend that patients with asymptomatic severe AS including these parameters and lower risk of surgery should have AVR (5, 32). One of the additions in the 2021 ESC/ EACTS guidelines include surgery, as a class I recommendation, for asymptomatic patients resting LVEF ≤ 50% without another differential diagnosis. In addition, early intervention in asymptomatic patients (Class IIa) with a LVEF < 55% and a normal exercise test if the stenosis is severe; i.e. mean gradient > 60 mmHg or Vmax >5 m/s, severe calcification on cardiac computed tomography and Vmax progression > 0.3 m/s/year, or elevated BNP that is three times the normal range (6). In addition, moderate to severe calcification of the aortic valve (28), reduced global longitudinal strain (GLS) (33), and impaired left atrial function (left atrial dilatation and reduced peak late diastolic annular velocity) have also been reported to be risk factors of AS progression (29, 34). Therefore, early and appropriate surgical aortic valve replacement (AVR) is recommended for high-risk severe AS patients in the stage C1, rather than clinical observation (26, 35, 36).
Several studies have demonstrated favorable impacts of DI and Zva on the clinical management of patients with asymptomatic severe AS (18, 37, 38). In the cohort of asymptomatic and minimally symptomatic severe AS, lower DI was significantly associated with poor mortality (18, 38). Rusinaru et al. reported that the risk of all-cause mortality and AVR increased by about 25% for every 0.05 decrease in the DI (18). Interestingly, patients with very low DI (< 0.2) had particularly increased adverse events, compared to that of severe AS and higher DI (18, 39). Oh et al. also reported that low DI (DI ≤ 0.25) was significantly related to the higher severity of AS (AVA ≤ 0.75 cm2) measured by cardiac catheterization (40). In addition, the combination of DI, AVA, and AV peak velocity was proven to be more sensitive indicators of cardiac adverse events, compared to AVA alone or a combination of AVA and AV peak velocity (18). Thus, the combination of DI and other hemodynamic parameters can be a promising clinical predictor for the timing of AVR.
Increased Zva is a useful predictor of LV hemodynamic afterload (37, 41). These myocardial changes instigate cardiac diastolic and systolic dysfunction, symptomatic heart failure, and cardiovascular death (37, 41–44). In addition, it is becoming evident that a higher global LV load which Zva quantifies is associated with the progression of LV dysfunction. Cramariuc et al. demonstrated that abnormal stress corrected mid-wall shortening and LV longitudinal strain was seen in 10% of patients in the lowest vs. 63% of patients in the highest tertile of Zva (P < 0.001) in 1,591 patients with asymptomatic severe AS and normal LVEF in the Simvastatin Ezetimibe in Aortic Stenosis trial (16). Currently, Zva > 5.0 mmHg/mL·m2 is considered to be an indicator of severe AS (45). A previous study reported that Zva value of > 3.5 mmHg/mL·m2 was able to predict AS progression or mortality in the patients with asymptomatic AS (41). Taken together, Zva may be used to improve risk stratification and clinical decision-making in patients with asymptomatic AS. To determine the timing of surgical intervention, the appropriate value of Zva should be further investigated.
Moderate AS is defined hemodynamically as an AV velocity of 3.0–3.9 m/s or mean pressure gradient (PG) of 20–39 mmHg, with AVA ≥ 1.0 cm2 or normal AV flow (SVI ≥ 35 L/m2) (5, 43). When a patient is classified as moderate AS with lower SVI (<35 mL/m2), additional examinations should be performed to assess whether the severity of AS is severe or moderate. In addition, if patients with moderate AS may have heart failure symptoms such as dyspnea on effort, chest pain, or fainting, the next two points need to be reconsidered: (1) Is the severity of AS really moderate, but not severe? and (2) Are there any other diseases related to the heart failure symptoms other than AS?
