- 1Nanjing University of Chinese Medicine, Nanjing, China
- 2Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, China
Background: Previous understanding holds that rotational atherectomy and modified balloons remain the default strategy for severely calcified coronary stenoses. In recent years, coronary intravascular lithotripsy (IVL) provides new ideas. This study was conducted to evaluate the safety and efficacy of IVL for the treatment of severely calcified coronary stenoses.
Methods: The serial Disrupt CAD trials (Disrupt CAD I, Disrupt CAD II, Disrupt CAD III, and Disrupt CAD IV) were included in this study. The safety endpoint was freedom from major adverse cardiovascular events (MACE) in hospital, at 30 days, and at 6 months following the index procedure. The efficacy endpoints included procedural success and angiographic success. Optical coherence tomography (OCT) was used to evaluate the mechanism of action of IVL quantifying the coronary artery calcification (CAC) characteristics and calcium plaque fracture.
Results: We enrolled a total of 628 patients with a mean age of 71.8 years, 77.1% males. In these patients, the left anterior descending artery and right coronary artery were the most vulnerable vessels. The diameter stenosis was 64.6 ± 11.6% and the lesion length was 24.2 ± 11.4 mm. IVL had a favorable efficacy (93.0% procedural success, 97.5% angiographic success, and 100.0% stent delivery). Among the 628 patients, 568, 568, and 60 reported MACE endpoints in hospital, at 30 days, and at 6 months, respectively. The results showed that 528, 514, and 55 patients were free from MACE in hospital, at 30 days, and at 6 months, respectively. OCT measurements demonstrated that calcium fracture was the underlying mechanism of action for coronary IVL.
Conclusions: IVL is safe and efficient for severely calcified coronary stenoses, and, importantly, calcium fracture facilitated increased vessel compliance and favorable stent expansion.
Background
In the process of coronary atherosclerosis, coronary artery calcification (CAC) is often accompanied. Even though CAC might have no specific clinical manifestation, this asymptomatic phenomenon is highly prevalent in patients with severe coronary artery disease (CAD) and is associated with major adverse cardiovascular events (MACE) (1). With the help of computed tomography coronary angiography (CTCA), intravascular ultrasound (IVUS), and optical coherence tomography (OCT), the detection of CAC has been greatly improved, but severe CAC still significantly increases the difficulty of percutaneous coronary intervention (PCI) (1, 2). The treatment of calcified lesions includes modified (scoring or cutting) balloons and the application of rotational atherectomy (RA) (3). RA, a complex method utilizing a diamond-tipped burr, can effectively fracture the calcium plaque through the high-speed rotation of its burr and then selectively act on the calcified tissue, resulting in the debulking of calcium plaques (4). RA is a technically demanding procedure reliant on operator experience, and the safety of RA has improved with accumulated experience and maturation of the technique (4, 5). The randomized ROTAXUS (Rotational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease) (3) and PREPARE-CALC (Comparison of Strategies to Prepare Severely Calcified Coronary Lesions) (6) trials implied that RA seems to be successful and is not associated with excessive late lumen loss. However, angiographic and clinical complications of RA are persistent (7) and emphasize the potential for further progress in technology and technique (8, 9). How to treat severely calcified coronary stenoses succinctly and effectively is still a great challenge facing interventional therapy. Coronary intravascular lithotripsy (IVL), based on an established treatment strategy for the renal calculi to disrupt vascular calcium, facilitates the PCI of severely calcified coronary stenoses by using high-pressure ultrasonic energy. IVL is the newest adjunctive method for calcium modification, and recently it is being applied in the clinic and generates promise and encouraging results as its users gain more experience and it becomes readily available worldwide (10). Since the sample sizes of the previously published studies involving IVL for severely calcified coronary stenoses were generally small, we here integrate the serial Disrupt CAD trials (11–14) to further evaluate the safety and efficacy of IVL for the treatment of severely calcified coronary stenoses.
Methods
The data were extracted from the serial Disrupt CAD trials by two reviewers independently and were crossed checked. All data indicators from these trials can be used for subsequent analysis. After detecting the skewness (15), we estimated the sample mean and standard deviation from the sample size, median, and interquartile range according to previous studies (16, 17), and then we pooled the corresponding data. All the results were descriptive analysis, as described previously (18).
The safety endpoint was freedom from MACE in hospital, at 30 days, and at 6 months following the index procedure. The efficacy endpoints included procedural success and angiographic success (19). MACE composited the occurrence of cardiac death, myocardial infarction (MI), or target vessel revascularization (TVR) (20). MI was defined as a creatine kinase MB isoform (CK-MB) level more than three times the upper limit of lab normal value with or without new pathologic Q waves at discharge (periprocedural MI) and using the Fourth Universal Definition of Myocardial Infarction beyond discharge (spontaneous MI). TVR was revascularization at the target vessel (inclusive of the target lesion) after the completion of the index procedure. Procedural success was defined as the ability of IVL to produce residual diameter stenosis <50% after stenting with no evidence of in-hospital MACE (21). The diameter stenosis was calculated by the fractional flow reserve or the quantitative flow ratio (22–24). Angiographic success was defined as success in facilitating stent delivery with less than 50% residual stenosis and without serious angiographic complications [severe dissection impairing flow (types D–F), perforation, abrupt closure, persistent slow flow, or no reflow] (19). Coronary artery dissections were categorized using the National Heart Lung and Blood Institute classification (25–27).
The OCT guide for calcified coronary lesions resulted in a larger percent stent expansion compared to the IVUS guide (28). In this study, OCT was used to evaluate the mechanism of action of IVL quantifying the CAC characteristics and calcium plaque fracture. Calcium plaque fracture is considered as a new rupture and/or discontinuity of the calcium plaque found on OCT after IVL or stent implantation (29). In order to determine the number of fractures in each lesion, the continuity of calcium plaque fracture was tracked in the whole lesion frame by frame and cross-examined with longitudinal OCT images (12, 30). OCT imaging was planned at three time points (pre-IVL, post-IVL, and following stent deployment at the end of the procedure) to more accurately characterize the extent of calcification and provide insights into the mechanism of IVL in facilitating stent expansion.
