- 1Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine and Cardiovascular Center, Yongin Severance Hospital, Yongin, South Korea
- 2Division of Cardiology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, South Korea
Background: Left ventricular diastolic function (LVDF) evaluation using a combination of several echocardiographic parameters is an important predictor of adverse events in patients with acute myocardial infarction (AMI). To date, the clinical impact of each individual LVDF marker is well-known, but the clinical significance of the sum of the abnormal diastolic function markers and the long-term clinical outcome are not well-known. This study aimed to investigate the usefulness of LVDF score in predicting clinical outcomes of patients with AMI.
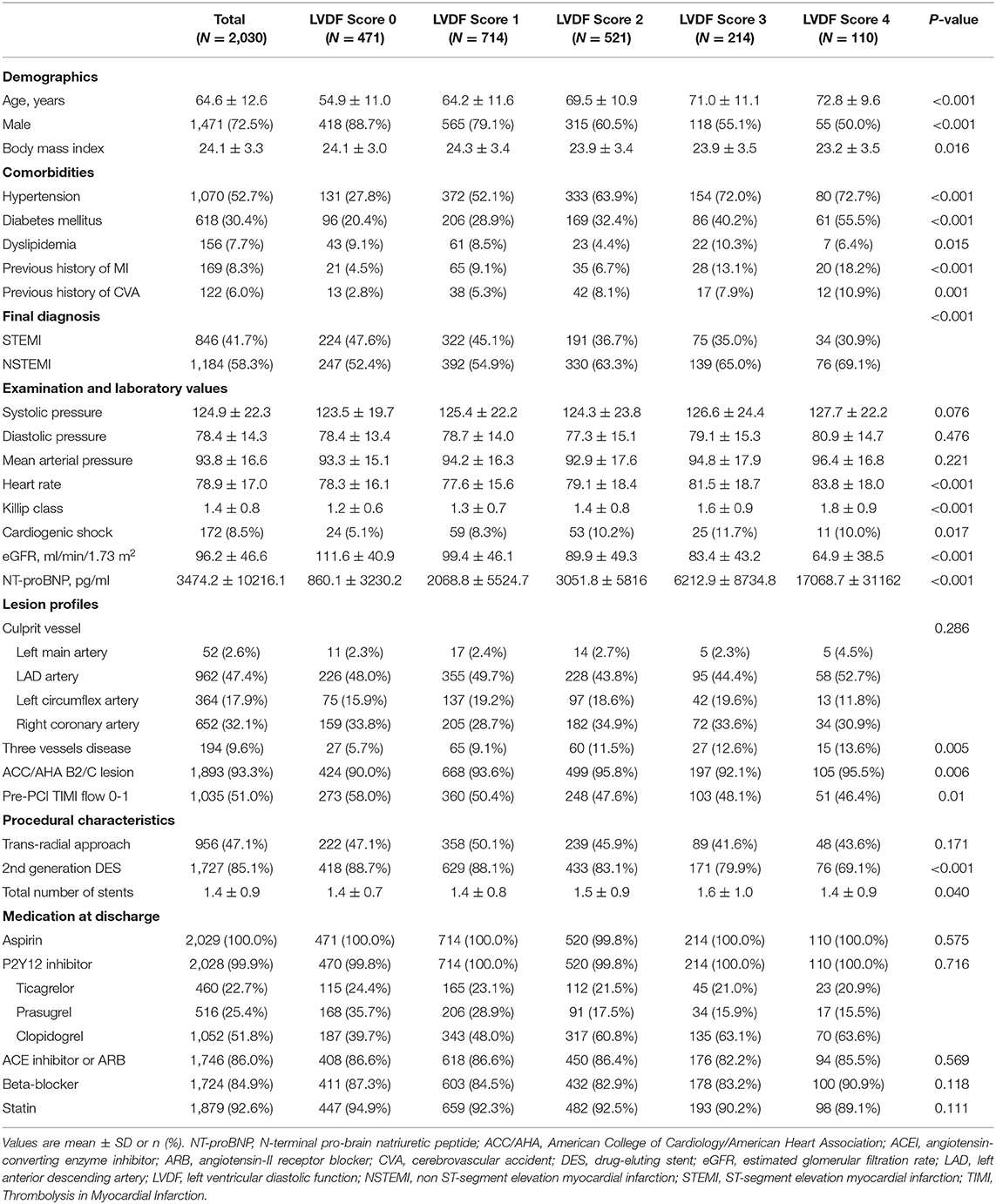
Methods: LVDF scores were measured in a 2,030 patients with AMI who underwent successful percutaneous coronary intervention from 2012 to 2015. Four LVDF parameters (septal e′ ≥ 7 cm/s, septal E/e′ ≤ 15, TR velocity ≤ 2.8 m/s, and LAVI ≤ 34 ml/m2) were used for LVDF scoring. The presence of each abnormal LVDF parameter was scored as 1, and the total LVDF score ranged from 0 to 4. Mortality and hospitalization due to heart failure (HHF) in relation to LVDF score were evaluated. To compare the predictive ability of LVDF scores and left ventricular ejection fraction (LVEF) for mortality and HHF, receiver operating characteristic (ROC) curve and landmark analyses were performed.
Results: Over the 3-year clinical follow-up, all-cause mortality occurred in 278 patients (13.7%), while 91 patients (4.5%) developed HHF. All-cause mortality and HHF significantly increased as LVDF scores increased (all-cause mortality–LVDF score 0: 2.3%, score 1: 8.8%, score 2: 16.7%, score 3: 31.8%, and score 4: 44.5%, p < 0.001; HHF–LVDF score 0: 0.6%, score 1: 1.8%, score 2: 6.3%, score 3: 10.3%, and score 4: 18.2%, p < 0.001). In multivariate analysis, a higher LVDF score was associated with significantly higher adjusted hazard ratios for all-cause mortality and HHF. In landmark analysis, LVDF score was a better predictor of long-term mortality than LVEF (area under the ROC curve: 0.739 vs. 0.640, p < 0.001).
Conclusion: The present study demonstrated that LVDF score was a significant predictor of mortality and HHF in patients with AMI. LVDF scores are useful for risk stratification of patients with AMI; therefore, careful monitoring and management should be performed for patients with AMI with higher LVDF scores.
Introduction
Acute myocardial infarction (AMI) is characterized by regional myocardial injury that may lead to systolic and diastolic dysfunction due to left ventricular (LV) remodeling and dysfunction. Left ventricular diastolic function (LVDF), an aftermath of AMI, is an important predictor for major adverse events (1–3). The 2009 guidelines for diastolic dysfunction included many parameters and was perceived as overly complex (4). In 2016, the guidelines were revised to simplify the measurement of LVDF, thereby enhancing the usefulness of the guidelines in routine practice (5, 6). It recommended two separate algorithms. For patients with maintained left ventricular ejection fraction (LVEF ≥ 50%) and unknown diastolic function, Algorithm A is primarily used to classify normal and abnormal diastolic function, while Algorithm B is designed to estimate LV filling pressure and grade diastolic function of patients with a reduced (<50%) or preserved LVEF and known or suspected diastolic dysfunction. However, if the patient's diagnosis is unknown or the LVEF is marginal (45–55%), there are problems in selecting an algorithm for LVDF evaluation. Therefore, there is a need for an LVDF assessment that can be easily applied to clinical practice by providers with different levels of expertise.
