
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 November 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.731261
 Lele Cheng1,2,3,4†
Lele Cheng1,2,3,4† Zixuan Meng1†
Zixuan Meng1† Qi Wang5
Qi Wang5 Zhijie Jian2
Zhijie Jian2 Pengcheng Fan1
Pengcheng Fan1 Xinxin Feng1
Xinxin Feng1 Xiangrui Qiao1,3,4
Xiangrui Qiao1,3,4 Jian Yang2
Jian Yang2 Zuyi Yuan1,3,4
Zuyi Yuan1,3,4 Bolin Li1,3,4*
Bolin Li1,3,4* Yue Wu1*
Yue Wu1*Inflammation and nutrition as main factors can affect the prognosis of patients with chronic total coronary occlusion (CTO) undergoing percutaneous coronary intervention (PCI). The C-reactive protein to albumin ratio (CAR) can clarify the inflammation and nutrition status, which are highly related to clinical outcomes. This study aims to investigate the association between CAR and adverse cardiovascular events in patients with CTO undergoing PCI. For this study, 664 patients were divided into three groups based on the tertiles of CAR. The primary endpoint was all-cause mortality and the secondary endpoint was major adverse cardiovascular events (MACE). Over a median follow-up of 33.7 months, the primary endpoint occurred in 64 patients (9.6%) and the secondary endpoint occurred in 170 patients (25.6%). The patients with higher CAR represented a worse prognosis with all-cause death and cardiovascular death after the adjustment for the baseline risk factors. Adding the CAR values raised the predictive value for the incidence of the all-cause death and cardiovascular death but not MACE. The capacity of prognosis prediction was improved after the addition of the CAR value to the traditional prediction model.
C-reactive protein, synthesized by hepatocytes, can be used as a biomarker for inflammation, infection and a predictive factor for cardiovascular events (1, 2) Albumin is also synthesized by hepatocytes, has a negative correlation with inflammation, and its levels in plasma can reflect nutrition status which is strongly associated with the prognosis of patients. With the decrease of albumin as well as the increase of C-reactive protein (CRP), it can show impaired immune function, severe inflammation, and worsened prognosis in patients (3, 4).
Among patients with chronic coronary total occlusion (CTO) undergoing percutaneous coronary intervention (PCI), several factors can affect its prognosis, mainly two of which are inflammation and nutrition status. It is known that inflammation plays a key role in coronary heart disease, especially in plaque formation and destabilization, leading to acute coronary syndromes (ACS) or CTO. More than that, it can lead to restenosis in patients with CTO-PCI, causing adverse cardiovascular outcomes and worsening the prognosis (5). In addition, malnutrition is associated with impaired immune function and weak healing ability which are highly related to the mortality of patients with cardiovascular heart disease or other chronic diseases (6, 7). It has been clarified that cytokines and metabolites can be released at malnourished status. Systemic inflammation can activate immune cell transdifferentiation into pro-inflammatory and pro-fibrotic subsets, leading to cardiac hypertrophy and fibrosis (8). For instance, TNF-β as one of the most important inflammatory cytokines increased significantly in patients with malnutrition and its increase resulted in potential negative inotropic effects, worsening ventricular functions, and eventually progressing to heart failure (9). Malnutrition also induced gut microbiota dysbiosis which plays a critical role in cardiovascular disease (CVD), making a difference in the prognosis of patients (10). Moreover, malnutrition usually comes along with the deficiency of micronutrients defined as vitamins and minerals. The lack of Vitamin D is related to the increase of CVD risks resulting from its far-ranging physiological effects (11–14). Supplementation with Vitamin D can prevent CVD by modifying risk factors such as high blood pressure, elevated parathyroid hormone, dyslipidemia, and inflammation (15). Therefore, it is necessary to make an overall consideration of inflammation and nutrition status to better access the prognosis of patients with CTO-PCI.
The emergence of CRP to albumin ratio (CAR) can reflect the inflammation and nutrition status in a better way than either CRP or albumin alone. It has been reported that the elevation of CAR is related to poor prognosis in sepsis, pancreatic cancer, acute pancreatitis, colorectal cancer, and other diseases (16). Studies revealed that CAR is independently associated with no-reflow, acute kidney injury, and coronary thrombus burden in patients with ACS (17–19). C-reactive protein to albumin ratio can be a more practical predictor of the higher presence of left ventricular thrombus formation among survivors of anterior myocardial infarction (20). Nevertheless, the prognostic effect of CAR in CTO remains largely unknown. The presents study aims to investigate whether CAR can be a credible prognostic indicator for patients with CTO undergoing PCI.
Between June 2013 to October 2017, 859 patients were screened in the First Affiliated Hospital of Xi'an Jiaotong University and 762 patients received the CTO-PCI strategy. The final follow-up status was ascertained between 2018 and 2019. After the exclusion of 91 patients who were unable to follow-up and 7 patients who missed CAR values, 664 patients were enrolled in the present study (Figure 1).
