- 1Post-graduate Program in Medicine and Health (PPgMS)/Federal University of Bahia (UFBA), Salvador, Brazil
- 2Federal University of Bahia, UFBA, Salvador, Brazil
- 3Federal University of Uberlandia, Uberlândia, Brazil
- 4University of São Paulo Medical School, HCFMUSP, São Paulo, Brazil
- 5Heart Institute, InCor, São Paulo, Brazil
Background: Direct oral anticoagulants (DOACS) are approved for use in non-valvular atrial fibrillation (AF). This systematic review and meta-analysis aimed to evaluate the efficacy and safety of DOACs vs. warfarin and update the evidence for treatment of AF and valvular heart disease (VHD).
Methods: We identified randomized clinical trials (RCTs) and post-hoc analyses comparing the use of DOACS and Warfarin in AF and VHD, including biological and mechanical heart valves (MHV), updating from 2010 to 2020. Through systematic review and meta-analysis, by using the “Rev Man” program 5.3, the primary effectiveness endpoints were stroke and systemic embolism (SE). The primary safety outcome was major bleeding, while the secondary outcome included intracranial hemorrhage. We performed prespecified subgroup analyses. Data were analyzed by risk ratio (RR) and 95% confidence interval (CI) and the I-square (I2) statistic as a quantitative measure of inconsistency. Risk of bias and methodological quality assessment of included trials was evaluated with the modified Cochrane risk-of-bias tool.
Results: We screened 326 articles and included 8 RCTs (n = 14.902). DOACs significantly reduced the risk of stroke/SE (RR 0.80, 95% CI: 0.68–0.94; P = 0.008; moderate quality evidence; I2 = 2%) and intracranial hemorrhage (RR 0.40, 95% CI: 0.24–0.66; P = 0.0004; I2 = 49%) with a similar risk of major bleeding (RR 0.83, 95% CI: 0.56–1.24; P = 0.36; I2 = 88%) compared to Warfarin.
Conclusions: In this update, DOACs remained with similar efficacy and safety compared to warfarin in thromboprophylaxis for AF and VHD.
Introduction
Direct oral anticoagulants (DOACs) have been developed as a alternative to vitamin k antagonist (VKA) and emerged as the preferred treatment option for atrial fibrillation (AF) in the general population (class I, LOE: B), as well as in the prophylaxis or treatment of deep vein thrombosis and pulmonary embolism (1). Prior studies in non-valvular AF using DOACs demonstrated their non-inferiority compared to the use of warfarin, but in some trials, superior (2).
Recent guidelines supported the use of DOACs in native valve diseases (CHA2DS2-VASc score of 2 or greater). However, in moderate to severe mitral stenosis (MS) and mechanical heart valves (MHV), the use of VKA in the prevention of thromboembolic events is the only established option (class I, LOE: A) (3), A low time in therapeutic range (TTR) was observed in MHV populations in use of warfarin, which can increase the cardiac and thromboembolic risk. (4) In contrast, the TTR control was even lower in use of acenocoumarol than warfarin in the same population. (5) In the presence of bioprosthetic valves (≥ 3 months postoperatively) and AF, non-vitamin K antagonist oral anticoagulants are a reasonable option to VKAs (class I, LOE: A) (6).
Previous meta-analyses compared DOACs to warfarin for the treatment of AF and valvular heart disease (VHD). The study aggregated data from 4 or 5–of the current eight available–randomized controlled trials (RCTs), suggesting that DOACs have similar safety and efficacy compared to VKA (7–9). Based on this knowledge gap, this systematic review and updated meta-analysis aimed to compare the efficacy and safety of DOACs vs. warfarin in adult patients with AF and VHD by aggregating results from all available RCTs and to assess their relative benefit in specific subgroups.
Methods
We conducted a systematic review of the literature and meta-analysis carried out to the standards established by the PRISMA recommendation (“Preferred Reporting Items for Systematic Reviews and Meta-Analyses”) (10). More details are available in Supplementary File 1 (Supplementary Table E1).
Literature Research and Study Selection
The databases included PubMed, LILACS, MEDLINE, SciELO and Cochrane Library (November 2020—December 2020), with year restriction for 2010 to 2020. Researchers used predefined search terms combined with filters to identify RCTs (complete search strategy in Supplementary File 2. To be eligible for inclusion, studies had to fulfill all predefined inclusion criteria that were defined as follows: RCTs that compared DOACS (Dabigatran, Rivaroxaban, Apixaban Edoxaban, and/or Betrixaban) to Warfarin in adult humans (aged ≥18 years) with AF and VHD (including patients with bioprosthesis and MHV ≥ 3 months postoperatively). Exclusion criteria were as follows: articles not focused on the use of DOACS in VHD and AF; inclusion of patients <18 years of age; observational studies; non-randomized clinical trials; studies performed in animals; reviews; duplicate publications reporting the same trials.
Data Collection
Two reviewers independently (B, Y, and D, A) evaluated the list of titles and abstracts from each data source. For articles considered eligible, researchers accessed the full text to verify if they met inclusion criteria, prior to data extraction. Any disagreements were resolved through a consensus discussion among reviewers. (More details in Supplementary File 2).