Recently, Oh et al. proposed a simplified and unified algorithm for LVDF assessment (7). This algorithm benefited by simplifying the assessment in clinical practice and avoiding problems with discordance and false calls of diastolic dysfunction to achieve high specificity. To date, the clinical impact of each individual LVDF marker is well-known, but the clinical significance of the sum of the abnormal diastolic function markers and the long-term clinical outcome are not well-known (8). This study aimed to investigate the usefulness of LVDF score in predicting clinical outcomes of patients with AMI.
Materials and Methods
Patient Population
All patients with AMI registered at Chonnam National University Hospital from 2011 to 2015 were included in the study. Of the initial 3,009 patients, 2,030 patients who underwent successful primary percutaneous intervention (PCI) and transthoracic echocardiography (TTE) were selected. Patients with moderate to severe mitral regurgitation (MR), mitral annular calcification, atrial fibrillation, those who did not undergo PCI, those who underwent suboptimal or failed PCI, those with no echocardiography findings, and those with insufficient TTE imaging or loss to follow-up were excluded (Supplementary Figure 1). AMI is defined as cardiomyocyte necrosis in a clinical setting consistent with acute myocardial ischemia (9). It was diagnosed by clinical presentation, serial changes on echocardiography suggesting infarction, and an increase in cardiac markers, preferably cardiac troponins, with at least one value above the 99th percentile of the upper reference limit. The study complies with the Declaration of Helsinki, and the local institutional review board (IRB) of the study center approved the study protocol (CNUH 05-49). Written informed consent was obtained from each study patient.
Echocardiographic Data and Study Definition
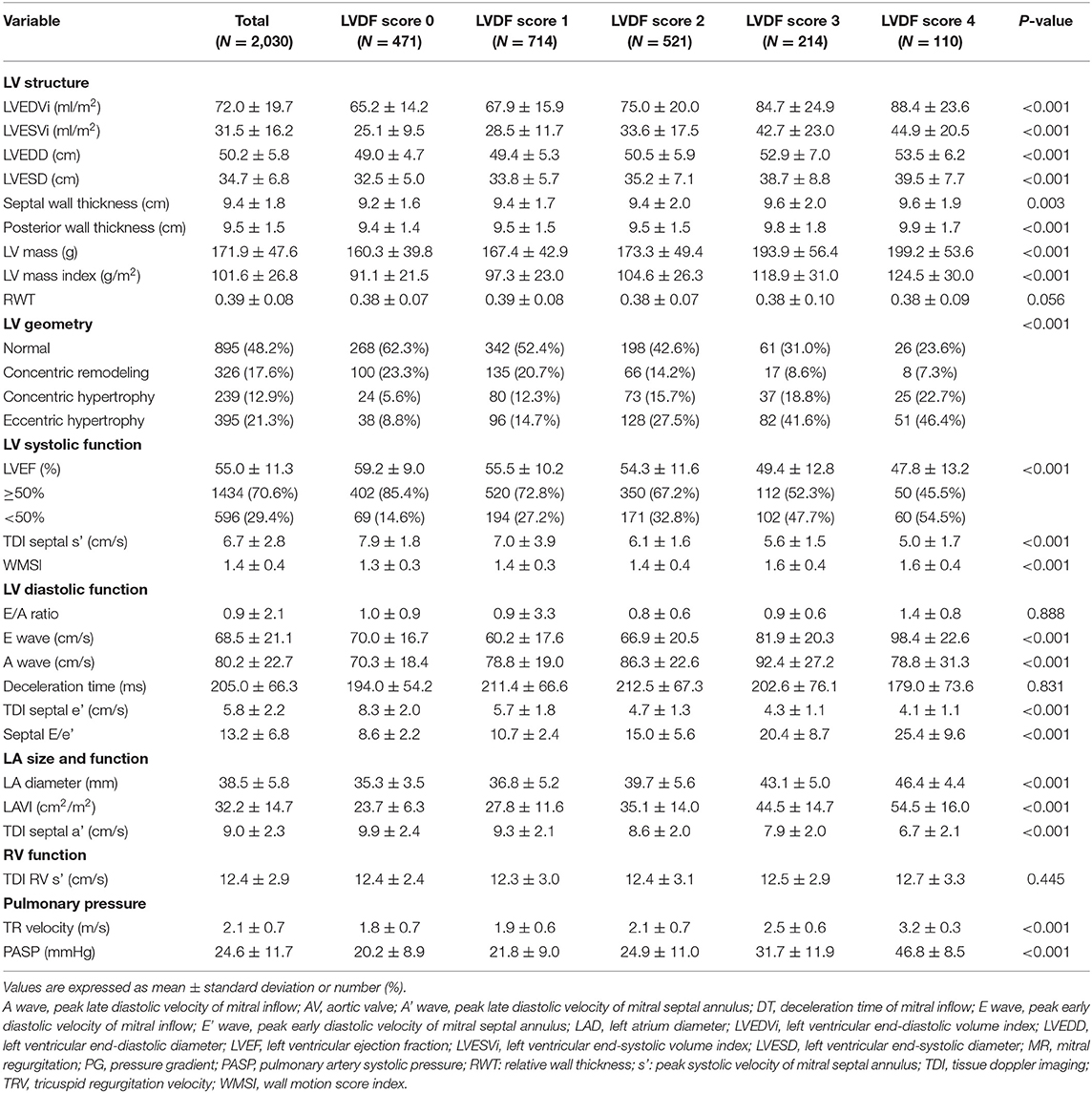
A comprehensive transthoracic echocardiogram was obtained within 48 h of admission for all patients. All TTE measurements were recorded during routine clinical practice according to the current American Society of Echocardiography (ASE/EACVI) recommendations (10). Left ventricular systolic function was assessed by LVEF obtained using the biplane method of disk summation, from the apical 2- and 4-chamber views, according to the modified biplane Simpson's method. To calculate the wall motion score index (WMSI), the LV was divided into 16 segments. Each segment was assessed and scored based on its motion and systolic thickening (1 = normokinesia, 2 = hypokinesia, 3 = akinesia, 4 = dyskinesia). The WMSI was calculated as the sum of the individual segment scores divided by the number of segments (11). Left atrial (LA) volume was assessed using the modified biplane Simpson's method, from the apical 2- and 4-chamber views, at end-systole and indexed to body surface area. In cases in which the Simpson's method could not be used due to missing or poor quality apical views, LA volume index (LAVI) was calculated using the Cube method (12). Peak early diastolic tissue velocity (e') was measured from the septal aspects of the mitral annulus, while mitral inflow velocity was assessed using the pulsed-wave Doppler from the apical 4-chamber view (5). The right ventricular (RV) functional measures were tricuspid annulus systolic tissue Doppler velocity (s') and RV dysfunction, which was defined as s' < 10 cm/s. Peak tricuspid regurgitation (TR) velocity was measured, and pulmonary artery systolic pressure (PASP) was estimated as 4 × (peak TR velocity)2 + 5 (5).
