- Department of Cardiology, Tianjin Chest Hospital, Tianjin, China
Background and Aims: Studies have highlighted the role of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio on subsequent cardiovascular events. However, the association of the TG/HDL-C ratio with survival outcomes in diabetic patients with coronary artery disease (CAD) treated with statins remains unknown. This study aimed to assess the predictive value of the TG/HDL-C ratio for all-cause mortality and cardiovascular death in diabetic patients with CAD treated with statins.
Methods: The data of patients with type 2 diabetes and angiographically-confirmed CAD who were undergoing statin therapy and visited Tianjin Chest Hospital between January 2016 and September 2016 were retrospectively collected. The patients were categorized based on the baseline TG/HDL-C ratio tertile. Kaplan-Meier analysis and multivariate Cox proportional hazard regression were applied to assess the role of the TG/HDL-C ratio in predicting all-cause mortality and cardiovascular death.
Results: A total of 2,080 patients were included. During the 4-year follow-up, 209 patients died, 136 of whom from cardiovascular death. The Kaplan-Meier analyses showed that an increased TG/HDL-C ratio was associated with an increased risk of all-cause mortality (P < 0.001) and cardiovascular death (P < 0.001). The multivariate cox hazard regression analysis revealed a similar effect of the TG/HDL-C ratio on the risk of all-cause mortality (P = 0.046) and cardiovascular death (P = 0.009). The role of the TG/HDL-C ratio in predicting all-cause mortality and cardiovascular death was similar among all subgroups (P > 0.050). For all-cause mortality, the TG/HDL-C ratio significantly improved the C-statistic from 0.799 to 0.812 (P = 0.018), and the net reclassification index (NRI) and integrated discrimination index (IDI) were 0.252 (95% CI: 0.112–0.392; P < 0.001) and 0.012 (95% CI: 0.003–0.022; P = 0.012), respectively. Similarly, for cardiovascular death, the TG/HDL-C ratio significantly improved the C-statistic from 0.771 to 0.804 (P < 0.001), and the NRI and IDI were 0.508 (95% CI: 0.335–0.680; P < 0.001) and 0.033 (95% CI: 0.015–0.050; P < 0.001).
Conclusion: TG/HDL-C ratio might be useful for predicting all-cause mortality and cardiovascular death in diabetic patients with CAD treated with statins.
Introduction
The role of diabetes mellitus (DM) on subsequent coronary artery disease (CAD) is well-illustrated (1), and studies have demonstrated that the use of statins could reduce the risk of major cardiovascular events (MACEs) in diabetic patients (2–5). However, patients with CAD have a higher prevalence of type 2 DM, and the risk of mortality remains high even in those treated with statins. The residual risk could be attributed to abnormal lipoprotein and lipid levels (6). Therefore, it is necessary that the lipid status be re-evaluated in diabetic patients with CAD treated with statins to identify those with higher residual risk such that tailored risk reduction strategies can be developed.
Dyslipidemia is characterized by elevated triglyceride (TG) and reduced dense high-density lipoprotein cholesterol particles levels, and lower high-density lipoprotein cholesterol (HDL-C) levels in diabetic patients (7, 8). Elevated TG and lower HDL-C are associated with poor prognosis in diabetic patients (9–12), but the use TG or HDL-C alone does not reflect the risk of atherosclerosis and cardiovascular disease (CVD) (13). The TG/HDL-C ratio may reflect the actual lipid profiles, and is considered an important marker of plasma atherosclerosis (14). Moreover, studies found that the TG/HDL-C ratio was an important predictor of insulin resistance and could evaluate the degree of abnormal glucose metabolism (15–17).
Numerous studies have reported a positive relationship between the TG/HDL-C ratio and hypertension (18–20), obesity (21), metabolic syndrome (22–24), hyperuricemia (25), and non-alcoholic fatty liver disease (26, 27). Moreover, an elevated TG/HDL-C ratio plays an important role on heart rate recovery after exercise (28), increased arterial stiffness (29, 30) and increased carotid atherosclerosis (31). Studies have indicated that the TG/HDL-C ratio should be considered as an important primary prevention cardiovascular risk factor, while the strength of the predictive value differs for patients undergoing various status (32–43). Furthermore, the predictive value of the TG/HDL-C ratio for all-cause mortality and cardiovascular death in diabetic patients with CAD treated with statins is unknown. This retrospective cohort study was therefore performed to assess the potential role of the TG/HDL-C ratio in the prediction of all-cause mortality and cardiovascular death in diabetic patients with CAD who were treated with statins.
Methods
Study Population
Patients who were admitted to Tianjin Chest Hospital between January 2016 and September 2016 were recruited in this retrospective cohort study. A total of 2,678 patients with T2DM and angiographically-confirmed CAD were included. CAD comprised stable angina pectoris (SAP) and acute coronary syndrome (ACS). ACS included unstable angina pectoris, non-ST-segment elevation myocardial infarction, and ST-segment elevation myocardial infarction (STEMI). Patients were excluded if they met any of the following criteria: (1) aged < 18.0 or >80.0 years (n = 72), (2) severe valvular heart disease or congenital heart disease (n = 34), (3) alanine aminotransferase level > 3-fold greater than the normal upper limit (n = 15), (4) serum creatinine level > 1.5-fold greater than the normal upper limit (n = 96), (5) hyperthyroidism or hypothyroidism (n = 16), (6) incomplete clinical data (n = 75), and (7) not treated with statins (n = 99). The remaining 2,271 patients were recruited, and 2,080 patients with full clinical data after 4-year follow-up were included in the final analysis. The patients were categorized based on the tertiles of the baseline TG/HDL-C ratio, as follows: tertile 1 (n = 693, TG/HDL-C ratio ≤ 1.20), tertile 2 (n = 693, 1.20 < TG/HDL-C ratio ≤ 1.92), and tertile 3 (n = 694, TG/HDL-C ratio > 1.92). The study was approved by the Ethical Committee of Tianjin Chest Hospital (NO:2021LW-006), and the need to obtain informed consent requirement was waived as the study comprised a retrospective analysis of clinical data.
