
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 30 September 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.732349
Background: A large number of studies have shown that the arrhythmia risks may be the potential causes of death among chronic obstructive pulmonary disease (COPD) patients. However, the association of COPD with risks of arrhythmias has never been systematically reviewed. Therefore, we performed a meta-analysis to assess the relationship between COPD and arrhythmia risks.
Methods: An updated systematic retrieval was carried out within the databases of Embase and PubMed until June 27, 2021.The random-effects model was used to pool studies due to the potential heterogeneity across the included studies. The risk ratios (RRs) with 95% confidence intervals (CIs) were regarded as effect estimates.
Results: A total of 21 studies were included in our meta-analysis. In the pooled analysis by the random-effects model, the results showed that COPD was significantly related to the risk of atrial fibrillation (AF) (RR = 1.99, 95% CI: 1.46–2.70), ventricular arrhythmias (VA) (RR = 2.01, 95% CI: 1.42–2.85), and sudden cardiac death (SCD) (RR = 1.68, 95% CI: 1.28–2.21). The corresponding results were not changed after exclusion one study at a time. The pooled results were also stable when we re-performed the analysis using the fixed-effects model.
Conclusions: Our current data suggested that COPD was associated with increased risks of AF, VA, and SCD.
Chronic obstructive pulmonary disease (COPD) is a chronic condition and imposes a heavy burden on both public health and the social economy. Available evidence (1) indicates that the worldwide incidence rate of COPD is estimated at 4–5 million cases per year, and nearly 10% of adults aged 40 years or older suffer from COPD. Moreover, the mortality rate of COPD has been increasing during the past few years. Not only COPD itself but also COPD-related complications account for a sizable proportion of the rising mortality, and one-third of deaths are associated with cardiovascular diseases (2). Indeed, COPD patients are more likely to develop cardiovascular diseases such as arrhythmia, ischemic heart disease, [200mm][-13mm] Q10heart failure, pulmonary circulation disease, and arterial disease (3). In addition, COPD and cardiovascular diseases share common risk factors such as smoking and obesity (4). Systemic inflammatory states in patients with COPD can aggravate the development of atherosclerosis, giving rise to the risk of heart failure (5).
Recent studies (6, 7) have shown COPD may be responsible for the development of arrhythmias. A comprehensive study conducted by Rusinowicz et al. (8) has shown that COPD exacerbation is related to a high incidence of arrhythmias, and COPD patients receiving conventional therapy can increase the risk of arrhythmias. Rusnak et al. (9) pointed out that COPD patients, who were presented with ventricular arrhythmias (VA), were associated with a higher rate of all-cause mortality. The lower forced expiratory volume at 1 s (FEV1) and forced vital capacity (FVC) are related to a higher risk of atrial fibrillation (AF) (10). In addition, COPD is found to be associated with a high risk of sudden cardiac death (SCD) in the community (11). Researching on the associations of COPD with the arrhythmia risks is deemed important since it may have implications for the management of these patients. In a word, the relationship between COPD and arrhythmia has not been fully elucidated and high-quality evidence is needed. The objective of this study is to prove the association between COPD and arrhythmia risks clearly, this allows medical personnel to adjust the treatment of patients with COPD.
Herein, we conducted a systematic review and meta-analysis to evaluate the relationship between COPD and arrhythmia risks among individuals without preexisting arrhythmias.
Our current meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (12). We did not need to provide ethical approval because only published articles were included in this meta-analysis. Readers can obtain data, methods, and materials by contacting the corresponding author to reproduce the results or the program.
We systematically searched the databases of Embase and PubMed until June 27, 2021 for studies reporting the relationship between COPD and the development of AF, VA, and SCD. We applied the following keywords and searched terms to found out the relevant studies: (1) COPD OR chronic obstructive pulmonary disease OR lung function AND (2) arrhythmia OR atrial fibrillation OR ventricular tachycardia OR ventricular arrhythmia OR sudden cardiac death. To avoid missing the potential studies, the reference lists of the included studies were checked. No language restriction was applied in the searching process.
We included studies that reported the relationship between COPD and the risk of AF, VA, and SCD with no restriction of study type. The definitions of COPD and outcomes (AF, VA, or SCD) were based on the original pieces of literature. Studies would be excluded if they had no data (e.g., comments, case reports, reviews, editorials, letters). Conference abstracts were also excluded because they did not have enough information to assess the study quality.
