
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 26 October 2021
Sec. Cardiovascular Imaging
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.734820
This article is part of the Research TopicMultimodality Imaging in CardiomyopathyView all 15 articles
 Giv Heidari-Bateni1
Giv Heidari-Bateni1 Jean-Bernard Durand2
Jean-Bernard Durand2 Cezar Iliescu2
Cezar Iliescu2 Greg Gladish3
Greg Gladish3 Anita Deswal2
Anita Deswal2 Amit R. Patel4
Amit R. Patel4 Peter Kim2
Peter Kim2 Juhee Song5
Juhee Song5 Saamir Hassan2
Saamir Hassan2 Nicolas Palaskas2
Nicolas Palaskas2 Lauren A. Baldassarre6
Lauren A. Baldassarre6 Chiara Bucciarelli-Ducci7
Chiara Bucciarelli-Ducci7 Juan Lopez-Mattei2,3*
Juan Lopez-Mattei2,3*Objectives: To assess the clinical impact of Cardiovascular Magnetic Resonance (CMR) in clinical decision making of cancer patients with a suspected cardiomyopathy in a tertiary cancer center.
Background: Cardiomyopathies of diverse etiologies are frequently encountered in a Cardio-Oncology practice. The clinical impact of CMR after a presumptive diagnosis of cardiomyopathy has not been studied in cancer patients.
Methods: We reviewed data on cancer patients with presumptive diagnosis of cardiomyopathy who underwent CMR in a tertiary cancer center. The clinical impact of CMR was defined as either change in clinical diagnosis or management post CMR results. Univariate and multivariate logistic regression models were used to assess whether any of the baseline characteristics were predictive of the clinical impact of CMR.
Results: A total of 110 consecutive patients were identified. Clinical impact of CMR was seen in 68 (62%) patients. Change in the clinical diagnosis and management was seen in 56 (51%) and 41 (37%) of patients, respectively. The most common change was prevention of endomyocardial biopsy in 26 patients (24%). Overall, patients with higher left ventricular ejection fraction (LVEF) by echocardiography (echo), clinical impact was influenced more by CMR (LVEF of 37.2 ± 12.3% vs. 51.5 ± 11.6%, p < 0.001). Cancer diagnosis of multiple myeloma was associated with change in the management post CMR (adjusted OR of 25.6, 95% CI 4.0–162.4, p = 0.001). Suspicion of infiltrative cardiomyopathy was associated with a higher likelihood of change in diagnosis. Having an LVEF≥40 by echo was associated with change in diagnosis and management by CMR.
Conclusions: Utilization of CMR has a significant clinical impact in cancer patients with suspected cardiomyopathy. Patients with cancer diagnosis of multiple myeloma, suspicion of infiltrative cardiomyopathy and those with higher LVEF by echo seem to benefit more from CMR.
The diagnosis and management of cardiomyopathies are important components of a Cardio-Oncology practice, given cancer patients are at an increased risk of heart failure (HF) (1) due to co-existing risk factors as well as cardiotoxic cancer therapeutics. The advancement of newer cancer therapies and improvement of survival rates, has led to an increase of the number of patients with cancer related cardiomyopathy (2, 3). In survivors of breast cancer, for instance, the adjusted 3-year cumulative incidence of anthracycline associated cardiomyopathy was calculated at 20.2 per 100 patients with an estimated increase by 21.7 per 100 patients with addition of trastuzumab (4).
Cardiovascular magnetic resonance (CMR) has been deemed appropriate for initial and sequential evaluation of patients with cardiomyopathies (specifically infiltrative, hypertrophic, and any cardiomyopathies of unclear etiology) based on appropriateness criteria from multimodality imaging scientific societies (5, 6). Moreover, CMR has an emergent role in detecting cardiotoxicity-related cardiomyopathy and other cardiovascular effects in patients undergoing anti-cancer therapy. Data from EuroCMR has shown that CMR has a strong impact on patient management, showing 62% of its findings impacting patient management (7). However, no data in cancer patients has been published in this regard.
In the current study, we aim to assess the clinical impact of CMR in clinical decision making for cancer patients with suspected cardiomyopathy in a large tertiary cancer center.