Since all the Disrupt CAD trials were single-arm studies, we cannot obtain anything about a control. Considering that RA is widely used for lesion preparation before stent implantation, as confirmed by the European Association of Percutaneous Cardiovascular Interventions (5), we also included two high-quality studies on RA [ROTAXUS (3) and PREPARE-CALC (6), which were performed at three and at two high-volume, experienced interventional study sites in Germany, respectively]. Similarly, the data were extracted by two reviewers independently and pooled as described above.
We pooled Disrupt CAD I (11), Disrupt CAD II (12), Disrupt CAD III (13), and Disrupt CAD IV (14) to the Disrupt CAD group and pooled ROTAXUS (3) and PREPARE-CALC (6) to the RA group. We only selected the same data in the two groups for comparison. The continuous variables, in accordance with the normality distribution, are represented as the mean and standard deviation; otherwise, they are represented as the median and interquartile range. The categorical variables are presented as counts and proportions. All the results were descriptive analysis, as described previously (18).
Results
Study Characteristics
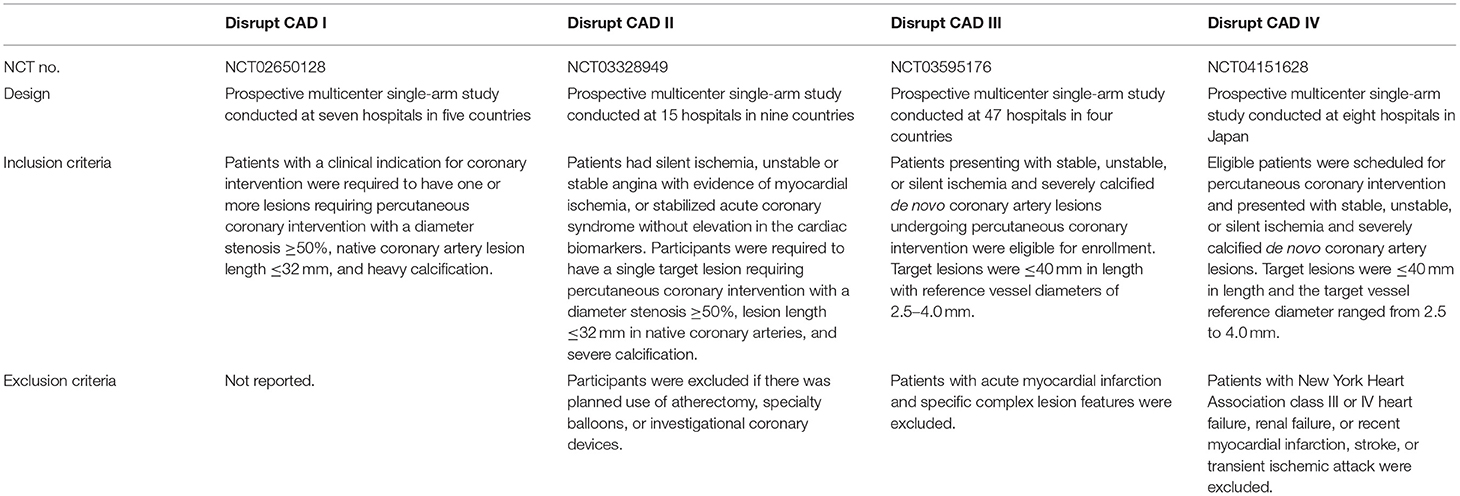
Four serial Disrupt CAD trials [Disrupt CAD I (11), Disrupt CAD II (12), Disrupt CAD III (13), and Disrupt CAD IV (14)] were included in our study. All of them were prospective, multicenter, single-arm studies and were conducted in multiple hospitals. Except for the Disrupt CAD IV, the others were all multi-country studies. The inclusion and exclusion criteria for the four studies are listed in Table 1.
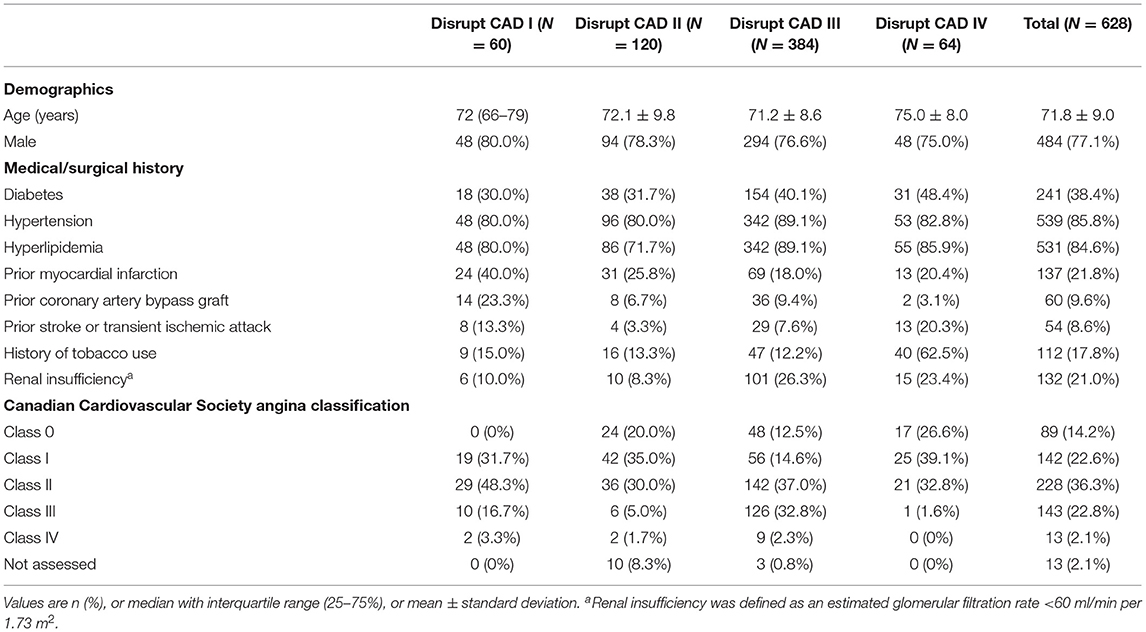
The studies included a total of 628 patients with a clinical indication for coronary intervention. The sample size in the trials ranged from 60 patients in the Disrupt CAD I to 384 patients in the Disrupt CAD III. The mean/median age of participants ranged from 70 to 75 years, with the Disrupt CAD IV trial including older patients. Males accounted for the majority of each study (range, 75.0–80.0%). The majority of the population included in our study had hypertension and hyperlipidemia; nearly half had diabetes and previous myocardial infarction; and a small number had previous coronary artery bypass graft, stroke or transient ischemic attack, history of smoking, and renal insufficiency (defined as an estimated glomerular filtration rate <60 ml/min per 1.73 m2). According to the angina classification of the Canadian Cardiovascular Society, most patients were in classes I and II (Table 2).