Data Collection and Definitions
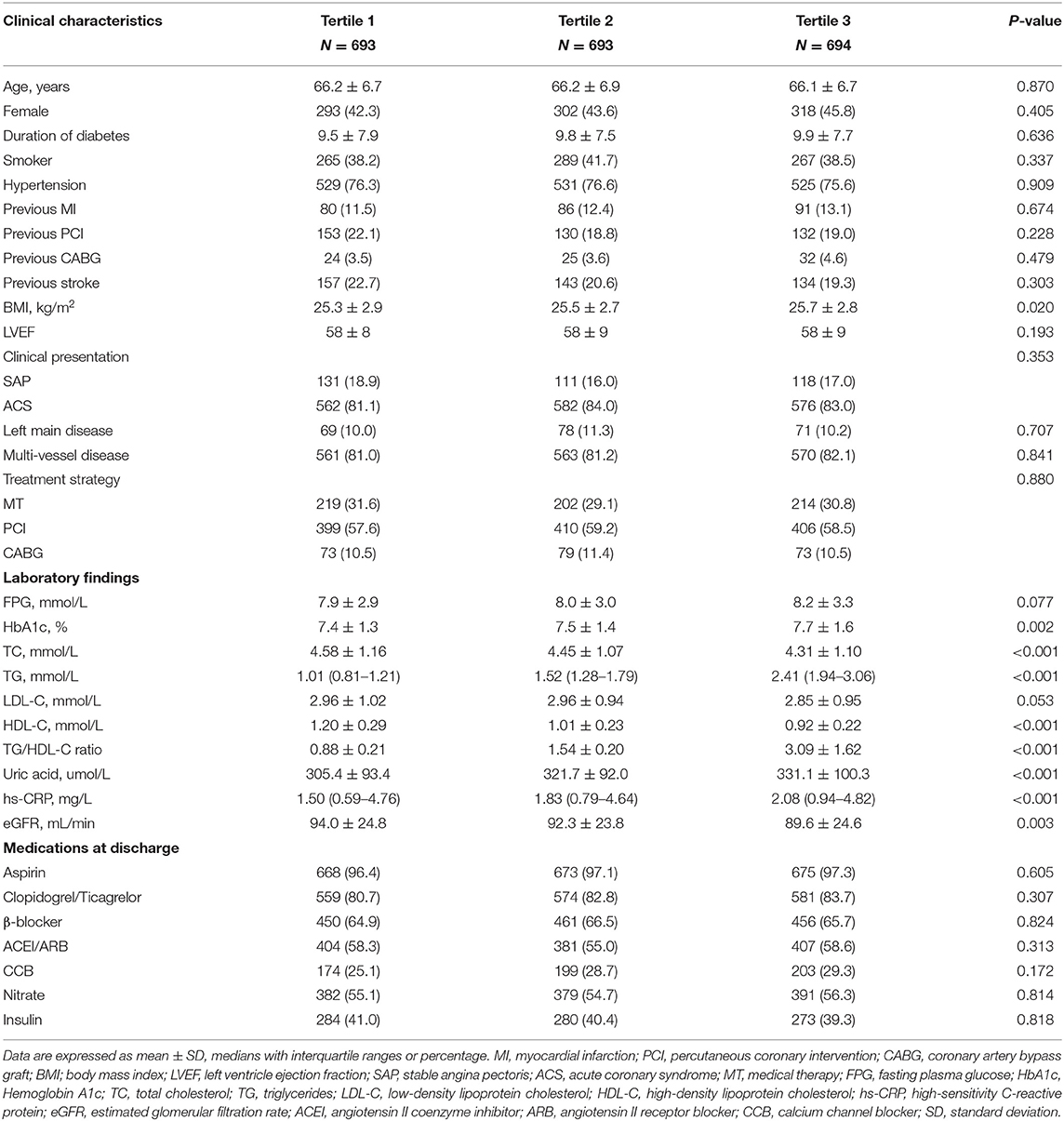
Baseline demographic characteristics, clinical presentation, cardiac function, extent of lesion, treatment strategy, laboratory findings at fasting status, and medication data at discharge were collected from medical records and the data managers were blinded to the study purpose. The demographic characteristics included age; sex ratio; duration of diabetes; smoker proportion; hypertension; prior myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), or stroke; and body mass index (BMI). The cardiac function included left ventricle ejection fraction (LVEF). The clinical presentation included SAP and ACS, and the extent of lesion included left main disease and multi-vessel disease (>2 vessels with ≥50% diameter stenosis in major coronary arteries). The treatment strategies included medical therapy, PCI, and CABG. Laboratory findings included fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), HDL-C, the TG/HDL-C ratio, serum uric acid, high-sensitivity C-reactive protein (hs-CRP), and estimated glomerular filtration rate (eGFR). The medications at discharge included aspirin, clopidogrel/ticagrelor, β-blocker, angiotensin II coenzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), calcium channel blocker (CCB), nitrate, and insulin.
Endpoints and Follow-Up Data
The investigated endpoints included all-cause mortality and cardiovascular death. All-cause mortality was defined as death from any cause, and cardiovascular death was defined as death caused by acute MI, heart failure, cardiac arrhythmia, or stroke. The follow-up information was collected by telephone or electronic medical record review.
Statistical Analysis
Continuous variables are presented as the mean [standard deviation (SD)] and median (interquartile) based on data distribution, and the differences among groups were compared using an analysis of variance or the Kruskal-Wallis test. Categorical variables are presented as frequencies and proportions, and the differences among groups were compared using the Chi-square or Fisher's exact tests. The association between the TG/HDL-C ratio and subsequent all-cause mortality and cardiovascular death were assessed using Kaplan-Meier analysis and the log-rank test. Multivariate Cox regression analysis was performed to identify the independent predictors of all-cause mortality and cardiovascular death. All the variables in Table 1 were listed in univariate model and then were introduced into the multivariate model if the P-value was <0.10. The possible factors included age, duration of diabetes, hypertension, previous MI, previous PCI, previous stroke, LVEF, left main disease, multi-vessel disease, FPG, TC, LDL-C, uric acid, hs-CRP, and eGFR. Sensitivity analyses were performed for all-cause mortality and cardiovascular death by sequential adjustment of potential confounders. The C-statistics, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were applied to assess the incremental predictive value of the TG/HDL-C ratio over the established model (including age, duration of diabetes, previous PCI, LVEF, left main disease, multi-vessel disease, FPG, and eGFR). The optimal cut-off values of the TG/HDL-C ratio for predicting all-cause mortality and cardiovascular death were determined using receiver operating characteristic (ROC) curves. Subgroup analyses for all-cause mortality and cardiovascular death were conducted according to sex (male or female), smoker (yes or no), BMI (≤ 28 or >28 kg/m2), duration of DM (≤ 10 or >10 years), ACS (yes or no), HbA1c (≤ 7.0 or >7.0%), LDL-C (≤ 1.8 or >1.8 mmol/L), insulin treatment (yes or no), and revascularization (yes or no). The differences between subgroup analyses were also compared using the interaction t-test. All P-values are two-sided, and the inspection level was 0.050. The statistical analyses in this study were performed using SPSS version 20.0 (IBM Corp, Armonk, New York) and SAS version 9.1.3 (Cary, NC, USA).