Evaluated Outcomes
We considered the primary endpoint of efficacy, stroke composition and SE, while the primary safety outcome was the presence of major bleeding (according to the International Society of Thrombosis and Haemostasis—ISTH–definition) (11). Intracranial hemorrhage was characterized as a secondary outcome. Risk of bias and methodological quality assessment of included trials was evaluated with the modified Cochrane risk-of-bias tool (Supplementary File 2)–version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) (12). The assessment involved five items: risk of bias, imprecision, inconsistency, indirectness, and publication bias. The quality of evidence was downgraded by one level for risk of bias when more than a quarter of the studies included in meta-analysis were considered at high risk of bias (Studies without allocation concealment, random allocation, and/or sample size calculation). Results were considered imprecise if the pooled sample size was <300 for dichotomous or <400 for continuous outcomes, and inconsistent if the heterogeneity between RCTs was substantial (i.e., I2 > 40%).
Statistical Analysis
Statistical analysis was performed using the Cochrane Collaboration Review Manager Software (RevMan version 5.3, 2011). We used the random-effects meta-analysis model, and forest plots were used to present the pooled stimates of the risk ratio (RR) and 95% confidence interval (CI). A p-value ≤ 0.05 was considered statistically significant. Researchers independently grouped, in different estimates, the studies that presented the use of different dosages, using the random-effects model in the meta-analysis. The I-square (I2) statistic was used as a quantitative measure of inconsistency. We used the GRADEpro software (Grading of Recommendations, Assessment, Development and Evaluation profiler) to assess the quality of evidence across studies and minimize bias in our findings and recommendations. GRADEpro classified the level of evidence as very low, low, moderate, or high (13).
Results
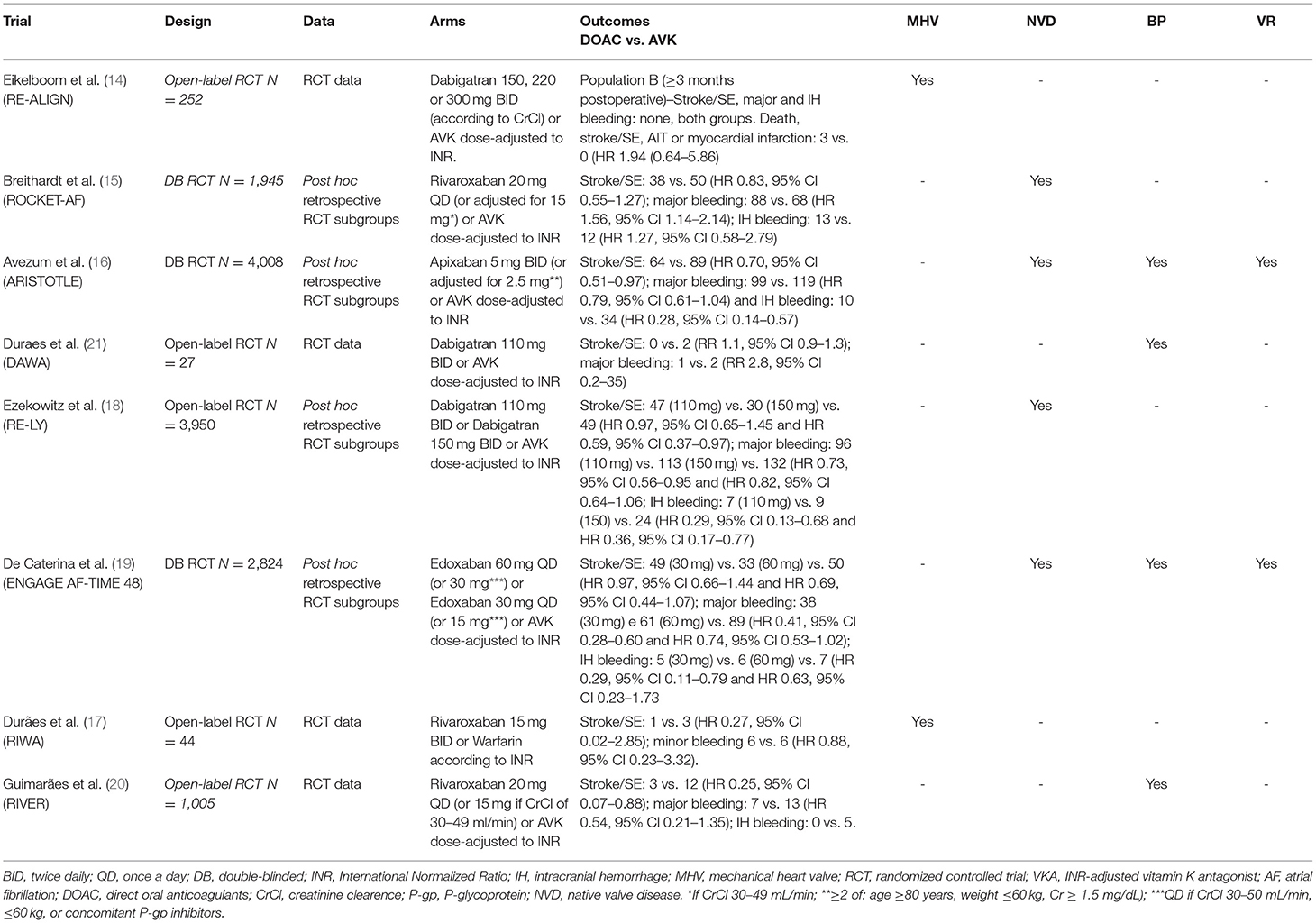
A total of 326 published records matched the predefined search terms. In our review, we identified a total of 10 studies that met eligibility criteria based on the screening process. Of those, eight were RCTs (n = 14.902), contributing to 10 different publications, including six publications on specific subgroups or post hoc analysis (14–21). In two studies, we found two different tested doses. Therefore, we performed specific analyses for each one. Figure 1 showed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram, which summarizes the study selection process. Table 1 presented the different types of study design, outcomes, and VHD included in this analysis.