Lesion Characteristics
For lesion characteristics, the most frequently involved target vessel was the left anterior descending artery, followed by the circumflex artery. The reference vessel diameter was about 3 mm and the minimum lumen diameter was about 1 mm (Disrupt CAD IV did not report the minimum lumen diameter). The incidence of diameter stenosis was more than 60.0% in all trials. The lesion length and the calcified length of the Disrupt CAD III and Disrupt CAD IV were longer than those of the Disrupt CAD I and Disrupt CAD II. Except for 94.2% of severe calcification in the Disrupt CAD II, all other studies achieved 100%. In all studies, the side branch involvement rate was about 30% (Table 3).
Procedural Details
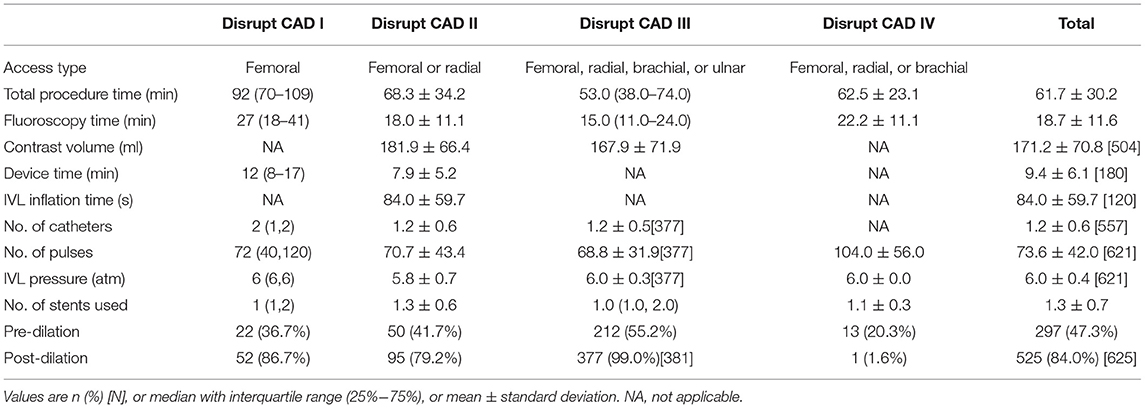
Regarding the total procedure time, the Disrupt CAD I took the longest, up to an hour and a half, and the rest took about an hour. Similarly, the Disrupt CAD I also had a longer fluoroscopy time than did others. The Disrupt CAD II and the Disrupt CAD III reported contrast volumes of 181.9 ± 66.4 and 167.9 ± 71.9 ml, respectively. Only the Disrupt CAD I and the Disrupt CAD II reported device times of 8 and 7.9 min, respectively. The Disrupt CAD II reported an IVL inflation time of 84.0 ± 59.7 s. The number of catheters was 1.2–2 (Disrupt CAD IV did not report). The number of pulses in the four trials ranged from 68.8 in the Disrupt CAD III to 104 in the Disrupt CAD IV, and IVL pressure was 6 atm. The number of stents used in IVL was about 1. The proportion of dilation has changed greatly: pre-dilation ranged from 20.3% in the Disrupt CAD IV and from 55.2% in the Disrupt CAD III, and post-dilation ranged from 1.6% in the Disrupt CAD IV and from 99.0% in the Disrupt CAD III (Table 4).
Safety Endpoint
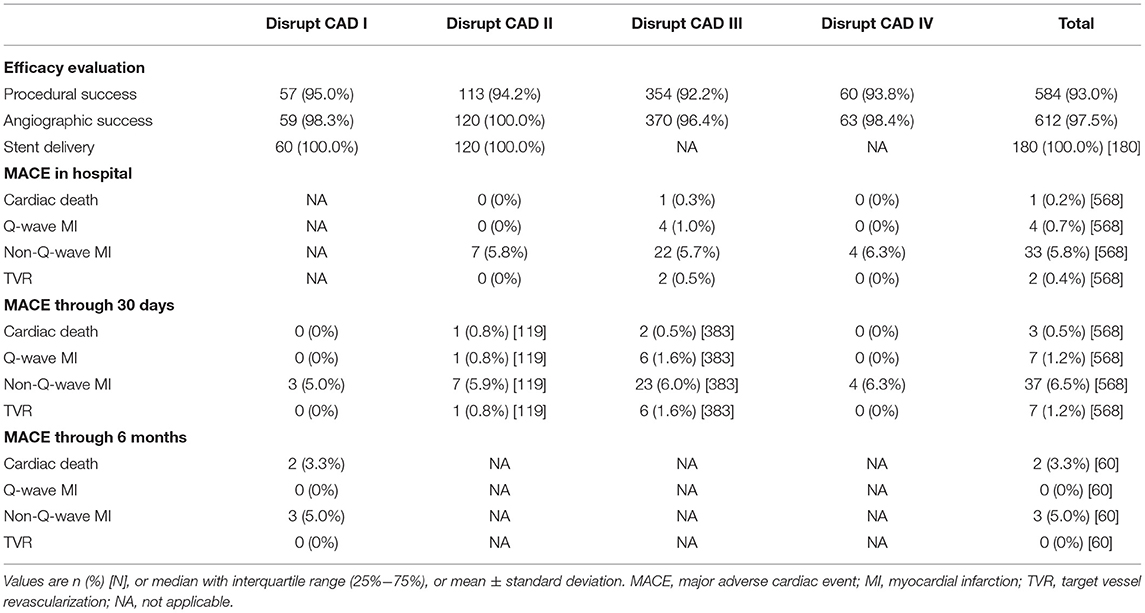
Different trials reported MACE at different times. The Disrupt CAD I reported MACE through 30 days and 6 months and found that there were three (5.0%) patients who had non-Q-wave MI at 30 days and that two (3.3%) patients had cardiac death and three (5.0%) patients had non-Q-wave MI at 6 months. The Disrupt CAD II, Disrupt CAD III, and Disrupt CAD IV reported MACE in hospital and at 30 days. In the Disrupt CAD II, there were seven non-Q-wave MI in hospital and 10 MACE (one cardiac death, one Q-wave MI, seven non-Q-wave MI, and one TVR) at 30 days. In the Disrupt CAD III, there were 29 MACE (one cardiac death, four Q-wave MI, 22 non-Q-wave MI, and two TVR) in hospital and 37 MACE (two cardiac death, six Q-wave MI, 23 non-Q-wave MI, and six TVR) at 30 days. In the Disrupt CAD IV, there were four MACE (four non-Q-wave MI) in hospital and four MACE (four non-Q-wave MI) at 30 days (Table 5).