Four LVDF parameters (septal e′ ≥ 7 cm/s, septal E/e′ ≤ 15, TR velocity ≤ 2.8 m/s, and LAVI ≤ 34 ml/m2) were used for LVDF scoring (7). The presence of each abnormal LVDF parameter was scored as 1, and the total LVDF score ranged from 0 to 4 (normal filling pressure: LVDF score 0–1, indeterminate: LVDF score 2, increased filling pressure: LVDF score 3–4).
Clinical Data Collection
Demographic features and cardiovascular risk factors were obtained via patient interviews or review of medical records. During admission, findings of coronary angiography and detailed procedural characteristics of PCI, as well as data on discharge medications were collected. Patient treatment was performed according to current standard practice. After PCI, all patients were recommended to take aspirin indefinitely with clopidogrel or a potent P2Y12 inhibitor, such as prasugrel or ticagrelor, for at least 1 year.
Clinical Outcomes
The incidence of mortality and hospitalization due to heart failure (HHF) in relation with the LVDF score over the 3-year study period were evaluated. All causes of death were considered cardiac unless an apparent non-cardiac cause was otherwise stated. Readmission for HF was defined as the patient showing signs and symptoms of HF upon admission and was treated with medications, including diuretic therapy (either intravenous diuretics or augmentation of oral diuretics), vasodilators, inotropic support, or ultrafiltration for HF during admission. All end points followed the definitions of the Academic Research Consortium (13).
Statistical Analyses
Continuous variables are presented as means ± standard deviations or medians with interquartile ranges and compared using an unpaired t-test or Mann–Whitney rank sum test. Categorical variables are expressed as numbers with percentages and compared using Pearson's chi-square test or Fisher's exact test. Mortality and HHF were assessed using Kaplan–Meier curves according to the LVDF score. A multivariate Cox regression model was used for each of the above-mentioned cut-offs, with covariates that had P < 0.05 on univariate analysis or had predictive values [age ≥ 65 years, male sex, previous MI, estimated glomerular filtration rate (eGFR) ≤60%, LVEF, and cardiogenic shock, LV end-diastolic volume index, LV end-systolic volume index, LV geometry]. To compare the predictive abilities of LVDF scores and LVEF for mortality and HHF, receiver operating characteristic (ROC) curve analysis and DeLong's test were performed. In addition, comparisons of all-cause mortality between the LVDF score and LVEF according to the exploratory subgroups of interest were assessed using an ROC curve. For ROC curves, landmark analyses were used to compare LVDF scores and LVEF before and after 30 days of follow-up because 30 days following primary reperfusion is a critical period where the greatest degree of cardiac remodeling occurs (14).
All probability values were two-sided, and p-values <0.05 were considered statistically significant. All statistical analyses were performed using R Core Team (2015). R: A language and environment for statistical computing (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
Baseline Characteristics
Of the 3,009 patients with AMI recruited for the study, 979 patients were excluded for following reasons: 39 with moderate to severe MR; 68 with mitral annular calcification; 194 with atrial fibrillation; 23 with failed or suboptimal PCI; 380 with no PCI; 93 with no TTE; 172 with insufficient TTE imaging; and 10 with follow-up loss (Supplementary Figure 1). The proportion of LVDF scores were as follows: 23.2% (score 0), 35.2% (score 1), 25.7% (score 2), 10.5% (score 3), and 5.4% (score 4) (Figure 1A). An LVDF score of 1 was the most prominent (35%), with an abnormal septal e' being the most common feature. E/e' > 15 showed an increasing pattern as the LVDF score increased (Figure 1B). The baseline demographics, final diagnosis, and risk factors were found to be significantly varied with the LVDF score (Table 1). A total of 2,030 patients with a mean age of 64.6 ± 12.6 years, including 1,471 males (72.5%), were included in this study. Co-morbidities such as hypertension and diabetes mellitus were found in 52.7 and 41.0% of patients, respectively. As the LVDF score increased, eGFR decreased while N-terminal pro-brain natriuretic peptide levels increased (p < 0.001). Second-generation drug-eluting stent was chosen as the most implanted intervention (85.1%), and the total number of stents was 1.4 ± 0.9. Most patients were receiving aspirin, a P2Y12 inhibitor, ACE inhibitor or ARB, beta-blocker, or a statin.
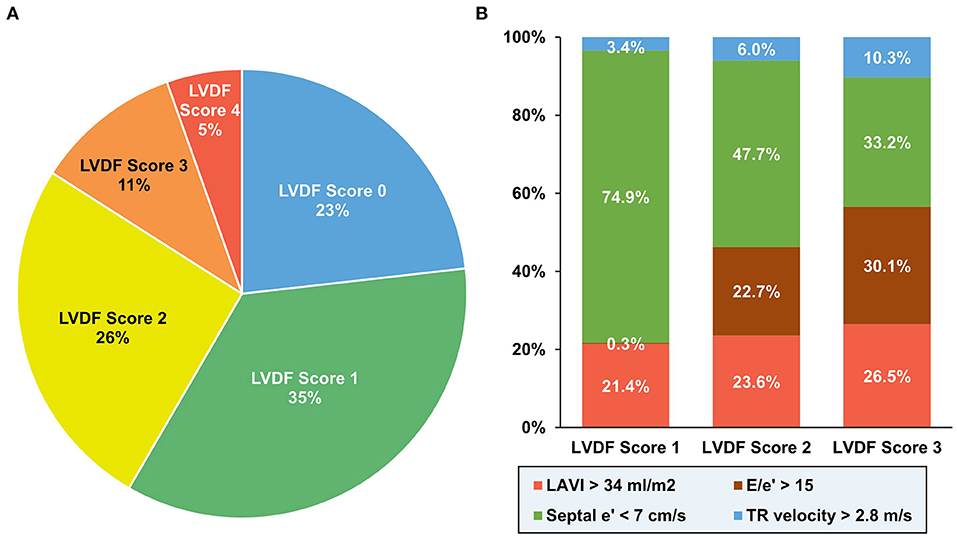
Figure 1. Distribution of diastolic function according to LVDF score. An LVDF score of 1 was the most prominent (35%) (A), while an abnormal septal e' was the most common feature (B). LAVI, left atrial volume index; LVDF, left ventricular diastolic function; TR, tricuspid regurgitation.