The patients received standard treatments during the PCI procedure and hospitalization. Coronary total occlusion was defined as total coronary artery occlusion (thrombolysis in myocardial infarction [TIMI] flow grade 0) of more than 3 months in duration (21, 22). The success of the CTO-PCI procedure was defined as the complete revascularization of the lesion with residual stenosis <30% and TIMI flow grade 3 (23). The exclusion criteria were patients with severe renal and hepatobiliary disease diseases, autoimmune diseases, malignant tumors, prior coronary artery bypass grafting, and prior history of surgical treatment within 1 month of this study. The primary endpoint was all-cause mortality and the secondary endpoint was major adverse cardiovascular events (MACE), including all-cause death, non-fatal acute myocardial infarction, revascularization, and stroke. All the participants gave their written informed consent for the enrollment in this study, and the study was approved by the ethics committee approval of the First Affiliated Hospital of Xi'an Jiaotong University.
We collected several demographics, cardiovascular risk factors, and laboratory data for all the participants. The demographics included age, gender, and body mass index (BMI). The cardiovascular risk factors included smoking, drinking, hypertension, diabetes mellitus (DM), prior myocardial infarction, and prior stroke, all of which came from the medical records of the participants. Diabetes mellitus was diagnosed according to the criteria by the American Diabetes Association (24). Triglycerides, cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), albumin, creatinine, creatine kinase isoenzymes MB (CKMB), and pro-B-type natriuretic peptide (pro-BNP) were assayed using the Cobas Integra automated chemistry analyzer (Roche Cobas Integra 400 Plus, Roche Diagnostics, United States). High-sensitivity CRP was tested using an enzyme-linked immunosorbent assay according to the instructions of the manufacturer (EIA-3954, DRG International Inc., Springfield Township, United States). Blood samples were drawn from the patients within 24 h of admission. All of these laboratory tests were implemented using standard methods. Echocardiographs were performed on admission by experienced cardiologists.
We divided all the patients into three groups based on the tertiles of CAR (Group 1,0.0022–0.0385; Group 2,0.0323–0.0392; Group 3,0.0394–2.1831), and we used the tertile categories in the following analysis. Continuous variables are presented as the mean ± SD if normally distributed or median (lower quartile, upper quartile) otherwise. The categorical variables are presented as numbers and percentages. Differences in the parameters among groups were analyzed using ANOVA for normally distributed variables, the Kruskal-Wallis test was used for non-normally distributed continuous variables, and the chi-square test was used for categorical variables. Spearman correlation coefficients were calculated to assess the association between continuous non-normally distributed variables.
For the prognostic analysis, a Kaplan-Meier curve was applied to compare the prognosis between the three groups based on tertile of CAR. The prognostic value of CAR was assessed by the univariate and multivariate Cox proportional hazards model. Model 1 was adjusted for age and sex. Model 2 included the same variables as model 1, additionally including lifestyle factors (smoking, alcohol drinks), hypertension, DM, cholesterol level, and stent numbers. Model 3 was additionally adjusted for left ventricular ejection fraction (LVEF), pro-BNP, creatinine, heart rate, revascularization, cholesterol, triglyceride, and thyroid-stimulating hormone (TSH). A Cox proportional hazards regression analysis was performed to estimate the hazard ratios (HRs) and 95% CIs for adverse outcomes among the tertiles of CAR. Survival analyses were performed using the R package “survival” (https://CRAN.R-project.org/package=survival) and “survminer” (https://CRAN.R-project.org/package=survminer).
Aiming to assess whether the accuracy of predicting clinical outcomes improved after the addition of CAR to a baseline model with established risk factors, including age, gender, smoking, alcohol drinks, hypertension, diabetes mellitus, prior myocardial infarction, cholesterol, CKMB, LVEF, and pro-BNP, the C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated. The IDI and NRI were performed using the R package “PredictABEL” (https://CRAN.R-project.org/package=PredictABEL). Receiver operating characteristic curve analyses were performed using the R package “Proc” (https://CRAN.R-project.org/package=pROC).
Restricted cubic splines were applied using the R package “rms” (https://CRAN.R-project.org/package=rms) to explore the relationship of CAR with the HR of all-cause death, cardiovascular death, and MACE adjusted by potential confounding factors as Model 3 used in Cox regression analysis. The reference value was the CAR−L group.
The p < 0.05 was regarded as statistically significant for all statistical tests. All statistical analyses were performed using R version 4.0.2 software (Vienna, Austria).
The CAR ranged from 0.0022 to 2.1831. The patients were divided into 3 groups according to the tertile of CAR: CAR−L (n = 221), CAR−M (n = 222), CAR−H (n = 221). The clinical characteristics and medications of the patients are summarized in Table 1. The mean patient age was 65.31 ± 10.00 years, 83.4% of patients were men, 36.0% had diabetes mellitus, and 55.6% had hypertension. Compared with the groups with low CAR values, the patients in the CAR-H group were older with higher hs-CRP, cholesterol, LDL, pro-BNP level, and lower serum albumin. There were no significant differences in other cardiovascular risk factors among the three groups. The success rate of the revascularization was similar. The compliance with medication among the medication use of the patients with CTO after PCI are as follows: aspirin (72.6%), clopidogrel (37.9%), statin (61.1%), ACEI/ARB (28.6%), β-blocker (52.7%), and CCB (21.7%).