Results
Baseline Characteristics
A total of 2,080 diabetic patients with CAD who were treated with statins were selected for analysis. The baseline characteristics of the patients in the three TG/HDL-C ratio categories are summarized in Table 1. Most variables did not significantly differ among the groups, including age; sex ratio; duration of diabetes; smoker proportion; hypertension; prior MI, PCI, CABG, or stroke; LVEF; clinical presentation; left main disease; multi-vessel disease; treatment strategy; FPG; LDL-C; aspirin; clopidogrel/ticagrelor; β-blocker; ACEI or ARB; CCB; nitrate; and insulin (P > 0.050). However, there were significant differences among the three groups in BMI (P = 0.020), HbA1c (P = 0.002), TC (P < 0.001), TG (P < 0.001), HDL-C (P < 0.001), TG/HDL-C ratio (P < 0.001), serum uric acid (P < 0.001), hs-CRP (P < 0.001), and eGFR (P = 0.003).
TG/HDL-C Ratio and All-Cause Mortality
A total of 209 patients died during the 4-year follow-up, and the proportions of all-cause mortality in tertiles 1, 2, and 3 were 6.6, 10.1, and 13.4%, respectively. Kaplan-Meier analysis indicated that an increased TG/HDL-C ratio was associated with an increased risk of all-cause mortality (P < 0.001; Figure 1). The Cox proportional hazard regression indicated that an increased TG/HDL-C ratio tertile was associated with an increased risk of all-cause mortality, irrespective of whether the unadjusted (P < 0.001) or adjusted (P = 0.046) was used. Moreover, per SD increment in the TG/HDL-C ratio was associated with an increased risk of all-cause mortality in both the unadjusted model (HR: 1.17; 95% CI: 1.10–1.24; P < 0.001) and the adjusted model (HR: 1.20; 95% CI: 1.11–1.30; P < 0.001) (Table 2). The role of the TG/HDL-C ratio in predicting the risk of all-cause mortality was robust after sequential adjustment for potential confounders (Table 3).
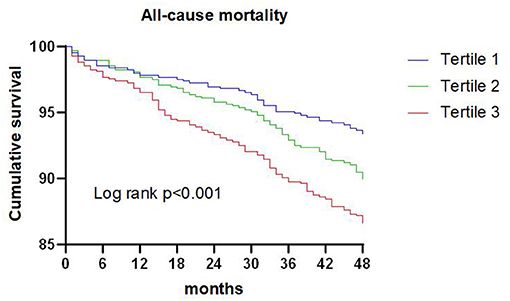
Figure 1. Kaplan-Meier survival curve for all-cause mortality across triglyceride to high density lipoprotein-C ratio tertiles.
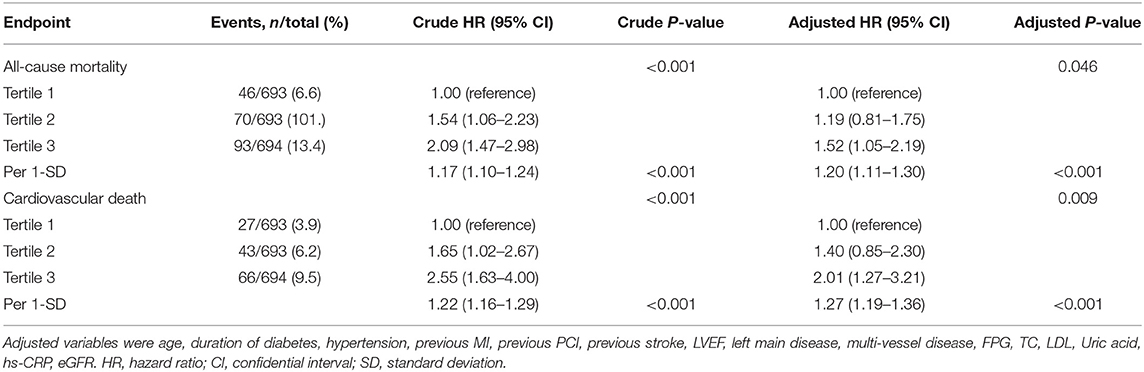
Table 2. Cox regression models in the prediction of all-cause mortality and cardiovascular death according to the triglyceride to high density lipoprotein-C ratio at baseline.
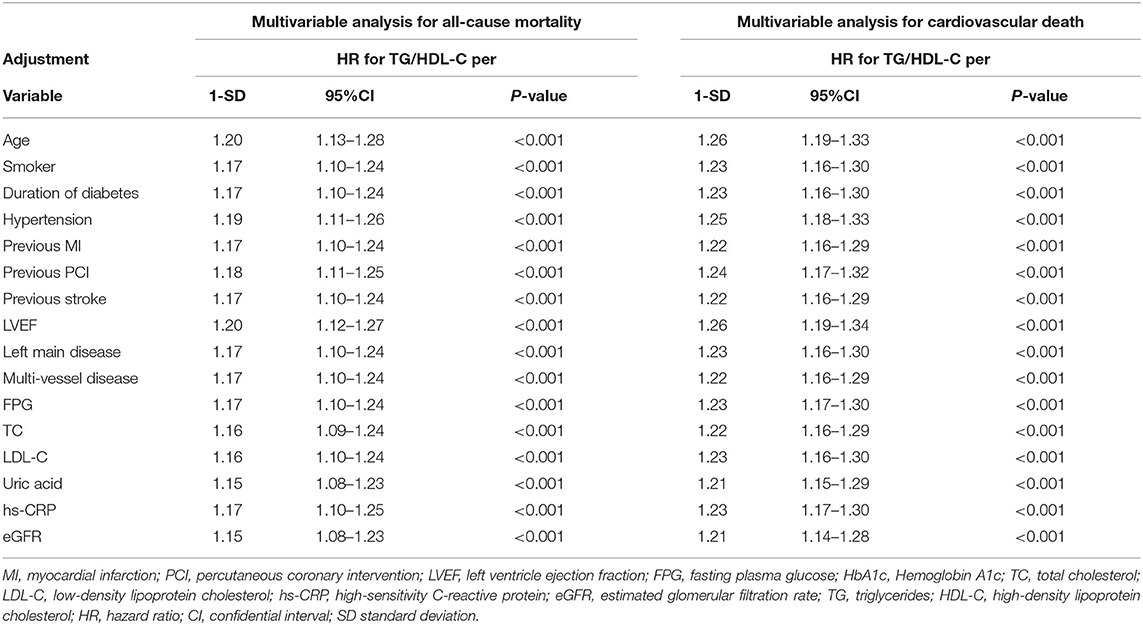
Table 3. Sensitivity analysis of the association of the triglyceride to high density lipoprotein-C ratio per 1 standard deviation with mortality after separate adjustment for each of the other significant variables.
