- 1Department of Endocrinology & Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Diabetology, Guangzhou, China
- 2VIP Medical Service Center, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Department of Clinical Immunology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background: Chronic inflammation in type 2 diabetes mellitus (T2DM) is an essential contributor to the development of diabetic retinopathy (DR). The monocyte–to–high-density lipoprotein cholesterol (HDL-C) ratio (MHR) is a novel and simple measure related to inflammatory and oxidative stress status. However, little is known regarding the role of the MHR in evaluating the development of DR.
Methods: A total of 771 patients with T2DM and 607 healthy controls were enrolled in this cross-sectional study. MHR determination and eye examination were performed. The association of MHR with the prevalence of DR in T2DM patients was analyzed.
Results: The MHR in patients with DR was significantly higher than that in both non-DR diabetic patients (P < 0.05) and healthy controls (P < 0.01). No significance was observed in the MHR of different DR severity grades. Moreover, the MHR was similar between patients with non-macular oedema and those with macular oedema. Logistic regression analysis demonstrated that MHR was independently associated with the prevalence of DR in diabetic patients [odds ratio (OR) = 1.438, 95% confidence interval (CI): 1.249–1.655, P < 0.01]. After additional stratification by HbA1c level and diabetic duration, the MHR was still independently associated with the prevalence of DR.
Conclusions: Our study suggests that the MHR can be used as a marker to indicate the prevalence of DR in patients with T2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is a highly and rapidly evolving global health issue (1, 2), even in patients with prediabetes, the risk of macrovascular and microvascular disease were increased (3–5). Diabetic retinopathy (DR) is one of the most important diabetic microvascular complications and a leading cause of irreversible blindness among the working-age population around the world (6). Although the underlying molecular mechanisms of DR are not yet fully understood, abundant evidence indicates that inflammation plays a key role in the pathophysiology of DR (7). Various inflammatory cytokines and chemokines, such as ICAM-1, IL-1β, IL-6, IL-8, TNF-α, and MCP-1, have been reported to be elevated in the serum and vitreous and aqueous humor from diabetic patients with DR (8).
Monocytes are released from their precursors in bone marrow into the circulation and migrate to tissues and release proinflammatory cytokines at sites of inflammation, thereby affecting the severity of inflammation, which is considered an inflammatory biomarker (9). Additionally, plasma high-density lipoprotein cholesterol (HDL-C) has an inverse relationship with DR risk (10, 11). In addition, HDL-C has antioxidant efficacy to protect endothelial functions (12, 13). Therefore, the monocyte count–to–HDL-C ratio (MHR) can reflect the inflammatory status and is related to the development of disease associated with chronic inflammation. The MHR has been found to be associated with the occurrence and prognosis of cardiovascular diseases (CVDs), diabetic nephropathy and diabetic peripheral neuropathy (14–17). However, to the best of our knowledge, there are a few studies with small sample size that evaluated the associations of the MHR with DR and got contradictory conclusion (18, 19). Further studies investigating the relationship between MHR and DR in larger patient groups are needed. Thus, in the present study, we aimed to investigate the associations between the MHR and the prevalence of DR in adults with T2DM.
Methods
Study Population
A total of 1,378 subjects between the ages of 18 and 70 years, including 771 patients with T2DM and 607 healthy individuals, were included consecutively in this observational cross-sectional study between January 2016 and December 2018. T2DM patients were diagnosed based on the 1999 criteria of the World Health Organization (WHO) (20). The exclusion criteria were as follows: active or chronic inflammation, active infection, autoimmune diseases, hematological disorder, recent blood transfusion before enrolment, malignancy, acute or chronic renal/hepatic diseases, or coronary artery disease. Ethics approval was obtained from the Third Affiliated Hospital of Sun Yat-sen University Network Ethics Committee.
Data Collection and Laboratory Analysis
Baseline information, including age, sex, comorbidities, smoking status, alcohol intake, medications, body height, weight, and blood pressure, was collected from medical records. Laboratory assessments consisted of fasting blood glucose, liver and renal function, uric acid, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), which were examined by a HITACHI (Tokyo, Japan) 7180 Automatic Analyser using 8-h overnight fasting blood samples. HbA1c was measured by high-performance liquid chromatography (HPLC) with a D-10 hemoglobin testing program (Bio-Rad). White blood cell measurement was performed with an automated hematology analyser XE-1200 (Sysmex, Kobe, Japan). The MHR ratio was calculated by dividing the monocyte count by HDL-C.
Eye Examination
Eye examinations were performed on all participants according to standard operation procedures by trained ophthalmologists. The eye examinations included visual acuity measurements, tonometry, intraocular pressure, an anterior ocular structure, and fundus examination using a standard protocol. The external and anterior ocular segments were examined by slit lamp biomicroscopy (BQ900; Haag-Streit, Bern, Switzerland). Two 45° field digital, color, non-stereoscopic fundal photographs of each eye were taken in the macula-centered and posterior pole by a non-mydriatic auto-fundus camera (TRC-NW400 Non-Mydriatic Retinal Camera, Topcon, Tokyo, Japan).
