
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 17 November 2021
Sec. Hypertension
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.710023
 Yang Wang1,2†
Yang Wang1,2† Ming-Fei Du1†
Ming-Fei Du1† Shi Yao3†
Shi Yao3† Ting Zou1
Ting Zou1 Xiao-Yu Zhang4
Xiao-Yu Zhang4 Gui-Lin Hu1
Gui-Lin Hu1 Chao Chu1,2
Chao Chu1,2 Yue-Yuan Liao1,2
Yue-Yuan Liao1,2 Chen Chen1
Chen Chen1 Dan Wang1,2
Dan Wang1,2 Qiong Ma1,2
Qiong Ma1,2 Ke-Ke Wang1,2
Ke-Ke Wang1,2 Yue Sun1,2
Yue Sun1,2 Ze-Jiaxin Niu1,2
Ze-Jiaxin Niu1,2 Rui-Chen Yan1,2
Rui-Chen Yan1,2 Yu Yan1,2
Yu Yan1,2 Hao-Wei Zhou1
Hao-Wei Zhou1 Hao Jia1
Hao Jia1 Wei-Hua Gao5
Wei-Hua Gao5 Hao Li6
Hao Li6 Chun-Hua Li7
Chun-Hua Li7 Fang-Yao Chen8
Fang-Yao Chen8 Ke Gao1
Ke Gao1 Jie Zhang9
Jie Zhang9 Robert Safirstein10
Robert Safirstein10 Feng Wang11
Feng Wang11 Tie-Lin Yang3*
Tie-Lin Yang3* Jian-Jun Mu1,2*
Jian-Jun Mu1,2*Background: Uromodulin, also named Tamm Horsfall protein, has been associated with renal function and regulation of sodium homeostasis. We aimed to examine the associations of serum uromodulin levels and its genetic variants with longitudinal blood pressure (BP) changes and hypertension incidence/risk.
Methods: A total of 514 participants from the original Baoji Salt-Sensitive Study cohort were genotyped to examine the associations of genetic variations in uromodulin gene with the longitudinal BP changes and the incidence of hypertension over 8 years of follow-up. In addition, 2,210 subjects from the cohort of Hanzhong Adolescent Hypertension Study were used to investigate the relationships between serum uromodulin levels and the risk of hypertension.
Results: SNPs rs12917707 and rs12708631 in the uromodulin gene were significantly associated with the longitudinal BP changes over 8 years of follow-up. SNP rs12708631 was significantly associated with the incidence of hypertension over 8 years. In addition, gene-based analyses supported the associations of uromodulin gene with the longitudinal BP changes and hypertension incidence in Baoji Salt-Sensitive Study cohort. Furthermore, serum uromodulin levels in the hypertensive subjects were lower than in the normotensive subjects (25.5 ± 1.1 vs. 34.7 ± 0.7 ng/mL). Serum uromodulin levels decreased gradually as BP levels increased (34.6, 33.2, 27.8, and 25.0 ng/mL for subjects with normotension, high-normal, grade 1 hypertension, and grade 2 hypertension, respectively). Serum uromodulin was significantly associated with the lower risk of hypertension [0.978 (0.972–0.984)] in Hanzhong Adolescent Hypertension Study cohort.
Conclusion: This study shows that uromodulin is associated with blood pressure progression and development of hypertension.
Hypertension, due to its high prevalence and its associated risk of cardiovascular disease and all-cause mortality, is considered as a major worldwide public health challenge (1, 2). Even a slight increase in blood pressure (BP) increases the risk of cardiovascular events (3). Sodium (Na+) homeostasis plays an important role in the regulation of BP, and small changes in the rate of its reabsorption may cause significant changes in Na+ excretion, leading to disturbances in the Na+ balance and extracellular fluid volume and ultimately to hypertension (4, 5).
Uromodulin, also known as Tamm-Horsfall protein, is a 95 kDa glycoprotein. Encoded by the UMOD gene located on chromosome 16p12.3 (6, 7), uromodulin is synthesized mainly by the thick ascending limb (TAL) and early distal convoluted tubule in the kidney (8). A large amount of uromodulin is secreted into the urinary tract and exerts anti-inflammatory, anti-infective and electrolyte treatment effects (9–12). In addition to its apical secretion, a small proportion of uromodulin is secreted from the basolateral side into the interstitial space and enters the blood (13). The physiological function of blood uromodulin is unknown, but it has emerged as a potential biomarker for renal function (14, 15). In addition, prior studies showed that genetic variants in the UMOD gene were associated with BP phenotypes and hypertension (16–18). However, no study has yet explored the associations of common variants in the UMOD gene with the longitudinal BP changes or the incidence of hypertension. Furthermore, data are scarce about the relationship between serum uromodulin levels and the risk of hypertension.
In the present study we used single marker–and gene–based analyses to prospectively evaluate the associations of UMOD gene with the longitudinal BP changes and the incidence of hypertension in a family-based cohort. In addition, we also used our large cross-sectional cohort to explore the possible relationships between serum uromodulin levels and the risk of hypertension.
The entire study consisted of two parts: (1) a longitudinal cohort study to explore the associations of UMOD gene with longitudinal BP changes and hypertension incidence; and (2) a cross-sectional cohort study to examine the relationships between serum uromodulin levels and the risk of hypertension.
Assembled from April to November 2004, the Baoji Salt-Sensitive Study cohort consists of 514 adults from 124 families in seven villages in Baoji, Shaanxi, China. The detailed design of this study has been published previously (19–24). To explore the associations of UMOD genetic variations with longitudinal BP changes and hypertension incidence, we followed up this cohort in 2009 and 2012. During each follow-up visit, a 3-day examination was performed as in 2004. Three BP measurements were obtained during each 3-day follow-up visit, and the mean of the nine BP measurements was used for the current analysis.