Echocardiographic Characteristics
Based on the left ventricular mass index (LVMi) results and relative wall thickness, LV hypertrophy (LVH) was observed in 34.2% of patients (concentric: 12.9%, eccentric: 21.3%; Table 2). The prevalence of LVH increased as the LVDF score increased. The mean LVEF was 55.0 ± 11.3%. As the LVDF score increased, the LVEF decreased and WMSI increased. The higher the LVDF score of patients, the higher the LAVI and septal E/e' TR velocity but the lower the septal e'.
Clinical Outcome
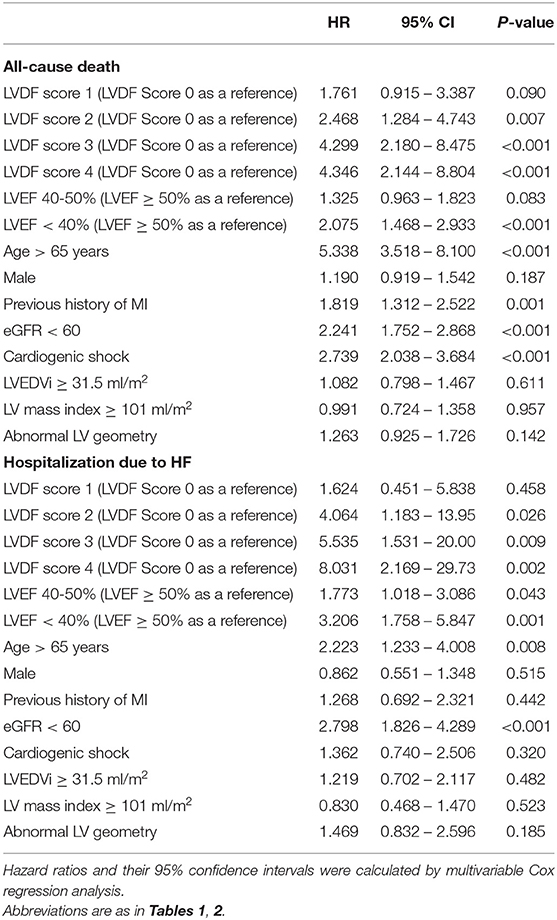
During a median follow-up period of 1,099 (interquartile range: 1,063–1,124) days, 278 patients (13.7%) died and 91 patients (4.5%) were readmitted for HF. All-cause mortality and HHF significantly increased as the LVDF score increased (all-cause mortality–LVDF score 0: 2.3%, score 1: 8.8%, score 2: 16.7%, score 3: 31.8%, and score 4: 44.5%, p < 0.001; HHF–LVDF score 0: 0.6%, score 1: 1.8%, score 2: 6.3%, score 3: 10.3%, and score 4: 18.2%, p < 0.001; Figure 2A). In multivariate analysis, a higher LVDF score was associated with significantly higher adjusted hazard ratios (HR) for all-cause mortality and HHF (Figure 2B). Kaplan-Meier survival analysis revealed that patients with higher LVDF scores had a higher rate of all-cause mortality, cardiac death, and HHF than those with lower LVDF scores (log-rank p < 0.001; Figure 3). In various subgroups, the LVDF scores stratification had incrementally higher adjusted hazard ratio (HR) for all-cause mortality (Supplementary Figure 2).
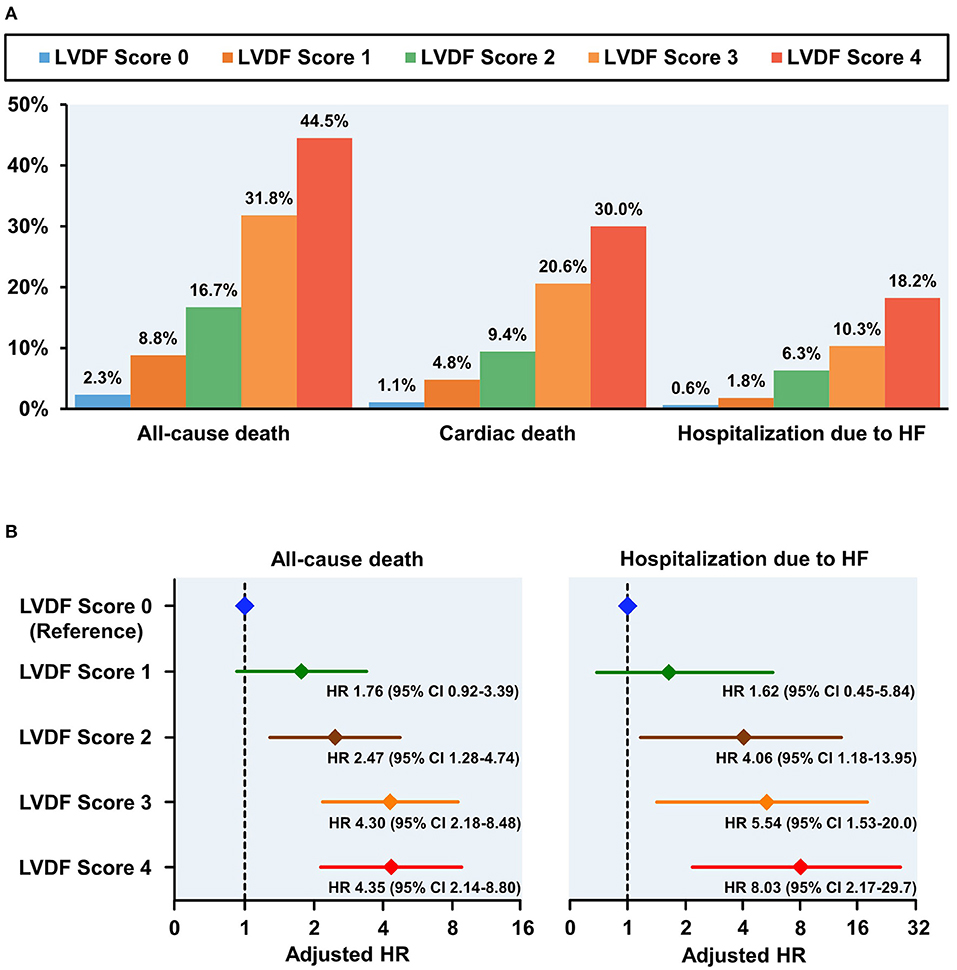
Figure 2. Clinical outcomes according to LVDF scores (A) and adjusted HR plot for all-cause mortality and hospitalization due to HF (B). All-cause mortality, cardiac death, and heart failure (HF) rehospitalization rates increased in a stepwise fashion from 2.3% (LVDF score 0) to 44.5% (LVEF score 4) (p < 0.001). Higher LVDF scores had incrementally higher adjusted HRs for all-cause mortality and hospitalization due to HF. CI, confidence interval; HF, heart failure; HR, hazard ratio; LVDF, left ventricular diastolic function.