The associations between conventional cardiovascular risk factors and CAR are shown in Supplementary Table 1. C-reactive protein to albumin ratio is predominantly associated with hs-CRP (p < 0.001), serum albumin (p < 0.001), and cholesterol (p = 0.046). In contrast, other cardiovascular risk factors including age, LDL, HDL, triglycerides, creatinine, pro-BNP, BMI, LVEF, and TSH showed no associations with CAR.
The median duration of follow-up was 33.7 months (interquartile range, 22.5 to 48.9 months) in the whole population. The analyses of the primary and secondary endpoints are provided in Table 2. During the follow-up, the primary endpoint occurred in 64 patients (9.6%) while the secondary endpoint occurred in 170 patients (25.6%).
As demonstrated in the Kaplan-Meier plots, the patients with higher CAR represented worse prognosis than the other two groups with all-cause death (log-rank test, p < 0.0001), cardiovascular death (log-rank test, p < 0.0001), and MACE (log-rank test, p = 0.021) (Figure 2).
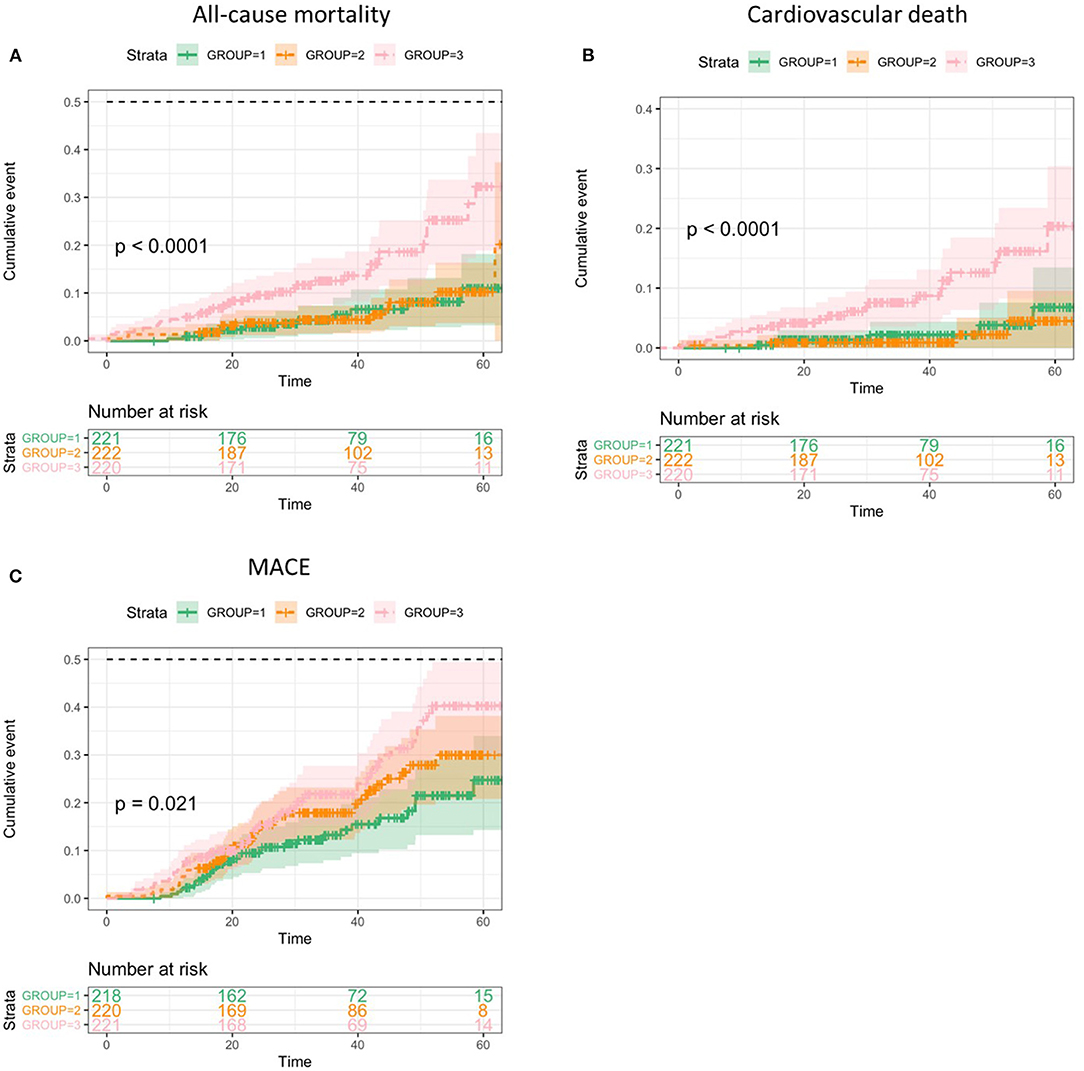
Figure 2. Kaplan-Meier analyses of clinical outcomes categorized by C-reactive protein to albumin ratio (CAR) values. (A), All-cause death. (B), Cardiovascular death. (C), Major adverse cardiovascular events (MACE).