To assess the AVA, maximum AV velocity should be selected from the highest velocity signal in multiple echocardiographic windows (43). However, obtaining an appropriate view is sometimes difficult in patients with poor echocardiographic delineation due to obesity, emaciation, emphysema, artificial breasts, or post-breast surgery. In these patients, AS severity may tend to be underestimated. In addition, there may be a discordance between the estimated LVOT by echocardiogram and its actual area due to the elliptical geometry of the LVOT (46). In contrast, DI does not require measurement of LVOT size and is less variable than the estimated AVA. Moreover, because DI is just a ratio, DI has the potential to assess the true severity of AS in patients with poor imaging in the echocardiogram. When AS severity is classified as moderate even after using the DI method, other reasons for the symptoms should be considered.
Evaluation of LV outflow tract stenosis (LVOTS) is also important. Abnormal LV septal thickening is observed about 10% in patients with AS (46). For patients with LVOTS, medications such as beta-blocker and cibenzoline are useful (47). In the condition of moderate AS with LVOTS, the DI can be useful for assessing AS severity (43). Zva can represent the effective global afterload against the LV (15, 17) (Figure 1). Indeed, the Zva is an independent predictor of syncope in patients with AS, which suggests that Zva may be able to detect hemodynamic changes in patients with AS (48). Thus, in identifying the causes of symptoms in patients with moderate AS, DI can play an important role in assessing the true severity of AS. In addition, Zva also can provide an accurate evaluation of global LV afterload in patients with moderate AS and LVOTS.
In patients with symptomatic moderate AS, coronary artery disease (CAD) should be carefully assessed. In patients with AS, CAD events occur at a rate of up to 20% per 5 years (49). Management of CAD risk factors, such as hyperlipidemia, hypertension, or diabetic mellitus, does not necessarily impede the progression of AS, but can prevent cardiac ischemic events (50, 51). In current guidelines, surgical AVR for moderate AS should be considered when another cardiac surgery is required (recommendation: class 2b) (52). When coronary artery bypass graft (CABG) is suitable, surgicalAVR with CABG is an indication.
In patients with severe AS and preserved ejection fraction, deciding between clinical observation vs. early intervention to AS remains disputable (28, 52). The clinical challenges are (1) to predict precise prognosis and (2) to determine the appropriate timing of intervention in asymptomatic patients with severe AS. Current guidelines recommend that AVR is appropriate in asymptomatic patients with very severe AS; however, the definition of severe AS based on peak aortic jet velocity (Vmax) remains unclear with a 5.0 m/s cutoff in ACC/AHA guidelines (7) and 5.5 m/s in European guidelines (4). Approximately 20% of patients with severe AS and preserved LVEF have Vmax in this range between 5.0 and 5.5 m/s (53). Therefore, additional clinical indicators to make the clinical decision must be evaluated.
Regarding the usefulness of DI for evaluating the severity of AS in patients with preserved EF, Otto et al. reported that the DI was more sensitive than aortic valve pressure gradient (AV-PG) in identifying severe AS (sensitivity 97 vs 81%) (20). Moreover, Oh et al. also demonstrated that DI < 0.25 was also useful in identifying severe AS (40). Rusinaru et al. evaluated the relation between DI value and 5-year survival free of the events including cardiovascular death or need for AVR in AS patients without symptoms (18). This study reported that the DI was a reliable marker for assessing the severity of AS and that DI < 0.25 was a useful parameter in predicting prognosis. Thus, the DI can assess the severity and predict the prognosis of AS in severe AS patients with preserved ejection fraction.
AS is affected by the global hemodynamic loads imposed on the LV and arterial afterload (14, 54). Especially in patients with degenerative AS, the coexistence of arteriosclerosis and hypertension is common, and these coexisting diseases increase systemic vascular resistance. Therefore, a comprehensive evaluation of the “actual” global hemodynamic load by Zva is even more useful than conventional AS parameters. Hachica et al. reported that Zva predicted adverse outcomes in asymptomatic AS patients with preserved LVEF, even after adjustment for standard indexes of LV geometry and function. Indeed, Zva > 3.5 mmHg/mL·m2 can detect a poor prognosis in these patients (45). Zito et al. also investigated the role of the Zva in patients of asymptomatic severe AS with preserved LVEF and demonstrated that Zva was independently associated with death, AVR, and heart failure symptoms including dyspnea, angina, and syncope (36). They also reported that Zva ≥ 4.7 mmHg/ml·m2 was identified as the best cutoff value associated with the cardiovascular events (sensitivity 100% and specificity 91%) (36). Based on these results, the Zva is a useful parameter for performing risk stratification and clinical decisions in severe AS with preserved LVEF.
