- 1Western Sydney Nursing and Midwifery Research Centre, Western Sydney University & Western Sydney Local Health District, Blacktown, NSW, Australia
- 2School of Nursing and Midwifery, Deakin University, Geelong, VIC, Australia
- 3Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
- 4Pharmacy Department, Blacktown Hospital, Western Sydney Local Health District, Blacktown, NSW, Australia
- 5Improving Palliative, Aged and Chronic Care Through Clinical Research and Translation (IMPACCT), University of Technology Sydney, Sydney, NSW, Australia
Background: Atrial Fibrillation (AF) is the most common sustained cardiac arrhythmia. Obesity is an independent risk factor for AF. Anticoagulants have been strongly recommended by all international guidelines to prevent stroke. However, altered pathophysiology in obese adults may influence anticoagulant pharmacology. Direct oral anticoagulants (DOACs) in the context of obesity and AF have been examined in recent systematic reviews. Despite the similarities in included studies, their results and conclusions do not agree.
Methods and Results: The protocol for this review was registered with PROSPERO (CRD42020181510). Seven key electronic databases were searched using search terms such as “atrial fibrillation,” “obese,*” “overweight,” “novel oral anticoagulant,” “direct oral anticoagulant,” “DOAC,” “NOAC,” “apixaban,” dabigatran,” “rivaroxaban,” and “edoxaban” to locate published and unpublished studies. Only systematic reviews with meta-analyses that examined the effect of DOACs in overweight or obese adults with AF, published in the English language, were included. A total of 9,547 articles were initially retrieved. After removing the duplicates, title and abstract review and full-text review, five articles were included in the systematic review. From these only RCTs were included in the meta-analyses. There was disagreement within the published systematic reviews on DOACs in obesity. The results from our meta-analysis did not show any significant difference between all body mass index (BMI) groups for all outcomes at both 12 months and for the entire trial duration. Non-significant differences were seen among the different types of DOACs.
Conclusion: There was no difference between the BMI classes in any of the outcomes assessed. This may be due to the limited number of people in the trial that were in the obese class, especially obese class III. There is a need for large prospective trials to confirm which DOACs are safe and efficacious in the obese class III adults and at which dose.
Introduction
Atrial Fibrillation (AF) is the most common sustained cardiac arrythmia. Major clinical sequela of AF includes systemic embolism, stroke, impaired cardiac function and heart failure (1, 2). Obesity is an independent risk factor for AF with underlying mechanisms that have a pathophysiological impact on AF (3–6). It is estimated that almost one in five cases of AF are attributed to obesity, to the extent that there is a 4 to 5% increase in AF risk for each incremental increase in body mass index (BMI) (7, 8).
The use of anticoagulants has been strongly recommended by all international guidelines, for AF patients that have a high risk of stroke (CHA2DS2-VASc score ≥ 2) (9–11). These guidelines recommend the use of direct oral anticoagulants (DOACs) rather than warfarin due to the significant association with higher rates of major bleeding, multiple food and drug interactions and the need for frequent monitoring (9, 10, 12–17). The altered pathophysiology in obese adults can influence the pharmacology of anticoagulants such as warfarin, thus requiring a higher dose and a longer time to reach therapeutic targets when compared to adults of normal weight (18). This may contribute to adverse events such as stroke and hospitalization because of anticoagulant under-dosing.
Despite the well-recognized cardiovascular consequences of obesity, there is a counterintuitive phenomenon known as the obesity paradox that has been hypothesized in some systematic reviews and meta-analyses (19, 20). In this phenomenon, overweight and mildly obese (BMI < 35 kg/m2) participants that were in the DOAC group, appear to have lower all-cause mortality in studies with longer-term follow up. Despite this finding, several studies have critiqued the assertion based on the potential for spurious associations with rhythm control strategies, unreported confounders, limitations of anthropometric markers such as BMI in assessing adiposity and selection bias in observational or cohort studies (6, 8, 21–23).
DOACs have been the focus of attention in several systematic reviews (19, 20, 24–27), exploring their use in obesity. Recommendations from these studies appear to be conflicting. The effect of the obesity paradox in the context of AF, or robust data comparing the effectiveness of DOACs with warfarin, remain elusive. Product information documents supporting DOAC use indicate that dose adjustment is not required for any of the DOACs (28–30). However, in the clinical trials conducted to inform the product information documents, such as ARISTOTLE, RE-LY and ROCKET-AF (31–33), weight classes were not equally distributed. For example, most of the participants enrolled in the dabigatran clinical trials (up to 80%) were between 50 and 100 kg (29). Participants in the ARISTOTLE trial (34) that were >140 kg were under-represented comprising only 1.4% of the entire trial population. Both the International Society on Thrombosis and Haemostasis (ISTH) and the European Society of Cardiology (ESC) Working Group on Thrombosis have questioned the use of DOACs in morbidly obese adults (i.e., BMI ≥ 40 kg/m2), due to the extremely limited or absent clinical data (35). The ISTH have suggested that DOACs should not be used in BMI of >40 kg/m2 or >120 kg (36). Although guidance from ISTH provides an alternative option for DOAC use in obesity, there have been no original research studies that have examined its effectiveness in the obese population or compared the effectiveness of DOACs exclusively according to BMI category. Given the high-risk clinical consequences of anticoagulants, a better understanding of the safety and efficacy of DOACs in obese adults with AF is warranted. The aim of this systematic review is to evaluate the current evidence on the safety and effectiveness of direct oral anticoagulants (DOACs) in obese adults with AF.
Methods
This systematic review was conducted in accordance with gold-standard systematic review and meta-analysis methodology informed by the Cochrane Collaboration and the Joanna Briggs Institute (JBI) methodology for systematic reviews of effectiveness evidence (37, 38). The review protocol has been registered with the PROSPERO register (CRD42020181510).
Search Strategy
The search strategy used key search terms such as “atrial fibrillation,” “obese,*” “overweight,” “novel oral anticoagulant,” “direct oral anticoagulant,” “DOAC,” “NOAC,” “apixaban,” dabigatran,” “rivaroxaban,” and “edoxaban” (see Supplementary Table 5 for full search strategy). It was designed to locate published and unpublished studies. Text words contained in the titles and abstracts of relevant articles and the index terms used to describe the articles were used to develop a full search strategy. The reference lists of all studies selected for critical appraisal were screened for additional studies that were then included in this study.
Inclusion and Exclusion Criteria
Only systematic reviews with meta-analyses that examined the effect of DOAC in overweight or obese adults with AF, published in the English language, were included. Studies were excluded if they were related to interventional studies (for example, cardioversion, catheter ablation and gastric bypass) and not a systematic review or a systematic review with meta-analysis (for example, post-hoc analysis, abstracts, conference proceedings, review paper, observational or retrospective cohort studies, editorials, and commentaries) (see Supplementary Table 1). Any non-RCT such as post-hoc analysis of a RCT, observational studies included in the systematic reviews and/or meta-analysis were excluded in this meta-analysis (see Supplementary Table 2). Studies that were published before 2005 were also excluded as prior to this time no DOAC trials had commenced.
Outcomes
Primary outcomes assessed were stroke (ischemic or hemorrhagic) or systemic or pulmonary embolism. Secondary outcomes assessed included all-cause mortality, transient ischemic attack, myocardial infarction, major bleed, all-cause hospitalization, and cardiovascular mortality. Outcomes were assessed at 12 months and for the entire trial duration.
Data Sources
Seven key electronic databases were searched including Medline, CINAHL, Scopus, Web of Science, Cochrane Database of Systematic Reviews, Johanna Briggs Institute and Embase. Clinical trial registries were checked to ensure all relevant trials were identified. The fidelity of the search strategy was tested and confirmed by two investigators (FS, CF) who independently implemented the search and compared findings from each database. Search findings were downloaded into EndNote X9.3 (39) citation management software.