In the univariate Cox proportional hazards analysis, the patients in the CAR−H group had increased risk of all-cause death (HR:2.96; 95% CI:1.55–5.52; p = 0.001, p for trend < 0.001), cardiovascular death (HR:3.87; 95% CI:1.57–9.54; p = 0.003, p for trend = 0.001), and MACE (HR:1.84; 95% CI:1.19–2.87; p = 0.006, p for trend = 0.006) as compared with the CAR−L group. In the multivariate analysis, the CAR−H group remained associated with increased risk for all-cause death, cardiovascular death, and MACE after the adjustment for models 1, 2, and 3 (Table 2). Additionally, the results of the subgroup analyses for the primary endpoint are provided in Table 3. The results indicated that patients with a high level of CAR implied a worse prognosis in subgroups such as age ≥ 65 years, male, without diabetes mellitus, baseline LDL < 2.46 mmol/L, HDL ≤ 1.04 mmol/L, and triglycerides < 1.68 mmol/L. No significant interaction between the subgroup factors and the effect of CAR was observed (Table 3). Then we chose the patients who have the risk characteristics including age ≥65 years, male, without diabetes mellitus, and baseline LDL < 2.46 mmol/L and found that these patients with high CAR had a 15.23-fold increased risk of having primary endpoint (HR:15.23; 95% CI:1.71–135.95, p = 0.015) as compared with those with low CAR values.
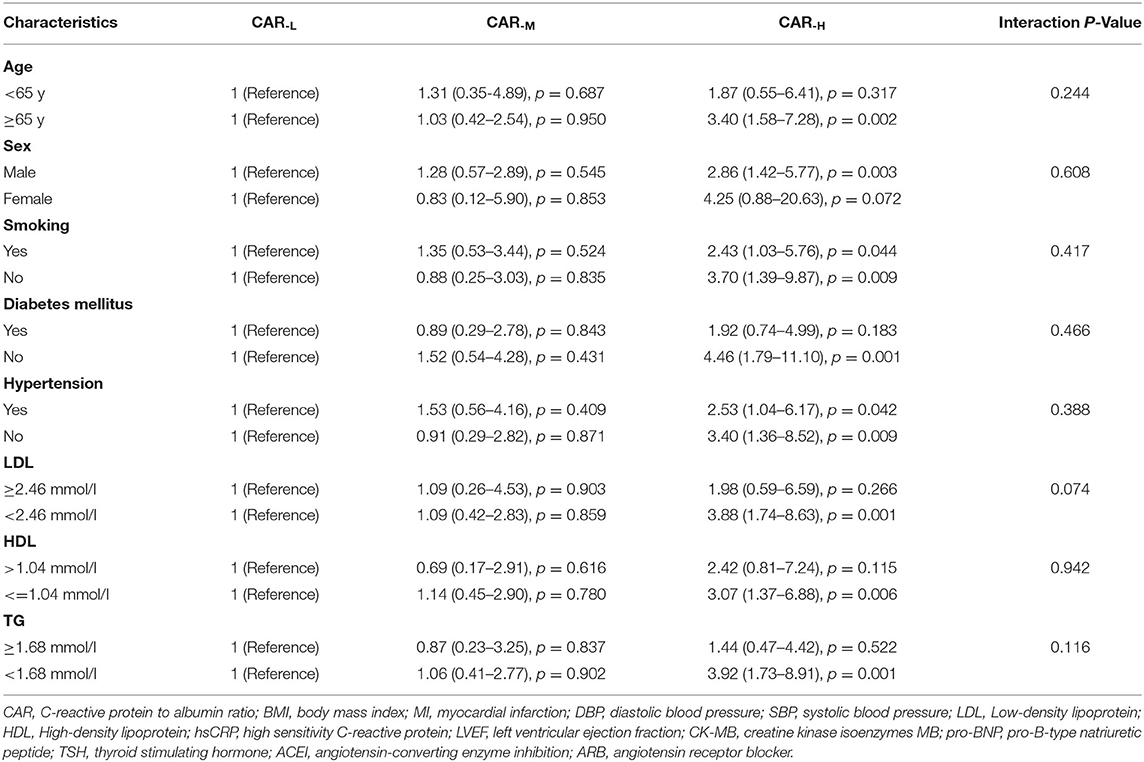
Table 3. CAR level and risk of all-cause death of CTO-PCI patients, stratified by various baseline characteristics.
The C-index of established risk factors including age, sex, smoking, alcohol drinks, hypertension, diabetes mellitus, prior myocardial infarction, cholesterol, CKMB, LVEF, and pro-BNP, for all-cause death (0.767, p < 0.001), cardiovascular death (0.812, p < 0.001), and MACE (0.587, p = 0.003) were revealed. As expected, the C-index was elevated with the addition of CAR,0.776 for all-cause death (p < 0.001),0.833 for cardiovascular death (p < 0.001), and 0.603 for MACE (p < 0.001) (Table 4). The CAR had incremental values for predicting the incidence of the all-cause death (0.451, p = 0.001 and 0.013, p = 0.049, NRI and IDI, respectively) and cardiovascular death (0.386, p = 0.006 and 0.010, p = 0.042, NRI and IDI, respectively). Whereas, the NRI and IDI for MACE were not significant after adding CAR (0.182, p = 0.129 and 0.002, p = 0.443, NRI and IDI, respectively).