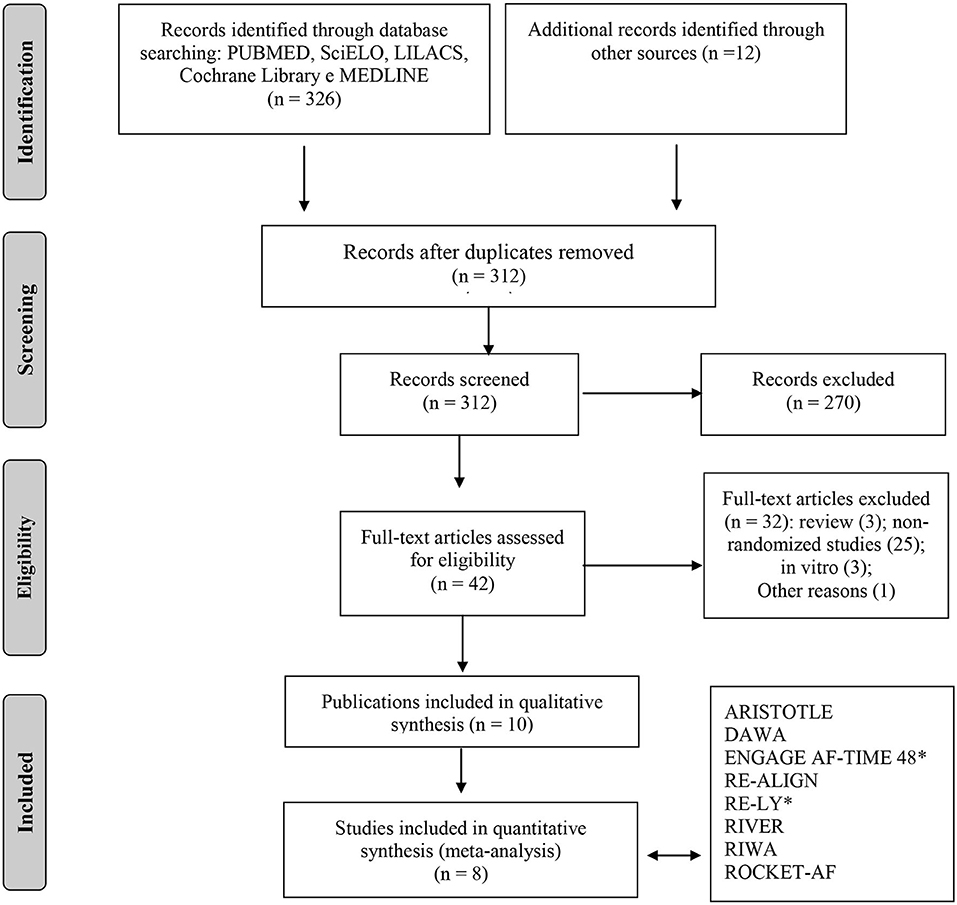
Figure 1. Flowchart of study selection adapted from the PRISMA recommendation (“Preferred Reporting Items for Systematic Reviews and Meta-Analyzes”). *For quantitative analysis, the study was separated into two subgroups (two different doses of NOACS was tested).
Study Characteristics
Among the included studies, we identified phase II (3) and III (5) RCTs (see more details in Supplementary Tables E1, 2). Three publications evaluated the use of Dabigatran (the RE-ALIGN study—with MHV only (14), post-hoc analysis of the RE-LY study (16) and the DAWA study (17)—with bioprosthetic valves only). The remaining studies are as follows: one evaluated the use of Apixaban by the post-hoc study of ARISTOTLE (16), three evaluated the use of Rivaroxaban (post-hoc analysis of the ROCKET-AF study (15), the RIWA study (21)—with MHV and the RIVER study (20)—with bioprosthetic valves in mitral position only) and one analyzed the use of Edoxaban (post-hoc analysis of the ENGAGE AF-TIME-48 trial) (19). We did not identify RCTs comparing betrixaban with warfarin and VHD.
The main clinical characteristics and risk factors for bleeding and (thromboembolic events) TE in patients with AF and VHD are detailed in Supplementary Table E2. The most frequent subtype of VHD identified in the enrolled population in these studies were as follows: 7.842 individuals with MR and aortic regurgitation (AR) 2.559, 3.303 with TR, 1.235 with AS, 708 with MS, 393 had some type of valve repair or repair, 296 patients with MHV and 1.223 with bioprostheses.