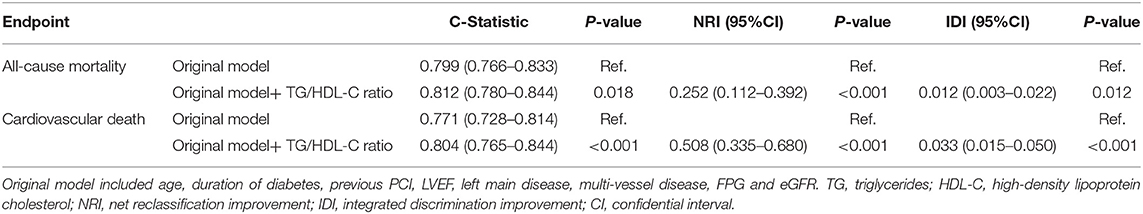
ROC analysis indicated that the optimal cutoff value of the TG/HDL-C ratio for predicting all-cause mortality was 1.77 (sensitivity: 53.1% and specificity: 62.8%), and the area under the curve (AUC) was 0.601 (95% CI: 0.561–0.640; P < 0.001). Adding the TG/HDL-C ratio to the model of established risk factors including age, duration of diabetes, previous PCI, LVEF, left main disease, multi-vessel disease, FBG, and eGFR improved the prediction of all-cause mortality in terms of the C-statistic (from 0.799 to 0.812; P = 0.018), and the NRI and IDI were 0.252 (95% CI: 0.112–0.392; P < 0.001) and 0.012 (95% CI: 0.003–0.022; P = 0.012), respectively (Table 4).
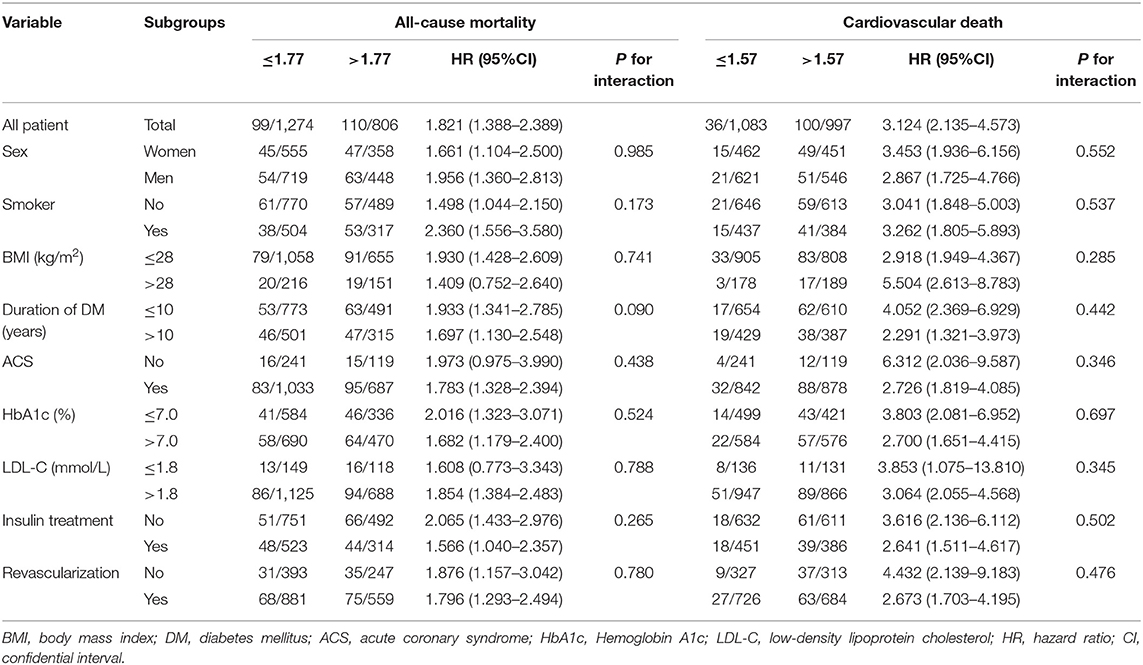
The results of subgroup analyses for all-cause mortality are illustrated in Table 5. An elevated TG/HDL-C ratio was associated with an increased risk of all-cause mortality in all subgroups, and the differences between subgroups were not significant based on sex (P = 0.985), smoker (P = 0.173), BMI (P = 0.741), duration of DM (P = 0.090), ACS (P = 0.438), HbA1c (P = 0.524), LDL-C (P = 0.788), insulin treatment (P = 0.265), and revascularization (P = 0.780).
TG/HDL-C Ratio and Cardiovascular Death
A total of 136 patients died from cardiovascular death during the 4-year follow-up, and the proportion of cardiovascular death in tertiles 1, 2, and 3 were 3.9, 6.2, and 9.5%, respectively. Kaplan-Meier analysis suggested that the risk of cardiovascular death was significantly increased with an elevated TG/HDL-C ratio (P < 0.001; Figure 2).
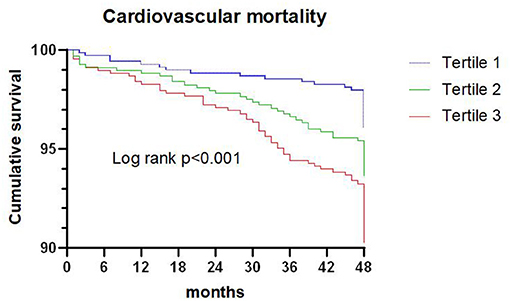
Figure 2. Kaplan-Meier survival curve for cardiovascular death across triglyceride to high density lipoprotein-C ratio tertiles.
Cox proportional hazard regression indicated that an increased TG/HDL-C ratio tertile was associated with an increased risk of cardiovascular death in both the unadjusted model (P < 0.001) and the adjusted model (P = 0.009). Furthermore, the risk of cardiovascular death was significantly increased per SD increment in the TG/HDL-C ratio in both the unadjusted model (HR: 1.22; 95% CI: 1.16–1.29; P < 0.001) and the adjusted model (HR: 1.27; 95% CI: 1.19–1.36; P < 0.001) (Table 2). Sensitivity analysis revealed that the association between the TG/HDL-C ratio and the risk of cardiovascular death was robust and not altered by sequential adjustment for potential confounders (Table 3).
ROC analysis indicated that the optimal cutoff value of the TG/HDL-C ratio for predicting cardiovascular death was 1.57 (sensitivity: 74.3% and specificity: 53.8%), with an AUC of 0.672 (95% CI: 0.625–0.718; P < 0.001). Adding the TG/HDL-C ratio to the established model improved the prediction of cardiovascular death in terms of the C-statistic (from 0.771 to 0.804; P < 0.001), and the NRI and IDI were 0.508 (95% CI: 0.335–0.680; P < 0.001) and 0.033 (95% CI: 0.015–0.050; P < 0.001), respectively (Table 4).