Low-flow (LF) severe AS (SVI ≤ 35 mL/m2) and low-gradient (LG) severe AS (transvalvular mean PG ≤ 40 mmHg) are partially overlapping categories in severe AS (Table 4). Severe AS with PG < 40 mmHg due to a decrease in cardiac output (SVI ≤ 35 mL/m2) is referred to as LF-LG severe AS (55). The type of LF-LG severe AS is separated into two types: (1) classical LF-LG severe AS and (2) paradoxical LF-LG severe AS. (1) The classical LF-LG severe AS is often observed in patients with the decreased LVEF (<40%). (2) The paradoxical LF-LG AS is due to both the LV narrowing cavity and LV diastolic dysfunction, despite preserved LVEF (LVEF ≥ 50%). The assessment of the actual type of LF-LG severe AS is important to decide therapeutic strategies.
There are two main causes of LV systolic dysfunction in classic LF-LG AS: (1) impacts from a progression of AS per se and (2) miscellaneous myocardial impairments such as myocardial ischemia or cardiomyopathy. Given LV systolic function is impaired by increased afterload due to severe AS per se, the peak aortic velocity and mean pressure gradient can be categorized in a moderate subset because the aortic velocity depends on the reduced flow rate. The effective orifice area (EOA) may be misinterpreted as a smaller area because the LV cannot provide sufficient stroke volume to generate the inertial force required to maximize the aortic valve opening. Therefore, patients with LF-LG AS need to be tested with increased SV to accurately assess EOA. Interestingly, it is well-known that the prognosis of classical LF-LG severe AS is worse than that of normal flow high gradient severe AS (56, 57). Therefore, it is important to distinguish “true-AS” or “pseudo-AS” in patients with LF-LG severe AS. As a useful evaluation method, dobutamine stress-echocardiography is recommended in guidelines (58). Dobutamine stress-echocardiography or invasive hemodynamic study with dobutamine can also evaluate the LV contractile reserve, determining whether it is true-AS or pseudo-AS and predicting patients' prognosis.
No studies have evaluated the impact of DI on clinical outcomes in patients with classical LF-LG severe AS. In assessing the severity of classical LF-LG severe AS, the AVA may be underestimated due to the reduced LV contractility and low flow rate (43). As being the LVOT measurement error, AVA calculated by the continuity equation might lead to measuring incorrectly. Thus, a multiparametric assessment is crucial in these patients. An ancillary study showed that an ejection dynamics parameter, the ratio of the acceleration time to ejection time >0.36, was associated with an increased risk of mortality in patients with LG severe AS (59). Multiple factors such as LV geometry, function, and systolic blood pressure the influence acceleration time to ejection time ratio. Interestingly, Bradley et al. looked at the accuracy of echocardiographic measures of AS severity in 77,067 patients where they found that a multiparameter assessment including using peak velocity, mean gradient, and DI provided the best sensitivity (92%) and specificity (99%) for diagnosis of severe AS compared with any single measure alone including AVA (60). Furthermore, when considering the relation between DI and the companion (Figure 2), DI may indicate the true severity of AS regardless of the size of companion, that is, flow size. A detailed evaluation of this relation is necessary in the future. The measurement of projected AVA under the dobutamine stress-echocardiography in classical LF-LG AS has been proposed in clinical guidelines (43, 61). However, it also includes the same problem caused by the measurement error of LVOT. In this respect, the DI, which excluded the effect of LVOT-CSA, might be useful as a value to support the change in AVA.