This protocol was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University, and adhered to the principles of the Declaration of Helsinki. Written informed consents were obtained from each participant (Trial registration number: NCT02915315. ClinicalTrials.gov).
In 1987, we established the cohort of Hanzhong Adolescent Hypertension Study, which was an ongoing prospective, population-based cohort study of 4,623 adolescents aimed to screen for cardiovascular risk factors that originate in childhood and adolescence. Detailed information of study has been published elsewhere (25–30). To explore the association of serum uromodulin levels with the risk of hypertension, we used cross-sectional analysis of the latest follow-up data in 2017. The participant selection process is described in Supplementary Figure 1. 2,780 participants were examined in 2017. After excluding 566 participants with missing data (e.g., serum or urinary biochemistry) and four participants with self-reported history of coronary heart disease, renal failure or stroke, 2,210 participants were included for the final analysis.
The study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University. This study followed the principles of the Declaration of Helsinki, and informed consent was obtained from study participants. The trial registration number was NCT02915315.
BP was measured in the sitting position by trained and certified observers using mercury sphygmomanometers. Smoking, drinking, coffee/tea and strenuous exercise were strictly prohibited for all participants at least 30 min before BP measurements. Systolic BP (SBP) and diastolic BP (DBP) were recorded at the first and fifth phases of the Korotkoff sounds. During the baseline survey and follow-ups, each subject needed to measure BP three times, and the average of nine BP measurements was calculated and recorded. The mean arterial pressure (MAP) was defined as DBP + [1/3 × (SBP – DBP)]. Pulse pressure (PP) was calculated as SBP – DBP. Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg or use of antihypertensive drugs (31). We categorized all subjects into normotension, high-normal BP, grade 1 and grade 2 hypertension according to the 2020 International Society of Hypertension global hypertension practice guidelines (31). The subtypes of hypertension were further defined as isolated systolic hypertension (ISH), isolated diastolic hypertension (IDH), and systolic diastolic hypertension (SDH) in the absence of antihypertensive treatment (31).
Total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine, serum uric acid (SUA) and serum glucose levels were analyzed by an automatic biochemical analyzer (Hitachi, Tokyo, Japan) as described previously (23, 32–34). Serum uromodulin levels were measured by commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotech, Wuhan, China). Five samples were randomly selected to evaluate intra and inter-assay coefficients of variation for uromodulin, which ranged from 2.3 to 5.9% and 3.7 to 6.8%, respectively.
Based on the Genome Variation Server database (http://gvs.gs.washington.edu/GVS147/) and the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/projects/SNP), we selected 13 tagged SNPs in the UMOD gene (rs77875418, rs4293393, rs7193058, rs4997081, rs11859916, rs13333226, rs79245268, rs4632135, rs4383153, rs12708631, rs7198000, rs6497476 and rs12917707). Genomic DNA was isolated and purified from the whole blood samples by the GoldMag-Mini kits (GoldMag Co. Ltd. Xian, China). All the genotyping experiments were completed by CapitalBio (CapitalBio Corp, Beijing, China) as previously described (19–22, 24).
For the analyses in the longitudinal cohort study, we used PLINK software (version 1.9, http://zzz.bwh.harvard.edu/plink/) to test the quality control, including genotyping call rate, Mendelian consistency, minor allele frequency and Hardy-Weinberg equilibrium on parental SNP data. The associations of each SNP with BP phenotypes were examined in three genetic models (dominant, recessive and additive) by mixed-effect regression models using lme function in nlme R package. For the analyses of the incidence of hypertension, 51 participants with hypertension diagnosed at baseline were excluded. The additive associations of each SNP with hypertension incidence were examined by a generalized linear mixed model, which allows multi-level modeling when the response variable follows a binary distribution (e.g., incident hypertension). Baseline age, gender and body mass index (BMI) as fixed effects and familial correlation as a random effect were adjusted in the multivariable analysis using glmer function in lme4 R package.
In addition, we used the truncated product method (TPM), which combines P values from each SNP association analysis, to evaluate the overall associations of UMOD gene with longitudinal BP changes and the incidence of hypertension (19, 24, 35). Gene-based analysis is a method that can evaluate the association between a trait and a candidate gene, and is performed with R software (version 3.0.1; http://www.r-project.org).
For the analyses of the cross-sectional cohort study, χ2-test, Student's t-test and Mann–Whitney test were used to compare the difference between groups as appropriate. One-way ANOVA was used to test the linearity across different hypertension grades and subtypes. We performed linear and logistic regression analyses to examine the associations of serum uromodulin levels with BP levels and the risk of hypertension. Multivariable models were adjusted for traditional cardiovascular risk factors and potential confounders. All statistical analyses were performed using SPSS 16.0 for windows (SPSS, Inc., Chicago, IL). P < 0.05 was considered statistically significant.
At baseline, the age of the participants was 48.6 years, BMI was 22.2 kg/m2, and the mean SBP, DBP, and MAP were 115.2, 71.3, and 86.0 mmHg, respectively. 51 (9.9%) subjects were diagnosed with hypertension at baseline. After 8 years of follow-up, the mean SBP, DBP, and MAP increased by 14.4, 6.6, and 9.1 mmHg, respectively, and 103 (28.9%) subjects developed hypertension (Table 1).