The results of the subgroup analyses for cardiovascular death based on pre-defined variables are shown in Table 5. An elevated TG/HDL-C ratio was associated with an increased risk of cardiovascular death in all subgroups, and sex (P = 0.552), smoker (P = 0.537), BMI (P = 0.285), duration of DM (P = 0.442), ACS (P = 0.346), HbA1c (P = 0.697), LDL-C (P = 0.345), insulin treatment (P = 0.502), and revascularization (P = 0.476) did not affect the role of TG/HDL-C ratio in predicting the risk of cardiovascular death.
Discussion
This study systematically analyzed the predictive value of the TG/HDL-C ratio for subsequent all-cause mortality and cardiovascular death in diabetic patients with CAD who were treated with statins. An elevated TG/HDL-C ratio was associated with an increased risk of all-cause mortality and cardiovascular death. Sensitivity analyses indicated that the role of TG/HDL-C ratio in predicting subsequent all-cause mortality and cardiovascular death was robust and not altered by sequential adjusted potential confounders. Furthermore, adding the TG/HDL-C ratio to the established model resulted in a significant enhancement of the predictive value. The risk of all-cause mortality and cardiovascular death was significantly increased when the TG/HDL-C ratio was increased in all subgroups, and these associations were not affected by sex, smoker, BMI, duration of DM, ACS, HbA1c, LDL-C, insulin treatment, or revascularization. The above results indicate that the TG/HDL-C ratio is a marker of poor prognosis even in the era of statin treatment and may contribute to the early identification of high-risk diabetic patients and CAD. Furthermore, routine TG/HDL-C ratio calculation may further improve risk stratification for all-cause mortality and cardiovascular death.
LDL-C plays a key role in the development and progression of atherosclerotic CVD (ASCVD) and statins are the first-line therapy for lowering LDL-C levels to reduce ASCVD risk. However, diabetic patients with CAD remain at high cardiovascular risk even after LDL-C reduction, which indicates that there are residual cardiovascular risk factors other than LDL-C. One study found that diabetic patients treated with statins had a high prevalence of persistent atherogenic dyslipidemia (13). Elevated TG levels and lower HDL-C levels, as typical lipid features of diabetes, are considered to indicate atherogenic dyslipidemia in diabetic patients (44, 45). However, the levels of TG and HDL-C are mutually independent, and the single lipid parameter could not reflect the actual status of plasma atherogenicity and CVD risk in the absence of insulin resistance (13). Therefore, the TG/HDL-C ratio could reflect TG and HDL-C simultaneously, and is regarded as a better marker in primary and secondary prevention of CVD (34, 36, 46). A study conducted by Edwards et al. suggested that the TG/HDL-C ratio has better predictive value for mortality than that of individual lipid parameters (47). Furthermore, a high TG/HDL-C ratio may strongly predict the extent of coronary lesions (48, 49). Moreover, the TG/HDL-C ratio is significantly related to vulnerable plaque features in diabetic patients treated with statins (50). Routine lipid examinations do not reflect the actual compositional changes of lipid parameters in diabetic patients with CAD. Therefore, evaluation of the TG/HDL-C ratio may have great clinical significance with regards to risk stratification for diabetic patients with CAD who are treated with statins.
Although previous studies have demonstrated the role of the TG/HDL-C ratio in predicting adverse cardiovascular events in patients with CAD (51–55), the potential role of TG/HDL-C ratio as a prognostic marker for patients with diabetes is still debated. The Swedish National Diabetes Register found that elevated TG/HDL-C ratio could increase the risk of CVD independent of the LDL-C level in obese T2DM patients (56). Yang et al. reported that the TG/HDL-C ratio was an important predictor of MACEs in patients with diabetes and CAD (42). Contrary to these studies, several other studies did not find significant associations between the TG/HDL-C ratio and the prognosis of T2DM. Tohidi et al. demonstrated that the TG/HDL-C ratio was not an independent predictor of cardiovascular events in diabetic patients without CVD (57). The sub analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) study was not able to establish an independent association between TG/HDL ration and CVD risk in patients with DM and without history of CVD (43). The potential reasons for this discrepancy could be the variation in definition of endpoints, patient characteristics among studies.
This study is the first to focus on the role of the TG/HDL-C ratio in the prediction of prognosis in diabetic patients with CAD who were treated with statins. Compared with previous studies focusing on patients with diabetes or CAD, this large cohort study included higher risk patients with a higher prevalence of a history of CVD. This study demonstrated that an elevated TG/HDL-C ratio was associated with poor prognosis in diabetic patients with CAD treated with statins. Although higher TG/LDL-C ratio were relevant for chronic kidney disease (CKD) in patients with diabetes (58), TG/LDL-C ratio remained a significant and independent predictor of all-cause mortality and cardiovascular death after adjustment for potential confounders including renal function measures (eGFR). This finding suggested that the association between TG/HDL-C ratio and the risk of mortality might not be mediated by the presence of kidney dysfunction. These associations were persistent in sensitivity and subgroup analyses. An elevated TG/HDL-C ratio was still associated with an increased risk of mortality in patients with LDL-C levels of ≤ 1.80 mmol/L, suggesting that the ratio may explain part of the residual cardiovascular risk. The use of statins has less impact on the prognostic value of the TG/HDL-C ratio in diabetic patients with CAD. Several potential mechanisms may account for the association of the TG/HDL-C ratio with all-cause mortality and cardiovascular death in diabetic patients with CAD: (1) an elevated TG level and lower HDL-C plays an important role in endothelial dysfunction and atherosclerosis. Combined TG and HDL-C are significantly related to other atherogenic lipid phenotypes, characterized by higher levels of small dense LDL particles along with higher levels of remnant particle cholesterol and non-HDL-C, which contribute to the progression of atherosclerosis (14, 58, 59); (2) the TG/HDL-C ratio is significantly related to insulin resistance and glycemic control in diabetic patients (15, 16, 60, 61). Insulin resistance is related to the progression of atherosclerosis, vulnerability of coronary plaques, and MACEs in patients with CAD (62–64). Moreover, a hyperglycemic environment could induce the progression of macrovascular and microvascular disease in diabetic patients, including diabetic nephropathy, CAD and peripheral artery disease, which could cause excess risk of all-cause mortality and cardiovascular death (65, 66).
Additionally, the addition of the TG/HDL-C ratio in the risk prediction model for subsequent all-cause mortality and cardiovascular death was associated with a high predictive value. These results suggest that the use of TG/HDL-C ratio could refine risk stratification for all-cause mortality and cardiovascular death in diabetic patients with CAD who are treated with statins. Moreover, this study identified the optimal cutoff value of the TG/HDL-C ratio in this context, suggesting that the ratio should be maintained at <1.57 to reduce the risk of all-cause mortality and cardiovascular death. The results of this study provide new evidence to reduce all-cause mortality and cardiovascular death in diabetic patients with CAD treated with statins. Further large-scale prospective cohort studies should be performed to verify whether implementation of screening TG/HDL-C ratio will change the prognosis of diabetic patients with CAD.