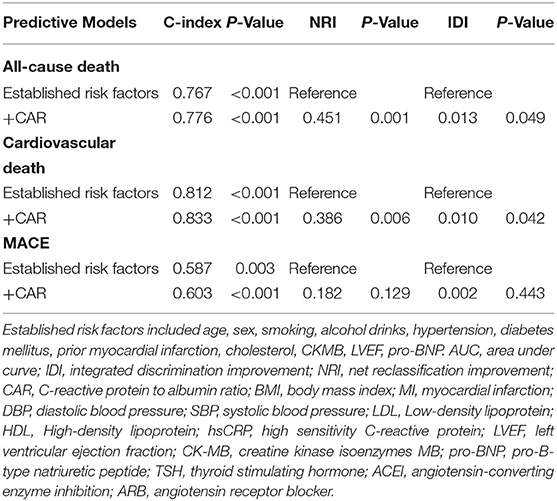
Table 4. Discrimination of each predictive model for outcomes using C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI).
Further, restricted cubic splines were applied to explore the association of CAR, which was treated as a continuous variable, with the HR of all-cause death, cardiovascular death, and MACE after adjusting as model 3 used in Cox regression analysis. The HR of all-cause death increased sharply until the CAR reached ~0.1. Similar relationships between CAR and cardiovascular death and CAR and MACE were found in the patients with CTO-PCI (Figure 3).
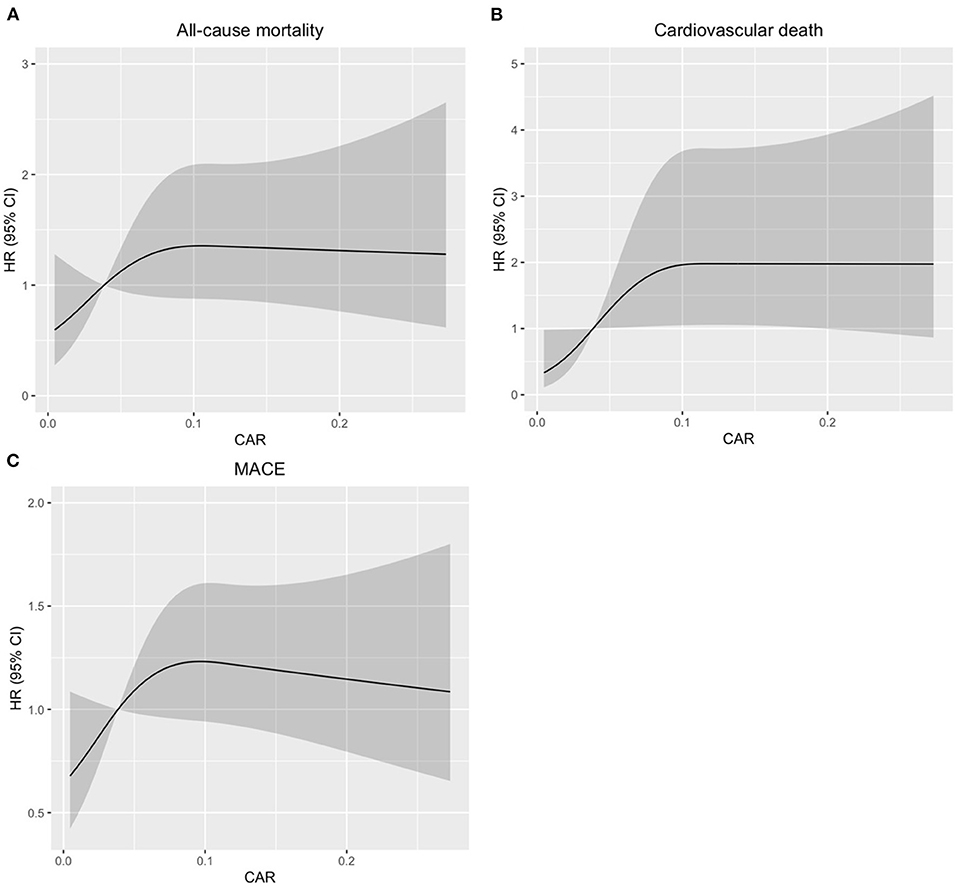
Figure 3. Restricted spline curves for the associations between CAR and all-cause mortality, cardiovascular death, or MACE in patients with coronary total occlusion (CTO) undergoing percutaneous coronary intervention (PCI). Black lines represent the hazard ratio (HR), gray areas represent the 95% CIs. (A), Association between CAR and all-cause mortality. (B), Association between CAR and cardiovascular death. (C), Association between CAR and MACE. The HR (95% CI) were all adjusted according to Model 3 in the Cox analysis.