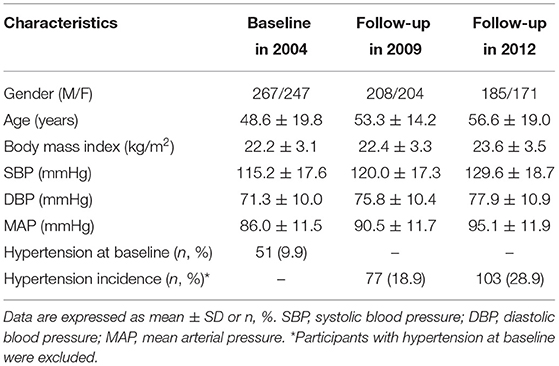
Table 1. Characteristics of the study participants at baseline and during follow-ups in the longitudinal cohort study.
Information on genotyped SNPs, including the genomic location, minor allele frequency, Hardy-Weinberg test and potential function prediction, are shown in Supplementary Table 1. No SNP deviated significantly from Hardy-Weinberg equilibrium.
The associations of each SNP in UMOD gene with the 5-year (2004–2009) and 8-year (2004–2012) BP changes are presented (Table 2). UMOD SNP rs12917707 and rs12708631 were significantly associated with the longitudinal changes in SBP, DBP, MAP and PP at both follow-ups. SNP rs11859916 was significantly associated with the 8-year change in PP. In addition, SNP rs12708631 was significantly associated with the incidence of hypertension (OR = 1.344, P = 0.035) over 8 years (Table 3). Gene-based analyses further showed that UMOD gene was significantly associated with the longitudinal SBP changes (PTPM = 0.004), MAP changes (PTPM = 0.035), PP changes (PTPM = 0.019) and hypertension incidence (PTPM = 0.044) over the 8-year follow-up after adjustment for multiple testing.
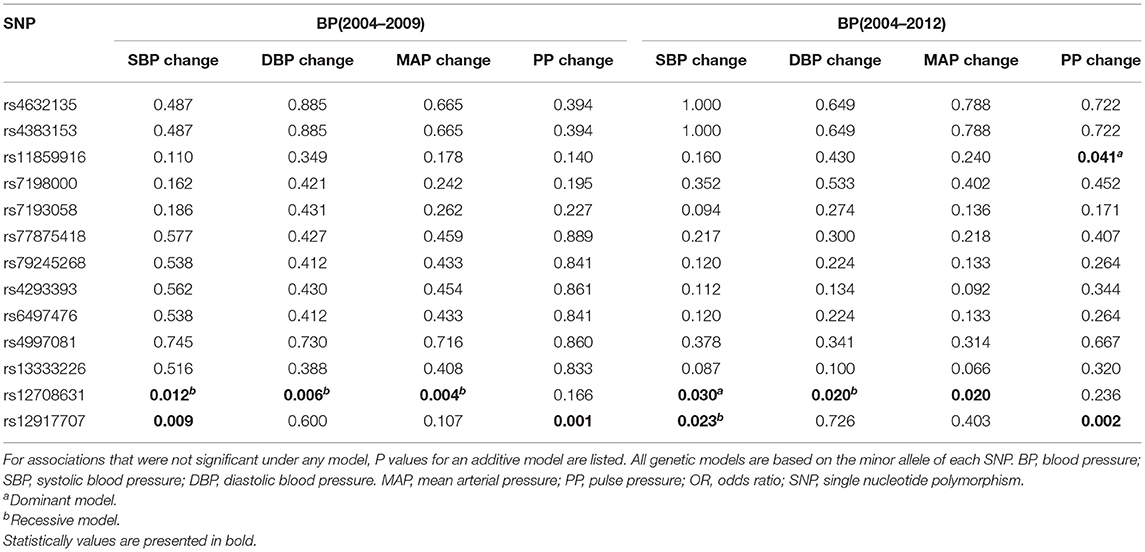
Table 2. Association of UMOD SNPs with longitudinal blood pressure changes changes blood pressure from baseline to the follow-ups.
Participants with hypertension were older, and are more likely to be men and to have higher BMI. Smoking, alcohol consumption, diabetes, and family history of hypertension were more common in the hypertension vs. normotension group. Compared with the normotension group, the following biochemical markers were higher in the hypertension group: SUA; blood glucose; ALT; AST; total cholesterol; triglycerides; LDL; urinary albumin/creatinine; and serum creatinine. In contrast, eGFR and HDL were lower in the hypertensive group (Table 4).