However, several limitations of this study should be acknowledged. First, the current study was retrospective. The lack of information of waist circumference made it difficult to calculate the fatty liver index. Therefore, fatty liver index was not included in the analysis. Second, the follow-up information was collected by telephone or electronic medical record review. The follow-up information mainly included survival data. Baseline data after 4-year follow-up was not collected. Third, the information about glycemic control optimization and changes in medications was not collected during follow-up. The effect of changes in medications should be taken into consideration in the future, prospective study. Fourth, the complications and severity of T2DM and CAD differ, which could have affected the risk of all-cause mortality and cardiovascular death. Finally, as lipid levels vary among different ethnicities, it is not known whether these findings can be applicable to other ethnicities.
Conclusion
An elevated TG/HDL-C ratio was associated with an increased risk of all-cause mortality and cardiovascular death in diabetic patients with CAD who were treated with statins. Moreover, the addition of the TG/HDL-C ratio into the traditional risk model increased the predictive value for subsequent all-cause mortality and cardiovascular death. Therefore, the TG/HDL-C ratio may be a useful marker for evaluating the prognosis in diabetic patients with CAD who are treated with statins.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Committee of Tianjin Chest Hospital. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
LW, HC, and JZ participated in the study design. LW, YH, AW, YZ, HY, LR, WQ, and WL participated in data collection. LW, HY, and LR performed the statistical analysis. LW drafted the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all of the investigators and patients who participated in this project.
References
1. Norhammar A, Malmberg K, Diderholm E, Lagerqvist B, Lindahl B, Rydén L, et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. J Am Coll Cardiol. (2004) 43:585–91. doi: 10.1016/j.jacc.2003.08.050
2. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet. (2004) 364:685–96. doi: 10.1016/S0140-6736(04)16895-5
3. Sever PS, Poulter NR, Dahlöf B, Wedel H, Collins R, Beevers G, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: anglo-scandinavian cardiac outcomes trial–lipid-lowering arm (ASCOT-LLA). Diabetes Care. (2005) 28:1151–7. doi: 10.2337/diacare.28.5.1151
4. Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the atorvastatin study for prevention of coronary heart disease endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. (2006) 29:1478–85. doi: 10.2337/dc05-2415
5. Tajima N, Kurata H, Nakaya N, Mizuno K, Ohashi Y, Kushiro T, et al. Pravastatin reduces the risk for cardiovascular disease in Japanese hypercholesterolemic patients with impaired fasting glucose or diabetes: diabetes subanalysis of the management of elevated cholesterol in the primary prevention group of adult Japanese (MEGA) study. Atherosclerosis. (2008) 199:455–62. doi: 10.1016/j.atherosclerosis.2008.05.027
6. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2018) 379:633–44. doi: 10.1056/NEJMoa1800256
7. Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. (2015) 58:886–99. doi: 10.1007/s00125-015-3525-8
8. Filippatos T, Tsimihodimos V, Pappa E, Elisaf M. Pathophysiology of diabetic dyslipidaemia. Curr Vasc Pharmacol. (2017) 15:566–75. doi: 10.2174/1570161115666170201105425
9. Chan WB, Tong PC, Chow CC, So WY, Ng MC, Ma RC, et al. Triglyceride predicts cardiovascular mortality and its relationship with glycaemia and obesity in Chinese type 2 diabetic patients. Diabetes Metab Res Rev. (2005) 21:183–8. doi: 10.1002/dmrr.497
10. Joo HJ, Cho SA, Hong SJ, Hur SH, Bae JH, Choi DJ, et al. Impact of low high-density lipoprotein-cholesterol level on 2-year clinical outcomes after acute myocardial infarction in patients with diabetes mellitus. Lipids Health Dis. (2016) 15:197. doi: 10.1186/s12944-016-0374-5
11. Li XL, Hong LF, Luo SH, Guo YL, Zhu CG, Sun J, et al. Impact of admission triglyceride for early outcome in diabetic patients with stable coronary artery disease. Lipids Health Dis. (2014) 13:73. doi: 10.1186/1476-511X-13-73
12. Ogita M, Miyauchi K, Miyazaki T, Naito R, Konishi H, Tsuboi S, et al. Low high-density lipoprotein cholesterol is a residual risk factor associated with long-term clinical outcomes in diabetic patients with stable coronary artery disease who achieve optimal control of low-density lipoprotein cholesterol. Heart Vessels. (2014) 29:35–41. doi: 10.1007/s00380-013-0330-5
13. Quispe R, Martin SS, Jones SR. Triglycerides to high-density lipoprotein-cholesterol ratio, glycemic control and cardiovascular risk in obese patients with type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. (2016) 23:150–6. doi: 10.1097/MED.0000000000000241
14. Yokoyama K, Tani S, Matsuo R, Matsumoto N. Increased triglyceride/high-density lipoprotein cholesterol ratio may be associated with reduction in the low-density lipoprotein particle size: assessment of atherosclerotic cardiovascular disease risk. Heart Vessels. (2019) 34:227–36. doi: 10.1007/s00380-018-1247-9
15. Ren X, Chen ZA, Zheng S, Han T, Li Y, Liu W, et al. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE. (2016) 11:e0154345. doi: 10.1371/journal.pone.0154345
16. Zhou M, Zhu L, Cui X, Feng L, Zhao X, He S, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of beta cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. (2016) 15:104. doi: 10.1186/s12944-016-0270-z
17. Uruska A, Zozulinska-Ziolkiewicz D, Niedzwiecki P, Pietrzak M, Wierusz-Wysocka B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J Clin Lipidol. (2018) 12:734–40. doi: 10.1016/j.jacl.2018.01.005
18. Liu D, Guan L, Zhao Y, Liu Y, Sun X, Li H, et al. Association of triglycerides to high-density lipoprotein-cholesterol ratio with risk of incident hypertension. Hypertens Res. (2020) 43:948–55. doi: 10.1038/s41440-020-0439-8