As one of the most prevalent diseases in both developed and developing countries, coronary artery disease (CAD) is still the dominant cause of mortality, especially in elder people (25, 26). With the morbidity of CAD increasing annually, the economic burden gets heavier (27) and CTO, represented by the narrowing of the coronary artery and completely interrupting coronary flow for more than 3 months, is a special form of CAD. Recent research revealed that about 50% of patients with significant CAD on angiography have at least one CTO lesion (21, 28). Although CTO-PCI is regarded as a valid procedure for the technical advancement of angioplasty equipment, it is still full of uncertainties regarding clinical prognosis, mainly due to its complex procedures and high dependence on both the health status of patients and the experience of operators (29).
Inflammation plays a vital role in disease progression and the prognosis of patients, it can not only accelerate the formation of plaque lesions but also promote the progression of complex re-occlusion lesions in elderly patients with very long stent implantations (5, 30). C-reactive protein acts as a biomarker of inflammation and a hallmark of acute-phase response, which can strongly reflect the inflammation status of patients. Previous studies have revealed its pathological role in vascular diseases and the correlation between CRP and the risk of cardiovascular events. C-reactive protein concentration is log linearly related to the risk of CAD (31–33). A moderately elevated CRP is proved to be an independent risk factor for CAD in healthy populations (34–36). Serum albumin levels are negatively related to inflammatory reactions, low serum albumin level is associated with the increased risk of several CVDs in abundant prospective studies (37–40). Up to 55% of elders with STsegment elevation myocardial infractions were malnourished. In addition, malnutrition is quite common in patients with CTO-PCI (41). To evaluate the health status of patients after processing PCI, it is essential to find an effective predictor for the prognosis of patients with CTO-PCI. C-reactive protein to albumin ratio combines these two crucial biomarkers, showing a strong point to clarify the inflammation and nutrition status, both of which are highly related to the disease progression and clinical outcomes of patients. The present study revealed that patients with CTO-PCI with higher CAR values had increased risk for all-cause death, cardiovascular death, and MACE as compared with the traditional prediction model. The addition of CAR remarkably enhanced the ability to predict the risk of adverse cardiovascular events in patients with CTO-PCI. Hence, CAR can be a valid predictor for adverse events in patients with CTO after PCI. To our knowledge, this is the first study to uncover the association between CAR and CTO and the addition of CAR to a clinical prediction model significantly improved the risk prediction for adverse cardiovascular events.
The specific mechanisms of inflammation and malnutrition influencing the prognosis of patients are still unknown. One possibility is that inflammation can restrain the immune system and retard the recovery progression by suppressing the protein synthesis, promoting protein degradation, and lowering plasma albumin. The inflammation cytokines activated by CRP can affect multiple organ functions, worsening the prognosis of patients. C-reactive protein to albumin ratio can also bind to LDL and contribute to the development of atherosclerotic plaques, leading to the incidence of restenosis (42). Serum albumin has anti-inflammatory, anticoagulant, and antiplatelet aggregation activity effects, and low serum albumin levels can reduce serum anti-oxidant activity and impair endothelium-derived relaxing factor-like activities (43, 44). Additionally, albumin can bind to homocysteine to lower the serum levels of homocysteine, which is a risk factor for atherosclerosis (45). Another possibility is that serum albumin can reflect the nutrition status of patients; lower serum albumin is often an indicator of malnutrition. In the state of malnutrition, immune system activity is impaired, leading to multiple complications and a worse prognosis (46). Whereupon, inflammation and malnutrition together form a vicious circle.
It is noteworthy that high CAR had better predictive value in patients of age ≥ 65 years, male, without diabetes mellitus, baseline LDL < 2.46 mmol/L, HDL ≤ 1.04 mmol/L, and triglycerides < 1.68 mmol/L. We deduced that the possible reasons are as follows. Firstly, previous studies based on the population demonstrated that serum albumin levels decreased with aging, which might result from elders suffering worse immunity function, being more vulnerable to malnutrition and chronic inflammation status (46), leading to higher CAR values. Secondly, albumin can be taken as an efficient antioxidant, protecting the organisms from oxidative attack (47, 48). Glucose and free radicals can impair the antioxidant properties of serum albumin (49). That is to say, hyperglycemia may affect the predictive values of CAR. The underlying mechanisms still need to be studied further.
The current study has several limitations that should be considered. Firstly, the clinical data comes from a single-center, small-scale observational cohort study, and some confounding factors may affect the outcomes. Further, multi-center and larger-scale surveys are required to confirm the results from this study and to elucidate the precise mechanisms. Secondly, we only measured the CAR values at admission, without assessing the dynamic changes during the medication. Thirdly, we evaluated CRP as a prognostic biomarker for CVD but lacks data on other inflammatory markers. Moreover, the study only included patients with CTO undergoing PCI, which suggests that the study results may not be extended to all CAD patients.
In summary, our study reveals that the risk of all-cause death, cardiovascular death, and MACE was increased with high CAR values in patients with CTO undergoing PCI. After adding CAR to the traditional prediction model, the capacity of prognosis prediction was improved in these patients. Therefore, CAR can be an indicator to predict the prognosis of patients with CTO undergoing PCI and to better stratify these patients.