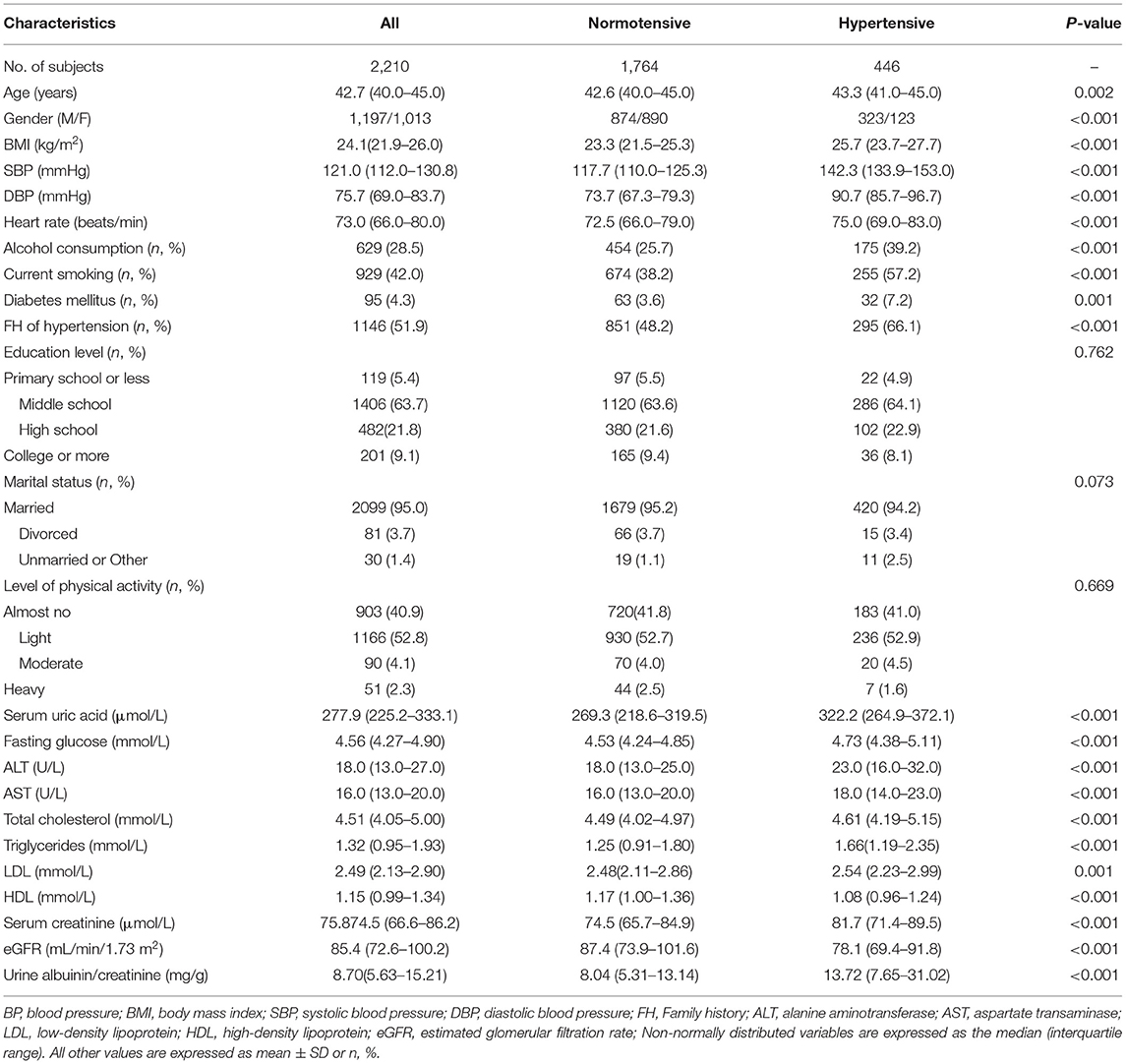
Table 4. Characteristics of participants categorized by BP status in the cross-sectional cohort study (n = 2,210).
Serum uromodulin levels were significantly lower in hypertensive subjects than in the normotensive subjects (25.5 ± 1.1 vs. 34.7 ± 0.7ng/mL, P < 0.001; Figure 1A). Next, we assessed serum uromodulin levels in different grades of hypertension, which was presented in Figure 1B. Serum uromodulin levels decreased gradually as BP levels increased (34.6, 33.2, 27.8, and 25.0 ng/mL for subjects with normotension, high-normal, grade 1 hypertension, and grade 2 hypertension, respectively, Pfortrend < 0.001). We further examined serum uromodulin in different groups with normotensive and hypertensive subtypes. Participants with ISH and SDH have lower serum uromodulin levels than normotensive subjects. Serum uromodulin was 34.1, 29.3, 26.2, and 27.1 ng/mL for subjects with normotension, IDH, ISH, and SDH, respectively (Pfortrend = 0.001, Figure 1C). In addition, we further divided all hypertensive and normotensive subjects into two groups according to the family history. No significant difference in serum uromodulin was found between any groups (Figure 1D).
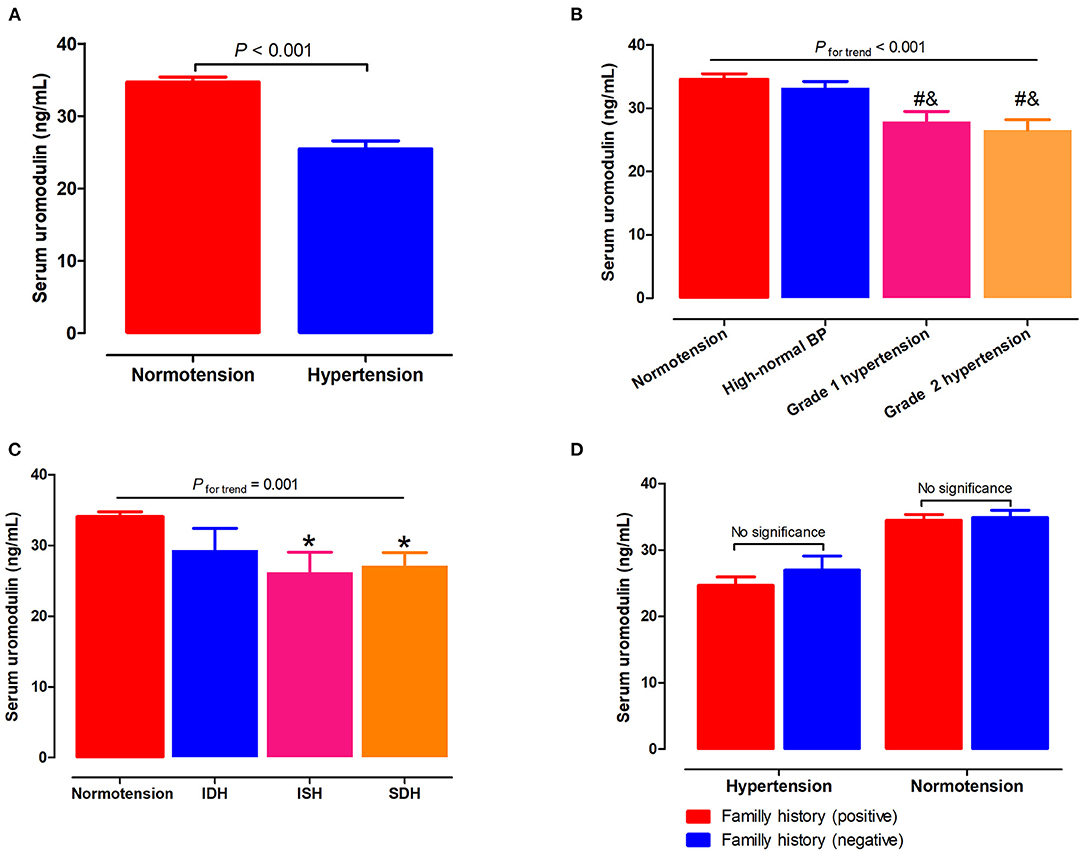
Figure 1. Associations of serum uromodulin with BP levels and hypertension. (A) Serum uromodulin levels in subjects with normotension and hypertension. (B) Serum uromodulin levels in subjects with different grades of BP, including normotension, high-normal, grade 1 hypertension, and grade 2 hypertension. #P < 0.05 vs. normotension, &P < 0.05 vs. high-normal BP. (C) Serum uromodulin levels in subjects with normotension and different hypertensive subtypes, including ISH, IDH, SDH, and those with controlled and uncontrolled BP in the