19. Yeom H, Kim HC, Lee JM, Jeon Y, Suh I. Triglyceride to high density lipoprotein cholesterol ratio among adolescents is associated with adult hypertension: the Kangwha study. Lipids Health Dis. (2018) 17:212. doi: 10.1186/s12944-018-0861-y
20. Tohidi M, Hatami M, Hadaegh F, Azizi F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J Hum Hypertens. (2012) 26:525–32. doi: 10.1038/jhh.2011.70
21. Karelis AD, Pasternyk SM, Messier L, St-Pierre DH, Lavoie JM, Garrel D, et al. Relationship between insulin sensitivity and the triglyceride-HDL-C ratio in overweight and obese postmenopausal women: a MONET study. Appl Physiol Nutr Metab. (2007) 32:1089–96. doi: 10.1139/H07-095
22. Ho CI, Chen JY, Chen SY, Tsai YW, Weng YM, Tsao YC, et al. Relationship between TG/HDL-C ratio and metabolic syndrome risk factors with chronic kidney disease in healthy adult population. Clin Nutr. (2015) 34:874–80. doi: 10.1016/j.clnu.2014.09.007
23. Shin HG, Kim YK, Kim YH, Jung YH, Kang HC. The relationship between the triglyceride to high-density lipoprotein cholesterol ratio and metabolic syndrome. Korean J Fam Med. (2017) 38:352–7. doi: 10.4082/kjfm.2017.38.6.352
24. Aslan Çin NN, Yardimci H, Koç N, Uçaktürk SA, Akçil Ok M. Triglycerides/high-density lipoprotein cholesterol is a predictor similar to the triglyceride-glucose index for the diagnosis of metabolic syndrome using international diabetes federation criteria of insulin resistance in obese adolescents: a cross-sectional study. J Pediatr Endocrinol Metab. (2020) 33:777–84. doi: 10.1515/jpem-2019-0310
25. Liu XY, Wu QY, Chen ZH, Yan GY, Lu Y, Dai HJ, et al. Elevated triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio increased risk of hyperuricemia: a 4-year cohort study in China. Endocrine. (2020) 68:71–80. doi: 10.1007/s12020-019-02176-5
26. Wu KT, Kuo PL, Su SB, Chen YY, Yeh ML, Huang CI, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol. (2016) 10:420–5.e1. doi: 10.1016/j.jacl.2015.12.026
27. Fan N, Peng L, Xia Z, Zhang L, Song Z, Wang Y, et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. (2019) 18:39. doi: 10.1186/s12944-019-0986-7
28. Shishehbor MH, Hoogwerf BJ, Lauer MS. Association of triglyceride-to-HDL cholesterol ratio with heart rate recovery. Diabetes Care. (2004) 27:936–41. doi: 10.2337/diacare.27.4.936
29. Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. (2018) 17:130. doi: 10.1186/s12944-018-0776-7
30. Chung TH, Shim JY, Kwon YJ, Lee YJ. High triglyceride to high-density lipoprotein cholesterol ratio and arterial stiffness in postmenopausal Korean women. J Clin Hypertens. (2019) 21:399–404. doi: 10.1111/jch.13484
31. Li X, Deng YP, Yang M, Wu YW, Sun SX, Sun JZ. Triglyceride to high-density lipoprotein cholesterol ratio and carotid intima-medial thickness in Chinese adolescents with newly diagnosed type 2 diabetes mellitus. Pediatr Diabetes. (2016) 17:87–92. doi: 10.1111/pedi.12250
32. Turak O, Afşar B, Ozcan F, Öksüz F, Mendi MA, Yayla Ç, et al. The role of plasma triglyceride/high-density lipoprotein cholesterol ratio to predict new cardiovascular events in essential hypertensive patients. J Clin Hypertens. (2016) 18:772–7. doi: 10.1111/jch.12758
33. Park JH, Lee J, Ovbiagele B. Nontraditional serum lipid variables and recurrent stroke risk. Stroke. (2014) 45:3269–74. doi: 10.1161/STROKEAHA.114.006827
34. Chen Z, Chen G, Qin H, Cai Z, Huang J, Chen H, et al. Higher triglyceride to high-density lipoprotein cholesterol ratio increases cardiovascular risk: 10-year prospective study in a cohort of Chinese adults. J Diabetes Investig. (2020) 11:475–81. doi: 10.1111/jdi.13118
35. Chang TI, Streja E, Soohoo M, Kim TW, Rhee CM, Kovesdy CP, et al. Association of serum triglyceride to HDL cholesterol ratio with all-cause and cardiovascular mortality in incident hemodialysis patients. Clin J Am Soc Nephrol. (2017) 12:591–602. doi: 10.2215/CJN.08730816
36. He S, Wang S, Chen X, Jiang L, Peng Y, Li L, et al. Higher ratio of triglyceride to high-density lipoprotein cholesterol may predispose to diabetes mellitus: 15-year prospective study in a general population. Metabolism. (2012) 61:30–6. doi: 10.1016/j.metabol.2011.05.007
37. Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. (2014) 62:345–9. doi: 10.2310/JIM.0000000000000044
38. Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, et al. Association of triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. (2020) 19:33. doi: 10.1186/s12944-020-01213-x
39. Zheng D, Li H, Ai F, Sun F, Singh M, Cao X, et al. Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of type 2 diabetes mellitus among Chinese elderly: the Beijing longitudinal study of aging. BMJ Open Diabetes Res Care. (2020) 8:e000811. doi: 10.1136/bmjdrc-2019-000811
40. Cheng C, Liu Y, Sun X, Yin Z, Li H, Zhang M, et al. Dose-response association between the triglycerides: high-density lipoprotein cholesterol ratio and type 2 diabetes mellitus risk: the rural Chinese cohort study and meta-analysis. J Diabetes. (2019) 11:183–92. doi: 10.1111/1753-0407.12836
41. Lee MY, Hsiao PJ, Huang JC, Hsu WH, Chen SC, Chang JM, et al. Associations between triglyceride/high-density lipoprotein cholesterol ratio and micro- and macroangiopathies in type 2 diabetes mellitus. Endocr Pract. (2018) 24:615–21. doi: 10.4158/EP-2017-0254
42. Yang SH, Du Y, Li XL, Zhang Y, Li S, Xu RX, et al. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in diabetics with coronary artery disease. Am J Med Sci. (2017) 354:117–24. doi: 10.1016/j.amjms.2017.03.032
43. Sone H, Nakagami T, Nishimura R, Tajima N, MEGA Study Group. Comparison of lipid parameters to predict cardiovascular events in Japanese mild-to-moderate hypercholesterolemic patients with and without type 2 diabetes: Subanalysis of the MEGA study. Diabetes Res Clin Pract. (2016) 113:14–22. doi: 10.1016/j.diabres.2015.12.002
44. Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. (2008) 51:724–30. doi: 10.1016/j.jacc.2007.10.038
45. Bos G, Dekker JM, Nijpels G, de Vegt F, Diamant M, Stehouwer CD, et al. A combination of high concentrations of serum triglyceride and non-high-density-lipoprotein-cholesterol is a risk factor for cardiovascular disease in subjects with abnormal glucose metabolism–The Hoorn Study. Diabetologia. (2003) 46:910–6. doi: 10.1007/s00125-003-1141-5