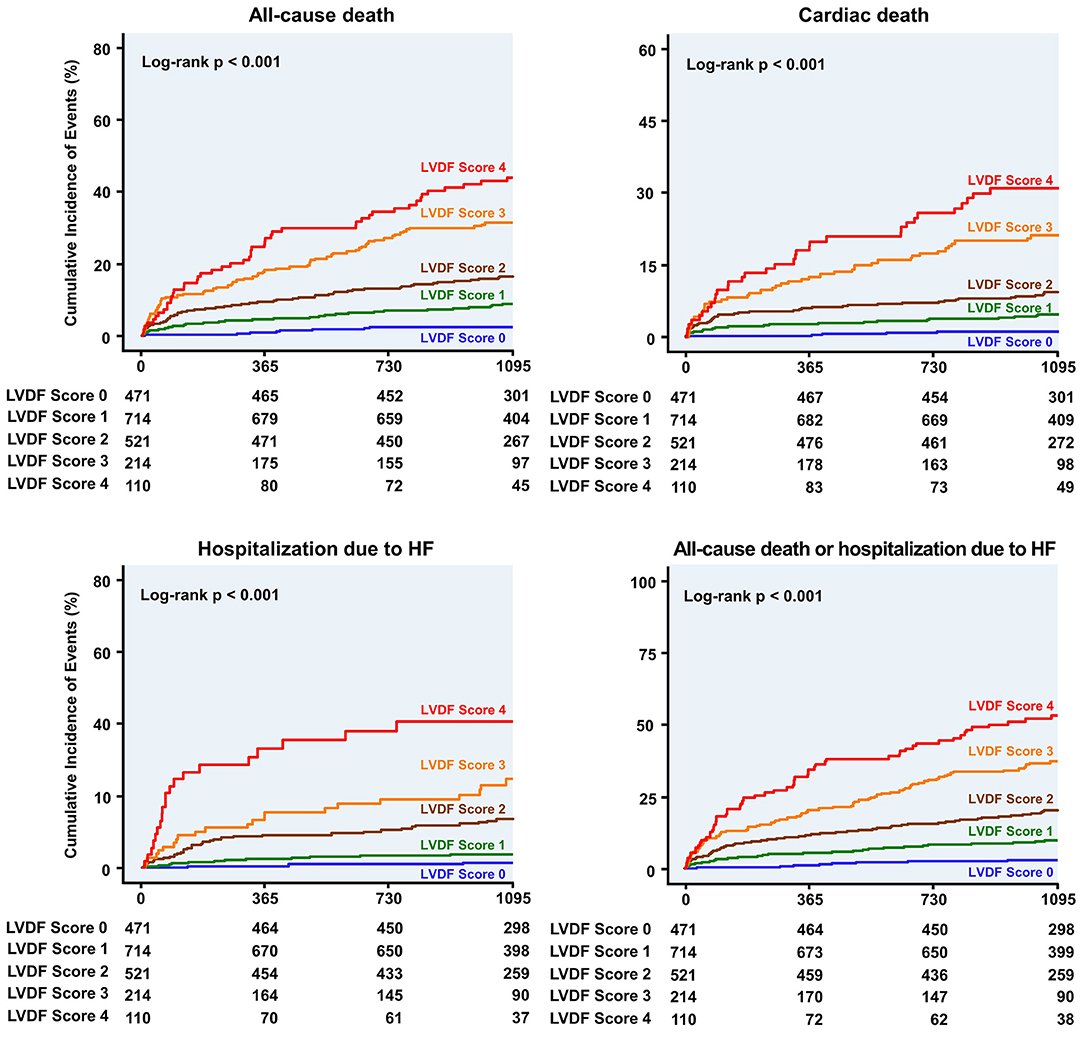
Figure 3. Kaplan–Meier curves for clinical outcome according to the LVDF score. Patients with higher LVDF scores had a higher rate of all-cause mortality, cardiac death, or hospitalization due to HF than patients with lower LVDF scores (log-rank p < 0.001). HF, heart failure; HR, hazard ratio; LVDF, left ventricular diastolic function.
LVDF score 2–4 and LVEF < 40% were independent predictors of all-cause death, and LVDF score 2–4, LVEF 40–50% and <40% were independent predictors of HHF (Table 3). In addition, HR values for all-cause death and HHF were higher in LVDF score 4 than in LVEF < 40% {all-cause death—LVDF score 4: HR 4.346 [95% confidence interval (CI) 1.468–2.933], p < 0.001) vs. LVEF < 40%: HR 2.075 (95% CI 1.468–2.933), p < 0.001; HHF–LVDF score 4: HR 8.031 (95% CI 2.169–29.73), p = 0.002 vs. LVEF < 40%: HR 3.206 (95% CI 1.758–5.847), p = 0.001}.
Prediction of Clinical Outcome: LVDF Score vs. LVEF
The ROC curve showed that the LVDF score was significantly better at predicting all-cause mortality and readmission for recurrent HF than LVEF (area under the ROC curve [AUC]: 0.743 vs. 0.676, Delong's test p = 0.003; AUC: 0.762 vs. 0.688, Delong's test p = 0.046; respectively; Figure 4).