Assessment of DR
Two physicians made the assessment of DR independently (Kappa index = 0.919, P < 0.0001, indicating an excellent agreement between two physician). DR was diagnosed if any characteristic lesions existed as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS). DR severity was further categorized into mild, moderate and severe non-proliferative DR (NPDR) and proliferative DR (PDR). Another important additional categorization in DR was diabetic macular oedema (DME) and non-DME (21).
Statistical Analyses
Database management and statistical analysis were performed using PASW 22.0 for Windows (IBM Inc., Armonk, USA). Continuous variables are presented as the means ± standard deviation or median (interquartile range), while categorical variables are expressed as numbers (percentages). One-way ANOVA was applied for the comparison of continuous variables among groups, and a post-hoc test using Fisher's least significant difference (LSD) was used to determine which means differed following ANOVA. Differences in categorical variables were evaluated by Pearson's chi-square test. Univariate logistic regression analysis was performed to assess the non-adjusted relationships between 10*MHR and the prevalence of DR. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for the association between DR and 10*MHR. Then, two multivariate logistic regression models were performed to adjust for confounding factors. Model 1 was adjusted for age, sex. Model 2 was additionally adjusted for body mass index, diabetes duration, smoking status, SBP, DBP, triglyceride, LDL-C, Cr, UA, FBG, HbA1c, and medications. To determine whether the duration of diabetes and glucose control status affect the relationship between MHR and the prevalence of DR, subgroup analyses were performed based on the duration of diabetes (<10 and ≥10 years) and HbA1c levels (<7.0 and ≥7.0%). A two-tailed P < 0.05 was considered statistically significant.
Results
The Clinical Characteristics of the Participants
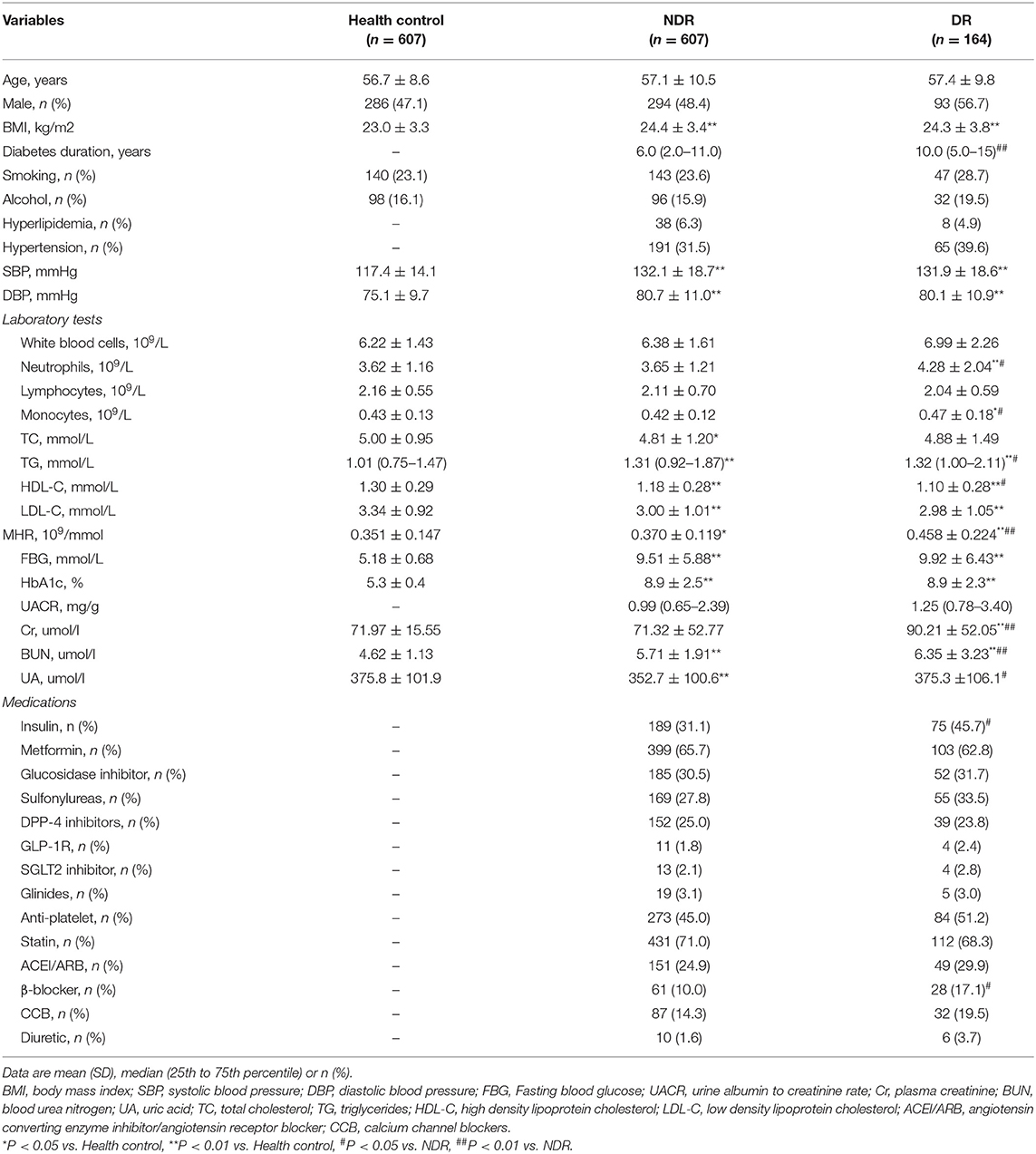