As mentioned, the impaired LV function in severe AS is affected by both the valvular and the arterial afterload. Therefore, Zva in classic LF-LG AS is theoretically high, especially in patients with lower SVI. Lewy et al. evaluated Zva in patients with severe symptomatic severe AS with LVEF ≤ 40% (62). The study demonstrated that higher Zva was associated with reduced LVEF. In this study, the Zva of patients with low LVEF-LG severe AS had a high value of 5.6 ± 1.7 mmHg/mL·m2. The assessment of contractile reserve in classical LF-LG severe AS is also important for the prognosis evaluation. This study also compared contractile reserve with Zva at rest by dobutamine stress-echocardiography and reported that patients with contractile reserve tended to have higher Zva (5.8 ± 1.8 vs 5.3 ± 1.3 mmHg/ml·m2; p = 0.07). There was also no statistical significance difference in Zva between resting and during dobutamine stress. Theoretically, pseudo-AS has a lower valvular afterload than true-AS. Therefore, it is ideal to evaluate the valvular and arterial afterload separately to distinguish between true-AS and pseudo-AS. However, it is difficult to separate these afterloads by Zva at rest. The Zva is flow-rate dependent (both mean PG [ΔPnet] and SVI, which are components of Zva) and can be changeable, especially in severe AS patients with low flow (63). In addition, the reduced arterial afterload due to the vasodilatory effect of low dose dobutamine may account for the differences in Zva between “true-AS” and “pseudo-AS,” Therefore, it might be useful to evaluate the change of Zva under dobutamine stress-echocardiography to distinguish between pseudo and true severe AS. However, this should be investigated further in future studies.
Paradoxical LF-LG severe AS has the preserved LVEF, but with the peak aortic velocity of <4.0 m/s and an average PG of <40 mmHg, and a valve area of fewer than 1 cm2 (34, 43) (Table 4). Patients with paradoxical LF-LG severe AS are more common in the elderly adults and characterized by markedly LV concentric hypertrophy and decreased stroke volume. Occasionally, technical errors may occur in the calculation of AVA by echocardiography, so careful assessment should be applied when diagnosing the severity of AS. It also remains challenging to distinguish between true- and pseudo-AS in patients with paradoxical LF-LG severe AS.
In paradoxical LF-LG severe AS, the LVOT often may be near obstructed due to LV hypertrophy and/or sigmoid septum. Both LV sigmoid septum and LVOT stenosis can increase apparent LVOT-VTI, leading to underestimation of DI. Hence, in the assessment of the severity of severe AS, it is necessary to consider that the morphology of LVOT may cause measurement errors related to the AVA and pressure gradient. DI, an AS severity index excluding the LVOT geometry, can be valuable for evaluating paradoxical LF-LG severe AS precisely as an alternative approach for reducing the measurement errors of the LVOT area. Indeed, Altes et al. reported that DI < 0.25 was a reliable parameter in the long-term mortality prediction of paradoxical LF-LG severe AS with or without symptoms (38). Furthermore, the patients with paradoxical LF-LG severe AS and DI < 0.25 were similar outcomes to the patients with high-gradient severe AS (38). As described in the above sections and Figure 2, DI may show the true severity regardless of the flow size. Therefore, further study in the LF-AS group is needed for the detailed evaluation.
Regarding Zva in paradoxical LF-LG AS, Hachicah et al. reported that Zva was higher than in patients with normal flow high gradient severe AS (17). In a multicenter prospective study, Adda et al. also reported that patients with paradoxical LF-LG severe AS had more severe AS (AVA 0.7 ± 0.12 vs. 0.86 ± 0.14 cm2) and higher Zva was seen in patients with normal-flow low-gradient AS (5.5 ± 1.1 vs. 4.0 ± 0.8 mmHg/mL·m2) (64). Those results suggested that the main mechanism of paradoxical LF-LG severe AS was elevated global afterload and that Zva may be useful in distinguishing the severity of paradoxical LF-LG AS. Regarding the effect of Zva on flow, unlike classical LF-LG AS, the SV index of preserved LF-LG AS is considered to be less affected by flow than that of classical LF-AS because the LVEF in paradoxical LF-LG AS is preserved, even if it is lower flow. Therefore, the influence of flow is considered smaller than that of classical LF-LG AS. However, as in the case of DI, the patient background of paradoxical LF-LG severe AS is sometimes accompanied by a sigmoid septum or LVOTS. This makes it difficult to assess the severity of AS because the ventricular outflow tract stenosis has an independent effect on left ventricular afterload. Therefore, a comprehensive evaluation of left ventricular geometry is required to assess the Zva in patients with paradoxical LF-LG AS.