Study Selection
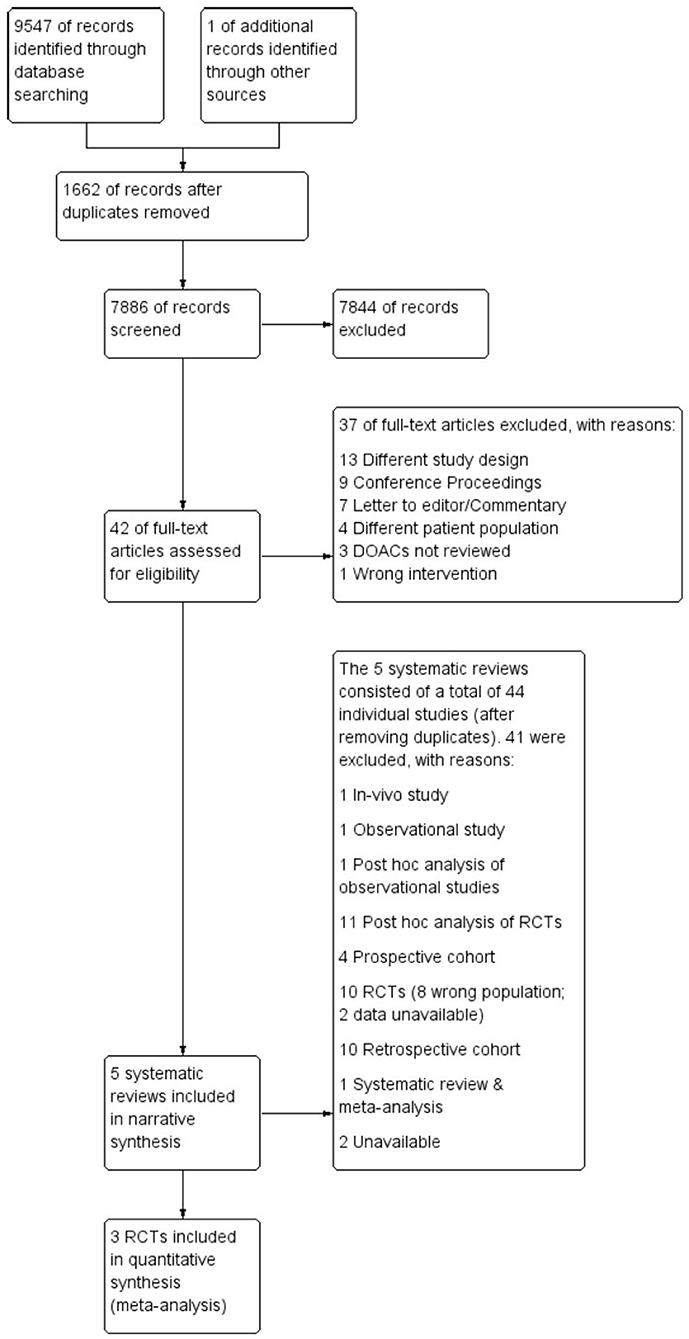
Following the search, all identified citations were uploaded into Covidence systematic review software (40) and duplicates removed. Titles and abstracts were screened for assessment against review inclusion and exclusion criteria. Full text of selected citations was assessed in detail against the inclusion and exclusion criteria. The entire screening process was undertaken by two investigators (FS, CF) at each stage of the study selection process and disagreements were resolved through consensus discussion with a third arbitrary investigator (RW). The results of the search are reported in full and presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram (41) as shown in Figure 1.
Assessment of Methodological Quality
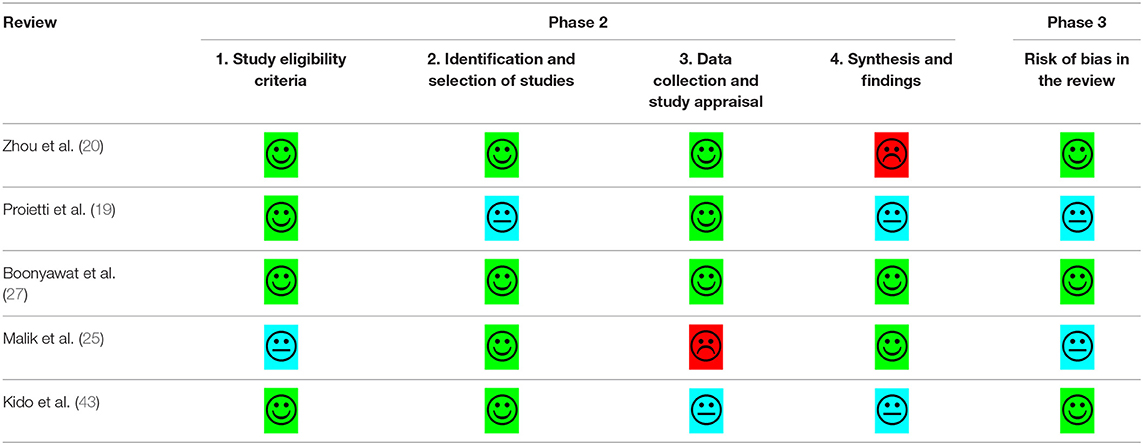
The quality of eligible studies was critically appraised by two investigators using a standardized critical appraisal instrument: The Assessment of Multiple Systematic Reviews (AMSTAR-2) tool (42). Any disagreements that arose were resolved through discussion, or review by a third investigator. The results of the critical appraisal are reported in narrative form in Table 1. Risk of bias was assessed using the ROBIS tool for risk of bias in systematic reviews (76).
Data Extraction
Data was extracted from studies included in the review using a standardized data extraction tool. The data extracted included specific details about the study population, methods, interventions, and outcomes of significance to the review objective. Any disagreements that arose between the reviewers (FS, CF) were resolved through discussion, or with a third investigator (RW). Authors of all the five DOAC trials that met our inclusion criteria for the meta-analysis were contacted by email to request the data as the published data did not enable stratification by BMI. Authors of three studies (RE-LY, AVERROES, and ENGAGE AF-TIMI 48) agreed to share data for the purposes of a meta-analysis. Two of the trials, ARISTOTLE, and ROCKET-AF, did not provide data stratified by BMI and were excluded from the meta-analysis. Data was analyzed using the intention to treat cohort in all trials to minimize any risk of bias.
Data Synthesis
Meta-analysis was performed using only RCTs from eligible systematic reviews to minimize risk of bias that can arise from other study designs. Data from only the DOAC group in the trials were pooled for statistical meta-analysis using RevMan 5.3 (77). Effect sizes were expressed as odds ratios (for dichotomous data) with 95% confidence intervals. Heterogeneity was assessed statistically using the standard chi-square and I2 tests. Statistical analyses were performed using the DerSimonian and Laird Method for random effects meta-analysis.
Deviation From Protocol
There have been three deviations from the registered protocol on PROSPERO. The first was that this paper also includes further analysis of the different BMI groups rather than the two groups noted in the registered protocol. The second major deviation was that a summary of findings is not provided as the risk of bias was only completed for systematic reviews, not primary studies, as these have previously been assessed for risk of bias when included in the original systematic reviews. The last deviation is that publication bias assessment was also excluded as it was not required, as per the Cochrane Handbook (38), due to the number and type of studies included in this systematic review.
Results
Search Results
As illustrated in Figure 1, a total of 9,547 articles were initially retrieved. After removing the duplicates (n = 1,662), 7,844 articles were excluded after title and abstract review, leaving 42 articles for full-text review. A further 37 articles were excluded for reasons listed in Supplementary Table 1, leaving five articles that met inclusion criteria. The five systematic reviews comprised 40 individual original studies after removing duplicates; 11 RCTs, 11 post-hoc analyses of RCTs, nine retrospective cohort studies, three prospective studies, one observational study, one post-hoc analysis of observational data, a systematic review and meta-analysis and a conference abstract (see Supplementary Table 2). As stated in the methods, only RCTs were included in meta-analyses. Six RCTs focused on Venous Thromboembolism (VTE) and Pulmonary Embolism (PE), hence were excluded from the meta-analysis. Of the remaining five trials that focused on AF, only three of the five authors of the trials agreed to share data for the meta-analysis. Thus, two of the trials, ARISTOTLE, and ROCKET-AF, were excluded from the meta-analysis and only the RE-LY, AVERROES and ENGAGE AF-TIMI 48 trials were included.
Description of Included Studies in Narrative Synthesis
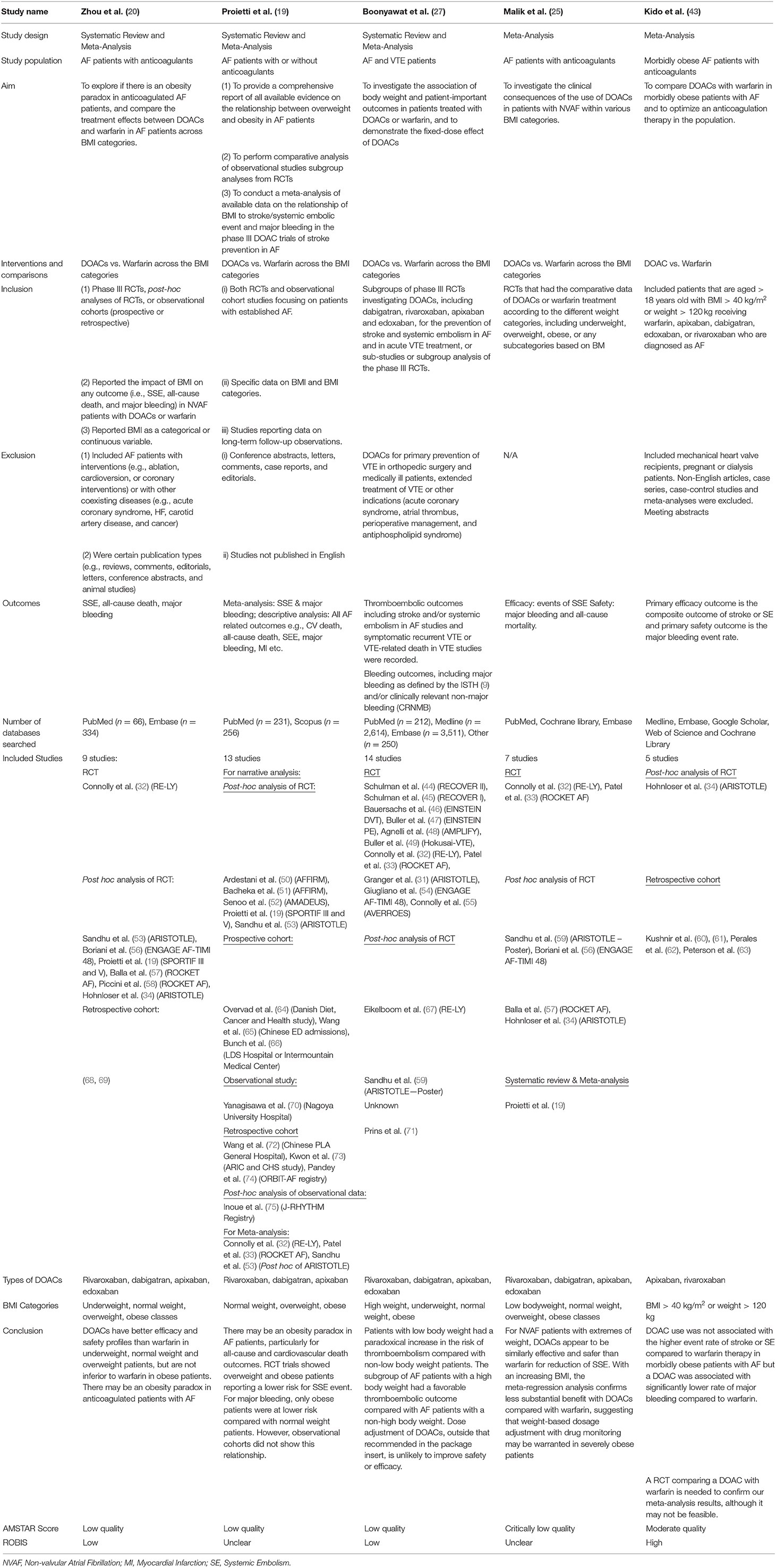
Table 1 provides a summary of the characteristics of the included reviews. In brief, all studies except for Kido et al. (43) evaluated the effect of DOACs vs. Warfarin across different weight groups. Kido et al. (43) only evaluated the effect of DOACs vs. Warfarin in obese groups (BMI > 40 or > 120 kg). Similarly, all studies evaluated the effected of DOACs vs. Warfarin in AF, apart from Boonyawat et al. (27) who also included VTE patients. Stroke or systemic embolism (SSE) and major bleeding were the primary efficacy and safety outcomes in all studies, however, some studies also reported outcomes such as all-cause death and cardiovascular death. Proeitti et al. (19) and Boonyawat et al. (27) provided the most comprehensive systematic reviews based on the number and type of included studies.