Efficacy Endpoint
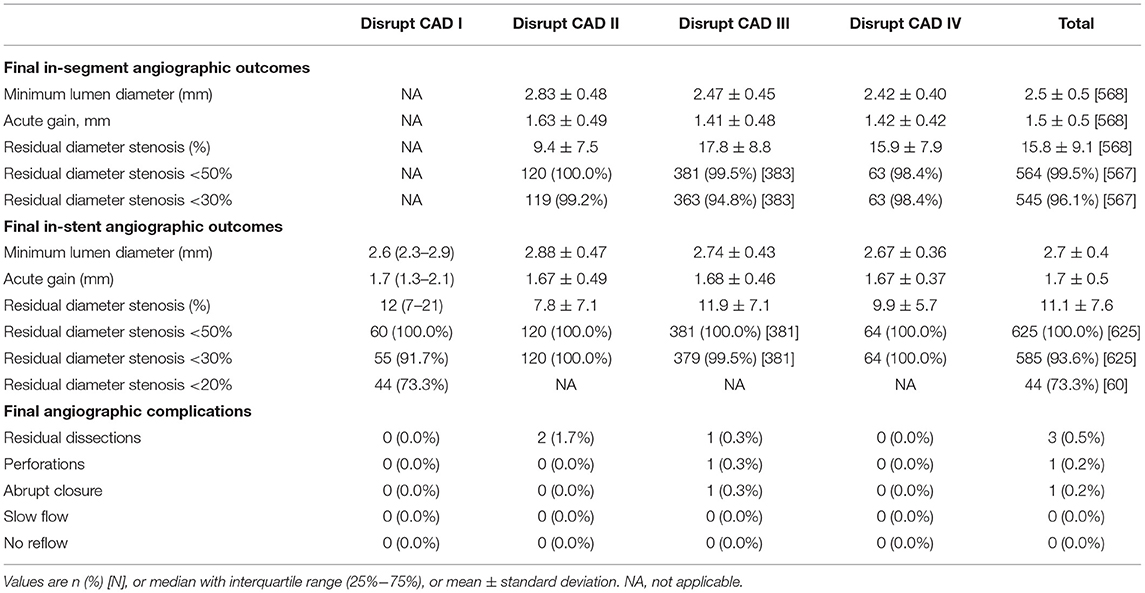
IVL showed good efficacy. The Disrupt CAD I, Disrupt CAD II, Disrupt CAD III, and Disrupt CAD IV achieved 95.0, 94.2, 92.2, and 93.8% procedural success, respectively. Moreover, the Disrupt CAD I, Disrupt CAD II, Disrupt CAD III, and Disrupt CAD IV achieved 98.3, 100, 96.4, and 98.4% angiographic success, respectively (Table 5). Angiographic outcomes included final in-segment angiographic outcomes, final in-stent angiographic outcomes, and final angiographic complications. The Disrupt CAD I did not report final in-segment angiographic outcomes. In the other three trials, the minimum lumen diameter ranged from 2.42 to 2.83 mm, acute gain ranged from 1.41 to 1.63 mm, and residual diameter stenosis ranged from 9.4% to 17.8% (more than 98.4% of patients obtained residual diameter stenosis <50 and 94.8% of patients obtained residual diameter stenosis <30%). All trials reported final in-stent angiographic outcomes. The minimum lumen diameter ranged from 2.60 to 2.88 mm, acute gain ranged from 1.67 to 1.70 mm, and residual diameter stenosis ranged from 7.8 to 12.0% (all patients obtained residual diameter stenosis <50% and more than 91.7% of patients obtained residual diameter stenosis <30%). The Disrupt CAD I and Disrupt CAD IV reported no final angiographic complications, whereas the Disrupt CAD II reported two residual dissections (one type B and one type C, respectively). The Disrupt CAD III reported three final angiographic complications [one residual dissection (types D–F), one perforation, and one abrupt closure] (Table 6).