Clinical assessment of AS severity requires calculated AVA, mean and peak PG, and transvalvular flow. Although the AS severity can be assessed and diagnosed by echocardiography, it may be underestimated if echocardiography image quality is poor, especially if color Doppler images are not well-aligned with the AV jet. In addition, the accurate evaluation of AS severity is difficult to assess when cardiac output is lower and LVEF is reduced. The LVOT radius is squared to obtain LVOT-CSA in AVA calculation, allowing inaccuracies and contributing substantially to error. In order to resolve these limitations, the dimensionless index, which is a simple ratio of LVOT-VTI to AV-VTI with removing CSA from the simplified continuity equation, can be used. Among patients with LF-LG severe AS, the low flow can result in incomplete valve opening, leading to overestimate AS severity. Patients with LF-LG severe AS usually have elevated valvulo-arterial impedance (Zva), the assessment of AS severity in consideration with the Zva should be paramount. Incorporating dimensionless index and Zva with standard practices can improve risk stratification and clinical decision-making in patients with severe AS.
YM, SF, SM, and MH wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
MH was supported in part by Clinical Research Promotion Foundation. MH was also supported by JSPS KAKENHI Grant-in-Aid for Young Scientists Number 21K17603.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pibarot P, Dumesnil JG. New concepts in valvular hemodynamics: implications for diagnosis and treatment of aortic stenosis. Can J Cardiol. (2007) 23(Suppl. B):40b−7. doi: 10.1016/S0828-282X(07)71009-7
2. Pibarot P, Dumesnil JG. Improving assessment of aortic stenosis. J Am Coll Cardiol. (2012) 60:169–80. doi: 10.1016/j.jacc.2011.11.078
3. Li JK, Zhu JY, Nanna M. Computer modeling of the effects of aortic valve stenosis and arterial system afterload on left ventricular hypertrophy. Comput Biol Med. (1997) 27:477–85. doi: 10.1016/S0010-4825(97)00027-9
4. Members ATF, Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2012) 33:2451–96. doi: 10.1093/eurheartj/ehs109
5. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
6. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: DEVELOPED by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2021) 1:72. doi: 10.1093/eurheartj/ehab395
7. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:e57–185. doi: 10.1016/j.jacc.2014.02.536
8. Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. (2010) 96:1463–8. doi: 10.1136/hrt.2009.181982
9. Skjaerpe T, Hegrenaes L, Hatle L. Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two-dimensional echocardiography. Circulation. (1985) 72:810–8. doi: 10.1161/01.CIR.72.4.810
10. Gorlin R, Gorlin SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I Am Heart J. (1951) 41:1–29. doi: 10.1016/0002-8703(51)90002-6
11. Otto CM, Nishimura RA. New ACC/AHA valve guidelines: aligning definitions of aortic stenosis severity with treatment recommendations. Heart. (2014) 100:902–4. doi: 10.1136/heartjnl-2013-305134
12. Jabbour A, Ismail TF, Moat N, Gulati A, Roussin I, Alpendurada F, et al. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol. (2011) 58:2165–73. doi: 10.1016/j.jacc.2011.09.010
13. Maes F, Pierard S, De Meester C, Boulif J, Amzulescu M, Vancraeynest D, et al. Impact of left ventricular outflow tract ellipticity on the grading of aortic stenosis in patients with normal ejection fraction. J Cardiovasc Magn Reson. (2017) 19:37. doi: 10.1186/s12968-017-0344-8