absence of antihypertensive treatment. *P < 0.05 vs. normotension. (D) Serum uromodulin levels in hypertensive or normotensive subjects with a family history of hypertension and those without. BP, blood pressure; ISH, isolated systolic hypertension (SBP ≥ 140 mmHg and DBP <90 mmHg); IDH, isolated diastolic hypertension (SBP <140 mmHg and DBP ≥ 90 mmHg); SDH, systolic diastolic hypertension (SBP ≥ 140 mmHg and DBP ≥ 90 mmHg).
SBP levels positively correlated with age, BMI, glucose, serum creatinine and total cholesterol, but inversely correlated with gender and serum uromodulin (β = −0.075, P = 0.001). In addition, DBP levels were also positively correlated with age, BMI, glucose, serum creatinine and total cholesterol, but negatively correlated with gender and serum uromodulin (β = −0.084, P < 0.001, Table 5). Furthermore, serum uromodulin was significantly associated with a lower risk of hypertension after adjusting for multiple confounders [0.978 (0.972–0.984), P < 0.001; Figure 2].
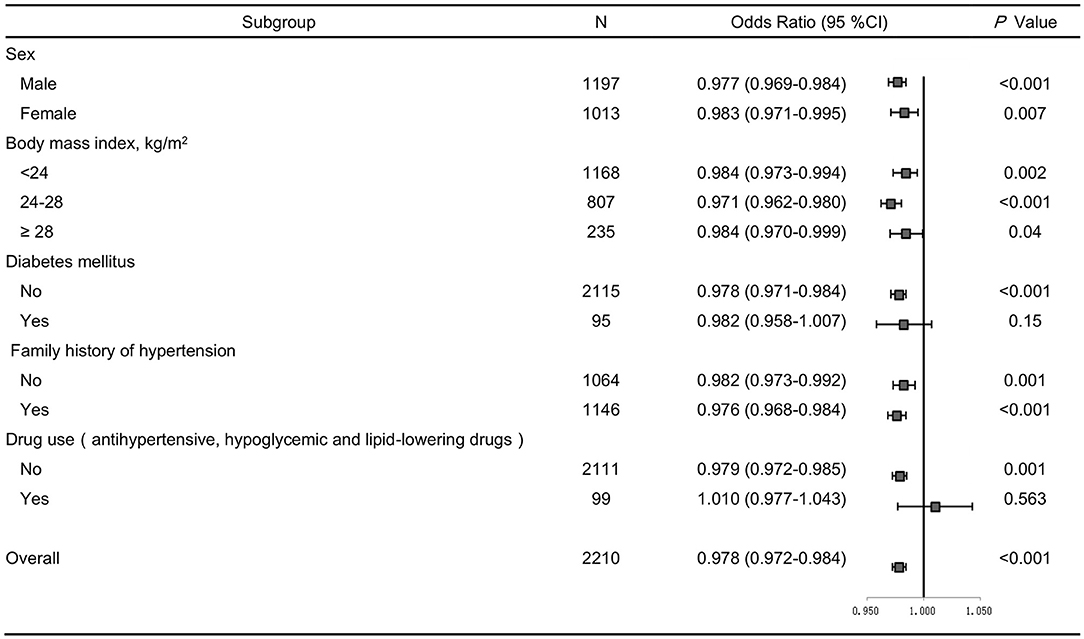
Figure 2. Forest plots of odds ratios (ORs) for serum uromodulin and risk of hypertension after adjustment. The adjustment model includes age, smoke, BMI, serum creatinine, total cholesterol, triglycerides, fasting glucose in subjects stratified by sex, BMI, diabetes, family history of hypertension, and drug use. Values are the OR (95% confidence interval [95% CI]).
We further performed several sensitivity analyses to test the robustness of the analysis. After excluding participants with diabetes, or those taking antihypertensive, hypoglycemic and lipid-lowering drugs, similar results were obtained (Figure 2). In addition, we stratified all participants by sex, BMI, family history of hypertension, and the results remained the same (Figure 2).