We designed a retrospective cohort study of patients treated at the MD Anderson Cancer Center in Houston, TX, United States. We queried a CMR imaging database from May 2015 to September 2017 to identify consecutive patients who underwent CMR for clinically suspected cardiomyopathy. All patients included were receiving cancer treatment at our institution. The study protocol was approved by the institutional review board of MD Anderson Cancer Center. We included 110 consecutive patients that underwent CMR in either inpatient or outpatient settings. The diagnosis of cardiomyopathy was pre-established clinically by chart review and available echocardiographic findings in all inpatient cases and in the majority of outpatient cases. For a minority of cases referred from outside centers, a recent outside hospital echocardiography record was used. Figure 1 illustrates a summary of study design algorithm. Given the diversity of cancer diagnosis in our cohort, we separated them into the following groups by cancer: solid tumors (most common was breast cancer, constituting 31% of the group), leukemia, lymphoma, myeloma and miscellaneous (more rare hematologic cancers).
All CMR images were acquired using a 1.5-T MRI scanner which was either Siemens Avanto (Siemens, Erlangen, Germany) or a 1.5-T GE AW (GE, Milwaukee, WI). All CMR exams were protocolled for cardiomyopathy, and included the following sequences: SSFP cines (real time cines if suspicion of constrictive pericarditis), T1 and T2 weighted double inversion recovery (IR) sequences, some included T2*, T1 (native and post contrast) and T2 mapping (Modified Look-Locker IR). Late gadolinium enhancement (LGE) was performed for tissue characterization using a segmented inversion-recovery sequence (in-plane spatial resolution, 1.8 × 1.3 mm; slice thickness, 8 mm; temporal resolution, 160–200 ms) 10–15 min after intravenous contrast administration (gadopentetate dimeglumine, 0.125 mmol/kg).
CMR images were interpreted independently by either a level 3 CMR board-certified cardiologist or a radiologist with level 3 equivalent training. Studies were reviewed to assess for image quality. Cases with poor quality studies, including those where gadolinium-based contrast was not administered, were excluded. For all patients, demographic and clinical variables including age, sex, type of malignancy, comorbidities, history of previous chemotherapy, and echocardiographic parameters were collected by electronic chart review. Patients with insufficient clinical data were excluded. The clinical impact of CMR was then assessed independently and determined upon consensus by two investigators (J.C.L., G.HB). We adopted the definitions per Abbasi et al. (8) where they defined significant clinical impact of CMR as either finding an entirely new diagnosis or if a change in clinical management occurred after CMR results. Change in diagnosis was defined as a diagnosis resulting from CMR that was previously unconfirmed or unsuspected. Change in management was defined as CMR results preventing or resulting in a procedure (invasive or medical), or admission or discharge from hospital (Figure 1). Changes in medical management that we considered as significant was either starting guideline directed medical treatment for heart failure or stopping it, starting or stopping anticoagulation, but no changes in cancer treatment were seen caused from CMR findings. Our outcomes were predefined as either clinical impact of CMR, depending on changes in diagnosis and changes in management. No survival analyses or hard endpoints were evaluated, just utilization endpoints as previously defined.
We evaluated patients' baseline clinical characteristics to assess if the clinical impact of CMR can be predicted by these characteristics. Univariate and multivariate logistic regression models were used to identify variables that were significantly associated with diagnosis change (change in diagnosis vs. no change), management change (change in management vs. no change), or either of them (any change vs. no change). Only variables with significant p-value in univariable analyses, or those with a trend toward a significant p value, were used in the multivariable analysis. Hosmer-Lemeshow test was used to check the model adequacy. A p-value less than 0.05 indicated a statistical significance. All statistical analyses were performed in SAS® Version 9.4 (SAS Institute, Cary, NC).
A total of 110 patients with clinical suspicion of cardiomyopathy were identified; of those 58 (53%) were female. The average age was 59 ± 15 years. Solid tumors were the most prevalent malignancies (40%), followed by myeloma (19%), and lymphoma (18%). Table 1 summarizes baseline demographic and clinical characteristics of the patients. Indications for CMR with respective percentage frequencies were the following: Routine CMR for cardiomyopathy (64%), suspected infiltrative cardiomyopathy (25%), hypertrophic cardiomyopathy (6%), viability (3%), suspected arrhythmogenic right ventricular dysplasia (2%) and carcinoid heart disease (1%) (see Table 2). Following CMR, cardiomyopathies were categorized into six different diagnostic groups as summarized in Table 3. In 27 (25%) patients with suspected iron overload cardiomyopathy, amyloidosis, or suspected myocarditis, CMR showed no evidence of cardiomyopathy (normal ejection fraction, normal T2* and absence of late gadolinium enhancement) despite clinical suspicion and suggestive echocardiographic findings.