OCT Measurements
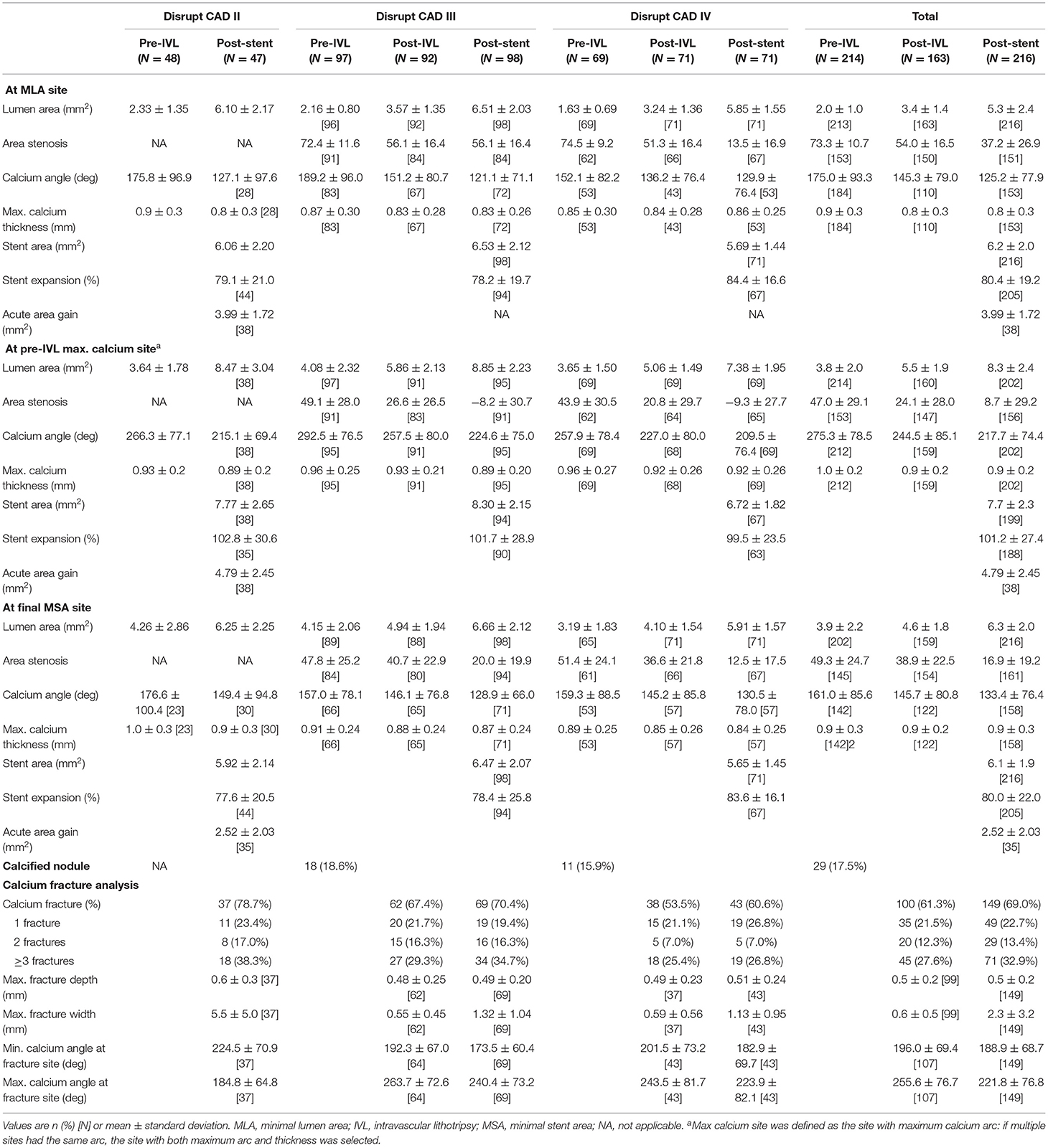
The Disrupt CAD II, Disrupt CAD III, and Disrupt CAD IV had OCT subgroup analysis.
Vessel preparation with IVL led to an increase in minimal luminal area from 2.33 ± 1.35 to 6.10 ± 2.17 mm2 in the Disrupt CAD II, from 2.16 ± 0.80 to 6.51 ± 2.03 mm2 in the Disrupt CAD III, and from 1.63 ± 0.69 to 5.85 ± 1.55 mm2 in the Disrupt CAD IV after drug-eluting stent (DES) implantation. The impact of IVL at the sites of the pre-IVL minimal luminal area, maximum calcium site, and final minimal stent area is shown in Table 7. IVL significantly increased the lumen area and decreased the calcium angle. Overall, calcium fracture was identified in 78.7% of lesions, with multiple fractures present in 55.3% in the Disrupt CAD II, in 70.4% of lesions with 51% multiple fractures in the Disrupt CAD III, and in 60.6% of lesions with 33.8% multiple fractures in the Disrupt CAD IV (Table 7). The maximum fracture depth and width and the maximum and minimum calcium angles at the fracture site are shown in Table 7.
Comparison With RA
We included ROTAXUS and PREPARE-CALC to pool the RA group, and the details of ROTAXUS and PREPARE-CALC are shown in Supplementary Table 1. We found more diabetes (38.4 vs. 30.0%), hyperlipidemia (84.6 vs. 72.3%), prior coronary artery bypass graft (9.6 vs. 6.8%), and patients with renal insufficiency (21.0 vs. 14.1%) in the Disrupt CAD group compared with the RA group (Supplementary Table 2). As for lesion characteristics, the target vessel involved more circumflex artery and right coronary artery and fewer protected left main artery and left anterior descending artery in the Disrupt CAD group than in the RA group (Supplementary Table 2). Moreover, since there were more severe calcification cases in the Disrupt CAD group (98.9 vs. 59.7%), the diameter stenosis was smaller (64.6 vs. 82.2%) than that in the RA group (Supplementary Table 2). Accordingly, the Disrupt CAD group had a shorter total procedure time (61.7 vs. 76.3 min), shorter fluoroscopy time (18.7 vs. 23.3 min), and smaller contrast volume (171.2 vs. 215.5 ml) than did the RA group (Supplementary Table 2). One case (0.2%) reported death and 37 cases (6.5%) reported MI among the 568 cases in the Disrupt CAD group, whereas two cases (0.9%) reported death and four cases (1.8%) reported MI among the 220 cases in the RA group. The procedural success, angiographic success, and stent delivery were 93.0, 97.5, and 100.0% in the Disrupt CAD group, and 95.0, 96.7, and 99.5% in the RA group, respectively (Supplementary Table 2). Angiographic outcomes are shown in Supplementary Table 2.
Discussion
The serial Disrupt CAD trials evaluated the utility of IVL for lesion preparation of severely calcified coronary stenoses prior to stent implantation. Several major findings were derived from the study results. Firstly, both the primary safety and efficacy endpoints were met among patients from different regions, including the USA, Europe, and Japan. Secondly, coronary IVL prior to stent implantation was well tolerated, with a low rate of major periprocedural clinical and angiographic complications. Thirdly, the rate of 30-day MACE was low. Moreover, IVL achieved acute luminal gains and residual stenosis. Finally, OCT imaging provided evidence that calcium fracture was the underlying mechanism of action for coronary IVL.