14. Antonini-Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R, et al. Symptomatic aortic stenosis: does systemic hypertension play an additional role? Hypertension. (2003) 41:1268–72. doi: 10.1161/01.HYP.0000070029.30058.59
15. Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. (2005) 46:291–8. doi: 10.1016/j.jacc.2004.10.081
16. Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, et al. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging. (2009) 2:390–9. doi: 10.1016/j.jcmg.2008.12.021
17. Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. (2007) 115:2856–64. doi: 10.1161/CIRCULATIONAHA.106.668681
18. Rusinaru D, Malaquin D, Maréchaux S, Debry N, Tribouilloy C. Relation of dimensionless index to long-term outcome in aortic stenosis with preserved LVEF. JACC Cardiovasc Imaging. (2015) 8:766–75. doi: 10.1016/j.jcmg.2015.01.023
19. Jander N, Hochholzer W, Kaufmann BA, Bahlmann E, Gerdts E, Boman K, et al. Velocity ratio predicts outcomes in patients with low gradient severe aortic stenosis and preserved EF. Heart. (2014) 100:1946–53. doi: 10.1136/heartjnl-2014-305763
20. Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol. (1986) 7:509–17. doi: 10.1016/S0735-1097(86)80460-0
21. Beringer JY, Kerkhof PL. A unifying representation of ventricular volumetric indexes. IEEE Trans Biomed Eng. (1998) 45:365–71. doi: 10.1109/10.661161
22. Kerkhof PLM, Konradi AO, Shlyakhto EV, Handly N, Li JK. Polar coordinate description of blood pressure measurements and implications for sex-specific and personalized analysis. Annu Int Conf IEEE Eng Med Biol Soc. (2019) 2019:502–5. doi: 10.1109/EMBC.2019.8857346
23. Kerkhof PLM, Osto E, Tona F, Heyndrickx GR, Handly N. Sex-specific interpretation of coronary flow reserve and fractional flow reserve metrics, including their companions. Annu Int Conf IEEE Eng Med Biol Soc. (2019) 2019:7006–9. doi: 10.1109/EMBC.2019.8857589
24. Kerkhof PL, Heyndrickx GR, Li JK. Hemodynamic determinants and ventriculo-arterial coupling are sex-associated in heart failure patients. Annu Int Conf IEEE Eng Med Biol Soc. (2016) 2016:3286–9. doi: 10.1109/EMBC.2016.7591430
25. Kerkhof PLM, Peace RA, Handly N. Ratiology and a complementary class of metrics for cardiovascular investigations. Physiology. (2019) 34:250–63. doi: 10.1152/physiol.00056.2018
26. Nistri S, Faggiano P, Olivotto I, Papesso B, Bordonali T, Vescovo G, et al. Hemodynamic progression and outcome of asymptomatic aortic stenosis in primary care. Am J Cardiol. (2012) 109:718–23. doi: 10.1016/j.amjcard.2011.10.035
27. Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. (2006) 82:2116–22. doi: 10.1016/j.athoracsur.2006.07.043
28. Kang DH, Park SJ, Rim JH, Yun SC, Kim DH, Song JM, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. (2010) 121:1502–9. doi: 10.1161/CIRCULATIONAHA.109.909903
29. Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, et al. Prospective study of asymptomatic valvular aortic stenosis. Clin Echocardiogr Exerc Predict Outcome Circ. (1997) 95:2262–70. doi: 10.1161/01.CIR.95.9.2262
30. Lancellotti P, Moonen M, Magne J, O'connor K, Cosyns B, Attena E, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol. (2010) 105:383–8. doi: 10.1016/j.amjcard.2009.09.043
31. Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. (2018) 3:1060–8. doi: 10.1001/jamacardio.2018.3152
32. Levy F, Bohbot Y, Sanhadji K, Rusinaru D, Ringle A, Delpierre Q, et al. Impact of pulmonary hypertension on long-term outcome in patients with severe aortic stenosis. Eur Heart J Cardiovasc Imaging. (2018) 19:553–61. doi: 10.1093/ehjci/jex166