Outcomes: Stroke/SES, Major Bleeding, and Intracranial Hemorrhage
DOACs were associated with a lower risk of stroke and SE in patients with VHD and AF (RR 0.80, 95% CI: 0.68–0.94; P = 0.008; moderate quality evidence) (Figure 2A). Heterogeneity among the studies evaluated was low (I2 = 2). Major bleeding was numerically lower among the DOAC 002group, showed a favorable effect of its use compared with warfarin (RR 0.83, 95% CI: 0.56–1.24; P = 0.36; low quality evidence), with I2 calculated at 88% (P < 0.00001), demonstrating high heterogeneity (Figure 2B). On the other hand, DOACs were associated with a significant reduction in the risk of intracranial hemorrhage in patients with VHD and AF in comparison with warfarin (RR 0.40, 95% CI: 0.24–0.66; P = 0.0004; low quality evidence), with an estimated I2 of 49% (P = 0.08) demonstrating moderate heterogeneity (Figure 2C).
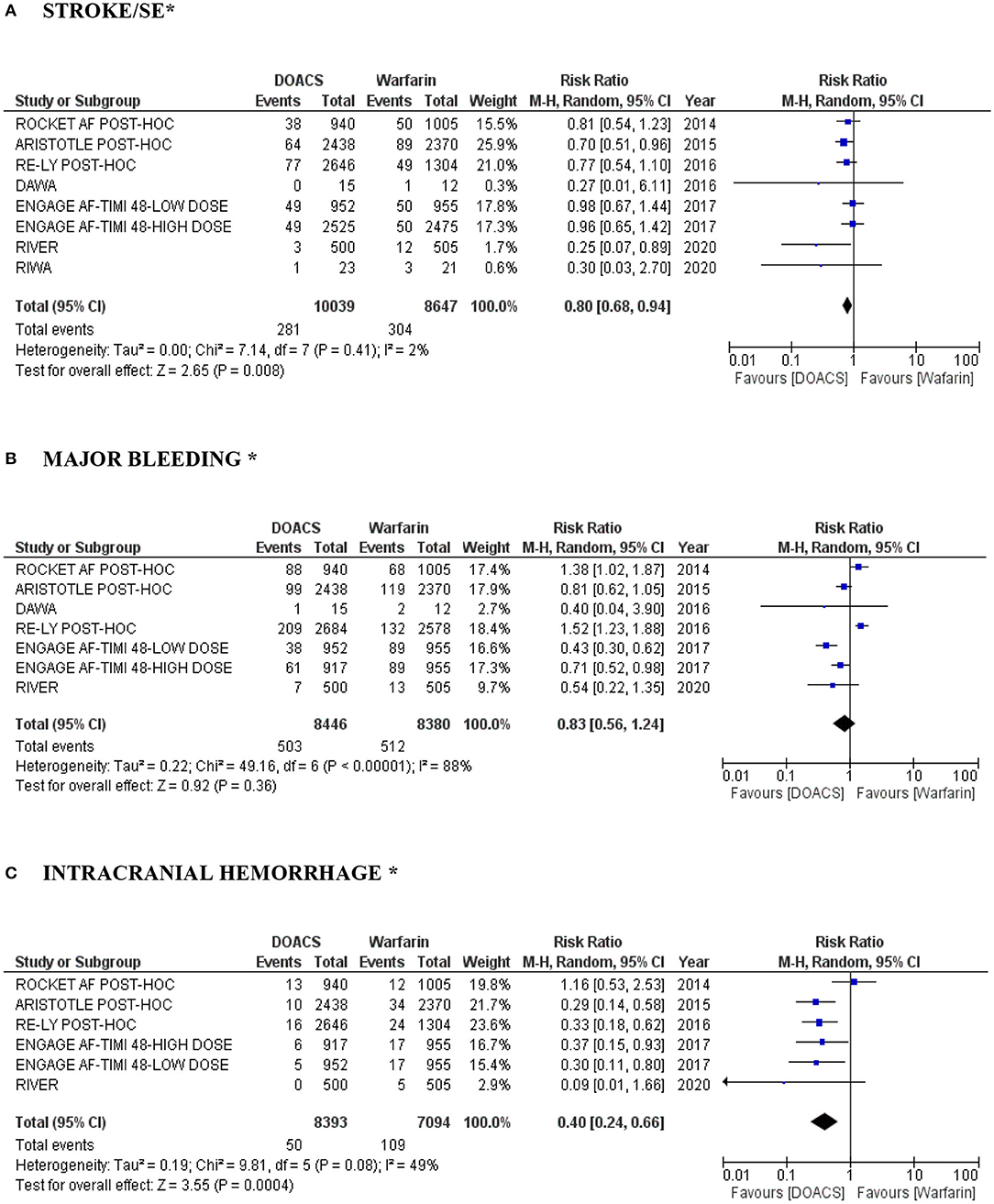
Figure 2. (A–C) “Forest plot” with individual and pooled estimates of the risk of stroke/SE, major bleeding and intracranial hemorrhage in patients with VHD and AF using DOACs (at different dosages) compared to warfarin. A random effects model was used to establish RR and 95% CI; SE, systemic embolism; AF, atrial fibrillation; VHD, valvular heart disease; DOAC, direct oral anticoagulant. *In the RE-ALIGN study performed by Eikelboom et al. (14), events in stroke/major bleeding/intracranial hemorrhage were not reported in the population B (MHV ≥ 3 months postoperatively). In addition, it was not possible to perform analysis by dose of the RE-LY study post-hoc performed by Ezekowitz et al. (18) (lack of data).