Overall, the clinical impact of CMR was seen in 68 (62%) patients. Results of CMR changed the diagnosis in 56 (51%), the management in 41 (37%), and both management and diagnosis in 29 (26%) patients. The most common clinical impact of CMR was prevention of endomyocardial biopsy in 26 (24%) patients by ruling out the working diagnosis of suspected infiltrative cardiomyopathy. One noticeable finding was that in 42 patients (38%) there was no change in diagnosis or management, and the mean LVEF in this group by TTE was 37 ± 12% in contrast to the 29 patients that had changes in both diagnosis and management, whose corresponding mean LVEF by TTE was 51 ± 11% (p < 0.001 by Chi-square test).
Table 4 summarizes all the management changes post CMR. Examples of patients with change in both diagnosis and management are summarized in Table 5. The clinical impact of CMR is illustrated in Figure 2. To assess whether any of the baseline characteristics can predict the clinical impact of CMR, univariate and multivariate logistic regression models were fitted, and they are summarized in Tables 6, 7. All analyses demonstrated that a higher ejection fraction by echocardiography (LVEF ≥ 40) predicts a greater clinical impact of CMR, adjusting for type of malignancy (adjusted OR 7.09, 95% CI, 2.09–24.11, p value = 0.002 and 6.16 with 95% CI 1.47–25.77, p value = 0.013 for change in diagnosis and management respectively). For change in diagnosis, a suspicion of infiltrative cardiomyopathy, when compared with routine indication of CMR for cardiomyopathy had a higher likelihood of change in diagnosis in the multivariate analysis for change in diagnosis (adjusted OR: 10.03, 95% CI, 1.91–52.69, p value = 0.006), but not in multivariate analysis for change in management.
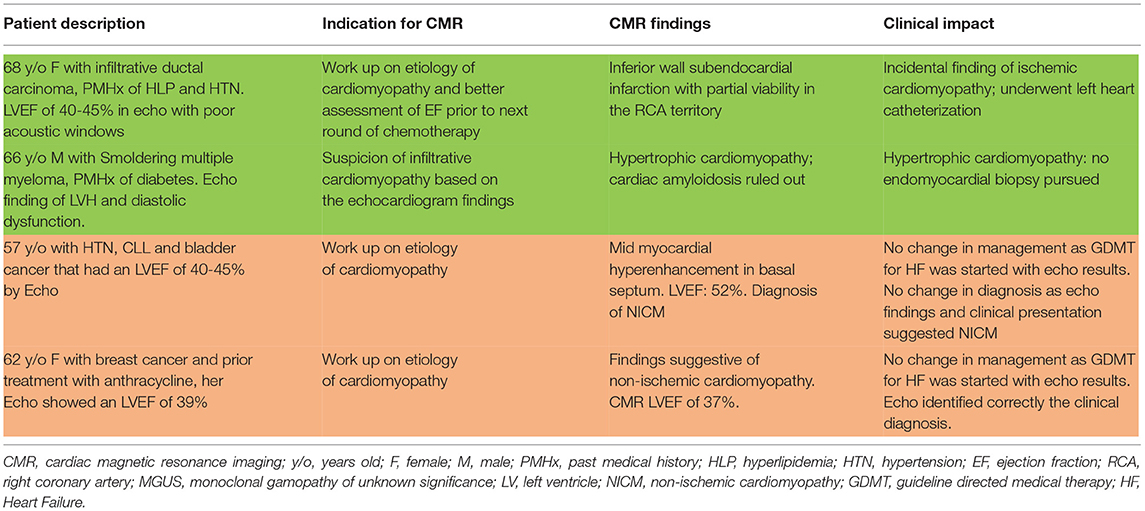
Table 5. Examples of patients with (green) and without (red) changes in both diagnosis and management after CMR.