Previous animal studies showed that uromodulin may be involved in BP regulation and the development of hypertension. Graham et al. (36) found that UMOD knockout (UMOD−/−) mice had significantly lower SBP than wild-type mice and were resistant to salt-induced BP changes. In the UMOD knockout mice, the pressure–natriuresis curve shifted to the left. By contrast, Trudu et al. (37) showed that UMOD overexpression increased BP in a dose-dependent manner due to the increased UMOD expression and excretion. These studies suggest that uromodulin may affect the development of hypertension by modifying sodium transport in the TAL. A previous clinical study also has shown a link between UMOD gene and hypertension. A large genome-wide association studies (GWAS) from the European cohorts showed that the minor G allele of rs13333226 had a lower risk of hypertension (17). By contrast, Algharably et al. (38) found that no significant associations between rs12917707 and mean 24 h SBP or DBP or any other BP phenotype were detected a cohort of 1,218 white individuals. Our long-term follow-up study showed for the first time that novel UMOD markers rs12708631 and rs12917707 were associated with longitudinal BP changes. In addition, this is the first study to explore the association between UMOD and the incidence of hypertension over time. UMOD variant rs12708631 was significantly associated with the incidence of hypertension during the 8-year follow-up. In the gene-based analyses, UMOD was aggregately associated with the incidence of hypertension. The different study populations, sample sizes, and racial differences among these various studies may be the causes of the discrepant results. The associations of UMOD gene variants with hypertension susceptibility may be linked to the role of uromodulin in regulating sodium-potassium-chloride cotransporter NKCC2 in the TAL and suggest that uromodulin is a potential therapeutic target for hypertension (39).
Several studies have shown that urinary uromodulin was independently associated with a rapid decline in renal function and the incidence of end-stage renal disease (ESRD) (40–42). While most studies in the past have focused on urinary uromodulin, few studies have looked at uromodulin in blood. Recently, Steubl et al. and Bostom et al. reported the associations of blood uromodulin levels with renal function and recommended its use as a potential kidney biomarker (14, 43, 44). In our study, subjects with hypertension had significantly lower serum uromodulin levels than those without hypertension. In contrast to the previous studies that used only the dichotomous definitions of hypertension, our study reports grade-specific hypertension pursuant to the 2020 ISH guideline classification. To our knowledge, ours is the first study to apply such definitions. We found that serum uromodulin showed a linear decrease from normotension to grade 2 hypertension, suggesting that circulating uromodulin may be a novel marker for identifying stages of hypertension. In addition, our study is the first to explore serum uromodulin levels in different hypertension subtypes. ISH is usually characterized as an aging phenomenon. Unlike DBP, SBP increases with age as arterial stiffness increases and arterial compliance decreases (45). SBP has been shown to be a more reliable predictor of adverse cardiovascular events than DBP (46, 47). In the present study, serum uromodulin in the ISH and SDH groups was significantly lower than the normotension group, but was similar to the IDH group. These data indicate serum uromodulin may be an independent marker of hypertension that identifies its subtypes and grades.
The current study has several strengths. First, we performed stringent quality control procedures in genotyping and data collection. At baseline and each follow-up survey, we used the mean values of 9 separate BP measurements for the final analysis, thereby reducing measurement errors. Furthermore, our study comprehensively examined the associations of serum uromodulin levels with hypertension and its subtypes and grades. However, several limitations should be acknowledged. The novel findings in our study need to be replicated in other cohorts of different genetic backgrounds. Additionally, due to the limited number of genotyped SNPs in the UMOD gene in this study, less frequent genetic variants may have been omitted.
In conclusion, based on both single-marker and gene-based analyses, we report for the first time that UMOD gene was associated with longitudinal BP phenotypes and hypertension incidence. Furthermore, our study also showed that serum uromodulin level was significantly associated with hypertension and its subtypes and grades. These findings suggest that serum uromodulin may serve as a biomarker marker for hypertension. In addition, this work contributes to the accumulating evidence that genomic differences regulate BP and the development of hypertension.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