Two authors independently screened the studies from the databases. We then read the titles and abstracts of the records to select potential studies that could be used. And then, we screened the full text of these potential studies to identify the final studies. Discrepancies and disagreements were resolved by consensus or discussion with other authors.
For each included study, we extracted the following characteristics: the first author and publication year, study design, data source, information of population (age, sex, and sample size), definitions of COPD, and outcomes of AF, VA, or SCD, follow-up duration, and effect estimates. Adjusted risk ratios (RRs) and confidence intervals (CIs) were extracted from each included study.
We assessed the study quality using the Newcastle-Ottawa Scale (NOS). The NOS tool included three major sections as follows: the selection of cohorts (0–4 points), the comparability of cohorts (0–2 points), and the assessment of the outcome (0–3 points). In this meta-analysis, a NOS score of ≥6 points was regarded as a moderate-to-high quality, while a NOS score of <6 points was regarded as a low-quality. The post-hoc analysis of RCT was regarded as a cohort study to conduct the quality assessment (13).
We assessed the heterogeneity across the included studies using the I2-values. In this study, I2 > 50% indicated significant heterogeneity. In the pooled analysis, we calculated the natural logarithm of the RR (Ln[RR]) and its standard error (SELn[RR]). The random-effects model was used to pool the Ln[RR] and SELn[RR] due to the potential heterogeneity across the included studies (14, 15). In the sensitivity analysis, we excluded one study at a time to examine the effect of each study on the pooled results. In addition, we used the fixed-effects model to re-perform the meta-analysis. The publication bias was checked by observing the symmetry characteristics of the funnel plots, a further assessed statistically using the Egger's and Begg's tests.
All statistical analyses were performed using the Stata software (version 15.0, Stata Corp LP, College Station, TX). In the pooled analysis, the statistical significance threshold was set at a P-value of < 0.05.
The process of the literature search is shown in Figure 1. A total of 21 studies (1 post-hoc analysis of RCT, and 20 observational studies) were included in our meta-analysis with a total of 7,916,582 participants (Supplementary Table 1).
The baseline characteristics of the included studies are illustrated in Table 1. These studies were published from 2002 to 2021, and the sample size ranged from 103 to 2,499,235 participants. Among the 21 included studies, 14 were prospective studies and 7 were retrospective studies. A total of 11, 8, and 5 studies provided the effect estimates for the risks of AF, VA, and SCD, respectively. The definition of COPD, AF, VA, and SCD in included studies were listed in Supplementary Tables 2–5. All the included studies had a moderate-to-high quality with a NOS score ranging from 6 to 8 points. The result of enrolled studies used the multivariable regression models (adjusting for confounders including age, sex, left atrial size, cardiovascular diseases, smoke) or propensity score-based analytical methods (balance baseline patient characteristics) (Supplementary Table 6).
A total of 11 included studies reported the association of COPD with AF risk. Li (16) reported the race- and sex-specific incidence rates of AF for airflow obstruction, separately. Lip et al. (19) reported the relationship between COPD and AF incidence at three distinct age cohorts (65, 70, and 75 years), separately. Grymonprez (23) studied this association according to the exacerbation frequency of COPD, and Konecny (27) reported the association between the severity of COPD and arrhythmias. Mapel et al. (17) provided the outcomes of AF and atrial flutter, respectively. Therefore, we combined the separate data of these studies as the final effect estimates in the pooled analysis. As shown in Figure 2, the pooled result from the random-effects model showed that COPD was associated with an increased risk of AF (RR = 1.99, 95% CI:1.46–2.70).