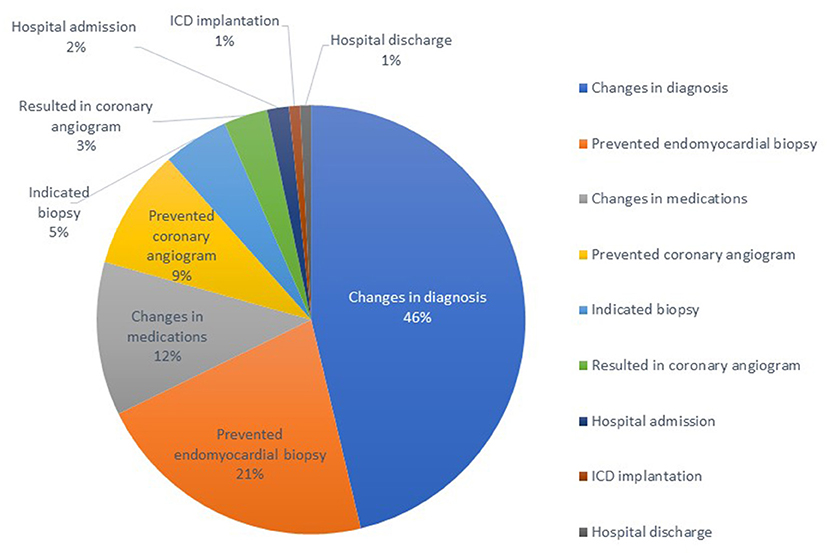
Figure 2. Clinical impact of utilization of cardiac magnetic resonance in a tertiary cancer center: 121 events in total of 66 patients.
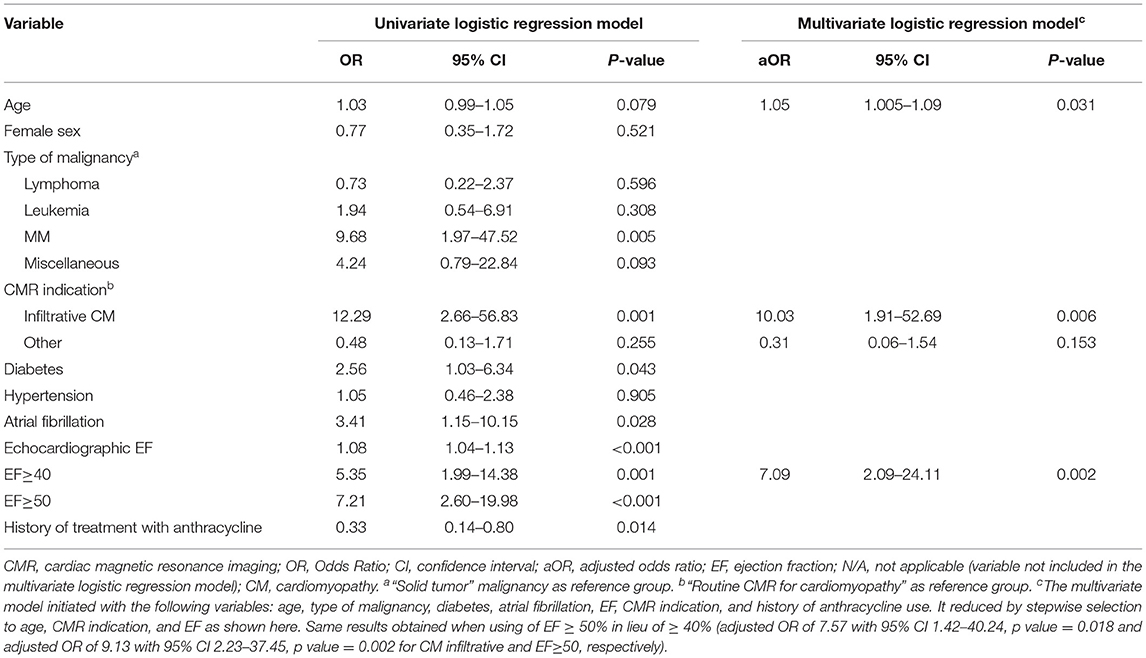
Table 6. Selected univariate and multivariate analysis of factors predicting diagnosis change following CMR.