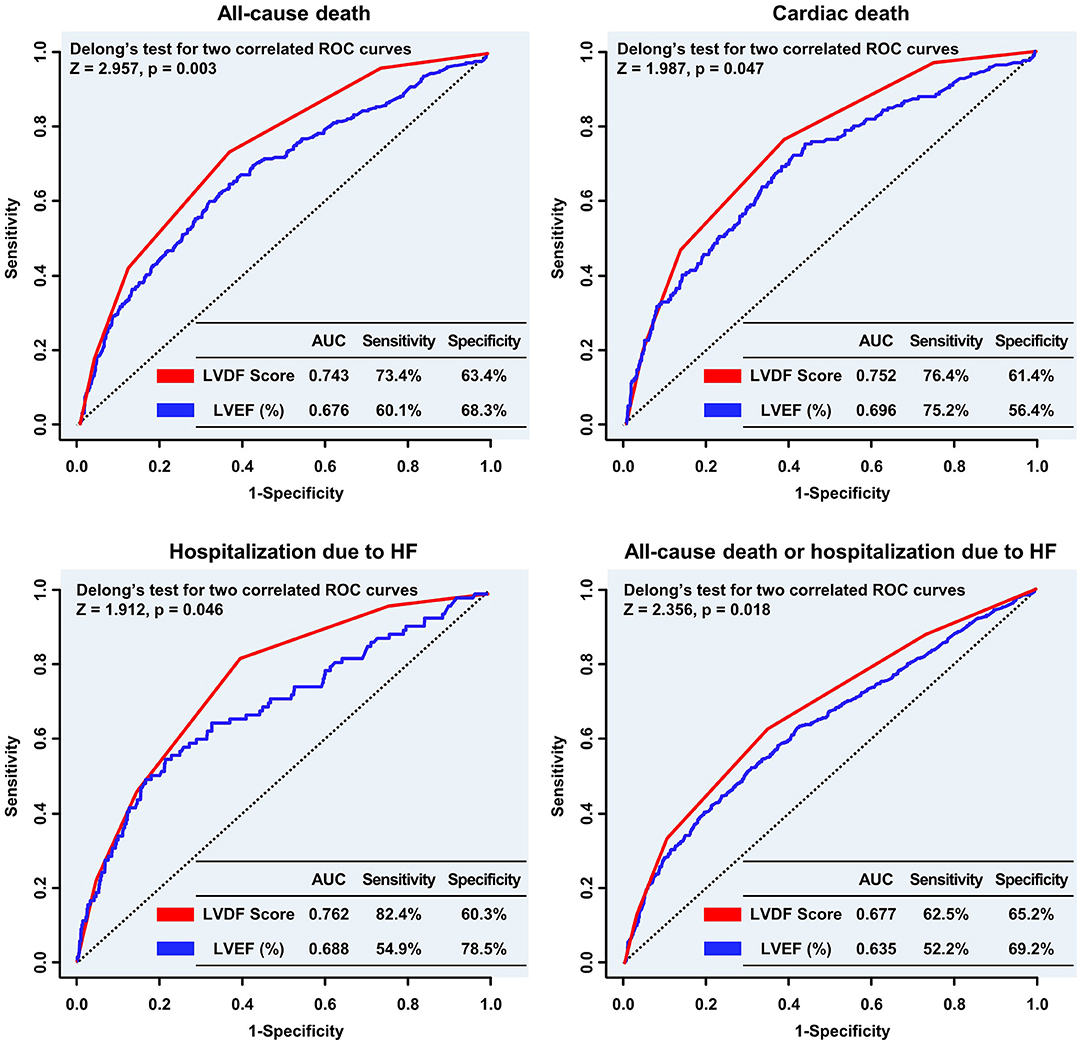
Figure 4. Comparison of the predictive performance of the LVDF score and LVEF for clinical outcomes. The LVDF score performed significantly better in predicting all-cause mortality, cardiac death, and hospitalization due to HF compared with LVEF. AUC, area under the receiver operating characteristic curve; HF, heart failure; LVDF, left ventricular diastolic function; LVEF, left ventricular ejection fraction; ROC, receiver operating characteristic.
Subgroup analysis showed that the LVDF score performed significantly better than LVEF in patients with ST-segment elevation myocardial infarction (STEMI), LVEF ≥ 50%, Killip class < 3, abnormal LV geometry (LV remodeling or LVH), and non-right coronary artery (RCA) target vessel (Supplementary Figure 3). Comparison of the predictive performance of the individual LVDF parameters and LVEF showed that E/e' ratio was the best predictor of all-cause death and HHF (Supplementary Figure 4).
In landmark analysis, LVEF was the most predictive parameter for all-cause mortality within the first 30 days of follow-up (AUC: 0.801 vs. 0.704, p = 0.045), but between 30 days and 3 years of follow-up, the LVDF score was the better predictor (AUC: 0.739 vs. 0.640, p < 0.001) (Figure 5).
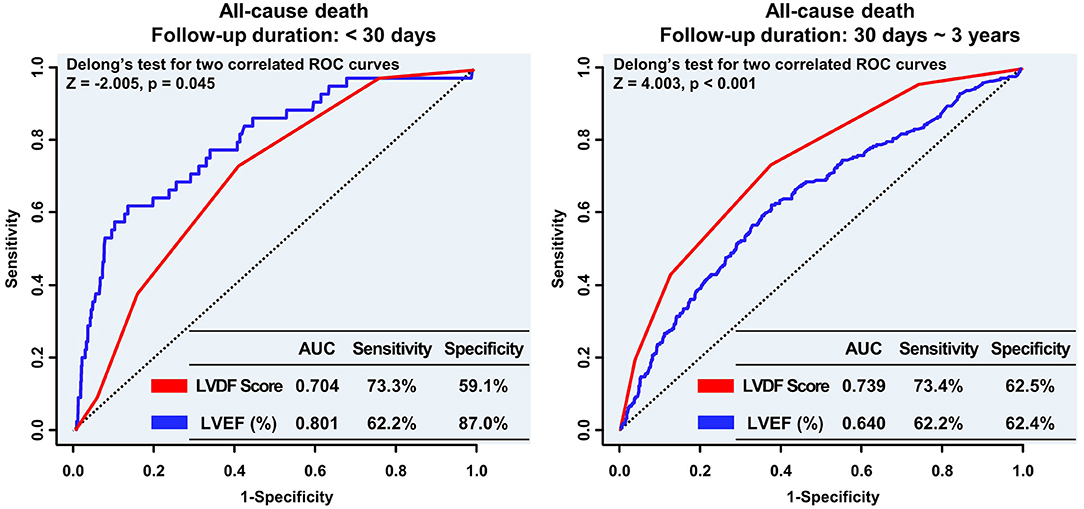
Figure 5. Landmark analyses of the ROC curve to compare the LVDF score and LVEF before and after the 30-day follow-up period. In the landmark analysis, LVEF was the strongest predictor of all-cause mortality during the short-term follow up; however, the LVDF score was a better predictor of mortality than LVEF during the long-term follow-up. AUC, area under the receiver operating characteristic curve; LVDF, left ventricular diastolic function; LVEF, left ventricular ejection fraction; ROC, receiver operating characteristic.
Discussion
This study examined the construct validity of the unified LVDF algorithm, by demonstrating the ability of a simplified LVDF score to outperform LV systolic function in predicting long-term clinical outcomes in 2,030 patients with AMI. Patients with high LVDF scores had a significantly higher risk of all-cause mortality or readmission for recurrent HF than patients with low LVDF scores, which was consistently observed even after adjusting for baseline differences. The LVDF score performed significantly better in predicting all-cause mortality and readmission for recurrent HF compared with LVEF. Subgroup analysis showed that LVDF scores performed significantly better than LVEF in patients with STEMI, LVEF ≥ 50%, Killip class < 3, abnormal LV geometry (LV remodeling or LVH), and non-RCA target vessels. In landmark analysis, LVDF scores were better in predicting all-cause mortality than LVEF in the long-term follow-up (30 days ~ 3 years).
Predicting Clinical Outcome Using the LVDF Score in Patients With Acute Myocardial Infarction
Previous studies have shown that in the 2016 ASE/EACVI guidelines, assessment of diastolic function was a strong independent predictor of outcomes for MI (15, 16). However, these studies excluded indeterminate variables and shock groups, had relatively smaller study subjects, and included limitations that were difficult to apply in a clinical setting. Contrastingly, the present study included patients with AMI with cardiogenic shock and indeterminate variables and found that the prognosis worsened as the LVDF score increased. Additionally, in the distribution of diastolic function, the most common in LVDF score 1 was septal e' < 7 cm/s. However, in LVDF score 2, the E/e' > 15 ratio increases, and thereafter, in LVDF score 3, the TR velocity > 2.8 m/s ratio increases. And among individual LVDF parameters, E/e' ratio and TR velocity were the best predictors of all-cause death and HHF. Therefore, clinical outcomes can be predicted simply by evaluating the LVDF scoring system, which is expected to be helpful in routine clinical practice for patients with AMI.