Despite the comprehensiveness with regards to the quantity and similarity of the included studies, the five systematic reviews did not have complete agreement in their results and conclusion, nor was the comprehensiveness reflected in the quality and risk of bias assessment, as discussed in the next section. Zhu et al. (26) and Proietti et al. (19) concluded that “… there appears to be an obesity paradox in obese adults with atrial fibrillation” and a superior efficacy and safety profile for DOACs in overweight and obese adults. Conclusions from Boonyawat et al. (27) were similar to the aforementioned studies but alluded to variability in baseline characteristics influencing outcome. Malik et al. (25) and Kido et al. (43) reached similar conclusions with no significant difference between DOACs and warfarin with regards to efficacy, however they reported better safety outcomes for DOACs compared to warfarin. Both reviews recommended further trials comparing DOACs to Warfarin to confirm their findings, in addition to suggesting the need for weight-based dosage adjustment with drug monitoring in such trials.
Methodological Quality and Risk of Bias Assessment
Quality assessment and risk of bias were undertaken using the AMSTAR-2© and ROBIS© tools (42, 76). Table 2 provides a summary of the risk of bias assessment. Three of the five systematic reviews were assessed as low quality. Zhou et al. (20) and Boonyawat et al. (27) had low risk of bias due to the thoroughness in their methodology and the quantity/quality of included studies. Zhou et al. (20) did not provide any justification for combining different study designs into the same analysis or why they had excluded some trials in the grouped analysis but included them in individual analysis. The review authors stated that they had extracted “underweight data from Hohnloser et al. (34) and overweight/obese data from Sandhu et al. (53).” However, these original studies used different definitions of weight groups, that is, Hohnloser et al. (34) stratified using actual weight and Sandhu et al. (53) used BMI. Boonyawat et al. (27) had used the Mantel-Haenszel method instead of the Laird Method to analyze the data which they determined to be of random effects and had defined high body weight as a minimum of 100 kg, which may have lacked clinical sensitivity.