Subgroup Analyses (Bioprosthetic Heart Valves)
We conducted a subgroup analysis of the risk of stroke, SE and major bleeding in patients with bioprosthetic valves and AF treated with DOACs compared to warfarin. There were four studies (overall 1.449 patients), with detailed data about this subgroup: another post hoc analysis of ARISTOTLE trial, this time, with only bioprosthesis (87 in the apixaban arm vs. 69 in the warfarin arm) (22) and of the ENGAGE AF-TIMI 48 study (high and low dose edoxaban vs. warfarin) (23), a pilot DAWA study (15 in the dabigatran arm vs. 12 in the warfarin arm) (17) and the RIVER study (500 in the rivaroxaban arm vs. 505 in the warfarin arm) (20). In our analyses, DOACs were more effective than warfarin with lower risk of stroke and SE (RR 0.49, 95% CI: 0.26–0.93; P = 0.03; moderate quality evidence) (Figure 3A) and with a lower risk of major bleeding (RR 0.53, 95% CI: 0.31–0.90; P = 0.02; moderate quality evidence) (Figure 3B). Heterogeneity among the studies evaluated was low (I2 = 0%).
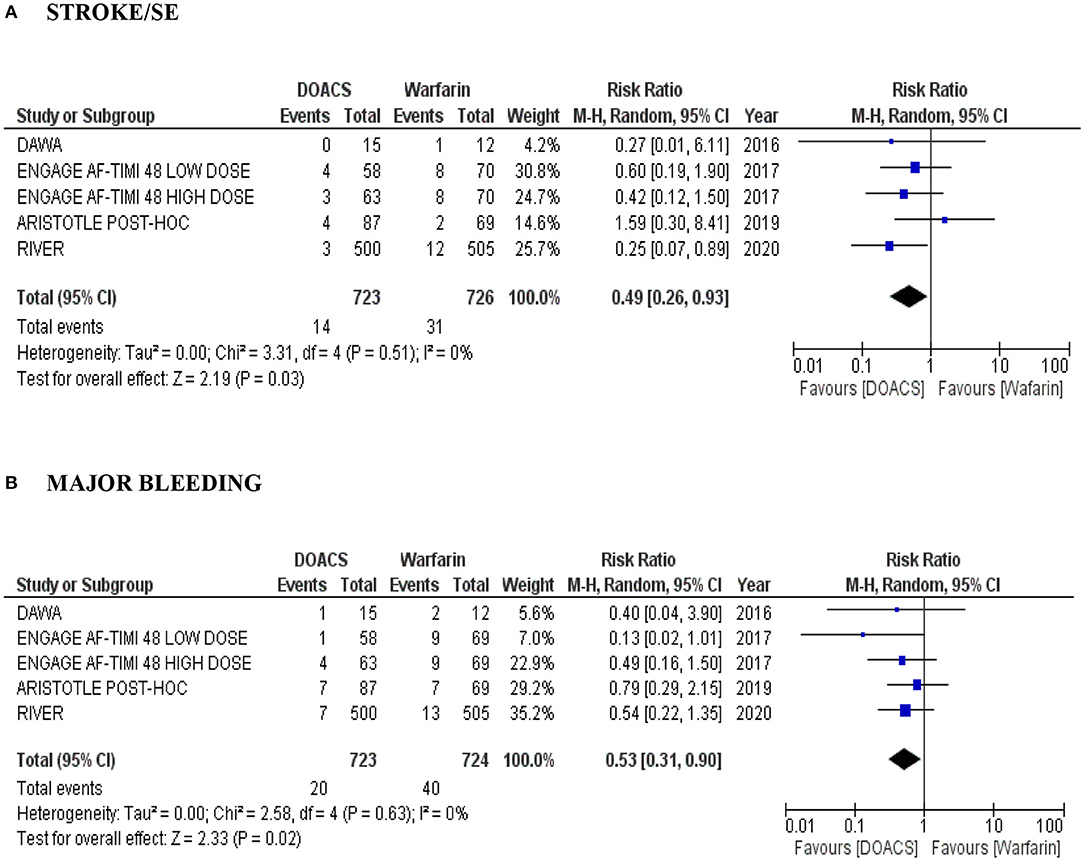
Figure 3. (A,B) Forest plot with pooled estimates of the risk of stroke/SE and major bleeding and intracranial hemorrhage in patients with AF and bioprosthesis with DOACs (at different dosages) compared to warfarin. CI, confidence interval; SE, systemic embolism; AF, atrial fibrillation; OR, odds ratio; VHD, valvular heart disease; DOAC, direct oral anticoagulant; A random effects model was used to establish RR and 95% CI.
Risk of Bias Across Studies and Quality of Evidence
The overall risk of reporting bias was low based on our analysis using the Cochrane Collaboration Tool (details in Supplementary File 5 and Supplementary Figure E1). The quality of evidence according to the GRADE system is presented in Supplementary File 6 (Supplementary Table E3). We summarize the main pharmacological characteristics and indications of DOACs approved by the FDA for use in the United States (U.S.) to date, (see more details in Supplementary Table E4).