Comparison of LVDF Score to LVEF for Mortality Prediction
Prognosis of LV systolic dysfunction after AMI has been a major research focus for several decades (3). The insights from these studies have led to several therapeutic interventions that have improved outcomes. In addition to depressed systolic function, clinical and radiological evidence of HF is a consistent and powerful predictor of outcomes in patients with AMI (17). However, there have been no studies comparing mortality between the two predictors, namely LV systolic function and current LVDF guidelines, in patients with AMI. In the present study, the LVDF score was found to be superior to LVEF in predicting mortality, especially in patients with AMI with STEMI, preserved LVEF (≥50%), hemodynamic stable state (Killip class <3), abnormal LV geometry, and non-RCA target vessels. LVEF is a strong predictor for clinical outcomes; however, since each LVDF parameter, including septal e', E/e', TR velocity, and LAVI, is known as a strong independent prognostic factor in HF and other diseases (18–21), the intersection of these four can be judged as a more powerful predictor for mortality. Especially, abnormal LV geometry (increased wall thickness and/or reduced end diastolic volume), which is a confounder for LVEF, makes it possible for LVEF to be unaltered despite significantly reduced LV function (22). In this study, as a result of analyzing the LV of patients with AMI, the normal LV geometry was less than half and the total LV mass index was 101.6 ± 26.8 g/m2, which was thicker compared with the LV wall of the normal population (69.9 ± 8.9 g/m2) (23); thus, LVDF is considered to be a better predictor than LVEF.
The Importance of Evaluating LV Diastolic Function
There are prognostic reasons why LVDF evaluation is clinically important. From a diagnostic point of view, elevated LV filling pressure is an important cause of HF in patients with AMI (24). There are several studies focusing on optimal non-invasive assessment of left ventricular filling pressures that compared natriuretic peptide levels with Doppler against mean wedge pressure. Studies have shown that Doppler had a stronger correlation with mean wedge pressure, and the E/e' ratio tracked with changes in mean wedge pressure, whereas B-type natriuretic peptide levels did not (25). Similar results were seen in patients with ambulatory HF in which the E/e' ratio successfully tracked with changes in LA pressure (26). In this study, there was no significant difference in MAP in all groups (p = 0.221), but the LVDF score increased with the E/e' ratio (p < 0.001), leading to a decrease in coronary perfusion pressure, which is thought to be the cause of increased mortality and readmission for recurrent HF in the long term.
Interestingly, LVEF was superior to the LVDF score for predicting all-cause mortality during the short-term follow-up period (<30 days), but the LVDF score was superior to LVEF during the long-term follow-up (30 days ~ 3 years). As a consequence of AMI, the measurement of changes in LV size, shape, and the thickness of both infarcted and non-infarcted segments of the ventricle, collectively referred to as ventricular remodeling, is important in evaluating ventricular function and prognosis (27); however, several studies have shown that measurement of lesion size and left ventricular systolic function (28, 29) or alterations in post-infarction left ventricular remodeling (30) do not explain why patients with AMI have an increased tendency to develop long-term adverse outcomes. Therefore, in the acute stage, assessing prognosis based on LVEF is reasonable, and it is desirable to assess the prognosis using the LVDF score for patients undergoing long-term follow-up.
Study Limitations
This study had several limitations. First, despite its large sample size and granular data, this study had the potential for unmeasured confounders and lack of some data. Second, echocardiography-based estimates of hemodynamic measurements, such as the E/e′ ratio, were used to measure LV filling pressures and TR velocity for pulmonary artery pressures, which are indirect measures. However, these correlate well with invasive measurements (31), and in clinical practice, diastolic function is evaluated mainly using echocardiography. Third, the 2016 ASE/EACVI guidelines recommended using the average of the lateral and septal velocities to measure LVDF, since these values are significantly different in certain situations such as left bundle branch block, regional wall motion abnormality, or significant right ventricular dysfunction, but only the septal e' velocity was used. However, there is no evidence that the average e' velocity provides a more reliable assessment for diastolic function (7). Moreover, septal E/e' was found to be associated with a poor outcome in TOPCAT trial (32), whereas lateral E/e' did not differ between patients with heart failure with preserved ejection fraction who were and were not hospitalized in I-Preserve (33). In the present study, comparison of the predictive performance of the individual LVDF parameters showed that E/e' ratio was the best predictor of all-cause death and hospitalization due to HF (Supplementary Figure 4). Subgroup analysis was performed for factors that could affect septal e' (WMSI ≥ 2, left bundle branch block or TDI RV s' < 9.5 cm/s) (Supplementary Figure 5). Therefore, consistent results were obtained.
Conclusion
The present study demonstrated that the LVDF score is a significant predictor of mortality and HHF in patients with AMI. The LVDF score can be useful in the risk stratification of patients with AMI; thus, careful monitoring and management should be provided to patients with AMI with higher LVDF scores.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the study complies with the Declaration of Helsinki, and the Chonnam national university hospital institutional review board of the study center approved the study protocol (CNUH 05-49). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SB performed the statistical analysis. SB and HY drafted the manuscript. KK, HK, HP, JC, MK, YK, YA, JC, and MJ reviewed/edited the manuscript and contributed to the interpretation of data. KK conceptualized the overall study design and supervised all aspects of the study and revised the manuscript critically. All authors have read and approved the manuscript.
Funding
This study was supported by a grant of Chonnam National University Hospital Biomedical Research Institute (BCRI19039).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.730872/full#supplementary-material
Abbreviations
AMI, acute myocardial infarction; AUC, area under the receiver operating characteristic curve; eGFR, estimated glomerular filtration rate; HF, heart failure; LAVI, left atrial volume index; LV, left ventricular; LVDF, left ventricular diastolic function; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; PCI, percutaneous coronary intervention; RCA, right coronary artery; ROC, receiver operating characteristic; RV, right ventricular; STEMI, ST-segment elevation myocardial infarction; TR, tricuspid regurgitation; TTE, transthoracic echocardiography; WMSI, wall motion score index.