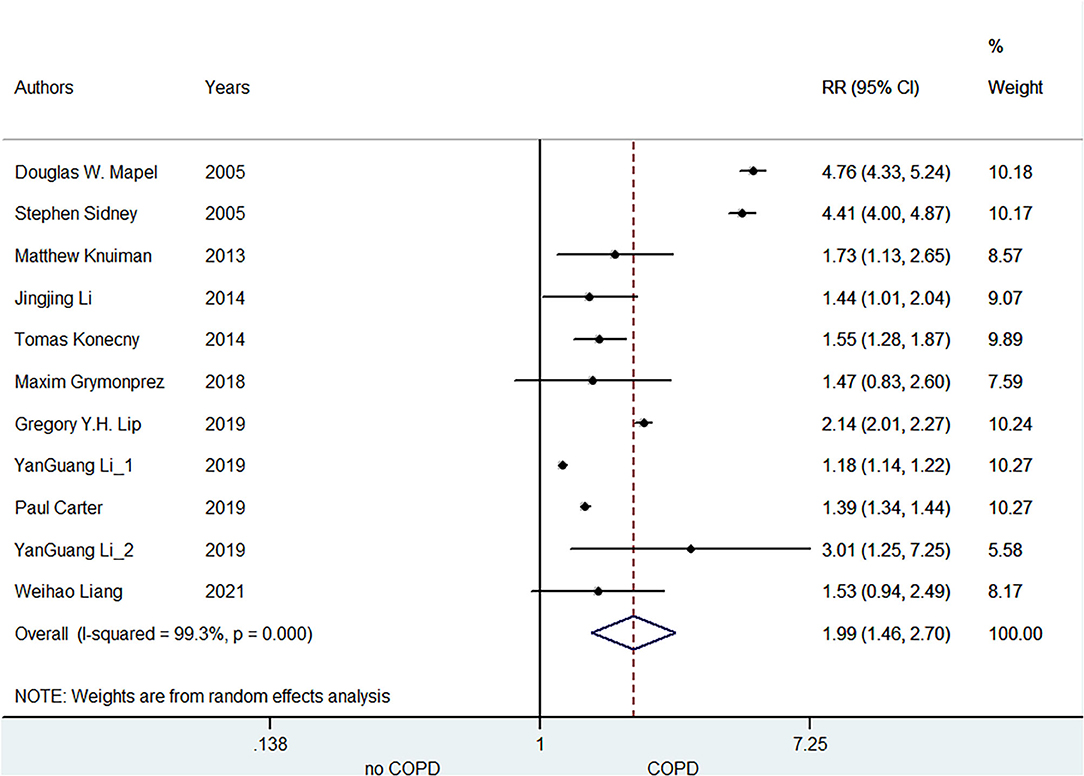
Figure 2. Meta-analysis of the pooled risk ratio of AF in COPD patients. AF, atrial fibrillation; RR, risk ratio.
A total of eight included studies focused on the relationship between COPD and VA risk. Konecny et al. (20) demonstrated that there was an independent correlation between the severity of COPD and arrhythmia. Kong (29) indicated this association based on heart rate deceleration runs. Chen (31) manifested the age- and sex-specific association of COPD with VA development. Kusunoki (7) points out that increased VA is likely related to the peculiar pathophysiology of COPD. As shown in Figure 3, the pooled result from the random-effects model showed that COPD was associated with an increased risk of VA (RR = 2.01, 95% CI: 1.42–2.85).
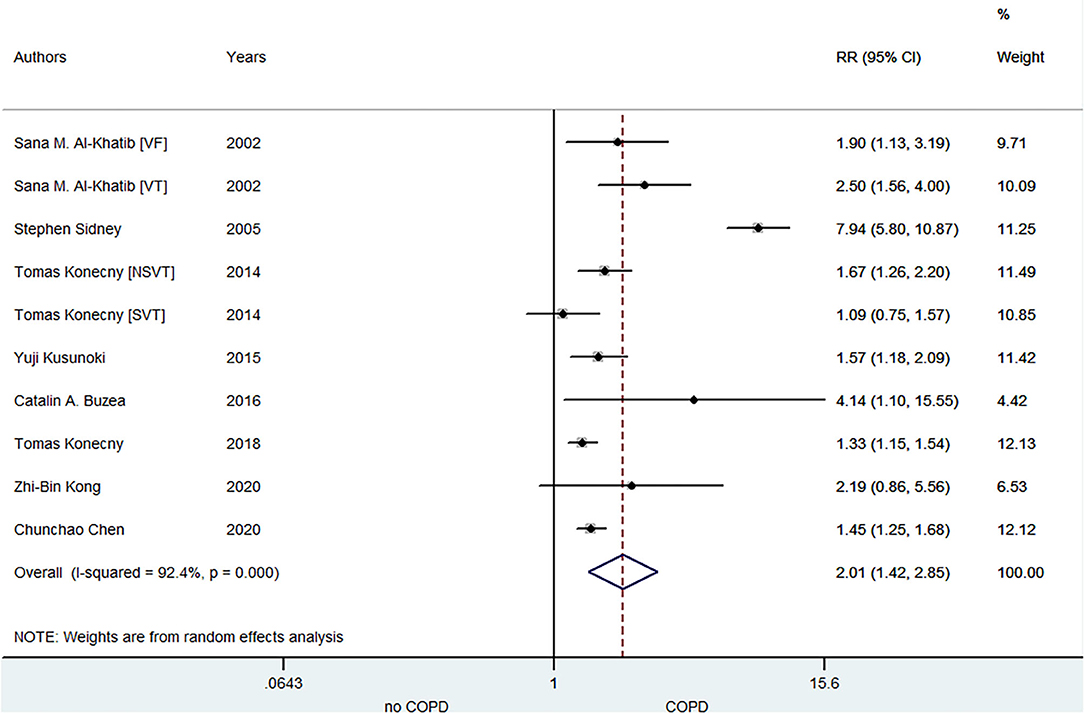
Figure 3. Meta-analysis of the pooled risk ratio of VA in COPD patients. VA, ventricular arrhythmia; RR, risk ratio; VF, ventricular fibrillation; VT, ventricular tachycardia; NSVT, non-sustained ventricular tachycardia; SVT, sustained ventricular tachycardia.