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by the Ethics Committee approval of the First Affiliated Hospital of Xi'an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
LC and BL: conceptualization and writing. YW: review and editing. QW, XQ, and BL: data curation. LC and ZM: formal analysis. ZY and YW: funding acquisition. LC, ZM, PF, and XF: investigation. BL: methodology. JY: project administration. ZY: resources. QW: software. ZY, YW, and BL: validation. LC and ZJ: visualization. ZM: writing—original draft. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key R&D Program of China (2019YFA0802300) and the National Natural Science Foundation of China (81822005, 91639301, 81500219).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.731261/full#supplementary-material
ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CAR, C-reactive protein to albumin ratio; CCB, calcium channel blockers; CIs, confidence intervals; CKMB, creatine kinase isoenzymes MB; CRP, C-reactive protein; CTO, chronic coronary total occlusion; CVD, cardiovascular disease; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein cholesterol; HRs, hazard ratios; IDI, integrated discrimination improvement; LDL, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; NRI, net reclassification improvement; pro-BNP, pro-B-type natriuretic peptide; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; TIMI, thrombolysis in myocardial infarction; TSH, thyroid-stimulating hormone.
1. Matowicka-Karna J. Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel diseases. Postepy Higieny Medycyny Doswiadczalnej. (2016) 70:305–12. doi: 10.5604/17322693.1199305
2. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. (2012) 367:1310–20. doi: 10.1056/NEJMoa1107477
3. Carriere I, Dupuy AM, Lacroux A, Cristol JP, Delcourt C. Biomarkers of inflammation and malnutrition associated with early death in healthy elderly people. J Am Geriatr Soc. (2008) 56:840–6. doi: 10.1111/j.1532-5415.2008.01677.x
4. Domínguez de Villota E, Mosquera JM, Rubio JJ, Galdos P, Díez Balda V, de la Serna JL, et al. Association of a low serum albumin with infection and increased mortality in critically ill patients. Intensive Care Med. (1980) 7:19–22. doi: 10.1007/BF01692917
5. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. doi: 10.1056/NEJMra043430
6. Pressoir M, Desné S, Berchery D, Rossignol G, Poiree B, Meslier M, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. (2010) 102:966–71. doi: 10.1038/sj.bjc.6605578
7. Takahashi H, Ito Y, Ishii H, Aoyama T, Kamoi D, Kasuga H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol. (2014) 64:32–6. doi: 10.1016/j.jjcc.2013.10.018
8. Li Z, Zhao H, Wang J. Metabolism and chronic inflammation: the links between chronic heart failure and comorbidities. Front Cardiovasc Med. (2021) 8:650278. doi: 10.3389/fcvm.2021.650278
9. Odeh M, Sabo E, Oliven A. Circulating levels of tumor necrosis factor-alpha correlate positively with severity of peripheral oedema in patients with right heart failure. Eur J Heart Fail. (2006) 8:141–6. doi: 10.1016/j.ejheart.2005.05.010
10. Pasini E, Aquilani R, Corsetti G, Dioguardi FS. Malnutrition and gut flora dysbiosis: specific therapies for emerging comorbidities in heart failure. Biomed Res Int. (2015) 2015:382585. doi: 10.1155/2015/382585
11. Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. (2016) 13:404–17. doi: 10.1038/nrcardio.2016.73
12. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. (2008) 29:726–76. doi: 10.1210/er.2008-0004
13. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. (2016) 96:365–408. doi: 10.1152/physrev.00014.2015
14. Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. (2014) 114:379–93. doi: 10.1161/CIRCRESAHA.113.301241
15. Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med. (2018) 5:87. doi: 10.3389/fcvm.2018.00087
16. Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. (2017) 24:561–8. doi: 10.1245/s10434-016-5579-3
17. Karabag Y, Çagdaş M, Rencuzogullari I, Karakoyun S, Artaç I, Iliş D, et al. Usefulness of the C-reactive protein/albumin ratio for predicting no-reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. (2018) 48:e12928. doi: 10.1111/eci.12928
18. Karabag Y, Çagdaş M, Rencuzogullari I, Karakoyun S, Artaç I, Iliş D, et al. The C-reactive protein to albumin ratio predicts acute kidney injury in patients with st-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Lung Circ. (2019) 28:1638–45. doi: 10.1016/j.hlc.2018.08.009
19. Duman H, Çinier G, Bakirci EM, Duman H, Simşek Z, Hamur H, et al. Relationship between C-reactive protein to albumin ratio and thrombus burden in patients with acute coronary syndrome. Clin Appl Thromb Hemost. (2019) 25:1076029618824418. doi: 10.1177/1076029618824418
20. Cirakoglu OF, Aslan AO, Yilmaz AS, Sahin S, Akyüz AR. Association between C-reactive protein to albumin ratio and left ventricular thrombus formation following acute anterior myocardial infarction. Angiology. (2020) 71:804–11. doi: 10.1177/0003319720933431