YW and J-JM conceived and designed the experiments. J-JM was responsible for subject recruitment. YW, X-YZ, TZ, CChu, CChen, DW, Y-YL, QM, K-KW, Z-JN, R-CY, YY, H-WZ, HJ, W-HG, HL, C-HL, KG, and JZ performed the experiments. T-LY, SY, F-YC, YW, and M-FD analyzed the data. YW, M-FD, and SY drafted the paper. FW, RS, and J-JM edited and revised manuscript. All authors have read, critically revised, and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China Nos. 81600327 (YW) and 81870319, 82070437 (J-JM), Natural Science Basic Research Program of Shaanxi Province (2021JM-257, 2021JM-588), Grants from China Postdoctoral Science Foundation funded project (Nos. 2018T111075 and 2018M631177), Institutional Foundation of the First Affiliated Hospital of Xi'an Jiaotong University No. 2019QN-06, Grants from the Major Chronic Non-communicable Disease Prevention and Control Research Key Project of the Ministry of Science and Technology of China (2017YFC1307604), and the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University of China No. XJTU1AF-CRF-2019-004.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would particularly like to thank Dr. John Chang (Section of Nephrology, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut, USA) for his language editing. The authors are grateful to the grassroots health staff in Hanzhong and Baoji for providing administrative and technical support during the follow-up. The abstract of this study has been presented at the Joint Meeting of the European Society of Hypertension (ESH) and the International Society of Hypertension (ISH) in 2021.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.710023/full#supplementary-material
1. Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 17 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. (2017) 390:2549–58. doi: 10.1016/S0140-6736(17)32478-9
2. Beaney T, Burrell LM, Castillo RR, Charchar FJ, Cro S, Damasceno A, et al. May measurement month 2018: a pragmatic global screening campaign to raise awareness of blood pressure by the International Society of Hypertension. Eur Heart J. (2019) 40:2006–17. doi: 10.1093/eurheartj/ehz300
3. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360:1903–13. doi: 10.1016/S0140-6736(02)11911-8
4. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. (2005) 85:679–715. doi: 10.1152/physrev.00056.2003
5. Frame AA, Wainford RD. Mechanisms of altered renal sodium handling in age-related hypertension. Am J Physiol Renal Physiol. (2018) 315:F1–6. doi: 10.1152/ajprenal.00594.2017
6. Devuyst O, Dahan K, Pirson Y. Tamm-Horsfall protein or uromodulin: new ideas about an old molecule. Nephrol Dial Transplant. (2005) 20:1290–4. doi: 10.1093/ndt/gfh851
7. Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. (2003) 42:658–76. doi: 10.1016/S0272-6386(03)00829-1
8. Pennica D, Kohr WJ, Kuang WJ, Glaister D, Aggarwal BB, Chen EY, et al. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. (1987) 236:83–8. doi: 10.1126/science.3453112
9. Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol. (2017) 13:525–44. doi: 10.1038/nrneph.2017.101
10. Raffi HS, Bates JM Jr, Laszik Z, Kumar S. Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J Urol. (2009) 181:2332–8. doi: 10.1016/j.juro.2009.01.014
11. Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. (2004) 66:1159–66. doi: 10.1111/j.1523-1755.2004.00867.x
12. Kreft B, Jabs WJ, Laskay T, Klinger M, Solbach W, Kumar S, et al. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun. (2002) 70:2650–6. doi: 10.1128/IAI.70.5.2650-2656.2002
13. El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. (2013) 304:F1066–1075. doi: 10.1152/ajprenal.00543.2012
14. Bostom A, Steubl D, Garimella PS, Franceschini N, Roberts MB, Pasch A, et al. Serum uromodulin: a biomarker of long-term kidney allograft failure. Am J Nephrol. (2018) 47:275–82. doi: 10.1159/000489095
15. Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, et al. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant. (2018) 33:284–95. doi: 10.1093/ndt/gfw422
16. Han J, Chen Y, Liu Y, Liang Y, Wang X, Liu L, et al. Common variants of the UMOD promoter associated with blood pressure in a community-based Chinese cohort. Hypertens Res. (2012) 35:769–74. doi: 10.1038/hr.2012.51
17. Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. (2010) 6:e1001177. doi: 10.1371/journal.pgen.1001177
18. Ahluwalia TS, Lindholm E, Groop L, Melander O. Uromodulin gene variant is associated with type 2 diabetic nephropathy. J Hypertens. (2011) 29:1731–4. doi: 10.1097/HJH.0b013e328349de25
19. Wang Y, Zhou Q, Gao WH, Yan Y, Chu C, Chen C, et al. Association of plasma cyclooxygenase-2 levels and genetic polymorphisms with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. J Hypertens. (2020) 38:1745–54. doi: 10.1097/HJH.0000000000002473
20. Chu C, Wang Y, Ren KY, Yan DY, Guo TS, Zheng WL, et al. Genetic variants in adiponectin and blood pressure responses to dietary sodium or potassium interventions: a family-based association study. J Hum Hypertens. (2016) 30:563–70. doi: 10.1038/jhh.2016.5
21. Chu C, Wang Y, Wang M, Mu JJ, Liu FQ, Wang L, et al. Common variants in Serum/Glucocorticoid Regulated Kinase 1 (SGK1) and blood pressure responses to dietary sodium or potassium interventions: a family-based association study. Kidney Blood Press Res. (2015) 40:424–34. doi: 10.1159/000368518
22. Liu F, Zheng S, Mu J, Chu C, Wang L, Wang Y, et al. Common variation in with no-lysine kinase 1 (WNK1) and blood pressure responses to dietary sodium or potassium interventions- family-based association study. Circ J. (2013) 77:169–74. doi: 10.1253/circj.CJ-12-0900
23. Wang Y, Chu C, Wang KK, Hu JW, Yan Y, Lv YB, et al. Effect of salt intake on plasma and urinary uric acid levels in Chinese adults: an interventional trial. Sci Rep. (2018) 8:1434. doi: 10.1097/01.hjh.0000539339.99882.e6
24. Wang Y, Jia H, Gao WH, Zou T, Yao S, Du MF, et al. Associations of plasma PAPP-A2 and genetic variations with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. J Hypertens. (2021). 39:1817–25. doi: 10.1097/HJH.0000000000002846
25. Zheng W, Mu J, Chu C, Hu J, Yan Y, Ma Q, et al. Association of blood pressure trajectories in early life with subclinical renal damage in middle age. J Am Soc Nephrol. (2018) 29:2835–46. doi: 10.1681/ASN.2018030263