A total of five included studies focused on the relationship between COPD and SCD risk. Lahousse (28) indicated that the risk of SCD especially increased in patients with frequent exacerbations within 5 years of the diagnosis of COPD. Narayanan (11) illustrated that COPD was associated with SCD risk in the community, and the medications, electrocardiographic risk markers and left ventricular ejection fraction did not influence this association. As shown in Figure 4, the pooled result from the random-effects model showed that COPD was associated with an increased risk of SCD (RR = 1.68, 95% CI: 1.28–2.21).
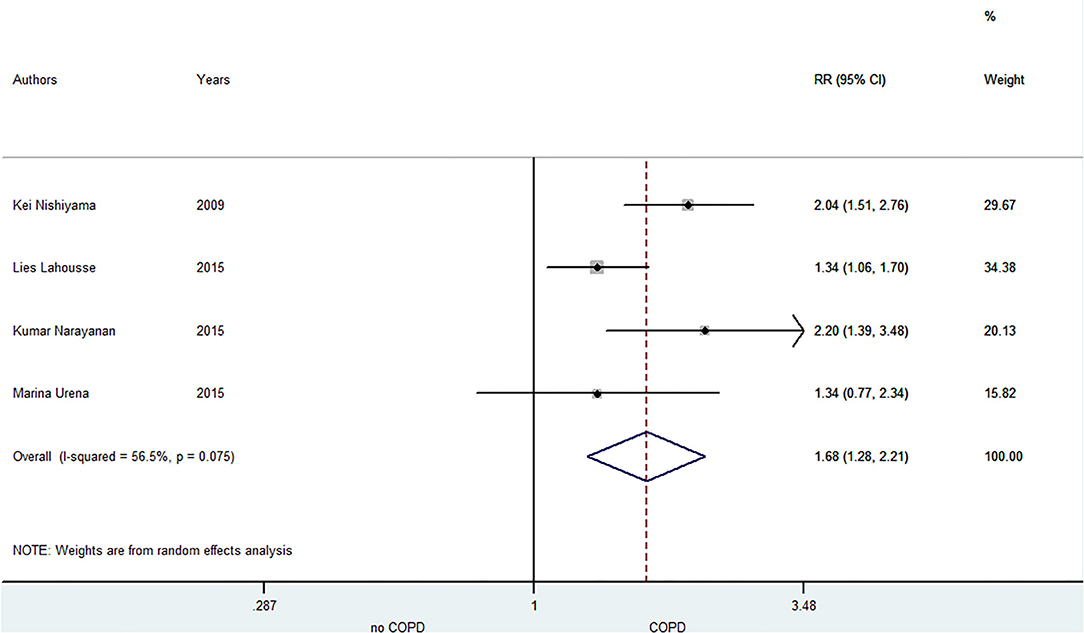
Figure 4. Meta-analysis of the pooled risk ratio of SCD in COPD patients. SCD, sudden cardiac death; RR, risk ratio.
In the sensitivity analysis, the pooled results were stable when we re-performed the above-mentioned analysis using the fixed-effects model (Supplementary Figures 1–3). In addition, the corresponding results were not changed after exclusion one study at a time (Supplementary Figures 7–9).
The publication biases assessed by the funnel plots are presented in Supplementary Figures 4–6. No potential publication biases were observed by inspecting the funnel plots. Furthermore, both the Egger's and Begg's tests showed no significant publication biases (all P > 0.1).