Discussion
The main findings from the our pooled analyses were: (i) DOACs significantly reducing the risk of stroke/systemic embolism and intracranial hemorrhage, even after the inclusion of patients with MHV ≥ 3 months postoperatively; (ii) the overall risk of major bleeding was lower; and (iii) the difference for stroke/systemic embolism and major bleeding was persistent in a subgroup analysis of bioprosthetic valves and AF. Prior systematic reviews and meta-analyses in the same direction, suggesting that DOACs are effective as warfarin in reducing the risk of TE in AF-associated VHD with a lower association with major bleeding (7, 8, 24). The robustness of our results was higher for intracranial hemorrhage followed by Stroke/SE.
In the context of heart valve surgery, the choice for MHV emerges as an attractive possibility due to its higher durability in comparison with bioprosthetic valves. On the other hand, the need for long-term anticoagulation exclusively with the use of VKA is relatively complex. There is the need for dose adjustment by laboratory hemostatic parameters, the possibility of interactions with nutrients, and drugs, which difficults the management of these drugs in clinical practice. These factors contribute to the instability of the international normalized ratio (INR), increasing the risks of thromboembolic and hemorrhagic disorders (25).
Small studies and post-hoc analyses of large RCTs presented promising results regarding the superiority of DOACs use compared to warfarin in the presence of bioprosthesis and AF in non-valvar AF. Based on these results, the RIVER study (Rivaroxaban in Patients with Atrial Fibrillation and a Bioprosthetic Mitral Valve) was a large randomized trial, prospective, non-inferiority, open-label, involving the enrollment of 1,005 individuals with 1-year follow-up (20). In this study, the authors identified a higher incidence of thrombotic events and major bleeding in the warfarin group, despite the absence of associated statistical significance. Rivaroxaban has not been inferior in this scenario.
Since 2015, the European Heart Rhythm Association (EHRA) stated that patients with AF and bioprosthesis (>3 months postoperative) could be eligible for DOACs (26). However, this use is still controversial. Studies to date show promising data for DOACs use in patients with bioprosthesis and AF. Future analyses are needed to evaluate the safety and efficacy of their use in other forms of VHD (eg, MS moderate to severe), including in patients with MHV. The current recommendations of the AHA/ACC and ESC/EACTS (2017) maintain the use of VKA until further studies can elucidate the safety and efficacy of DOACs in this population (27, 28).
After encouraging results from previously published preclinical studies and considering the high prevalence rates of MS in Asian countries, associated with the presence of high rates of intracranial hemorrhage in individuals using VKA, the University of Hong Kong is conducting the DAVID-MS study (DAbigatran for Stroke PreVention In Atrial Fibrillation in MoDerate or Severe Mitral Stenosis The DAVID-MS study is a randomized, prospective, open, phase IV study, which aims to evaluate the efficacy and safety of the use of Dabigatran (150 mg or 110 mg according to creatinine clearance level, twice daily) compared with warfarin (targeting to INR 2–3) for preventing thromboembolism in individuals diagnosed with AF associated with moderate to severe MS (29).
Based on the finds of preclinical studies, and the RE-ALIN (Dabigatran vs. Warfarin in Patients with Mechanical Heart Valves) study (14), the ideal candidate for the use of DOACs (especially FXa inhibitors) in MHV (recognized for its fundamental action activating the intrinsic coagulation pathway by contact) could be considered in case of postoperative valve replacement (>3 months), aortic position (minor thrombogenicity), absence of systolic dysfunction, low risk of bleeding, absence of hypercoagulability states, and good drug adherence (25).
An experimental model with MHV, using an in vitro thrombosis tester, evaluated the efficacy of dabigatran as compared to unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) in thromboembolism prevention (30). Dabigatran was similarly effective in preventing clot formation compared to UFH and LMWH. However, serum levels 10 times higher than the RE-ALIGN trial doses. Therefore, the clinical application is not possible due to the risk of serious adverse effects concerning the use of warfarin. Other studies with MHV, using in vitro or animal models, demonstrated the efficacy of DOACs. Both dabigatran and rivaroxaban prevented thrombus formation as enoxaparin (31–33).
In this scenario, where is a large knowledge gap, new studies involving humans' subjects are required to evaluate the applicability of the use of Factor Xa (FXa) inhibitors in MHV, for the feasibility of developing a new phase III RCTs. The PROACT Xa study is being conducted aiming to determine if patients with a mechanical On-X aortic heart valve or On-X ascending aortic prosthesis can be maintained safely on apixaban (5 mg twice daily) in comparison to warfarin (INR range of 2.0–3.0). The PROACT Xa is a multicenter, prospective, and open-label study, which is in the phase of recruitment. The study estimates the randomized enrollment of 1,000 patients from 60 sites in North America who underwent aortic valve replacement at least 3 months prior, with an expected duration of 2 years of follow-up (34). The RENOVATE study (Randomized, Evaluation of Long-term Anticoagulation With Oral Factor Xa Inhibitor vs. Vitamin K Antagonist After Mechanical Aortic Valve Replacement) is also being conducted. It is a prospective Korean study (Asan Medical Center), open-label, phase IV, estimating the enrollment of 1,374 participants, which is not recruiting participants yet (35).