Coronary revascularization is common. Researchers analyzed the preprocedural, in-hospital, and long-term data from the Coronary Revascularization Demonstrating Outcome Registry (Kyoto, Japan) and the Texas Heart Institute Research Database (Houston, Texas) of 16,100 patients who had undergone elective, initial percutaneous coronary intervention or coronary artery bypass grafting to compare the differences in the clinical characteristics and long-term outcomes of patients in these two countries. They found that the two registries showed similar crude outcomes, but for important differences in patient risk factors such as obesity, in the adjusted analysis, the Japanese patients had better outcomes than did the USA patients (31). The findings in this study including the serial Disrupt CAD trials, which cover a wide range of crowds, suggest that, despite underlying ethnic differences in the risk factors and the differing prevalences and morphologies of coronary artery plaques, the clinical outcomes of vessel preparation using IVL prior to stent placement are consistent among ethnic groups. In fact, in addition to the serial Disrupt CAD trials, other studies are exploring the safety and applicability of IVL in severely calcified coronary stenoses, but most of them are case reports and experience reports (32–37), so we did not include these low-quality studies, which are bound to have some impact on our conclusions.
IVL offers a novel option for severely calcified coronary stenoses. It is unique among all technologies due to its ability to modify calcium circumferentially and transmurally, which is provided by a diffuse acoustic pulse delivered through a low-pressure balloon as opposed to other devices that induce mechanical tissue injury, thus modifying transmural conduit compliance (2). The resulting potential clinical benefits of IVL include uniform plaque modification in which the fractured calcium remains in situ with no microcirculation embolization, thereby safely facilitating stent apposition and expansion (38). Previous long-term follow-up studies, such as ORBIT II (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions) (39) and COAST (Coronary Orbital Atherectomy System Study) (40), have confirmed that the incidence of MACE increases with the extension of the follow-up time. Here, we highlight its best clinical application through appropriate patient and lesion selection, with the main objective of optimizing stent delivery and implantation and, subsequently, improved outcomes. In view of the design of the serial Disrupt CAD trials, except for the Disrupt CAD I, the follow-up time was 6 months; the follow-up time of other studies was 30 days. In other words, we can only evaluate the immediate and short-term clinical utility, but not the long-term benefits of IVL. Secondly, IVL obviates the need for more complex lesion preparation strategies such as RA, except in severe undeletable cases where IVL is impossible (33). The serial Disrupt CAD trials are of a single-arm design, so we could not obtain data on the comparison between or the combination of IVL and RA or conventional balloon angioplasty (cutting or drug-coated balloons). Therefore, we introduced ROTAXUS and PREPARE-CALC to critically describe the basic information of these trials and the applicability and safety of IVL and RA. The previous retrospective study confirmed the high rate of procedural success and the low incidence of target lesion revascularization and MACE of RA in the European population (41), which are consistent with what we report here. Unfortunately, nearly one-third of the patients enrolled in ROTAXUS experienced MACE within a 2-year follow-up, with no differences between the patients treated with or without RA (42). However, because of the design and the statistical methods of these trials, we cannot directly compare IVL and RA. It is urgent and warranted to design high-quality trials to further directly compare the safety and efficacy between IVL and other methods. Thirdly, previous results have shown that radial access could reduce the hemorrhagic events and mortality compared to transfemoral access (43). In the Disrupt CAD I, PCI was all performed via femoral access; only femoral access was obtained in the Disrupt CAD II, whereas both femoral and radial access were obtained in the Disrupt CAD III and Disrupt CAD IV. Because the outcome indicators of the different approaches cannot be obtained alone, we regret that we cannot verify the superiority of the radial artery approach in prognosis. But it should be noted that femoral access is feasible in emergency, complications, or inability to use the radial access (43). Moreover, pivotal trials in acute coronary syndromes over the past several years have led to the introduction of novel antiplatelet agents, (44) such as prasugrel (45) and ticagrelor (46). The impact of these new agents on the complications of IVL is unknown and merits study. Finally, so far, no study has reported on the cost-effectiveness of IVL, which is necessary to be considered before clinical application.
Conclusions
Ultimately, IVL is an efficient vessel preparation strategy in the presence of a heavy coronary calcium burden, and these results appear to be consistent regardless of ethnicity or geography. Moreover, calcium fracture facilitated increased vessel compliance and a favorable stent expansion. In addition, the impact of this technology on the long-term prognosis of patients with severe calcification is also the focus of attention and expectation. More importantly, the advantage of IVL over the other methods in this particular population is still unknown. Enhancing the comparison of IVL would help guide the therapeutic decisions in these patients. We hope that one day this technology can eventually replace the other coronary calcification treatment technologies currently used in clinical practice. By then, we will have a safe, efficient, and simple treatment method to treat severe calcification lesions accurately, rapidly, and efficiently.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
BL and NG conceived, designed, or planned the idea. All authors acquired, analyzed and interpreted the data. BL drafted the manuscript. NG revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (81774229), the Research and Practice Innovation Plan for Postgraduates in Jiangsu, China (KYCX21_1641), Jiangsu Leading Talent Project of Traditional Chinese Medicine (Jiangsu TCM 2018 no. 4), and Jiangsu Universities Nursing Advantage Discipline Project (2019YSHL095).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all scientists and participants involved in IVL and severely calcified coronary stenoses.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.724481/full#supplementary-material
Abbreviations
CAC, coronary artery calcification; CAD, coronary artery disease; CK-MB, creatine kinase MB isoform; COAST, Coronary Orbital Atherectomy System Study; CTCA, computed tomography coronary angiography; IVUS, intravascular ultrasound; IVL, intravascular lithotripsy; MACE, major adverse cardiovascular events; MI, myocardial infarction; OCT, optical coherence tomography; ORBIT II, Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions; PCI, percutaneous coronary intervention; PREPARE-CALC, Comparison of Strategies to Prepare Severely Calcified Coronary Lesions; RA, rotational atherectomy; ROTAXUS, Rotational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease; TVR, target vessel revascularization.