References
1. Hillis GS, Møller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, et al. Noninvasive estimation of left ventricular filling pressure by E/e' is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. (2004) 43:360–7. doi: 10.1016/j.jacc.2003.07.044
2. Jons C, Joergensen RM, Hassager C, Gang UJ, Dixen U, Johannesen A, et al. Diastolic dysfunction predicts new-onset atrial fibrillation and cardiovascular events in patients with acute myocardial infarction and depressed left ventricular systolic function: a CARISMA substudy. Eur J Echocardiogr. (2010) 11:602–7. doi: 10.1093/ejechocard/jeq024
3. Møller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. (2006) 114:438–44. doi: 10.1161/CIRCULATIONAHA.105.601005
4. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. (2009) 22:107–33. doi: 10.1016/j.echo.2008.11.023
5. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
6. Nagueh SF. Left ventricular diastolic function: understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging. (2020) 13(1 Pt 2):228–44. doi: 10.1016/j.jcmg.2018.10.038
7. Oh JK, Miranda WR, Bird JG, Kane GC, Nagueh SF. The 2016 diastolic function guideline: is it already time to revisit or revise them? JACC Cardiovasc Imaging. (2020) 13(1 Pt 2):327–35. doi: 10.1016/j.jcmg.2019.12.004
8. Almeida JG, Fontes-Carvalho R, Sampaio F, Ribeiro J, Bettencourt P, Flachskampf FA, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging. (2018) 19:380–6. doi: 10.1093/ehjci/jex252
9. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. (2012) 33:2551–67. doi: 10.1093/eurheartj/ehs184
10. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. (2005) 18:1440–63. doi: 10.1016/j.echo.2005.10.005
11. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
12. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. (2006) 7:79–108. doi: 10.1016/j.euje.2005.12.014
13. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 Consensus Document. Circulation. (2018) 137:2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289
14. Daubert MA, White JA, Al-Khalidi HR, Velazquez EJ, Rao SV, Crowley AL, et al. Cardiac remodeling after large ST-elevation myocardial infarction in the current therapeutic era. Am Heart J. (2020) 223:87–97. doi: 10.1016/j.ahj.2020.02.017
15. Prasad SB, Lin AK, Guppy-Coles KB, Stanton T, Krishnasamy R, Whalley GA, et al. Diastolic dysfunction assessed using contemporary guidelines and prognosis following myocardial infarction. J Am Soc Echocardiogr. (2018) 31:1127–36. doi: 10.1016/j.echo.2018.05.016
16. Prasad SB, Guppy-Coles KB, Holland D, Stanton T, Krishnasamy R, Whalley G, et al. Echocardiographic predictors of all-cause mortality in patients with left ventricular ejection fraction >35%: value of guideline based assessment of diastolic dysfunction. Int J Cardiol Heart Vasc. (2019) 24:100407. doi: 10.1016/j.ijcha.2019.100407
17. Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. (1967) 20:457–64. doi: 10.1016/0002-9149(67)90023-9
18. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol. (2019) 74:2858–73. doi: 10.1016/j.jacc.2019.09.063
19. Benfari G, Miller WL, Antoine C, Rossi A, Lin G, Oh JK, et al. Diastolic determinants of excess mortality in heart failure with reduced ejection fraction. JACC Heart Fail. (2019) 7:808–17. doi: 10.1016/j.jchf.2019.04.024
20. Tennøe AH, Murbræch K, Andreassen JC, Fretheim H, Garen T, Gude E, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol. (2018) 72:1804–13. doi: 10.1016/j.jacc.2018.07.068
21. Bae S, Kim KH, Yoon HJ, Kim HY, Park H, Cho JY, et al. Clinical impact of echocardiography-defined pulmonary hypertension on the clinical outcome in patients with multiple myeloma. Medicine. (2020) 99:e22952. doi: 10.1097/MD.0000000000022952
22. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, et al. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol. (2017) 70:942–54. doi: 10.1016/j.jacc.2017.06.046
23. Mizukoshi K, Takeuchi M, Nagata Y, Addetia K, Lang RM, Akashi YJ, et al. Normal values of left ventricular mass index assessed by transthoracic three-dimensional echocardiography. J Am Soc Echocardiogr. (2016) 29:51–61. doi: 10.1016/j.echo.2015.09.009
24. Somaratne JB, Whalley GA, Gamble GD, Doughty RN. Restrictive filling pattern is a powerful predictor of heart failure events postacute myocardial infarction and in established heart failure: a literature-based meta-analysis. J Card Fail. (2007) 13:346–52. doi: 10.1016/j.cardfail.2007.01.010
25. Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, et al. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. (2004) 109:2432–9. doi: 10.1161/01.CIR.0000127882.58426.7A
26. Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. (2013) 309:781–91. doi: 10.1001/jama.2013.905
27. van der Laan AM, Nahrendorf M, Piek JJ. Healing and adverse remodelling after acute myocardial infarction: role of the cellular immune response. Heart. (2012) 98:1384–90. doi: 10.1136/heartjnl-2012-301623
28. Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. (1989) 14:49–57. doi: 10.1016/0735-1097(89)90053-3
29. Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, et al. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. (1996) 28:1661–9. doi: 10.1016/S0735-1097(96)00397-X
30. Solomon SD, St John Sutton M, Lamas GA, Plappert T, Rouleau JL, Skali H, et al. Ventricular remodeling does not accompany the development of heart failure in diabetic patients after myocardial infarction. Circulation. (2002) 106:1251–5. doi: 10.1161/01.CIR.0000032313.82552.E3
31. Kampaktsis PN, Kokkinidis DG, Wong SC, Vavuranakis M, Skubas NJ, Devereux RB. The role and clinical implications of diastolic dysfunction in aortic stenosis. Heart. (2017) 103:1481–7. doi: 10.1136/heartjnl-2017-311506
32. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. (2014) 7:740–51. doi: 10.1161/CIRCHEARTFAILURE.114.001583
33. Carson PE, Anand IS, Win S, Rector T, Haass M, Lopez-Sendon J. The hospitalization burden and post-hospitalization mortality risk in heart failure with preserved ejection fraction: results from the I-PRESERVE trial (Irbesartan in Heart Failure and Preserved Ejection Fraction). JACC Heart Fail. (2015) 3:429–41. doi: 10.1016/j.jchf.2014.12.017
Keywords: diastolic function, left ventricular ejection fraction, myocardial infarction, mortality, heart failure
Citation: Bae S, Yoon HJ, Kim KH, Kim HY, Park H, Cho JY, Kim MC, Kim Y, Ahn Y, Cho JG and Jeong MH (2021) Usefulness of Diastolic Function Score as a Predictor of Long-Term Prognosis in Patients With Acute Myocardial Infarction. Front. Cardiovasc. Med. 8:730872. doi: 10.3389/fcvm.2021.730872
Received: 25 June 2021; Accepted: 19 August 2021;
Published: 10 September 2021.
Edited by:
Minjie Lu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Kenya Kusunose, Tokushima University Hospital, JapanMariana Paiva, Centro Hospitalar Universitário de São João (CHUSJ), Portugal
Copyright © 2021 Bae, Yoon, Kim, Kim, Park, Cho, Kim, Kim, Ahn, Cho and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyun Ju Yoon, YW5uNDI2QGhhbm1haWwubmV0
 SungA Bae
SungA Bae Hyun Ju Yoon
Hyun Ju Yoon Kye Hun Kim2
Kye Hun Kim2 Hyung Yoon Kim
Hyung Yoon Kim Yongcheol Kim
Yongcheol Kim