33. Lancellotti P, Magne J, Donal E, O'connor K, Dulgheru R, Rosca M, et al. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation. (2012) 126:851–9. doi: 10.1161/CIRCULATIONAHA.111.088427
34. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1016/j.rec.2017.12.013
35. Lancellotti P, Donal E, Magne J, Moonen M, O'connor K, Daubert JC, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. (2010) 96:1364–71. doi: 10.1136/hrt.2009.190942
36. Zito C, Salvia J, Cusmà-Piccione M, Antonini-Canterin F, Lentini S, Oreto G, et al. Prognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valves. Am J Cardiol. (2011) 108:1463–9. doi: 10.1016/j.amjcard.2011.06.070
37. Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. (2003) 107:984–91. doi: 10.1161/01.CIR.0000051865.66123.B7
38. Altes A, Thellier N, Rusinaru D, Marsou W, Bohbot Y, Chadha G, et al. dimensionless index in patients with low-gradient severe aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. (2020) 13:e010925. doi: 10.1161/CIRCIMAGING.120.010925
39. Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, et al. Natural history of very severe aortic stenosis. Circulation. (2010) 121:151–6. doi: 10.1161/CIRCULATIONAHA.109.894170
40. Oh JK, Taliercio CP, Holmes DR Jr, Reeder GS, Bailey KR, Seward JB, et al. Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: prospective Doppler-catheterization correlation in 100 patients. J Am Coll Cardiol. (1988) 11:1227–34. doi: 10.1016/0735-1097(88)90286-0
41. Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. (2005) 26:1790–6. doi: 10.1093/eurheartj/ehi290
42. Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Edwards ST, Nowicki ER, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. (2014) 147:362–9.e8. doi: 10.1016/j.jtcvs.2012.12.016
43. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. (2017) 30:372–92. doi: 10.1016/j.echo.2017.02.009
44. Lindman BR, Dweck MR, Lancellotti P, Généreux P, Piérard LA, O'gara PT, et al. Management of asymptomatic severe aortic stenosis: evolving concepts in timing of valve replacement. JACC Cardiovasc Imaging. (2020) 13:481–93. doi: 10.1016/j.jcmg.2019.01.036
45. Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. (2009) 54:1003–11. doi: 10.1016/j.jacc.2009.04.079
46. Saitoh T, Shiota M, Izumo M, Gurudevan SV, Tolstrup K, Siegel RJ, et al. Comparison of left ventricular outflow geometry and aortic valve area in patients with aortic stenosis by 2-dimensional versus 3-dimensional echocardiography. Am J Cardiol. (2012) 109:1626–31. doi: 10.1016/j.amjcard.2012.01.391
47. Hamada M, Shigematsu Y, Ikeda S, Hara Y, Okayama H, Kodama K, et al. Class Ia antiarrhythmic drug cibenzoline: a new approach to the medical treatment of hypertrophic obstructive cardiomyopathy. Circulation. (1997) 96:1520–4. doi: 10.1161/01.CIR.96.5.1520
48. Harada K, Saitoh T, Tanaka J, Shibayama K, Berdejo J, Shiota T. Valvuloarterial impedance, but not aortic stenosis severity, predicts syncope in patients with aortic stenosis. Circ Cardiovasc Imaging. (2013) 6:1024–31. doi: 10.1161/CIRCIMAGING.113.000584
49. Maron BJ, Clark CE, Henry WL, Fukuda T, Edwards JE, Mathews EC Jr, et al. Prevalence and characteristics of disproportionate ventricular septal thickening in patients with acquired or congenital heart diseases: echocardiographic and morphologic findings. Circulation. (1977) 55:489–96. doi: 10.1161/01.CIR.55.3.489
50. Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. (2008) 359:1343–56. doi: 10.1056/NEJMoa0804602
51. Banovic M, Athithan L, Mccann GP. Aortic stenosis and diabetes mellitus: an ominous combination. Diab Vasc Dis Res. (2019) 16:310–23. doi: 10.1177/1479164118820657