References
1. Liu W, Zhang Y, Yu C-M, Ji Q-W, Cai M, Zhao Y-X, et al. Current understanding of coronary artery calcification. J Geriatr Cardiol. (2015) 12:668–75. doi: 10.11909/j.issn.1671-5411.2015.06.012
2. Kassimis G, Didagelos M, De Maria GL, Kontogiannis N, Karamasis GV, Katsikis A, et al. Shockwave intravascular lithotripsy for the treatment of severe vascular calcification. Angiology. (2020) 71:677–88. doi: 10.1177/0003319720932455
3. Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. (2013) 6:10–9. doi: 10.1016/j.jcin.2012.07.017
4. Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv. (2014) 7:345–53. doi: 10.1016/j.jcin.2013.12.196
5. Barbato E, Carrié D, Dardas P, Fajadet J, Gaul G, Haude M, et al. European expert consensus on rotational atherectomy. EuroIntervention. (2015) 11:30–6. doi: 10.4244/EIJV11I1A6
6. Abdel-Wahab M, Toelg R, Byrne RA, Geist V, El-Mawardy M, Allali A, et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. (2018) 11:e007415. doi: 10.1161/CIRCINTERVENTIONS.118.007415
7. Iannopollo G, Gallo F, Mangieri A, Laricchia A, Erriquez A, Tzanis G, et al. Tips and tricks for rotational atherectomy. J Invasive Cardiol. (2019) 31:E376–E83.
8. Cavusoglu E, Kini AS, Marmur JD, Sharma SK. Current status of rotational atherectomy. Cathet Cardiovasc Interv. (2004) 62:485–98. doi: 10.1002/ccd.20081
9. Sharma SK, Tomey MI, Teirstein PS, Kini AS, Reitman AB, Lee AC, et al. North American expert review of rotational atherectomy. Circ Cardiovasc Interv. (2019) 12:e007448. doi: 10.1161/CIRCINTERVENTIONS.118.007448
10. Yeoh J, Hill J. Intracoronary lithotripsy for the treatment of calcified plaque. Interv Cardiol Clin. (2019) 8:411–24. doi: 10.1016/j.iccl.2019.06.004
11. Brinton TJ, Ali ZA, Hill JM, Meredith IT, Maehara A, Illindala U. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. (2019) 139:834–6. doi: 10.1161/CIRCULATIONAHA.118.036531
12. Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. (2019) 12:e008434. doi: 10.1161/CIRCINTERVENTIONS.119.008434
13. Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJ, et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol. (2020) 76:2635–46. doi: 10.1016/j.jacc.2020.09.603
14. Saito S, Yamazaki S, Takahashi A, Namiki A, Kawasaki T, Otsuji S, et al. Intravascular lithotripsy for vessel preparation in severely calcified coronary arteries prior to stent placement - primary outcomes from the japanese disrupt CAD IV study. Circ J. (2021) 85:826–33. doi: 10.1253/circj.CJ-20-1174
15. Shi J, Luo D, Wan X, Liu Y, Liu J, Bian Z, et al. Detecting the skewness of data from the sample size and the five-number summary. arXiv [preprint]. arXiv:2010.05749 (2020).
16. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
17. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
18. Liang B, Gu N. Liraglutide in the treatment of heart failure: insight from FIGHT and LIVE. Cardiovasc Diabetol. (2020) 19:106. doi: 10.1186/s12933-020-01088-3
19. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. (2018) 137:2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289
20. Kereiakes DJ, Hill JM, Ben-Yehuda O, Maehara A, Alexander B, Stone GW. Evaluation of safety and efficacy of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: design and rationale for the Disrupt CAD III trial. Am Heart J. (2020) 225:10–8. doi: 10.1016/j.ahj.2020.04.005
21. Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. (2014) 7:510–8. doi: 10.1016/j.jcin.2014.01.158
22. Cesaro A, Gragnano F, Di Girolamo D, Moscarella E, Diana V, Pariggiano I, et al. Functional assessment of coronary stenosis: an overview of available techniques. Is quantitative flow ratio a step to the future? Expert Rev Cardiovasc Ther. (2018) 16:951–62. doi: 10.1080/14779072.2018.1540303
23. Moscarella E, Gragnano F, Cesaro A, Ielasi A, Diana V, Conte M, et al. Coronary physiology assessment for the diagnosis and treatment of coronary artery disease. Cardiol Clin. (2020) 38:575–88. doi: 10.1016/j.ccl.2020.07.003
24. Westra J, Sejr-Hansen M, Kołtowski Ł, Mejía-Rentería H, Tu S, Kochman J, et al. Reproducibility of quantitative flow ratio: the QREP study. EuroIntervention. (2021). doi: 10.4244/eij-d-21-00425. [Epub ahead of print].
25. Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. (2004) 16:493–9.
26. Eshtehardi P, Adorjan P, Togni M, Tevaearai H, Vogel R, Seiler C, et al. Iatrogenic left main coronary artery dissection: Incidence, classification, management, and long-term follow-up. Am Heart J. (2010) 159:1147–53. doi: 10.1016/j.ahj.2010.03.012
27. Goldstein JA, Casserly IP, Katsiyiannis WT, Lasala JM, Taniuchi M. Aortocoronary dissection complicating a percutaneous coronary intervention. J Invasive Cardiol. (2003) 15:89–92.