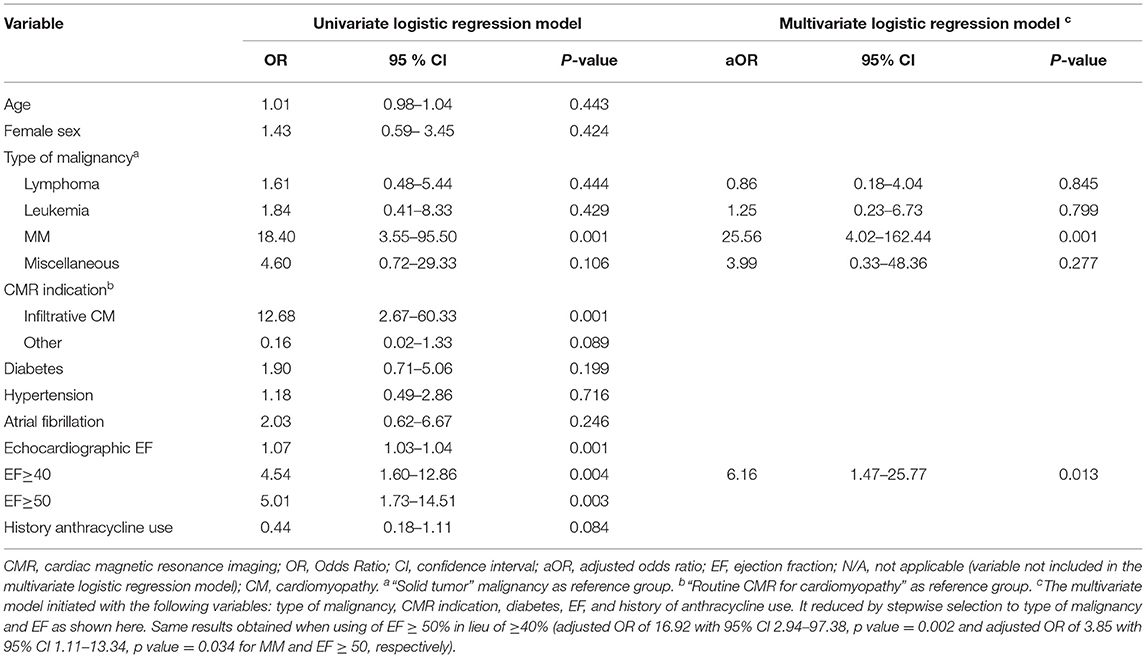
Table 7. Selected univariate and multivariate analysis of factors predicting management change following CMR.
We also found that “type of malignancy” predicted change in management post CMR. As shown in Table 7, multiple myeloma was the cancer group associated with significant change in the management post CMR (adjusted OR of 25.56 with 95% CI 4.02–162.44, p value = 0.001).
This study demonstrates a valuable role for CMR as part of the assessment for suspected or known cardiomyopathy in patients with cancer. In 62% of our patients there was a benefit from the addition of CMR imaging in their diagnostic work up, by achieving either a change in diagnosis or change in management. Furthermore, we identified baseline clinical characteristics that could predict the clinical impact of CMR. In particular, we showed that patients with higher left ventricular ejection fraction by echocardiography, patients with a diagnosis of multiple myeloma as the primary malignancy and those with suspicion of infiltrative cardiomyopathy were those in which CMR had the most clinical impact.
CMR has proven to have an additive role in diagnosis and management of patients with cardiomyopathy (8–10). However, in patients with non-ischemic cardiomyopathy, its value on routine use may be limited, given it may not yield more specific etiologies in majority of cases (11). Which was consistent with our findings, given routine CMR for cardiomyopathy didn't yield as much clinical impact as suspicion of infiltrative cardiomyopathy. Compared to echocardiography, CMR has a higher spatial resolution, larger field of view, highly reproducible ventricular volumes and ejection fraction quantification (12) with the ability for functional assessment and ability to perform tissue phenotyping using tissue characterization sequences such T1 weighted imaging with late gadolinium enhancement (LGE) (13), T2 weighted imaging and parametric mapping (14).
With the advancement of CMR techniques, recent multi-society expert consensus recommendations for multimodality imaging in cardiac amyloidosis have considered a central role for CMR in the non-invasive diagnosis of cardiac amyloidosis (15). CMR is a cornerstone test in the evaluation of patients with left ventricular hypertrophy (LVH) phenotype on echocardiography and suspected infiltrative cardiomyopathies, explaining why there was a significant clinical impact in cancer patients with suspected infiltrative cardiomyopathy particularly those with primary multiple myeloma, given the risk to develop cardiac amyloidosis. This might in part explain our findings of baseline multiple myeloma predicting the clinical impact of CMR.