46. Matsumoto I, Misaki A, Kurozumi M, Nanba T, Takagi Y. Impact of nonfasting triglycerides/high-density lipoprotein cholesterol ratio on secondary prevention in patients treated with statins. J Cardiol. (2018) 71:10–5. doi: 10.1016/j.jjcc.2017.07.012
47. Edwards MK, Blaha MJ, Loprinzi PD. Atherogenic index of plasma and triglyceride/high-density lipoprotein cholesterol ratio predict mortality risk better than individual cholesterol risk factors, among an older adult population. Mayo Clin Proc. (2017) 92:680–1. doi: 10.1016/j.mayocp.2016.12.018
48. da Luz PL, Favarato D, Faria-Neto JR Jr, Lemos P, Chagas AC. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics. (2008) 63:427–32. doi: 10.1590/S1807-59322008000400003
49. Yunke Z, Guoping L, Zhenyue C. Triglyceride-to-HDL cholesterol ratio. Predictive value for CHD severity and new-onset heart failure. Herz. (2014) 39:105–10. doi: 10.1007/s00059-013-3788-0
50. Takata K, Kataoka Y, Andrews J, Puri R, Hammadah M, Duggal B, et al. Triglyceride-to-high-density lipoprotein cholesterol ratio and vulnerable plaque features with statin therapy in diabetic patients with coronary artery disease: frequency-domain optical coherence tomography analysis. JACC Cardiovasc Imaging. (2018) 11:1721–3. doi: 10.1016/j.jcmg.2018.02.017
51. Wan K, Zhao J, Huang H, Zhang Q, Chen X, Zeng Z, et al. The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. PLoS ONE. (2015) 10:e0123521. doi: 10.1371/journal.pone.0123521
52. Dai XY, Zheng YY, Tang JN, Yang XM, Guo QQ, Zhang JC, et al. Triglyceride to high-density lipoprotein cholesterol ratio as a predictor of long-term mortality in patients with coronary artery disease after undergoing percutaneous coronary intervention: a retrospective cohort study. Lipids Health Dis. (2019) 18:210. doi: 10.1186/s12944-019-1152-y
53. Sultani R, Tong DC, Peverelle M, Lee YS, Baradi A, Wilson AM. Elevated triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) ratio predicts long-term mortality in high-risk patients. Heart Lung Circ. (2020) 29:414–21. doi: 10.1016/j.hlc.2019.03.019
54. Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, et al. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the women's ischemia syndrome evaluation (WISE). Am Heart J. (2009) 157:548–55. doi: 10.1016/j.ahj.2008.11.014
55. Prasad M, Sara J, Widmer RJ, Lennon R, Lerman LO, Lerman A. Triglyceride and triglyceride/ HDL (high density lipoprotein) ratio predict major adverse cardiovascular outcomes in women with non-obstructive coronary artery disease. J Am Heart Assoc. (2019) 8:e009442. doi: 10.1161/JAHA.118.009442
56. Eeg-Olofsson K, Gudbjörnsdottir S, Eliasson B, Zethelius B, Cederholm J; NDR. The triglycerides-to-HDL-cholesterol ratio and cardiovascular disease risk in obese patients with type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Res Clin Pract. (2014) 106:136–44. doi: 10.1016/j.diabres.2014.07.010
57. Tohidi M, Hatami M, Hadaegh F, Safarkhani M, Harati H, Azizi F. Lipid measures for prediction of incident cardiovascular disease in diabetic and non-diabetic adults: results of the 8.6 years follow-up of a population based cohort study. Lipids Health Dis. (2010) 9:6. doi: 10.1186/1476-511X-9-6
58. Tsuruya K, Yoshida H, Nagata M, Kitazono T, Hirakata H, Iseki K, et al. Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: analysis in a large Japanese population. Atherosclerosis. (2014) 233:260–7. doi: 10.1016/j.atherosclerosis.2013.12.037
59. Moriyama K. The association between the triglyceride to high-density lipoprotein cholesterol ratio and low-density lipoprotein subclasses. Intern Med. (2020) 59:2661–9. doi: 10.2169/internalmedicine.4954-20
60. Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the very large database of lipids-4 (VLDL-4) study. Atherosclerosis. (2015) 242:243–50. doi: 10.1016/j.atherosclerosis.2015.06.057
61. Zonszein J, Lombardero M, Ismail-Beigi F, Palumbo P, Foucher S, Groenewoud Y, et al. Triglyceride high-density lipoprotein ratios predict glycemia-lowering in response to insulin sensitizing drugs in type 2 diabetes: a post hoc analysis of the BARI 2D. J Diabetes Res. (2015) 2015:129891. doi: 10.1155/2015/129891
62. An X, Yu D, Zhang R, Zhu J, Du R, Shi Y, et al. Insulin resistance predicts progression of de novo atherosclerotic plaques in patients with coronary heart disease: a one-year follow-up study. Cardiovasc Diabetol. (2012) 11:71. doi: 10.1186/1475-2840-11-71
63. Nishimura M, Tokoro T, Nishida M, Hashimoto T, Kobayashi H, Yamazaki S, et al. Association of insulin resistance with de novo coronary stenosis after percutaneous coronary artery intervention in hemodialysis patients. Nephron Clin Pract. (2008) 109:c9–17. doi: 10.1159/000132391
64. Iguchi T, Hasegawa T, Otsuka K, Matsumoto K, Yamazaki T, Nishimura S, et al. Insulin resistance is associated with coronary plaque vulnerability: insight from optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging. (2014) 15:284–91. doi: 10.1093/ehjci/jet158
65. Sowers JR, Stump CS. Insights into the biology of diabetic vascular disease: what's new? Am J Hypertens. (2004) 17:2S−6S. doi: 10.1016/j.amjhyper.2004.08.007
Keywords: triglyceride to high-density lipoprotein cholesterol ratio, type 2 diabetes, coronary artery disease, statin, all-cause mortality, cardiovascular death
Citation: Wang L, Cong H, Zhang J, Hu Y, Wei A, Zhang Y, Yang H, Ren L, Qi W and Li W (2021) Predictive Value of the Triglyceride to High-Density Lipoprotein Cholesterol Ratio for All-Cause Mortality and Cardiovascular Death in Diabetic Patients With Coronary Artery Disease Treated With Statins. Front. Cardiovasc. Med. 8:718604. doi: 10.3389/fcvm.2021.718604
Received: 01 June 2021; Accepted: 29 June 2021;
Published: 21 July 2021.
Edited by:
Hanrui Zhang, Columbia University, United StatesReviewed by:
Jianting Shi, Columbia University, United StatesJosep Julve, Institut de Recerca de l'Hospital de la Santa Creu i Sant Pau, Spain
Copyright © 2021 Wang, Cong, Zhang, Hu, Wei, Zhang, Yang, Ren, Qi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongliang Cong, hongliangcong@126.com
 Le Wang
Le Wang Hongliang Cong
Hongliang Cong