To the best of our knowledge, this was the first meta-analysis to assess the relationship between COPD and arrhythmia risks. Our results based on the random-effect model analysis demonstrated that COPD was associated with the risks of AF, VA, and SCD. The corresponding results were not changed after exclusion one study at once. The pooled results were also stable when we re-performed the analysis by using the fixed-effects model.
Several studies have shown that COPD is associated with increased risks of cardiovascular diseases (e.g., arrhythmia, ischemic heart disease, heart failure, and arterial disease). Cosgun et al. (35) reported that the markers of arrhythmia (Tp-e, cTp-e intervals, Tp-e/QT ratio, and Tp-e/QTc ratio) were significantly higher in COPD patients compared with non-COPD individuals, suggesting the relationship between COPD and arrhythmia risks. Konecny et al. (27) evaluated the severity of COPD and found that it was an independent risk factor for VA in COPD patients. Cardiovascular diseases and COPD have a pro-inflammatory state characterized by oxidative stress and accelerated aging (36), which are considered to be a possible mechanism for the increased risk of cardiovascular diseases in COPD patients. However, compelling evidence to formally quantified the increased risks of arrhythmia in patients with COPD are still lacked. Herein, we summed up relevant studies to figure out the clear relationship between COPD and arrhythmia risks, suggesting that COPD was associated with the risks of AF, VA, and SCD.
Recent studies have explored the pathophysiological mechanisms between COPD and arrhythmias. As an important pathological manifestation of COPD, hypoxia has an impact on atrial electrophysiology, potentially resulting in the development of arrhythmias. Lammers et al. (37) have reported that hypoxia can cause reentrant arrhythmia in the experimental rabbits particularly at the apex of the heart. Both hypercapnia and hypoxemia caused by COPD can lead to the pulmonary arterioles contracting, which in turn increases pulmonary artery and right atrial pressure. The increased pressure of the right atrium will cause the expansion of the right atrium, the hemodynamic changes of the endocardial blood vessels, and the resetting of blood flow, resulting in the increased arrhythmia susceptibility (38).
Our results in this meta-analysis showed that patients with COPD were more likely to suffer from AF. A large population-based cohort study conducted by Mapel et al. (17) showed the risk of AF in COPD subjects was increased significantly, and patients with COPD might have sustained significant myocardial damage when AF is combined. AF in patients with COPD is more likely to worsen dramatically due to frequent deterioration and enlargement of the left atrium. Moreover, Stevenson illustrated that different recovery of effective refractory period and conduction after hypercapnia may be the reasons for the increased risk of AF in patients with COPD (39). In addition, the ventricular systolic dysfunction (40) and the increased systolic pressure caused by COPD could trigger AF (41), respectively.
We also found that COPD was associated with an increased risk of VA. In patients with COPD, there are a large number of risk factors that induce VA, such as hypoxia, acidosis, and decreased FEV1 (42). Mulloy et al. (43) showed that because of alveolar hypoventilation and imbalance of ventilation/perfusion ratio, COPD patients had a significant reduction in oxygen saturation during sleep. Besides, it was found by Shepard et al. (44) that patients with average SaO2 <0.80 had a particularly high frequency of VA. Therefore, we indicated that night hypoxia caused by COPD is one of the factors that increase the risk of VA.
In addition, similar results as above were observed in the relationship between COPD and SCD. Several typical and severe features of COPD were also key factors for SCD, including prolonged heart rate, hypoxia, hypoxemia, myocardial ischemia, and heart failure. Lahousse et al. (28) revealed that SCD incidence significantly increased in the case of reduced ventilation and weaken responses to hypercapnia. That's why the night was a time of high incidence of SCD in patients with COPD. Furthermore, the correlation between COPD and SCD was independent in smoking but involved in gender, lung function, QT interval variability, tissue hypoxia, and drug treatment (45). Even though there was no study to assess the common genetic risk factors between COPD and SCD, it may also play a key role in the relationship.