Multivariate analyses also revealed that the clinical impact of CMR in both diagnosis and management is more appreciated in patients' groups with an echo LVEF of 40% or higher and changes in diagnosis more likely with suspicion of infiltrative cardiomyopathy (adjusted OR 10.03, p = 0.006). This could be explained due to the high proportion of cases (25%) that CMR showed no evidence of cardiomyopathy despite clinical suspicion by TTE. This finding changes the management dramatically, as patients could be able to resume chemotherapy promptly and without the need for treatment of Heart failure. Conversely, the patients that did not have significant change in diagnosis and management had lower mean LVEF by TTE, which could suggest that in most of these cases LV systolic dysfunction was identified appropriately by TTE. In other words, in the context of a significant decrease in LVEF that could be detected by TTE, CMR might not add to the diagnosis or management. Anthracycline and trastuzumab cardiotoxicity and related cardiomyopathies are not well characterized in CMR by a specific patterns of LGE or specific findings in parametric mapping (16). Conversely, if an LVEF measurement by TTE is borderline low or in the realm of 40%, CMR accurately differentiates true decreases from cases of preserved LV systolic function because of its robustness in reproducibility and less interobserver variability in ventricular volumes and LVEF quantification. This suggest that routine utilization of CMR for cardiomyopathy evaluation based on echo findings might not offer clinical benefit if echo identifies well possible etiologies such as decreased LVEF from anthracyclines. Also suspicion of infiltrative cardiomyopathy was associated with increased of change in diagnosis related with CMR. Which suggests that clinical impact by CMR is higher in patients for which iron overload cardiomyopathy or cardiac amyloidosis must be evaluated. CMR ruled out cardiomyopathy in 25% of patients. This has an impact for patients' mental wellbeing, withdrawal of heart failure medications and resumption of lifesaving cancer therapies. In our study, prevention of endomyocardial biopsy was found to be the most common clinical impact of CMR comprising 24% of patients. Prevention of endomyocardial biopsy is important in the setting of cancer particularly for those actively receiving chemotherapy given the increased risk of complications, mainly vascular complications and bleeding in this vulnerable group. A cost effectiveness analysis may also enlighten the economic benefit of using CMR as a gatekeeper for myocardial biopsy.
In conclusion, application of CMR in Cardio-Oncology appears to have frequent clinical impact (62% patients) on the evaluation of confirmed or suspected cases of cardiomyopathy in a cohort of cancer patients. Baseline systolic function from TTE, suspicion of infiltrative cardiomyopathy and primary malignancy type increase the likelihood of clinical impact of the addition of CMR to the diagnostic approach. Our findings support an important role of CMR in a Cardio-Oncology practice. Further larger and multi-center studies looking at hard clinical endpoints and cost-effectiveness analyses are needed to quantify better the benefits of CMR in these patients.
We cannot exclude the role of selection and referral bias of the primary cardiologist when choosing the appropriate patient for CMR assessment; however, the studied patients represents a heterogeneous group either referred by outpatient centers or seen as an inpatient consult in a tertiary cardio-oncology practice.
Our study has some additional limitations. First, this is a retrospective study which can be skewed by limitations of medical documentation and the absence of a control group. Second, there are some known limitations in application of CMR such as patients with claustrophobia, prosthetic devices or foreign bodies. Also, 11 out of 121 (9%) of scans were excluded due to poor image quality or lack of contrast (see Figure 1), and therefore our results may overestimate the benefit of performing CMR.
Moreover, the economic value of CMR for all patients with suspected cardiomyopathy is uncertain. Similarly, there is no outcome data on survival benefit of using CMR in the management of cardiomyopathy in cancer patients. Undoubtedly a cost-effective analytical study or a comparative effectiveness study with focus on survival benefit can better highlight the value of this approach.
In patients with cancer and suspected cardiomyopathy, CMR may result in change in diagnosis and management in certain clinical scenarios. Patients with LVEF of 40% or more, those with suspicion of infiltrative cardiomyopathy and those with cancer diagnosis of multiple myeloma, have a higher likelihood to benefit from the use of CMR.