Lastly, FDA approved (2018) the use of betrixaban (a new FXa Inhibitor) for extended thromboprophylaxis, based on the results of the APEX study (Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients), a randomized, phase III, double-blind, double-dummy, active-controlled, and multinational clinical trial. In this study, the authors identified that extended prophylaxis with betrixaban led to a reduction in VTE compared with standard-duration enoxaparin, without an increase of hemorrhagic events (36). Betrixaban has the longest half-life among the DOACs, with an effective half-life of 19–27 h, and is mainly cleared via the hepatobiliary system. Therefore, its use is possible in case of severe renal insufficiency (37). To date, there is still no report of its use in valvular AF.
Limitations of the Study
Our updated meta-analysis has several limitations. First, most of our results were produced through information obtained in post-hoc analyses of large RCTs. The populations involved in the included studies in our analysis are relatively heterogeneous and analyze different drugs. Combined outcome analyses may overestimate or underestimate the benefit of the results found. Also, we did not included hard endpoints such as mortality. Further studies are required to establish the efficacy and safety of DOACs in valvular AF and the effects of their use in different valve diseases.
Conclusion
This study demonstrated that DOACs, compared to Warfarin, in patients with AF and VHD showed a reduction in thromboembolic events (stroke and systemic embolism), with a better safety profile (reduction in intracranial hemorrhage). Individual differences between each drug (rivaroxaban, apixaban, dagigatran, edoxaban) need to be clarified in further studies.
Data Availability Statement
The original contributions generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YB: conceptualization, methodology, and writing-original draft preparation. AD: supervision, investigation, and writing-original draft preparation. LR and MG: software, visualization, and investigation. LL-K: data curation, writing-review and editing, and validation. EB: writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.712585/full#supplementary-material
References
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
2. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000719
4. Pastori D, Lip G, Poli D, Antonucci E, Rubino L, Menichelli D, et al. Determinants of low-quality warfarin anticoagulation in patients with mechanical prosthetic heart valves The nationwide PLECTRUM study [published online ahead of print, 2020 Feb 20]. Br J Haematol. (2020) 190:588–93. doi: 10.1111/bjh.16528
5. Menichelli D, Poli D, Antonucci E, Cammisotto V, Testa S, Pignatelli P, et al. Comparison of anticoagulation quality between acenocoumarol and warfarin in patients with mechanical prosthetic heart valves: insights from the nationwide PLECTRUM study. Molecules. (2021) 26:1425. doi: 10.3390/molecules26051425
6. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. (2021) 143:e35–71. doi: 10.1016/j.jacc.2020.11.018
7. Caldeira D, David C, Costa J, Ferreira JJ, Pinto FJ. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease: systematic review and meta-analysis. Eur Hear J Cardiovasc Pharmacother. (2018) 4:111–8. doi: 10.1093/ehjcvp/pvx028
8. Pan K, Singer DE, Ovbiagele B, Wu Y, Ahmed MA, Lee M. Effects of non–vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. (2017) 6:e005835. doi: 10.1161/JAHA.117.005835
9. Renda G, Ricci F, Giugliano RP, Caterina R De. Non–vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Am Coll Cardiol. (2017) 69:1363–71. doi: 10.1016/j.jacc.2016.12.038
10. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
11. Schulman S, Anger SU, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. (2005) 8:202–4. doi: 10.1111/j.1538-7836.2009.03678.x
12. Higgins J, Altman D, Gotzsche P, Juni P, Moher D, Oxman A, et al. Chapter 8: assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collab (2011) 343:1–9.
13. Schünemann HJ, Schünemann AHJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. (2008) 336:1106–10. doi: 10.1136/bmj.39500.677199.AE
14. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. (2013) 369:1206–14. doi: 10.1056/NEJMoa1300615
15. Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Stevens SR, et al. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. (2014) 35:3377–85. doi: 10.1093/eurheartj/ehu305
16. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, et al. Apixaban compared with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the ARISTOTLE trial. Circulation. (2015) 132:624–32. doi: 10.1161/CIRCULATIONAHA.114.014807
17. Rodrigues Durães A, Pollianna de Souza Roriz B, Bianca de Almeida Nunes B, Pinho Albuquerque F, Fábio Vieira de Bulhões B, Andre Mauricio de Souza Fernandes B, et al. Dabigatran versus warfarin after bioprosthesis valve replacement for the management of atrial fibrillation postoperatively: DAWA pilot study. Drugs R D. (2016) 16:149–54. doi: 10.1007/s40268-016-0124-1
18. Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, et al. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart diseaseclinical perspective. Circulation. (2016) 134:589–98. doi: 10.1161/CIRCULATIONAHA.115.020950
19. Caterina R De, Renda G, Carnicelli AP, Nordio F, Trevisan M, Mercuri MF, et al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol. (2017) 69:1372–82. doi: 10.1016/j.jacc.2016.12.031
20. Guimarães H, Lopes R, Barros E, Silva P de, Liporace I, Sampaio R, Tarasoutchi F, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. (2020) 383:1–11. doi: 10.1056/NEJMoa2029603
21. Duraes AR, Souza Lima Bitar Y de, Schonhofen IS, Travassos KSO, Pereira LV, Filho JAL. Rivaroxaban versus warfarin in patients with mechanical heart valves: open-label, proof-of-concept trial—the RIWA STUDY. Am J Cardiovasc Drugs. (2021) 21:363–71. doi: 10.1007/s40256-020-00449-3
22. Guimarães PO, Pokorney SD, Lopes RD, Wojdyla DM, Gersh BJ, Giczewska A, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: Insights from the ARISTOTLE trial. Clin Cardiol. (2019) 42:568–71. doi: 10.1002/clc.23178
23. Carnicelli AP, Caterina R De, Halperin JL, Renda G, Ruff CT, Trevisan M, et al. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. (2017) 135:1273–5. doi: 10.1161/CIRCULATIONAHA.116.026714
24. Hicks T, Stewart F, Eisinga A. NOACs versus warfarin for stroke prevention in patients with AF: a systematic review and meta-analysis. Open Hear. (2016) 3:e000279. doi: 10.1136/openhrt-2015-000279
25. Aimo A, Giugliano RP, Caterina R De. Non–vitamin K antagonist oral anticoagulants for mechanical heart valves. Circulation. (2018) 138:1356–65. doi: 10.1161/CIRCULATIONAHA.118.035612
26. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener H-C, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. (2015) 17:1467–507. doi: 10.1093/europace/euv309
27. Baumgartner H, Falk V, Bax JJ, Bonis M De, Hamm C, Holm PJ, et al. ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2017) 38:2739–2791. doi: 10.1093/eurheartj/ehx391
28. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. (2017) 135:e1159–95. doi: 10.1161/CIR.0000000000000503
29. Zhou M, Chan EW, Hai JJ, Wong CK, Lau YM, Huang D, et al. Protocol, rationale and design of DAbigatran for Stroke PreVention In Atrial Fibrillation in MoDerate or Severe Mitral Stenosis (DAVID-MS): a randomised, open-label study. BMJ Open. (2020) 10:e038194. doi: 10.1136/bmjopen-2020-038194
30. Maegdefessel L, Linde T, Krapiec F, Hamilton K, Steinseifer U, Ryn J Van, et al. In vitro comparison of dabigatran, unfractionated heparin, and low-molecular-weight heparin in preventing thrombus formation on mechanical heart valves. Thromb Res. (2010) 126:e196–200. doi: 10.1016/j.thromres.2010.06.011
31. Forsberg P, DeSancho MT. Role of novel anticoagulants for patients with mechanical heart valves. Curr Atheroscler Rep. (2014) 16:448. doi: 10.1007/s11883-014-0448-7
32. Kaeberich A, Reindl I, Raaz U, Maegdefessel L, Vogt A, Linde T, et al. Comparison of unfractionated heparin, low-molecular-weight heparin, low-dose and high-dose rivaroxaban in preventing thrombus formation on mechanical heart valves: results of an in vitro study. J Thromb Thrombolysis. (2011) 32:417–25. doi: 10.1007/s11239-011-0621-6
33. Greiten LE, McKellar SH, Rysavy J, Schaff HV. Effectiveness of rivaroxaban for thromboprophylaxis of prosthetic heart valves in a porcine heterotopic valve model. Eur J Cardio-Thoracic Surg. (2014) 45:914–9. doi: 10.1093/ejcts/ezt545
34. Jawitz OK, Wang TY, Lopes RD, Chavez A, Boyer B, Kim H, et al. Rationale and design of PROACT Xa: a randomized, multicenter, open-label, clinical trial to evaluate the efficacy and safety of apixaban versus warfarin in patients with a mechanical On-X Aortic Heart Valve. Am Heart J. (2020) 227:91–9. doi: 10.1016/j.ahj.2020.06.014
35. NCT04258488. Long-term Anticoagulation With Oral Factor Xa Inhibitor Versus Vitamin K Antagonist After Mechanical Aortic Valve Replacement. Available online at: https://clinicaltrials.gov/show/NCT04258488 (accessed November 22, 2020).
36. Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. (2016) 375:534–44. doi: 10.1056/NEJMoa1601747
Keywords: valvular atrial fibrillation, valvular heart disease, warfarin, direct oral anticoagulants, anticoagulation
Citation: Bitar YdSL, Duraes AR, Roever L, Gomes Neto M, Lins-Kusterer L and Bocchi EA (2021) Comparison of the Direct Oral Anticoagulants and Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease: Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 8:712585. doi: 10.3389/fcvm.2021.712585
Received: 20 May 2021; Accepted: 29 July 2021;
Published: 22 September 2021.
Edited by:
Ying Hu Shen, Baylor College of Medicine, United StatesReviewed by:
Wei Luo, Fudan University, ChinaDanilo Menichelli, Sapienza University of Rome, Italy
Yinchuan Xu, Zhejiang University, China
Copyright © 2021 Bitar, Duraes, Roever, Gomes Neto, Lins-Kusterer and Bocchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasmin de Souza Lima Bitar, eWFzbWluLnVmYmFAZ21haWwuY29t
 Yasmin de Souza Lima Bitar
Yasmin de Souza Lima Bitar Andre Rodrigues Duraes
Andre Rodrigues Duraes Leonardo Roever
Leonardo Roever Mansueto Gomes Neto
Mansueto Gomes Neto Liliane Lins-Kusterer1,2
Liliane Lins-Kusterer1,2