Although a clear relationship between arrhythmia and COPD has been verified in our meta-analysis, the arrangement of COPD patients remains controversial. Konecny et al. (26) demonstrated that preventing the occurrence of COPD may help reduce the incidence of arrhythmia. Major inhalation medications have been shown to precipitate arrhythmia. Since β-adrenergic receptors mediate the distribution of potassium in cardiomyocytes during the action potential, β receptor agonists have a potential for arrhythmogenicity (46). Studies have shown that the new use of short-acting beta agonists and long-acting beta agonists will increase the risk of AF (47). Salpetera et al. noted that a single, regular dose of inhalation of salbutamol enhances atrioventricular nodal conduction and shortens the atrial refractory period, thereby increasing the incidence of arrhythmias (48). However, Chen et al. (31) reported that the combination of short-acting Beta2 agonists and short-acting muscarinic agonists helped reduce VA risk. Since available data could not draw a unanimous conclusion, a more detailed, larger sample study was expected to conduct for investigating a better treatment in COPD patients with arrhythmia.
Overall, our current meta-analysis still had several limitations. First, since most of the selected studies do not perform a bronchial reversibility test to distinguish COPD from asthma, the diagnosis of COPD is not accurate enough. Second, we did not register this research in the PROSPERO (International prospective register of systematic reviews) system. Nevertheless, no similar researches have appeared in this system. Third, although the result yielded statistically significant, clinical heterogeneity was still obvious. Potential causes included the lack of recent studies and the inconsistent definition of COPD. Fourth, we could not perform the subgroup analysis based on the classification of AF (paroxysmal and persistent) due to the limited data. In addition, we did not conduct subgroup analysis due to insufficient original data while some confounding factors (e.g. race, follow-up time, number of participants, and whether be received treatment) may affect the results.
Available data support that COPD was associated with increased risks of AF, VA, and SCD. Further high-quality studies would be required to confirm the relationship between COPD and arrhythmia risks.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
XL, SX, ZC, and SL conceived and designed the study. XL, ZC, and SL contributed to acquisition of data, quality assessment, and design of statistical analyses. XL and ZC interpreted the data and wrote the first draft of the study. All authors contributed to critical revision of the report for important intellectual content and approval of the final version to be published.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank Dr. Wengen Zhu (Department of Cardiology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou of Guangdong, China) for his directions in the revised version of our meta-analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.732349/full#supplementary-material
1. Global regional and national deaths prevalence disability-adjusted life years and years lived with disability for chronic obstructive pulmonary disease and asthma 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. (2017) 5:691–706. doi: 10.1016/s2213-2600(17)30293-x
2. André S, Conde B, Fragoso E, Boléo-Tomé JP, Areias V, Cardoso J. COPD and cardiovascular disease. Pulmonology. (2019) 25:168–76. doi: 10.1016/j.pulmoe.2018.09.006
3. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. (2015) 3:631–9. doi: 10.1016/s2213-2600(15)00241-6
4. Bourgeois D, Inquimbert C, Ottolenghi L, Carrouel F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-is there cause for consideration? Microorganisms. (2019) 7:424. doi: 10.3390/microorganisms7100424
5. de Miguel Díez J, Chancafe Morgan J, Jiménez García R. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. (2013) 8:305–12. doi: 10.2147/copd.S31236
6. Desai R, Patel U, Singh S, Bhuva R, Fong HK, Nunna P, et al. The burden and impact of arrhythmia in chronic obstructive pulmonary disease: insights from the National Inpatient Sample. Int J Cardiol. (2019) 281:49–55. doi: 10.1016/j.ijcard.2019.01.074
7. Kusunoki Y, Nakamura T, Hattori K, Motegi T, Ishii T, Gemma A, et al. Atrial and ventricular arrhythmia-associated factors in stable patients with chronic obstructive pulmonary disease. Respiration. (2016) 91:34–42. doi: 10.1159/000442447