Proietti et al. (19) was also assessed as low quality but had unclear risk of bias, due to several issues. Firstly, the authors mentioned that they had used I2 to determine if there was heterogeneity in the trial. However, given there were different doses and drugs used across the different trials, heterogeneity would have been intrinsic. Fixed method modeling instead of random with the Laird Method was used for their analysis which is not consistent with heterogeneity. Secondly, the event numbers that the authors presented in their forest plots did not correspond to the event numbers we received from the trial authors. The authors did not provide a justification for combining different study designs into the same analysis; observational studies were included. Risk of bias was only completed for the studies included in the meta-analysis, without any justification for excluding the studies included in the narrative synthesis. Lastly, the authors mentioned they also relied on data from regulatory submissions for dabigatran and rivaroxaban; however, they did not specify which trial was included as part of their data extraction.
Malik et al. (25) was assessed as critically low quality with an unclear risk of bias. This was predominantly due to the lack of clarity and risk of bias assessment, limited comprehensiveness in their literature search and justification behind its exclusion of articles. Additionally, in the methods, the authors stated that the RR would be reported, but ORs were reported throughout, with no justification for change in reporting measure.
Although the quality assessment of Kido et al. (43) was the highest of all the included reviews, a high risk of bias was revealed. This was due to the unjustified exclusion of all the DOAC trials and the post-hoc analysis of the RCTs, as well as other relevant key studies. Along with Zhou et al. (20) and Proietti et al. (19), Kido et al. (43) also used the Mantel-Haenszel method instead of the Laird Method to analyze the data, which they determined to be of random effects. There was no justification for combining different study designs into the same analysis and the data extracted from Hohnloser et al. (34) may not be accurate; in Figure 3, the DOAC event states 13/480, however, in the paper by Hohnloser et al. (34) the event rate is 13 per 100 per year. Kido et al. (43) had reported this number over a 4-year period.
Meta-Analysis of Data From DOAC Trials
Data were obtained by contacting the study authors of all five DOAC trials (31–33, 54, 55) as the data from the post-hoc analysis of the RCTs did not have adequate information to conduct a meta-analysis for our intended subgroup analysis. Only the ENGAGE AF-TIMI 48 trial reported transient ischemic attack (TIA) and only two trials, ENGAGE AF-TIMI 48 and RE-LY, reported all-cause hospitalization.
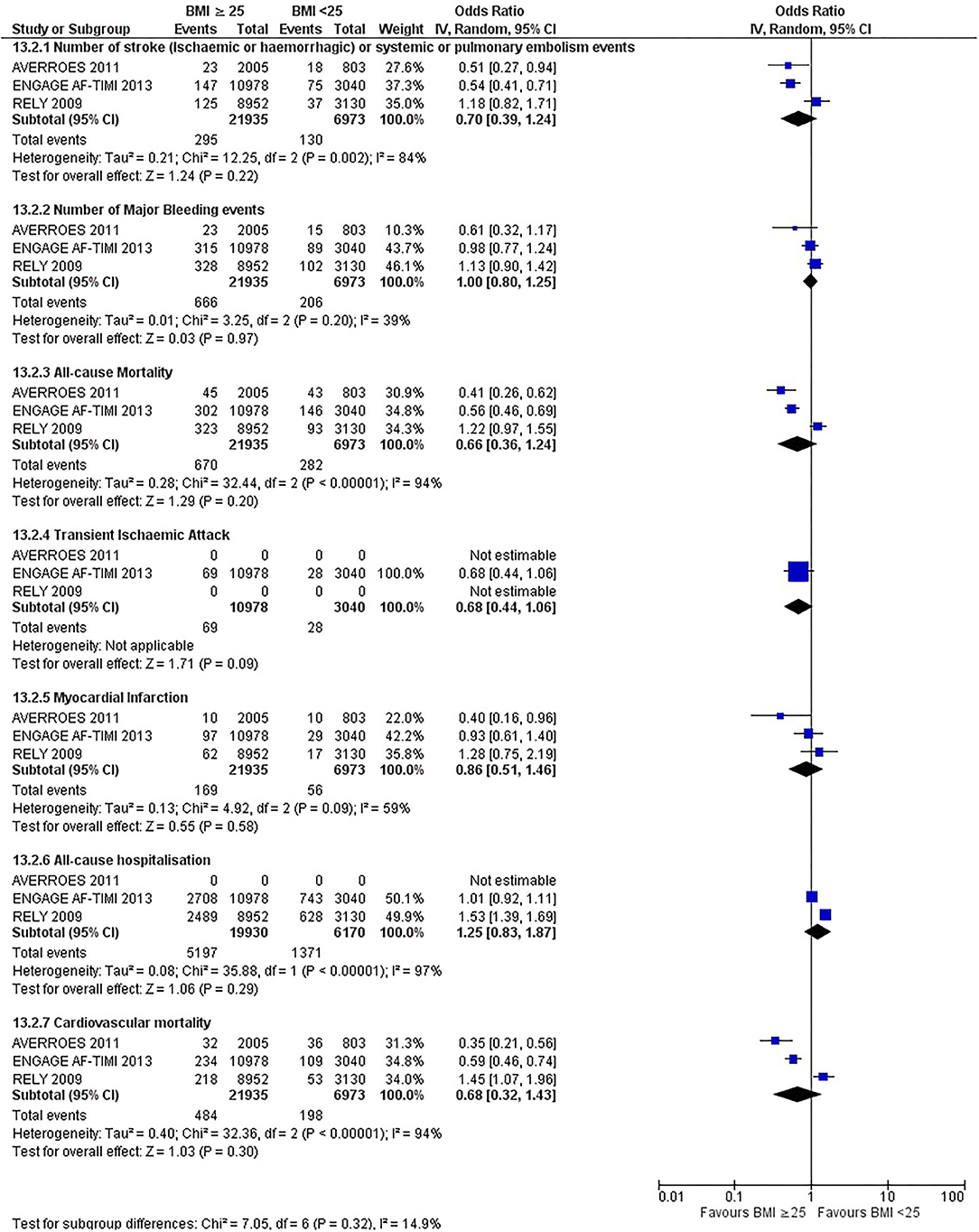
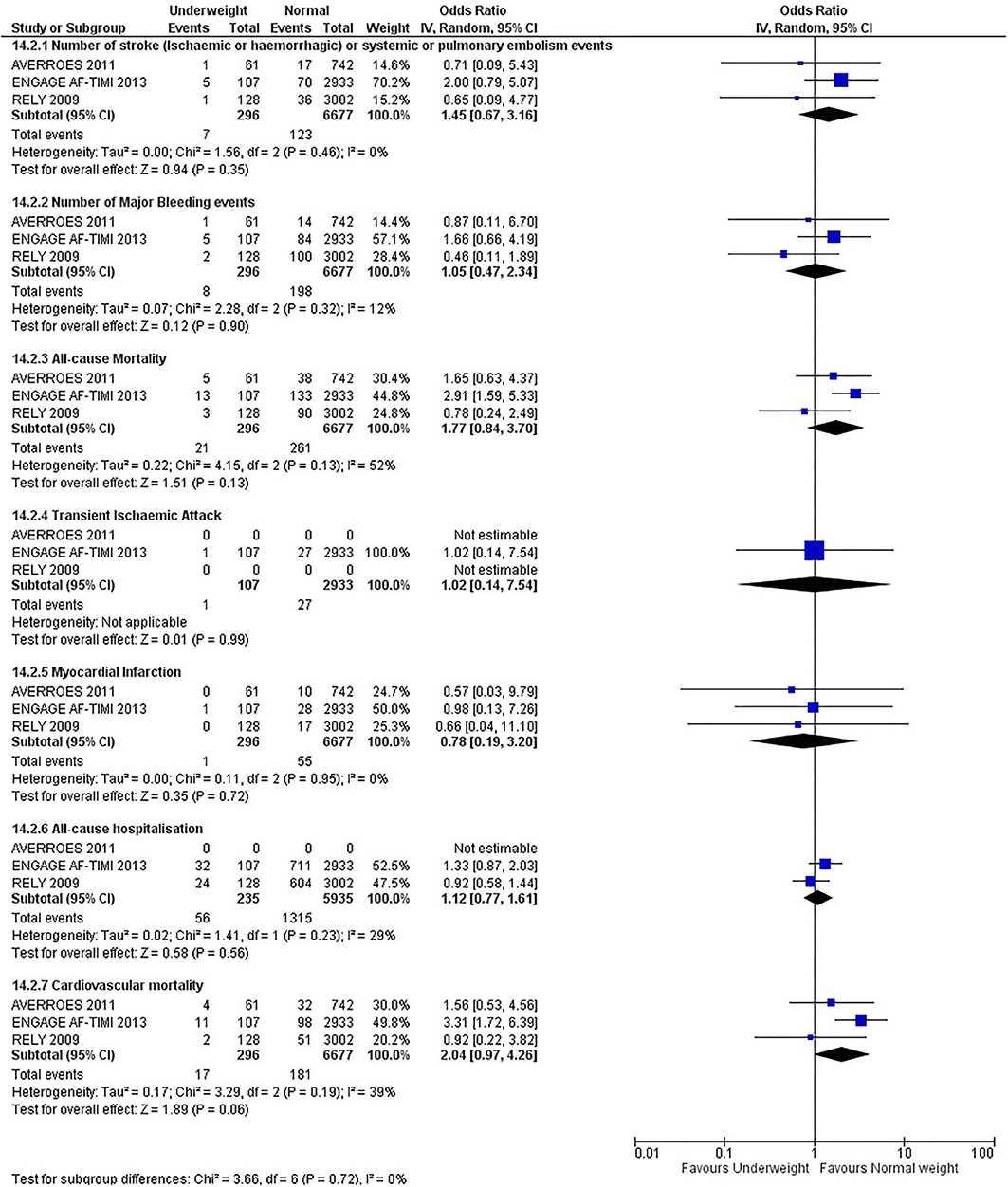
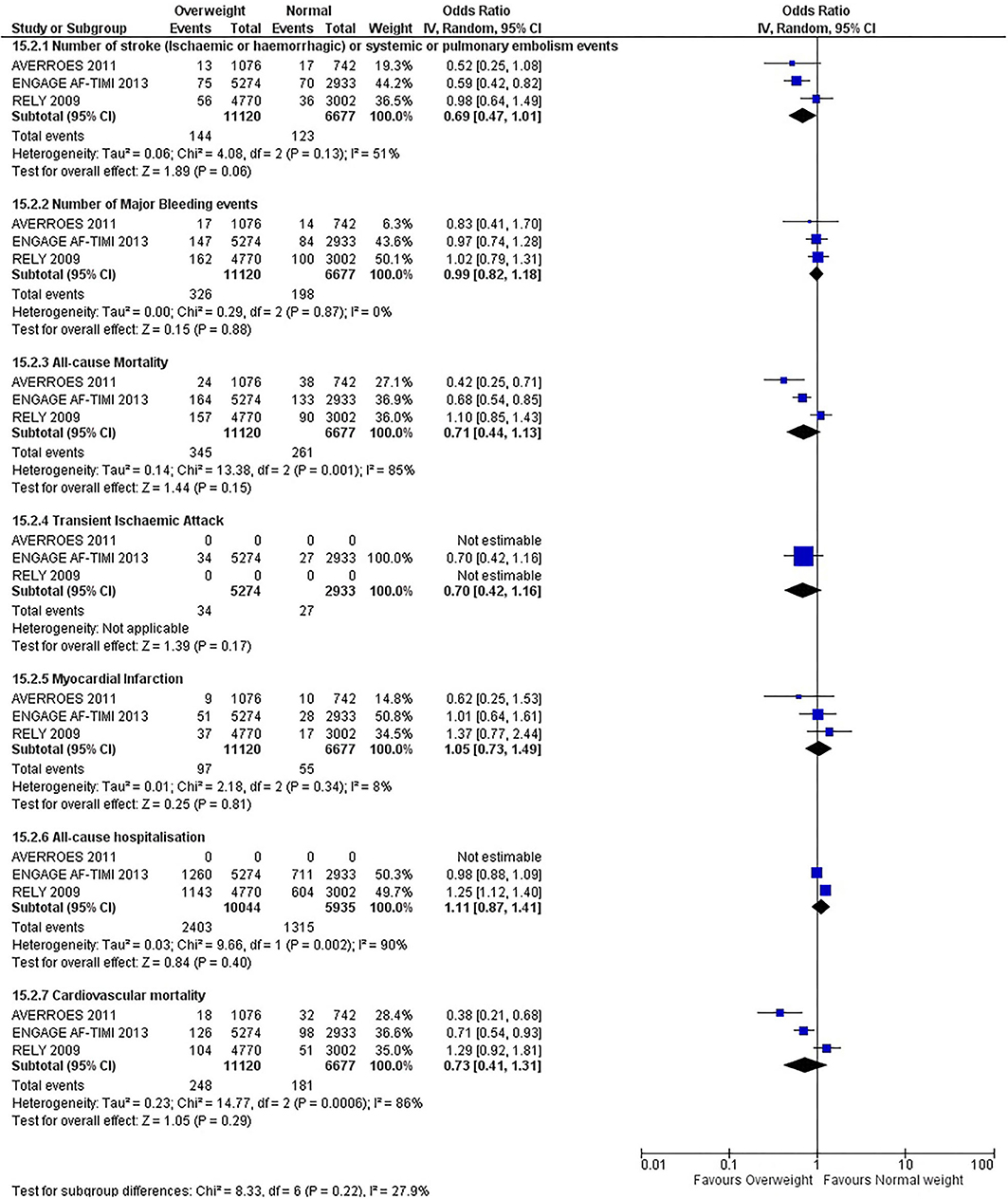
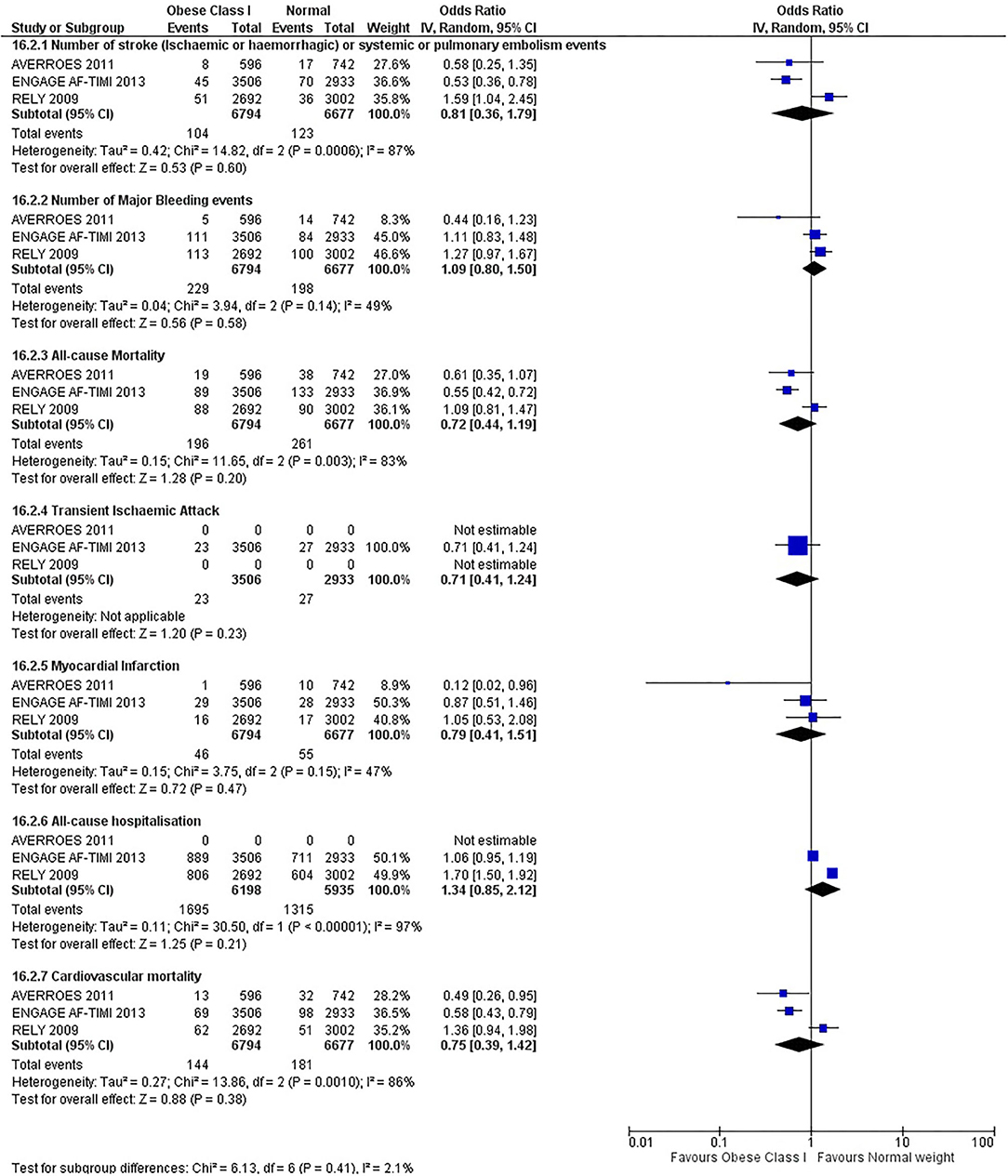
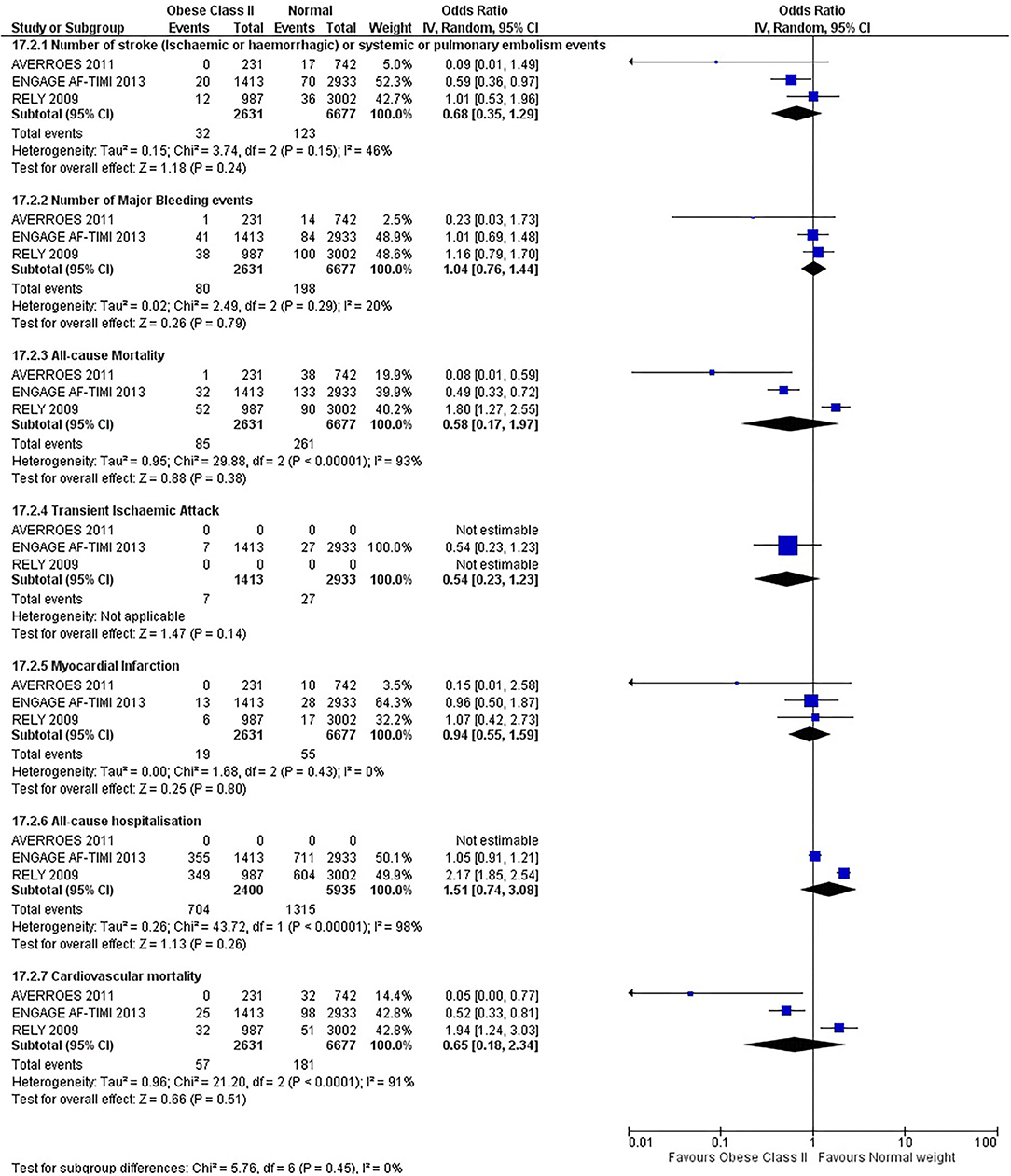
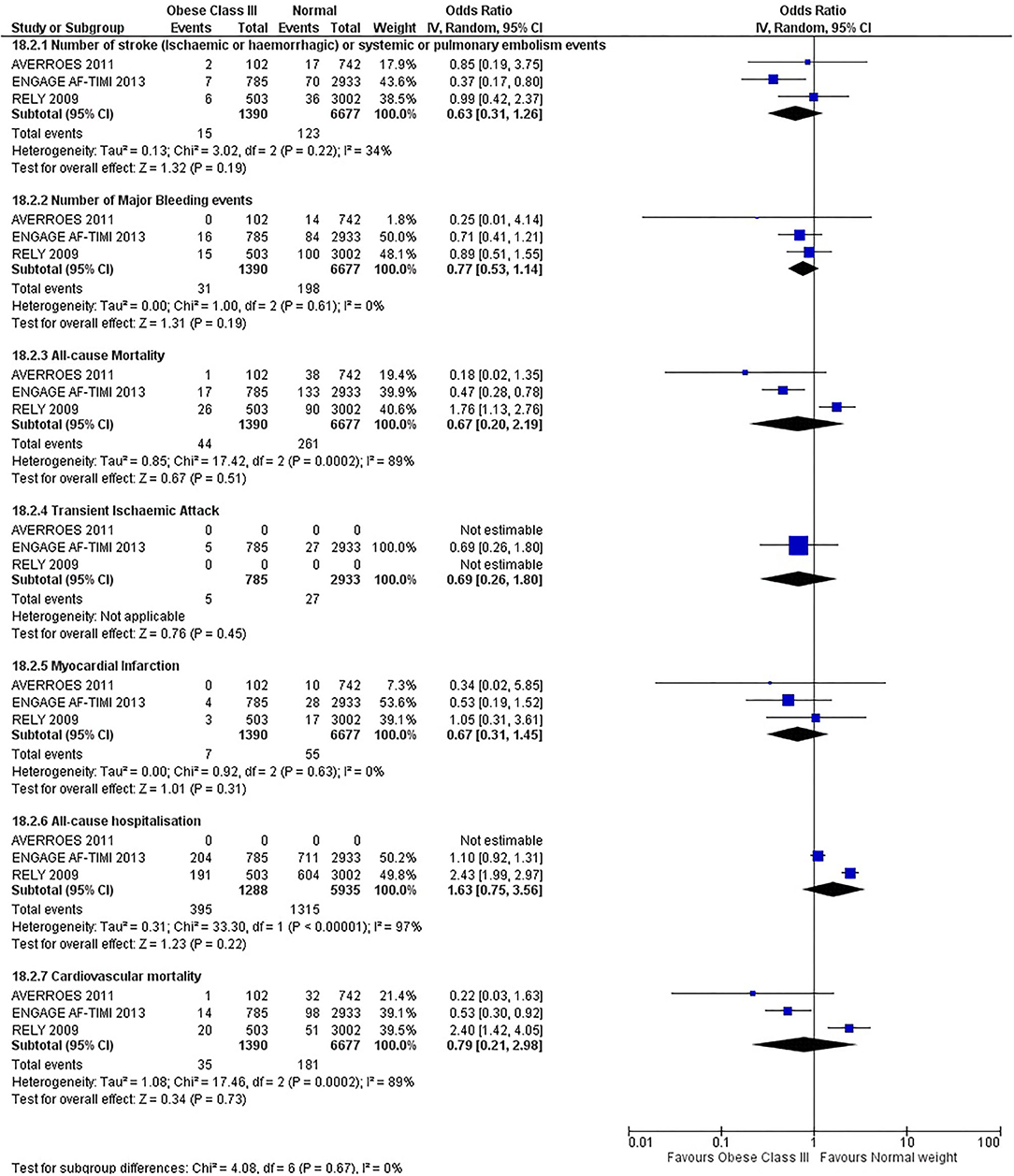
Our initial analysis had grouped the populations as either overweight/obese or normal/underweight. There was no significant difference between the two groups for any outcomes at 12 months (see Figures 2–7). Similarly, there was no significant difference between the different BMI groups when compared with normal BMI. However, we did notice a common trend across all analyses; there were differences in the results from the individual trials, suggesting there might be differences in the individual agents among the different weight groups. The primary efficacy outcome of stroke and primary safety outcome of major bleeding did not show any significant difference between any BMI groups.