28. Kobayashi N, Ito Y, Yamawaki M, Araki M, Obokata M, Sakamoto Y, et al. Optical coherence tomography-guided versus intravascular ultrasound-guided rotational atherectomy in patients with calcified coronary lesions. EuroIntervention. (2020) 16:e313–e21. doi: 10.4244/EIJ-D-19-00725
29. Kereiakes DJ, Virmani R, Hokama JY, Illindala U, Mena-Hurtado C, Holden A, et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Intervent. (2021) 14:1275–92. doi: 10.1016/j.jcin.2021.03.036
30. Karimi Galougahi K, Patel S, Shlofmitz RA, Maehara A, Kereiakes DJ, Hill JM, et al. Calcific plaque modification by acoustic shock waves: intravascular lithotripsy in coronary interventions. Circ Cardiov Intervent. (2021) 14:e009354. doi: 10.1161/CIRCINTERVENTIONS.120.009354
31. Kohsaka S, Kimura T, Goto M, Lee V-V, Elayda M, Furukawa Y, et al. Difference in patient profiles and outcomes in Japanese versus American patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the Texas Heart Institute Research Database). Am J Cardiol. (2010) 105:1698–704. doi: 10.1016/j.amjcard.2010.01.349
32. Aksoy A, Salazar C, Becher MU, Tiyerili V, Weber M, Jansen F, et al. Intravascular lithotripsy in calcified coronary lesions: a prospective, observational, multicenter registry. Circ Cardiov Intervent. (2019) 12:e008154. doi: 10.1161/CIRCINTERVENTIONS.119.008154
33. Wong B, El-Jack S, Newcombe R, Glenie T, Armstrong G, Khan A. Shockwave intravascular lithotripsy for calcified coronary lesions: first real-world experience. J Invasive Cardiol. (2019) 31:46–8. doi: 10.1016/j.hlc.2019.05.022
34. Vadalà G, Galassi AR, Nerla R, Micari A. Shockwave intravascular lithoplasty for the treatment of calcified carotid artery stenosis: A very early single-center experience. Cathet Cardiov Intervent. (2020) 96:E608–E13. doi: 10.1002/ccd.28963
35. Ocaranza-Sánchez R, Abellás-Sequeiros RA, Santás-Álvarez M, Bayón-Lorenzo J, Gonzalez-Juanatey C. First-in-man reported 12-months follow-up after intravascular lithotripsy in left main percutaneous revascularization. Coron Artery Dis. (2021). doi: 10.1097/MCA.0000000000001003. [Epub ahead of print].
36. Legutko J, Niewiara Ł, Tomala M, Zajdel W, Durak M, Tomaszewski P, et al. Successful shockwave intravascular lithotripsy for a severely calcified and undilatable left anterior descending coronary artery lesion in a patient with recurrent myocardial infarction. Kardiol Pol. (2019) 77:723–5. doi: 10.33963/KP.14859
37. Yeoh J, Hill J, Spratt JC. Intravascular lithotripsy assisted chronic total occlusion revascularization with reverse controlled antegrade retrograde tracking. Cathet Cardiovasc Interv. (2019) 93:1295–7. doi: 10.1002/ccd.28165
38. Ali ZA, Brinton TJ, Hill JM, Maehara A, Matsumura M, Karimi Galougahi K, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. (2017) 10:897–906. doi: 10.1016/j.jcmg.2017.05.012
39. Lee M, Généreux P, Shlofmitz R, Phillipson D, Anose BM, Martinsen BJ, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. (2017) 18:261–4. doi: 10.1016/j.carrev.2017.01.011
40. Redfors B, Sharma SK, Saito S, Kini AS, Lee AC, Moses JW, et al. Novel micro crown orbital atherectomy for severe lesion calcification: coronary orbital atherectomy system study (COAST). Circ Cardiovasc Intervent. (2020) 13:e008993. doi: 10.1161/CIRCINTERVENTIONS.120.008993
41. Abdel-Wahab M, Baev R, Dieker P, Kassner G, Khattab AA, Toelg R, et al. Long-term clinical outcome of rotational atherectomy followed by drug-eluting stent implantation in complex calcified coronary lesions. Catheterization Cardiovasc Intervent. (2013) 81:285–91. doi: 10.1002/ccd.24367
42. de Waha S, Allali A, Büttner H-J, Toelg R, Geist V, Neumann F-J, et al. Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: Two-year clinical outcome of the randomized ROTAXUS trial. Catheter Cardiovasc Interv. (2016) 87:691–700. doi: 10.1002/ccd.26290
43. Cesaro A, Moscarella E, Gragnano F, Perrotta R, Diana V, Pariggiano I, et al. Transradial access versus transfemoral access: a comparison of outcomes and efficacy in reducing hemorrhagic events. Expert Rev Cardiovasc Ther. (2019) 17:435–47. doi: 10.1080/14779072.2019.1627873
44. Valina C, Neumann F-J, Menichelli M, Mayer K, Wöhrle J, Bernlochner I, et al. Ticagrelor or prasugrel in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. (2020) 76:2436–46. doi: 10.1016/j.jacc.2020.09.584
45. Aytekin A, Ndrepepa G, Neumann F-J, Menichelli M, Mayer K, Wöhrle J, et al. Ticagrelor or prasugrel in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. (2020) 142:2329–37. doi: 10.1161/CIRCULATIONAHA.120.050244
46. Coughlan JJ, Aytekin A, Lahu S, Ndrepepa G, Menichelli M, Mayer K, et al. Ticagrelor or prasugrel for patients with acute coronary syndrome treated with percutaneous coronary intervention: a prespecified subgroup analysis of a randomized clinical trial. JAMA Cardiol. (2021). doi: 10.1001/jamacardio.2021.2228. [Epub ahead of print].
Keywords: coronary intravascular lithotripsy, severely calcified coronary stenoses, optical coherence tomography, Disrupt CAD, cardiology (clinical)
Citation: Liang B and Gu N (2021) Evaluation of the Safety and Efficacy of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: Evidence From the Serial Disrupt CAD Trials. Front. Cardiovasc. Med. 8:724481. doi: 10.3389/fcvm.2021.724481
Received: 13 June 2021; Accepted: 19 July 2021;
Published: 19 August 2021.
Edited by:
Angelo Silverio, University of Salerno, ItalyReviewed by:
Arturo Cesaro, University of Campania Luigi Vanvitelli, ItalyMichele Bellino, University of Salerno, Italy
Copyright © 2021 Liang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Gu, Z3VuaW5nQG5qdWNtLmVkdS5jbg==
†ORCID: Bo Liang orcid.org/0000-0002-1749-6976
Ning Gu orcid.org/0000-0003-0704-6768
 Bo Liang
Bo Liang Ning Gu2*†
Ning Gu2*†