8. Rusinowicz T, Zielonka TM, Zycinska K. cardiac arrhythmias in patients with exacerbation of COPD. Adv Exp Med Biol. (2017) 1022:53–62. doi: 10.1007/5584_2017_41
9. Rusnak J, Behnes M, Schupp T, Reiser L, Bollow A, Taton G, et al. COPD increases cardiac mortality in patients presenting with ventricular tachyarrhythmias and aborted cardiac arrest. Respir Med. (2018) 145:153–60. doi: 10.1016/j.rmed.2018.10.019
10. Chahal H, Heckbert SR, Barr RG, Bluemke DA, Jain A, Habibi M, et al. Ability of reduced lung function to predict development of atrial fibrillation in persons aged 45 to 84 years (from the multi-ethnic study of atherosclerosis-lung study). Amer Journal Cardiol. (2015) 115:1700–4. doi: 10.1016/j.amjcard.2015.03.018
11. Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Zhang L, Chugh H, et al. Chronic obstructive pulmonary disease and risk of sudden cardiac death. JACC Clin Electrophysiol. (2015) 1:381–7. doi: 10.1016/j.jacep.2015.06.005
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Deng Y, Tong Y, Deng Y, Zou L, Li S, Chen H. Non-vitamin K antagonist oral anticoagulants versus warfarin in patients with cancer and atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. (2019) 8:e012540. doi: 10.1161/jaha.119.012540
14. Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. doi: 10.1161/strokeaha.120.031007
15. Zhou Y, Ma J, Zhu W. Efficacy and Safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation across BMI categories: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020) 20:51–60. doi: 10.1007/s40256-019-00362-4
16. Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. (2014) 129:971–80. doi: 10.1161/circulationaha.113.004050
17. Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991-1999. COPD. (2005) 2:35–41. doi: 10.1081/copd-200050671
18. Knuiman M, Briffa T, Divitini M, Chew D, Eikelboom J, McQuillan B, et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur J Epidemiol. (2014) 29:181–90. doi: 10.1007/s10654-013-9875-y
19. Lip GYH, Skjøth F, Nielsen PB, Larsen TB. Evaluation of the C(2)HEST risk score as a possible opportunistic screening tool for incident atrial fibrillation in a healthy population (From a Nationwide Danish Cohort Study). Am J Cardiol. (2020) 125:48–54. doi: 10.1016/j.amjcard.2019.09.034
20. Li YG, Bisson A, Bodin A, Herbert J, Grammatico-Guillon L, Joung B, et al. C(2) HEST score and prediction of incident atrial fibrillation in poststroke patients: a french nationwide study. J Am Heart Assoc. (2019) 8:e012546. doi: 10.1161/jaha.119.012546
21. Li YG, Pastori D, Farcomeni A, Yang P-S, Jang E, Joung B, et al. A simple clinical risk score (C(2)HEST) for predicting incident atrial fibrillation in asian subjects: derivation in 471,446 chinese subjects, with internal validation and external application in 451,199 korean subjects. Chest. (2019) 155:510–518. doi: 10.1016/j.chest.2018.09.011
22. Carter P, Lagan J, Fortune C, Bhatt DL, Vestbo J, Niven R, et al. Association of Cardiovascular Disease With Respiratory Disease. J Am Coll Cardiol. (2019) 73:2166–177. doi: 10.1016/j.jacc.2018.11.063
23. Grymonprez M, Vakaet V, Kavousi M, Stricker BH, Ikram MA, Heeringa J, et al. Chronic obstructive pulmonary disease and the development of atrial fibrillation. Int J Cardiol. (2019) 276:118–24. doi: 10.1016/j.ijcard.2018.09.056
24. Liang W, Wu Y, Xue R, Wu Z, Wu D, He J, et al. C(2)HEST score predicts clinical outcomes in heart failure with preserved ejection fraction: a secondary analysis of the TOPCAT trial. BMC Med. (2021) 19:44. doi: 10.1186/s12916-021-01921-w
25. Buzea CA, Dan GA, Dan AR, Delcea C, Balea MI, Gologanu D, et al. Deceleration and acceleration capacities in risk stratification for arrhythmias in patients with chronic obstructive pulmonary disease. Am J Ther. (2017) 24:e44–e51. doi: 10.1097/MJT.0000000000000450
26. Konecny T, Somers KR, Park JY, John A, Orban M, Doshi R, et al. Chronic obstructive pulmonary disease as a risk factor for ventricular arrhythmias independent of left ventricular function. Heart rhythm. (2018) 15:832–8. doi: 10.1016/j.hrthm.2017.09.042
27. Konecny T, Park JY, Somers KR, Konecny D, Orban M, Soucek F, et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol. (2014) 114:272–7. doi: 10.1016/j.amjcard.2014.04.030