26. Wang Y, Chen C, Yan Y, Yuan Y, Wang KK, Chu C, et al. Association of uric acid in serum and urine with subclinical renal damage: Hanzhong adolescent hypertension study. PLoS ONE. (2019) 14:e0224680. doi: 10.1371/journal.pone.0224680
27. Wang Y, Zhang XY, Gao WH, Du MF, Chu C, Wang D, et al. Association of uric acid in serum and urine with arterial stiffness: Hanzhong adolescent hypertension study. Dis Markers. (2020) 2020:1638515. doi: 10.1155/2020/1638515
28. Liao YY, Chu C, Wang Y, Zheng WL, Ma Q, Hu JW, et al. Sex differences in impact of long-term burden and trends of body mass index and blood pressure from childhood to adulthood on arterial stiffness in adults: A 30-year cohort study. Atherosclerosis. (2020) 313:118–25. doi: 10.1016/j.atherosclerosis.2020.10.003
29. Wang Y, Du MF, Gao WH, Fu BW, Ma Q, Yan Y, et al. Risk factors for subclinical renal damage and its progression: Hanzhong adolescent hypertension study. Eur J Clin Nutr. (2021) 75:531–8. doi: 10.1038/s41430-020-00752-x
30. Liao YY, Ma Q, Chu C, Wang Y, Zheng WL, Hu JW, et al. The predictive value of repeated blood pressure measurements in childhood for cardiovascular risk in adults: the Hanzhong adolescent hypertension study. Hypertens Res. (2020) 43:969–78. doi: 10.1038/s41440-020-0480-7
31. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. J Hypertens. (2020) 38:982–1004. doi: 10.1097/HJH.0000000000002453
32. Wang Y, Hu JW, Qu PF, Wang KK, Yan Y, Chu C, et al. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci Rep. (2018) 8:7749. doi: 10.1038/s41598-018-26148-3
33. Wang Y, Yuan Y, Gao WH, Yan Y, Wang KK, Qu PF, et al. Predictors for progressions of brachial-ankle pulse wave velocity and carotid intima-media thickness over a 12-year follow-up: Hanzhong adolescent hypertension study. J Hypertens. (2019) 37:1167–75. doi: 10.1097/HJH.0000000000002020
34. Yuan Y, Chu C, Zheng WL, Ma Q, Hu JW, Wang Y, et al. Body mass index trajectories in early life is predictive of cardiometabolic risk. J Pediatr. (2020) 219:31–7.e6. doi: 10.1016/j.jpeds.2019.12.060
35. Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS. Truncated product method for combining P-values. Genet Epidemiol. (2002) 22:170–85. doi: 10.1002/gepi.0042
36. Graham LA, Padmanabhan S, Fraser NJ, Kumar S, Bates JM, Raffi HS, et al. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension. (2014) 63:551–8. doi: 10.1161/HYPERTENSIONAHA.113.01423
37. Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med. (2013) 19:1655–60. doi: 10.1038/nm.3384
38. Algharably E, Bolbrinker J, Lezius S, Reibis R, Wegscheider K, Völler H, et al. Uromodulin associates with cardiorenal function in patients with hypertension and cardiovascular disease. J Hypertens. (2017) 35:2053–8. doi: 10.1097/HJH.0000000000001432
39. Scolari F, Izzi C, Ghiggeri GM. Uromodulin: from monogenic to multifactorial diseases. Nephrol Dial Transplant. (2015) 30:1250–6. doi: 10.1093/ndt/gfu300
40. Garimella PS, Katz R, Ix JH, Fried LF, Kritchevsky SB, Devarajan P, et al. Association of urinary uromodulin with kidney function decline and mortality: the health ABC study. Clin Nephrol. (2017) 87:278–86. doi: 10.5414/CN109005
41. Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Kemmner S, et al. Urinary uromodulin independently predicts end-stage renal disease and rapid kidney function decline in a cohort of chronic kidney disease patients. Medicine (Baltimore). (2019) 98:e15808. doi: 10.1097/MD.0000000000015808
42. Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int. (2015) 88:1126–34. doi: 10.1038/ki.2015.192
43. Steubl D, Buzkova P, Garimella PS, Ix JH, Devarajan P, Bennett MR, et al. Association of serum uromodulin with ESKD and kidney function decline in the elderly: the cardiovascular health study. Am J Kidney Dis. (2019) 74:501–9. doi: 10.1053/j.ajkd.2019.02.024
44. Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, et al. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore). (2016) 95:e3011. doi: 10.1097/MD.0000000000003011
45. Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults:the 1999-2004 US National Health And Nutrition Examination Survey. J Hypertens. (2010) 28:15–23. doi: 10.1097/HJH.0b013e328331b7ff
46. Antikainen R, Jousilahti P, Tuomilehto J. Systolic blood pressure, isolated systolic hypertension and risk of coronary heart disease, strokes, cardiovascular disease and all-cause mortality in the middle-aged population. J Hypertens. (1998) 16:577–83. doi: 10.1097/00004872-199816050-00004
Keywords: gene polymorphism, hypertension, uromodulin (UMOD), longitudinal change, blood pressure
Citation: Wang Y, Du M-F, Yao S, Zou T, Zhang X-Y, Hu G-L, Chu C, Liao Y-Y, Chen C, Wang D, Ma Q, Wang K-K, Sun Y, Niu Z-J, Yan R-C, Yan Y, Zhou H-W, Jia H, Gao W-H, Li H, Li C-H, Chen F-Y, Gao K, Zhang J, Safirstein R, Wang F, Yang T-L and Mu J-J (2021) Associations of Serum Uromodulin and Its Genetic Variants With Blood Pressure and Hypertension in Chinese Adults. Front. Cardiovasc. Med. 8:710023. doi: 10.3389/fcvm.2021.710023
Received: 14 May 2021; Accepted: 26 October 2021;
Published: 17 November 2021.
Edited by:
Maddalena Illario, University of Naples Federico II, ItalyReviewed by:
Birsen Ucar, Eskişehir Osmangazi University, TurkeyCopyright © 2021 Wang, Du, Yao, Zou, Zhang, Hu, Chu, Liao, Chen, Wang, Ma, Wang, Sun, Niu, Yan, Yan, Zhou, Jia, Gao, Li, Li, Chen, Gao, Zhang, Safirstein, Wang, Yang and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tie-Lin Yang, eWFuZ3RpZWxpbkB4anR1LmVkdS5jbg==; Jian-Jun Mu, bXVqanVuQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.