There was, however, a difference between dabigatran (RE-LY 2009), apixaban (AVERROES 2011) and edoxaban (ENGAGE AF-TIMI 48 2013), where overall, dabigatran was favorable in the normal weight group when compared to overweight and obese classes for all-cause mortality (OR, 1.80; 95% CI, 1.27–2.55 [obese class II vs. normal]; OR, 1.76; 95% CI, 1.13–2.76 [obese class III vs. normal]), all-cause hospitalization (OR, 1.25; 95% CI, 1.12–1.40 [overweight vs. normal]; OR, 1.70; 95% CI, 1.50–1.92 [obese class I vs. normal]) OR, 2.17; 95% CI, 1.8–2.54 [obese class II vs. normal]) OR, 2.43; 95% CI, 1.99–2.97 [obese class III vs. normal]) and cardiovascular mortality (OR, 1.94; 95% CI, 1.24–3.03 [obese class II vs. normal]; OR, 2.40; 95% CI, 1.42–4.05 [obese class III vs. normal]). Dabigatran was also favorable in the BMI ≤ 25 group for all-cause hospitalization (OR, 1.53; 95% CI, 1.39, 1.69) and cardiovascular mortality (OR, 1.45; 95% CI, 1.07, 1.96) outcomes in the BMI ≥ 25 vs. BMI ≤ 25 comparison. Furthermore, data from the entire trial suggested that dabigatran was favorable in the normal group when compared to the obese class III for stroke (OR, 2.00; 95% CI, 1.23–3.27) and major bleeding (OR, 1.59; 95% CI, 1.11–2.26).
In contrast, apixaban was favorable in the overweight (OR, 0.42; 95% CI, 0.25, 0.71) and obese class II (OR, 0.08; 95% CI, 0.01, 0.59) group for all-cause mortality, and among the overweight (OR, 0.38; 95% CI, 0.21–0.68), obese class I (OR, 0.49; 95% CI, 0.26–0.95) and obese class II (OR, 0.05; 95% CI, 0.00–0.77) groups, for cardiovascular mortality in the overweight, obese class I and obese class II vs. normal weight comparisons. In the BMI ≥ 25 vs. BMI ≤ 25 comparison, apixaban was favorable in the BMI ≥ 25 group for stroke (OR, 0.51; 95% CI, 0.27–0.94), all-cause mortality (OR, 0.41; 95% CI, 0.26–0.62) and cardiovascular mortality (OR, 0.35; 95% CI, 0.21–0.56) outcomes.
Similarly, edoxaban (ENGAGE AF-TIMI 48) was favorable in the overweight and all obese classes for stroke (OR, 0.59; 95% CI, 0.42–0.82 [overweight vs. normal]; OR, 0.53; 95% CI, 0.36–0.78 [obese class I vs. normal]; OR, 0.59; 95% CI, 0.36–0.97 [obese class II vs. normal]; OR, 0.37; 95% CI, 0.17–0.80 [obese class III vs. normal]), all-cause mortality (OR, 0.68; 95% CI, 0.54–0.85 [overweight vs. normal]; OR, 0.55; 95% CI, 0.42–0.72 [obese class I vs. normal]; OR, 0.49; 95% CI, 0.33–0.72 [obese class II vs. normal]; OR, 0.47; 95% CI, 0.28–0.78 [obese class III vs. normal]) and cardiovascular mortality (OR, 0.71; 95% CI, 0.54–0.93 [overweight vs. normal]; OR, 0.58; 95% CI, 0.43–0.79 [obese class I vs. normal]; OR, 0.52; 95% CI, 0.33–0.81 [obese class II vs. normal]; OR, 0.53; 95% CI, 0.30–0.92 [obese class III vs. normal]) in the overweight and obese vs. normal comparisons. In the BMI ≥ 25 vs. BMI ≤ 25 comparison, edoxaban was favorable in the BMI ≥ 25 group for stroke (OR, 0.54; 95% CI, 0.41–0.71), all-cause mortality (OR, 0.56; 95% CI, 0.46–0.69) and cardiovascular mortality (OR, 0.59; 95% CI, 0.46–0.74) outcomes.
The analysis was repeated using data collected for the entire trial duration to explore differences resulting from a potential lack of power in data from 12 months (see Supplementary Figures 1–6). Our analysis revealed results similar to those reported at 12 months, where no significant difference was found between any of the subgroups. Additionally, we also noticed similar trends to that at 12 months, where there some difference with regards to the favorable subgroups when comparing the different DOACs. In summary, dabigatran was overall more favorable in the normal BMI group when compared to the different obese classes. This was in contrast with apixaban and edoxaban, where overall they were more favorable in the overweight/obese classes when compared to the normal BMI group. Supplementary Table 4 provides a summary of the differences between DOACs at both time points.
Discussion
There appears to be disagreement within the published systematic reviews on the use of DOACs in obese adults with AF. Data extraction inconsistencies and appropriateness of the statistical methods used in the analysis of the trials warrant further validation of the findings of the studies.
This meta-analysis did not show any significant difference between all BMI groups at 12 months or for the entire trial duration for all outcomes. The results do not indicate the presence of the obesity paradox for DOACs overall, although individual superiority may exist, which contrasts with the findings of Zhou et al. (20) and Proietti et al. (19).
We did, however, notice differences and trends, although not significant, among the different types of DOACs. Dabigatran was favorable overall in the normal weight group compared to overweight and obese classes predominately for stroke, major bleeding, all-cause mortality, all-cause hospitalization, and cardiovascular mortality. This contrasts with the results for apixaban and edoxaban, where these drugs were overall favorable in the overweight/obese classes. A similar observation was also found in a retrospective cohort study and a recent review of literature (61, 78).
Although our findings are not statistically significant or conclusive, the consistent trend across most of the analysis of the BMI groups, and new data from the literature, suggests there may be differences in the individual agents among the different weight groups. However, this would need to be further evaluated by future prospective trials and meta-analysis to contrast DOACs and evaluate the effect of dose differences of specific DOACs in obese adults.
While the original systematic reviews suggest the presence of an obesity paradox, they also point toward several underlying reasons for this. These include changes in baseline characteristics, that is, BMI, and dominance in data from subgroup analysis of RCTs, compared to data from observational studies after statistical adjustments for confounding factors (19, 27).
Over recent years, there have been numerous studies that have examined and alluded to the existence of the obesity paradox in multiple conditions such heart failure, diabetes, and now AF (22, 79). However, many of these studies fail to address or explore the possible reasons behind the “illusion” of the obesity paradox, despite the well-known consequences of obesity, which ironically is a risk factor of cardiovascular disease.
These findings are often found in post-hoc analysis of RCTs, where the authors also acknowledge the lack of recorded follow-up data regarding weight change or nutritional behavior as a limitation (19, 27, 79). This illuminates the importance of changes in baseline characteristics and lack of recording of any physical and nutritional changes that may occur in participants in RCTs. Lavie et al. (8) have also argued for the involvement of other confounding factors such as age and management disparity within the BMI groups, where higher BMI groups were significantly younger and had greater use of rhythm, rate and anticoagulant interventions compared to normal BMI groups (8).
Furthermore, due to the well-known complications and negative effects of obesity, over 50% of physicians advise patients to lose weight and to maintain a healthy diet (80). Studies have shown that physical activity can modify anticoagulation (warfarin) response by affecting blood fluidity (81–83). It has also been hypothesized that the presence of the obesity paradox is largely related to differences in cardiorespiratory fitness levels (8).
Although RCTs are considered the highest level of evidence for experimental studies, the lack of recording of any changes in baseline characteristics at follow up can influence the results, especially when post-hoc analyses are undertaken. Additionally, due to the strict inclusion and exclusion criteria many participants are not able to be included in the trial (84, 85). Studies have shown that up to 50–75% of patients that will end up being prescribed the same medications will not meet the inclusion criteria, implying that participants that are enrolled in the trial may not always be a true representation of the population (86, 87).
On the contrary, several recent studies (56, 88–90) have shown use of DOACs to be safe and effective in most obese adults compared to warfarin. These recent findings suggest that the previous threshold of 120 kg may have been conservative and generalized indicating all DOACs have a similar effect. Results from recent studies (61, 78, 91), including the results from this meta-analysis, however, suggest individual superiority of DOACs may exist within the obese adult populations. Further studies are warranted, however, to appreciate the true effect of obesity on DOACs.