28. Lahousse L, Niemeijer MN, van den Berg ME, Rijnbeek PR, Joos GF, Hofman A, et al. Chronic obstructive pulmonary disease and sudden cardiac death: the Rotterdam study. Eur Heart J. (2015) 36:1754–61. doi: 10.1093/eurheartj/ehv121
29. Kong ZB, Wang XD, Shen SR, Liu H, Zhou L, Chen B, et al. Risk prediction for arrhythmias by heart rate deceleration runs in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2020) 15:585–93. doi: 10.2147/copd.S234470
30. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser permanente medical care program. Chest. (2005) 128:2068–75. doi: 10.1378/chest.128.4.2068
31. Chen CC, Lin CH, Hao WR, Chiu CC, Fang YA, Liu JC, et al. Association between chronic obstructive pulmonary disease and ventricular arrhythmia: a nationwide population-based cohort study. NPJ Prim Care Respir Med. (2021) 31:8. doi: 10.1038/s41533-021-00221-3
32. Urena M, Webb JG, Eltchaninoff H, Muñoz-García AJ, Bouleti C, Tamburino C, et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol. (2015) 65:437–48. doi: 10.1016/j.jacc.2014.11.027
33. Nishiyama K, Shizuta S, Doi T, Morimoto T, Kimura T. Sudden cardiac death after PCI and CABG in the bare-metal stent era: Incidence, prevalence, and predictors. Int J Cardiol. (2010) 144:263–6. doi: 10.1016/j.ijcard.2009.01.040
34. Al-Khatib SM, Granger CB, Huang Y, Lee KL, Califf RM, Simoons ML, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. (2002) 106:309–12. doi: 10.1161/01.cir.0000022692.49934.e3
35. Cosgun A, Oren H, Turkkani MH. The relationship between systolic pulmonary arterial pressure and Tp-e interval, Tp-e/QT, and Tp-e/QTc ratios in patients with newly diagnosed chronic obstructive pulmonary disease. Ann Noninvas Electrocardiol. (2020) 25:e12691. doi: 10.1111/anec.12691
36. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. (2016) 138:16–27. doi: 10.1016/j.jaci.2016.05.011
37. Lammers WJ, Kirchhof C, Bonke FI, Allessie MA. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Amer J Physiol. (1992) 262:H47–55. doi: 10.1152/ajpheart.1992.262.1.H47
38. Terzano C, Romani S, Conti V, Paone G, Oriolo F, Vitarelli A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur. Rev Med Pharmacol Sci. (2014) 18:2908–17.
39. Stevenson IH, Roberts-Thomson KC, Kistler PM, Edwards GA, Spence S, Sanders P, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart rhythm. (2010) 7:1263–70. doi: 10.1016/j.hrthm.2010.03.020
40. Chatterjee NA, Shah RV, Murthy VL, Praestgaard A, Shah SJ, Ventetuolo CE, et al. Right ventricular structure and function are associated with incident atrial fibrillation: MESA-RV study (multi-ethnic study of atherosclerosis-right ventricle). Circ Arrhyth Electrophysiol. (2017) 10, e004738. doi: 10.1161/circep.116.004738
41. Gorter TM, van Melle JP, Rienstra M, Borlaug BA, Hummel YM, van Gelder IC, et al. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail. (2018) 24:177–85. doi: 10.1016/j.cardfail.2017.11.005
42. Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E Jr, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. (2006) 16:63–70. doi: 10.1016/j.annepidem.2005.04.008
43. Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest. (1996) 109:387–94. doi: 10.1378/chest.109.2.387
44. Shepard JW Jr, Garrison MW, Grither DA, Evans R, Schweitzer PK. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Amer J Med. (1985) 78:28–34. doi: 10.1016/0002-9343(85)90457-7
45. van den Berg ME, Stricker BH, Brusselle GG, Lahousse L. Chronic obstructive pulmonary disease and sudden cardiac death: a systematic review. Trends Cardiovasc Med. (2016) 26:606–13. doi: 10.1016/j.tcm.2016.04.001
46. Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 1: Saskatchewan cohort study. Chest. (2012) 142:298–304. doi: 10.1378/chest.10-2499
47. Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. (2012) 142:305–11. doi: 10.1378/chest.11-1597
Keywords: chronic obstructive pulmonary disease (COPD), arrhythmia, risk factor, meta-analysis, atrial fibrillation
Citation: Liu X, Chen Z, Li S and Xu S (2021) Association of Chronic Obstructive Pulmonary Disease With Arrhythmia Risks: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:732349. doi: 10.3389/fcvm.2021.732349
Received: 28 June 2021; Accepted: 06 September 2021;
Published: 30 September 2021.
Edited by:
Takatoshi Kasai, Juntendo University, JapanReviewed by:
Naoyuki Taniguchi, Osaka International Cancer Institute, JapanCopyright © 2021 Liu, Chen, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuo Xu, eHVzaHVvMjAxMzExMThAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.