21. Hoebers LP, Claessen BE, Dangas GD, Råmunddal T, Mehran R, Henriques JP. Contemporary overview and clinical perspectives of chronic total occlusions. Nat Rev Cardiol. (2014) 11:458–69. doi: 10.1038/nrcardio.2014.74
22. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. (2019) 139:1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313
23. Toma A, Gick M, Minners J, Ferenc M, Valina C, Löffelhardt N, et al. Survival after percutaneous coronary intervention for chronic total occlusion. Clin Res Cardiol. (2016) 105:921–9. doi: 10.1007/s00392-016-1000-2
24. Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endoc Pract. (2011) 17(Suppl. 2):1–53. doi: 10.4158/EP.17.S2.1
25. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
26. Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary artery disease in patients ≥80 years of age. J Am Coll Cardiol. (2018) 71:2015–40. doi: 10.1016/j.jacc.2017.12.068
27. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics−2014 update: a report from the American Heart Association. Circulation. (2014) 129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80
28. Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol. (2005) 95:1088–91. doi: 10.1016/j.amjcard.2004.12.065
29. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. (2015) 8:245–53. doi: 10.1016/j.jcin.2014.08.014
30. Li X, Guo D, Chen Y, Hu Y, Zhang F. Complex coronary instent chronic total occlusion lesions: oxidative stress, inflammation, and coronary stent lengths. Oxid Med Cell Longev. (2021) 2021:8815048. doi: 10.1155/2021/8815048
31. Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. (2003) 115(Suppl. 8A):99–106s. doi: 10.1016/j.amjmed.2003.09.016
32. Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, et al. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. (2003) 108:512–5. doi: 10.1161/01.CIR.0000085568.13915.1E
33. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375:132–40. doi: 10.1016/S0140-6736(09)61717-7
34. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. (2004) 350:1387–97. doi: 10.1056/NEJMoa032804
35. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. (2000) 342:836–43. doi: 10.1056/NEJM200003233421202
36. Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem. (2001) 47:403–11. doi: 10.1093/clinchem/47.3.403
37. Arques S. Human serum albumin in cardiovascular diseases. Eur J Internal Med. (2018) 52:8–12. doi: 10.1016/j.ejim.2018.04.014
38. Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. (1989) 2:1434–6. doi: 10.1016/S0140-6736(89)92042-4
39. Chien SC, Chen CY, Leu HB, Su CH, Yin WH, Tseng WK, et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int J Cardiol. (2017) 241:1–5. doi: 10.1016/j.ijcard.2017.04.003
40. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54. doi: 10.1056/NEJM199902113400607
41. Cheng L, Rong J, Zhuo X, Gao K, Meng Z, Wen X, et al. Prognostic value of malnutrition using geriatric nutritional risk index in patients with coronary chronic total occlusion after percutaneous coronary intervention. Clin Nutr. (2021) 40:4171–9. doi: 10.1016/j.clnu.2021.01.042
42. de Beer FC, Soutar AK, Baltz ML, Trayner IM, Feinstein A, Pepys MB. Low density lipoprotein and very low density lipoprotein are selectively bound by aggregated C-reactive protein. J Exp Med. (1982) 156:230–42. doi: 10.1084/jem.156.1.230
43. Ridker PM. C-reactive protein and risks of future myocardial infarction and thrombotic stroke. Eur Heart J. (1998) 19:1–3.
44. Keaney JF Jr., Simon DI, Stamler JS, Jaraki O, Scharfstein J, et al. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Investig. (1993) 91:1582–9. doi: 10.1172/JCI116364
45. Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. (2007) 9:1941–58. doi: 10.1089/ars.2007.1750
46. Gom I, Fukushima H, Shiraki M, Miwa Y, Ando T, Takai K, et al. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J Nutr Sci Vitaminol. (2007) 53:37–42. doi: 10.3177/jnsv.53.37
47. Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. (2005) 41:1211–9. doi: 10.1002/hep.20720
48. Soriani M, Pietraforte D, Minetti M. Antioxidant potential of anaerobic human plasma: role of serum albumin and thiols as scavengers of carbon radicals. Arch Biochem Biophys. (1994) 312:180–8. doi: 10.1006/abbi.1994.1297
Keywords: c-reactive protein to albumin ratio, percutaneous coronary intervention, chronic coronary total occlusion, adverse cardiovascular events, prognostic indicator
Citation: Cheng L, Meng Z, Wang Q, Jian Z, Fan P, Feng X, Qiao X, Yang J, Yuan Z, Li B and Wu Y (2021) The Usefulness of C-Reactive Protein to Albumin Ratio in the Prediction of Adverse Cardiovascular Events in Coronary Chronic Total Occlusion Undergoing Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 8:731261. doi: 10.3389/fcvm.2021.731261
Received: 26 June 2021; Accepted: 12 October 2021;
Published: 12 November 2021.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Owais Bhat, Virginia Commonwealth University, United StatesCopyright © 2021 Cheng, Meng, Wang, Jian, Fan, Feng, Qiao, Yang, Yuan, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Wu, eXVlLnd1QHhqdHUuZWR1LmNu; Bolin Li, YmVybGluMTAxQHN0dS54anR1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.