Limitations
This review has several limitations. A key limitation was that we were unable to include the ARISTOTLE and ROCKET-AF trials in our meta-analysis. This meant that we were unable to comment on rivaroxaban and to a certain degree apixaban. Secondly, we did not include non-AF clinical trials and other study designs in our meta-analysis, which may have an impact on the applicability of the results on other conditions, that is, VTE and PE.
Conclusions
There was no difference between the BMI classes in any of the outcomes assessed. This may be due to the limited number of people in the trial that were in the obese class, especially obese class III. There is an urgent need for large prospective trials with population stratification for the inclusion of obese adults, especially obese class III, to confirm which DOACs are safe and efficacious in these patients and at which dose.
Author Contributions
FS and CF conceived the study and developed the search strategy, screened, and reviewed articles. FS, CF, and RW wrote and edited the manuscript. RC and SI edited, reviewed the articles, and provided expert opinion. All authors contributed to the article and approved the submitted version.
Funding
FS was supported by the Australian Government Research Training Program (RTP) through a Western Sydney University Doctoral Scholarship. SI receives funding through a Heart Foundation Future Leader Fellowship by the Heart Foundation (Australia). CF receives funding through a Heart Foundation Postdoctoral Fellowship (Ref: 102168) from the Heart Foundation (Australia) and a NHMRC Emerging Leadership Fellowship (APP 1196262).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the contribution of Dr. Robert P. Giugliano, MD (ENGAGE AF-TIMI 48) and Dr. Stuart Connolly, MD (RE-LY & AVERROES) who provided original data toward this review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.732828/full#supplementary-material
References
2. Capucci A, Villani CQ, Aschieri D. Risk of complications of atrial fibrillation. Pacing Clin Electrophysiol. (1997) 20: 2684–91. doi: 10.1111/j.1540-8159.1997.tb06117.x
3. Wang TJ, Parise H, Levy D, D'Agostino RB, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. (2004) 292:2471–7. doi: 10.1001/jama.292.20.2471
4. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. (2007) 49: 565–71. doi: 10.1016/j.jacc.2006.08.060
5. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol. (2010) 55:2319–27. doi: 10.1016/j.jacc.2010.02.029
6. Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. (2019) 8:28–36. doi: 10.15420/aer.2018.76.2
7. Asghar O, Alam U, Hayat SA, Aghamohammadzadeh R, Heagerty AM, Malik RA. Obesity, diabetes and atrial fibrillation; epidemiology, mechanisms and interventions. Curr Cardiol Rev. (2012) 8:253–64. doi: 10.2174/157340312803760749
8. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. (2017) 70:2022–35. doi: 10.1016/j.jacc.2017.09.002
9. Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, et al. National heart foundation of Australia and the cardiac society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. (2018) 27:1209–66. doi: 10.1016/j.hlc.2018.06.1043
10. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000665
11. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio-thoracic surgery (EACTS). Eur Heart J. (2020). 42:373–498. doi: 10.1093/eurheartj/ehaa612
12. Deloitte Access Economics. Off Beat: Atrial Fibrillation and the Cost of Preventable Strokes (2011).
13. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C, et al. 2018 focused update of the canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. (2018) 34:1371–92. doi: 10.1016/j.cjca.2018.08.026
14. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64:e1–76. doi: 10.1161/CIR.0000000000000041
15. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ J. (2018) 48:1033–80. doi: 10.4070/kcj.2018.0339
16. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210
17. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. (2018) 154:1121–201. doi: 10.1016/j.chest.2018.07.040
18. Wallace JL, Reaves AB, Tolley EA, Oliphant CS, Hutchison L, Alabdan NA, et al. Comparison of initial warfarin response in obese patients versus non-obese patients. J Thromb Thrombolysis. (2013) 36:96–101. doi: 10.1007/s11239-012-0811-x
19. Proietti M, Guiducci E, Cheli P, Lip GYH. Is There an obesity paradox for outcomes in atrial fibrillation?: a systematic review and meta-analysis of non-vitamin k antagonist oral anticoagulant trials. Stroke. (2017) 48:857–66. doi: 10.1161/STROKEAHA.116.015984
20. Zhou Y, Ma J, Zhu W. Efficacy and safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation across BMI categories: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020) 20:51–60. doi: 10.1007/s40256-019-00362-4
21. Pouwels S, Topal B, Knook MT, Celik A, Sundbom M, Ribeiro R, et al. Interaction of obesity and atrial fibrillation: an overview of pathophysiology and clinical management. Expert Rev Cardiovasc Ther. (2019) 17:209–23. doi: 10.1080/14779072.2019.1581064
22. Lamelas P, Schwalm JD, Leong D, Jolly S, Mehta S, Bangdiwala S, et al. Varying effects of body mass index and mortality in different risk groups. AmJ Cardiol. (2018) 122:1155–60. doi: 10.1016/j.amjcard.2018.06.038
23. Javed S, Gupta D, Lip GYH. Obesity and atrial fibrillation: making inroads through fat. Eur Heart J Cardiovasc Pharmacother. (2021) 7:59–67. doi: 10.1093/ehjcvp/pvaa013
24. van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. (2014) 124:1968–75. doi: 10.1182/blood-2014-04-571232
25. Malik AH, Yandrapalli S, Shetty S, Aronow WS, Jain D, Frishman WH, et al. Impact of weight on the efficacy and safety of direct-acting oral anticoagulants in patients with non-valvular atrial fibrillation: a meta-analysis. Europace. (2020) 27:361–7. doi: 10.1093/europace/euz361
26. Zhu W, Wan R, Liu F, Hu J, Huang L, Li J, et al. Relation of body mass index with adverse outcomes among patients with atrial fibrillation: a meta-analysis and systematic review. J Am Heart Assoc. (2016) 5:e004006. doi: 10.1161/JAHA.116.004006
27. Boonyawat K, Caron F, Li A, Chai-Adisaksopha C, Lim W, Iorio A, et al. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: a systematic review and meta-analysis. J Thromb Haemost. (2017) 15:1322–33. doi: 10.1111/jth.13701
28. EliquisR (Apixaban). [Australian Product Information]. Mulgrave, VIC: Bristol-Myers Squibb Australia Pty. Ltd. (2020).
29. Pradaxa R (dabigatran etexilate). [Australian Product Information]. North Ryde, NSW: Boehringer Ingelheim Pty. Limited (2020).
31. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Eng J Med. (2011) 365:981–92. doi: 10.1056/NEJMoa1107039
32. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New Eng J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
33. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New Eng JMed. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
34. Hohnloser SH, Fudim M, Alexander JH, Wojdyla DM, Ezekowitz JA, Hanna M, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight: insights from the aristotle trial. Circulation. (2019) 139:2292–300. doi: 10.1161/CIRCULATIONAHA.118.037955
35. Rocca B, Fox KAA, Ajjan RA, Andreotti F, Baigent C, Collet JP, et al. Antithrombotic therapy and body mass: an expert position paper of the ESC working group on thrombosis. Eur Heart J. (2018) 39:1672–86. doi: 10.1093/eurheartj/ehy066
36. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. (2016) 14:1308–13. doi: 10.1111/jth.13323
37. Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI. (2020). doi: 10.46658/JBIMES-20-04
38. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane (2021).
40. Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, VIC: Veritas Health Innovation (2020).
41. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
42. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
43. Kido K, Shimizu M, Shiga T, Hashiguchi M. Meta-analysis comparing direct oral anticoagulants versus warfarin in morbidly obese patients with atrial fibrillation. Am J Cardiol. (2020) 126:23–28. doi: 10.1016/j.amjcard.2020.03.048
44. Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. (2014) 129:764–72. doi: 10.1161/CIRCULATIONAHA.113.004450
45. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New Eng J Med. (2009) 361:2342–52. doi: 10.1160/TH17-03-0176
46. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl JMed. (2010) 363:2499–510. doi: 10.1056/NEJMoa1007903
47. Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. (2012) 366:1287–97. doi: 10.1056/NEJMoa1113572
48. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. (2013) 369:799–808. doi: 10.1056/NEJMoa1302507
49. Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. (2013) 369:1406–15. doi: 10.1056/NEJMoa1306638
50. Ardestani A, Hoffman HJ, Cooper HA. Obesity and outcomes among patients with established atrial fibrillation. Am J Cardiol. (2010) 106:369–73. doi: 10.1016/j.amjcard.2010.03.036
51. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. (2010) 123:646–51. doi: 10.1016/j.amjmed.2009.11.026
52. Senoo K, Lip GY. Body mass index and adverse outcomes in elderly patients with atrial fibrillation: the AMADEUS trial. Stroke. (2016) 47:523–6. doi: 10.1161/STROKEAHA.115.011876
53. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, et al. The'obesity paradox'in atrial fibrillation: observations from the ARISTOTLE (Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. (2016) 37:2869–78. doi: 10.1093/eurheartj/ehw124
54. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
55. Connolly SJ, Eikelboom J, Joyner C, Diener H-C, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. New Eng J Med. (2011) 364:806–17. doi: 10.1056/NEJMoa1007432
56. Boriani G, Ruff CT, Kuder JF, Shi M, Lanz HJ, Rutman H, et al. Relationship between body mass index and outcomes in patients with atrial fibrillation treated with edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. Eur Heart J. (2019) 40:1541–50. doi: 10.1093/eurheartj/ehy861
57. Balla SR, Cyr DD, Lokhnygina Y, Becker RC, Berkowitz SD, Breithardt G, et al. Relation of risk of stroke in patients with atrial fibrillation to body mass index (from patients treated with rivaroxaban and warfarin in the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation trial). Am J Cardiol. (2017) 119:1989–96. doi: 10.1016/j.amjcard.2017.03.028
58. Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R2CHADS2 Index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonismfor prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) Study Cohorts. Circulation. (2013) 127:224–32. doi: 10.1161/CIRCULATIONAHA.112.107128
59. Sandhu RK, Ezekowitz J, Andersson U, Alexander J, Granger C, Halvorsen S, et al. Body mass index and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the aristotle (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. J Am College Cardiol. (2015) 65:A284. doi: 10.1016/S0735-1097(15)60284-4
60. Kushnir M, Choi Y, Eisenberg R, Rao D, Tolu S, Gao J, et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single-centre, retrospective analysis of chart data. Lancet Haematol. (2019) 6:e359–65. doi: 10.1016/S2352-3026(19)30086-9
61. Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother. (2019) 53:165–70. doi: 10.1177/1060028018796604
62. Perales IJ, San Agustin K, DeAngelo J, Campbell AM. Rivaroxaban versus warfarin for stroke prevention and venous thromboembolism treatment in extreme obesity and high body weight. Ann Pharmacother. (2020) 54:344–50. doi: 10.1177/1060028019886092
63. Peterson ED, Ashton V, Chen YW, Wu B, Spyropoulos AC. Comparative effectiveness, safety, and costs of rivaroxaban and warfarin among morbidly obese patients with atrial fibrillation. Am Heart J. (2019) 212:113–9. doi: 10.1016/j.ahj.2019.02.001
64. Overvad TF, Rasmussen LH, Skjøth F, Overvad K, Lip GY, Larsen TB. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med. (2013) 126:640.e9–17. doi: 10.1016/j.amjmed.2012.11.024
65. Wang J, Yang YM, Zhu J, Zhang H, Shao XH, Tian L, et al. Overweight is associated with improved survival and outcomes in patients with atrial fibrillation. Clin Res Cardiol. (2014) 103:533–42. doi: 10.1007/s00392-014-0681-7
66. Bunch TJ, May HT, Bair TL, Crandall BG, Cutler MJ, Jacobs V, et al. Long-term influence of body mass index on cardiovascular events after atrial fibrillation ablation. Jour of Intervent Card Electrop. (2016) 46:259–65. doi: 10.1007/s10840-016-0142-5
67. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. (2011) 123:2363–72. doi: 10.1161/CIRCULATIONAHA.110.004747
68. Park CS, Choi EK, Kim HM, Lee SR, Cha MJ, Oh S. Increased risk of major bleeding in underweight patients with atrial fibrillation who were prescribed non-vitamin K antagonist oral anticoagulants. Heart Rhythm. (2017) 14:501–7.
69. Lee SR, Choi EK, Park CS, Han KD, Jung JH, Oh S, et al. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am College Cardiol. (2019) 73:919–31. doi: 10.1016/j.jacc.2018.11.051
70. Yanagisawa S, Inden Y, Yoshida N, Kato H, Miyoshi-Fujii A, Mizutani Y, et al. Body mass index is associated with prognosis in Japanese elderly patients with atrial fibrillation: an observational study from the outpatient clinic. Heart Vessels. (2016) 31:1553–61. doi: 10.1007/s00380-015-0765-y
71. Prins M, Nisio M, Vedovati M, Riera-Mestre A, Mueller K, Cohen A, et al. Fixed-dose rivaroxaban is not associated with increased recurrent venous thromboembolism or major bleeding in patients with a high or low body weight: AS099. J Thrombosis Haemost. (2015) 13:35–6.
72. Wang HJ, Si QJ, Shan ZL, Guo YT, Lin K, Zhao XN, et al. Effects of body mass index on risks for ischemic stroke, thromboembolism, and mortality in Chinese atrial fibrillation patients: a single-center experience. PLoS ONE. (2015) 10:e0123516. doi: 10.1371/journal.pone.0123516
73. Kwon Y, Norby FL, Jensen PN, Agarwal SK, Soliman EZ, Lip GY, et al. Association of smoking, alcohol, and obesity with cardiovascular death and ischemic stroke in atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study and Cardiovascular Health Study (CHS). PLoS ONE. (2016) 11:e0147065. doi: 10.1371/journal.pone.0147065
74. Pandey A, Gersh BJ, McGuire DK, Shrader P, Thomas L, Kowey PR, et al. Association of body mass index with care and outcomes in patients with atrial fibrillation: results from the ORBIT-AF registry. JACC Clin Electrophysiol. (2016) 2:355–63. doi: 10.1016/j.jacep.2015.12.001
75. Inoue H, Kodani E, Atarashi H, Okumura K, Yamashita T, Origasa H, et al. Impact of body mass index on the prognosis of Japanese patients with non-valvular atrial fibrillation. Am J Cardiol. (2016) 118:215–21. doi: 10.1016/j.amjcard.2016.04.036
76. Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. (2016) 69:225–34. doi: 10.1016/j.jclinepi.2015.06.005
77. Collaboration TC. Review Manager (RevMan). 53 ed. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration. (2014).
78. Sebaaly J, Kelley D. Direct oral anticoagulants in obesity: an updated literature review. Ann Pharmacother. (2020) 54:1144. doi: 10.1177/1060028020923584
79. Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J. (2013) 34:268–77. doi: 10.1093/eurheartj/ehs340
80. Halbert CH, Jefferson M, Melvin CL, Rice L, Chukwuka KM. Provider advice about weight loss in a primary care sample of obese and overweight patients. J Prim Care Community Health. (2017) 8:239–46. doi: 10.1177/2150131917715336
81. Ernst E. Influence of regular physical activity on blood rheology. Eur Heart J. (1987) 8:59–62. doi: 10.1093/eurheartj/8.suppl_g.59
82. Shendre A, Beasley TM, Brown TM, Hill CE, Arnett DK, Limdi NA. Influence of regular physical activity on warfarin dose and risk of hemorrhagic complications: physical activity and warfarin response. Pharmacotherapy. (2014) 34:545–54. doi: 10.1002/phar.1401
83. Frey PM, Mean M, Limacher A, Jaeger K, Beer HJ, Frauchiger B, et al. Physical activity and risk of bleeding in elderly patients taking anticoagulants. J Thromb Haemost. (2015) 13:197–205. doi: 10.1111/jth.12793
84. Averitt AJ, Weng C, Ryan P, Perotte A. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med. (2020) 3:67. doi: 10.1038/s41746-020-0277-8
85. Tashkin DP, Amin AN, Kerwin EM. Comparing randomized controlled trials and real-world studies in chronic obstructive pulmonary disease pharmacotherapy. Int J Chron Obstruct Pulmon Dis. (2020) 15:1225. doi: 10.2147/COPD.S244942
86. Knudsen JS, Thomsen RW, Pottegard A, Knop FK, Sorensen HT. Differences between randomized clinical trial patients and real-world initiators of the glucagon-like peptide 1 receptor agonist liraglutide. Diabetes Care. (2018) 41:e13345. doi: 10.2337/dc18-0999
87. Munk NE, Knudsen JS, Pottegard A, Witte DR, Thomsen RW. Differences between randomized clinical trial participants and real-world empagliflozin users and the changes in their glycated hemoglobin levels. JAMA Netw Open. (2020) 3:e1920949. doi: 10.1001/jamanetworkopen.2019.20949
88. Kalani C, Awudi E, Alexander T, Udeani G, Surani S. Evaluation of the efficacy of direct oral anticoagulants (DOACs) in comparison to warfarin in morbidly obese patients. Hosp Pract. (2019) 47:181–85. doi: 10.1080/21548331.2019.1674586
89. Coons JC, Albert L, Bejjani A, Iasella CJ. Effectiveness and safety of direct oral anticoagulants versus warfarin in obese patients with acute venous thromboembolism. Pharmacotherapy. (2020) 40:204. doi: 10.1002/phar.2369
90. Martin AC, Thomas W, Mahir Z, Crowley MP, Dowling T, Breen K, et al. Direct oral anticoagulant concentrations in obese and high body weight patients: a cohort study. Thromb Haemost. (2021) 121:224–33. doi: 10.1055/s-0040-171583
Keywords: atrial fibrillation, obesity, anticoagulant, direct oral anticoagulants, body mass index, pharmacology
Citation: Shaikh F, Wynne R, Castelino RL, Inglis SC and Ferguson C (2021) Effectiveness of Direct Oral Anticoagulants in Obese Adults With Atrial Fibrillation: A Systematic Review of Systematic Reviews and Meta-Analysis. Front. Cardiovasc. Med. 8:732828. doi: 10.3389/fcvm.2021.732828
Received: 30 June 2021; Accepted: 13 September 2021;
Published: 08 October 2021.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Tanja Mueller, University of Strathclyde, United KingdomJohn Fanikos, Brigham and Women's Hospital and Harvard Medical School, United States
Benilde Cosmi, Università di Bologna, Italy
Copyright © 2021 Shaikh, Wynne, Castelino, Inglis and Ferguson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahad Shaikh, MTk1ODQ3NzVAc3R1ZGVudC53ZXN0ZXJuc3lkbmV5LmVkdS5hdQ==
 Fahad Shaikh
Fahad Shaikh Rochelle Wynne
Rochelle Wynne Ronald L. Castelino3,4
Ronald L. Castelino3,4 Caleb Ferguson
Caleb Ferguson