Further prospective research is needed to identify the value, including the economic burden, of the use of CMR for cancer patients with suspected cardiomyopathy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by MD Anderson IRB provided exemption due to retrospective review. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
GH-B and JL-M planned and wrote the manuscript. All other authors edited the manuscript.
The statistical analysis work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672). CB-D was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol.
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CMR, Cardiovascular Magnetic Resonance; TTE, Transthoracic echocardiogram; LVEF, Left ventricular ejection fraction; LGE, Late Gadolinium enhancement.
1. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res. (2019) 115:844–53. doi: 10.1093/cvr/cvz035
2. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. (2016) 66:309–25. doi: 10.3322/caac.21341
3. Araujo-Gutierrez R, Ibarra-Cortez SH, Estep JD, Bhimaraj A, Guha A, Hussain I, et al. Incidence and outcomes of cancer treatment-related cardiomyopathy among referrals for advanced heart failure. Cardio-Oncol. (2018) 4:3. doi: 10.1186/s40959-018-0029-y
4. Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. (2012) 60:2504–12. doi: 10.1016/j.jacc.2012.07.068
5. Patel MR, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson FJ, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. (2006) 48:1475–97. doi: 10.1016/j.jacc.2006.07.003
6. Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Soc Echocard. (2019) 32:553–79. doi: 10.1016/j.echo.2019.01.008
7. Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry–multi national results from 57 centers in 15 countries. J Cardiovasc Mag Resonan. (2013) 15:9. doi: 10.1186/1532-429X-15-S1-O96
8. Abbasi SA, Ertel A, Shah RV, Dandekar V, Chung J, Bhat G, et al. Impact of cardiovascular magnetic resonance on management and clinical decision-making in heart failure patients. J Cardiovasc Mag Resonan. (2013) 15:89. doi: 10.1186/1532-429X-15-89
9. Patel AR, Kramer CM. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. JACC Cardiovasc Imaging. (2017) 10:1180–93. doi: 10.1016/j.jcmg.2017.08.005
10. Kramer CM. Role of cardiac MR imaging in cardiomyopathies. J Nucl Med. (2015) 56:39s–45s. doi: 10.2967/jnumed.114.142729
11. Paterson DI, Wells G, Erthal F, Mielniczuk L, O'Meara E, White J, et al. OUTSMART HF: a randomized controlled trial of routine versus selective cardiac magnetic resonance for patients with nonischemic heart failure (IMAGE-HF 1B). Circulation. (2020) 141:818–27. doi: 10.1161/CIRCULATIONAHA.119.043964
12. Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Mag Reson. (2000) 2:271–8. doi: 10.3109/10976640009148691
13. Shah DJ, Judd RM, Kim RJ. Technology insight: MRI of the myocardium. Nat Clin Pract Cardiov Med. (2005) 2:597–605. doi: 10.1038/ncpcardio0352
14. Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiov Imag. (2013) 6:806–22. doi: 10.1016/j.jcmg.2013.05.005
15. Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol. (2019) 26:2065–123. doi: 10.1007/s12350-019-01760-6
Keywords: cardiovascular magnetic resonance, cardiomyopathy, Cardio-Oncology, clinical impact, echocardiography
Citation: Heidari-Bateni G, Durand J-B, Iliescu C, Gladish G, Deswal A, Patel AR, Kim P, Song J, Hassan S, Palaskas N, Baldassarre LA, Bucciarelli-Ducci C and Lopez-Mattei J (2021) Clinical Impact of Cardiovascular Magnetic Resonance in Cancer Patients With Suspected Cardiomyopathy. Front. Cardiovasc. Med. 8:734820. doi: 10.3389/fcvm.2021.734820
Received: 01 July 2021; Accepted: 27 September 2021;
Published: 26 October 2021.
Edited by:
Reza Nezafat, Harvard University, United StatesReviewed by:
Otávio R. Coelho-Filho, State University of Campinas, BrazilCopyright © 2021 Heidari-Bateni, Durand, Iliescu, Gladish, Deswal, Patel, Kim, Song, Hassan, Palaskas, Baldassarre, Bucciarelli-Ducci and Lopez-Mattei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Lopez-Mattei, amxvcGV6OUBtZGFuZGVyc29uLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.