52. Lancellotti P, Magne J, Donal E, Davin L, O'connor K, Rosca M, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. (2012) 59:235–43. doi: 10.1016/j.jacc.2011.08.072
53. Castel A-L, Maréchaux S, Laaouaj J, Rusinaru D, Levy F, Tribouilloy C. Relationship between cutoff values of peak aortic valve velocity and those of other doppler echocardiographic parameters of severity in patients with aortic stenosis and normal flow. Echocardiography. (2012) 29:1150–6. doi: 10.1111/j.1540-8175.2012.01790.x
54. Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart. (2005) 91:354–61. doi: 10.1136/hrt.2003.030601
55. Finegold JA, Manisty CH, Cecaro F, Sutaria N, Mayet J, Francis DP. Choosing between velocity-time-integral ratio and peak velocity ratio for calculation of the dimensionless index (or aortic valve area) in serial follow-up of aortic stenosis. Int J Cardiol. (2013) 167:1524–31. doi: 10.1016/j.ijcard.2012.04.105
56. Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. (2009) 53:1865–73. doi: 10.1016/j.jacc.2009.02.026
57. Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation. (2013) 127:2316–26. doi: 10.1161/CIRCULATIONAHA.112.001290
58. Baumgartner HC, Hung JC-C, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. (2017) 18:254–75. doi: 10.1093/ehjci/jew335
59. Altes A, Thellier N, Bohbot Y, Marsou W, Chadha G, Binda C, et al. Prognostic impact of the ratio of acceleration time to ejection time in patients with low gradient severe aortic stenosis and preserved ejection fraction. Am J Cardiol. (2019) 124:1594–600. doi: 10.1016/j.amjcard.2019.07.064
60. Bradley SM, Foag K, Monteagudo K, Rush P, Strauss CE, Gössl M, et al. Use of routinely captured echocardiographic data in the diagnosis of severe aortic stenosis. Heart. (2019) 105:112–6. doi: 10.1136/heartjnl-2018-313269
61. Blais C, Burwash IG, Mundigler G, Dumesnil JG, Loho N, Rader F, et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation. (2006) 113:711–21. doi: 10.1161/CIRCULATIONAHA.105.557678
62. Levy F, Luc Monin J, Rusinaru D, Petit-Eisenmann H, Lelguen C, Chauvel C, et al. Valvuloarterial impedance does not improve risk stratification in low-ejection fraction, low-gradient aortic stenosis: results from a multicentre study. Eur J Echocardiogr. (2011) 12:358–63. doi: 10.1093/ejechocard/jer022
63. Lancellotti P, Magne J. Valvuloarterial impedance in aortic stenosis: look at the load, but do not forget the flow. Eur J Echocardiogr. (2011) 12:354–7. doi: 10.1093/ejechocard/jer044
64. Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, et al. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: a multicenter study. Circ Cardiovasc Imaging. (2012) 5:27–35. doi: 10.1161/CIRCIMAGING.111.967554
Keywords: aortic stenosis, valvulo-arterial impedance, dimensionless index, paradoxical low-flow low-gradient severe AS, heart failure, valvular heart disease, global load, echocardiography
Citation: Mantha Y, Futami S, Moriyama S and Hieda M (2021) Valvulo-Arterial Impedance and Dimensionless Index for Risk Stratifying Patients With Severe Aortic Stenosis. Front. Cardiovasc. Med. 8:742297. doi: 10.3389/fcvm.2021.742297
Received: 16 July 2021; Accepted: 25 October 2021;
Published: 02 December 2021.
Edited by:
Peter L. Kerkhof, VU University Medical Center, NetherlandsReviewed by:
John K-J. Li, Rutgers, The State University of New Jersey, United StatesCopyright © 2021 Mantha, Futami, Moriyama and Hieda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michinari Hieda, aGllZGEubWljaGluYXJpLjI2NUBtLmt5dXNodS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.