Of 771 patients with T2DM, 164 (21%) were DR patients. The clinical characteristics of all participants are summarized in Table 1. No significant differences were observed in terms of age, sex, smoking status, or alcohol intake among all groups. Body mass index (BMI), blood pressure, monocyte counts, TGs, fasting blood glucose, HbA1c, and BUN in the healthy control group were lower than those in T2DM patients, while HDL-C and LDL-C were higher (all P < 0.05). Among all subjects with T2DM, the diabetic duration in the subjects with DR was much longer than that in the subjects with non-DR (P < 0.01). Higher neutrophil counts, monocyte counts, TGs, Cr, BUN, UA, and insulin use and lower levels of HDL-C were observed in patients with DR (all P < 0.05). However, no significant differences were observed in hyperlipidemia, blood pressure, fasting blood glucose, HbA1c, UACR, antiplatelet use, or statin use between subjects with DR and non-DR.
The Association Between MHR and DR
Compared to that in the healthy controls, the level of MHR was remarkably increased in patients with T2DM (P < 0.01), as shown in Figure 1A. The MHR in patients with DR was significantly higher than that of both the diabetic patients without DR and the healthy controls (P < 0.01). No significant difference was observed in DR subjects with different severity or between subjects with DME and non-DME subjects (Figures 1B,C).

Figure 1. The association between the MHR and patients with DR. (A) MHR levels in healthy controls, diabetic patients with and without DR; (B) MHR levels in DR subjects with different severity; (C) MHR levels between subjects with DME and non-DME.
Univariate and Multivariate Logistic Regression Analysis
Univariate logistic regression analysis demonstrated that 10*MHR was associated with the development of DR [OR (95% CI) = 1.435 (1.277–1.614), P < 0.001] (Table 2). After adjusting for age and sex, 10*MHR was still independently related to the development of DR [OR (95% CI) = 1.424 (1.263–1.605), P < 0.001]. When further adjusting for BMI, diabetes duration, smoking status, SBP, DBP, triglyceride, LDL-C, Cr, UA, FBG, HbA1c, and medications, 10*MHR remained independently associated with the prevalence of DR [OR (95% CI) = 1.438 (1.249–1.655), P < 0.001].
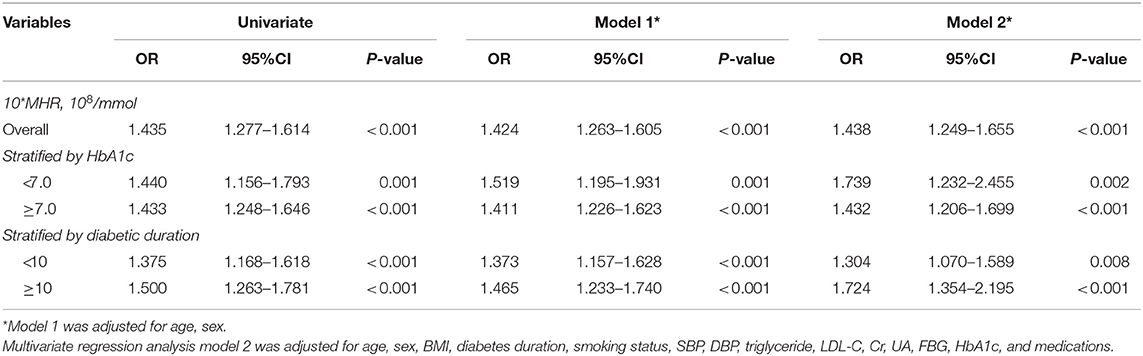
Table 2. Logistic regression analysis assessing the association of the MHR with diabetic retinopathy.
Subgroup Analysis
The most consistent risk factors for the development of DR are long duration of diabetes and hyperglycemia (6, 22, 23). A reasonable HbA1c level is below or around 7% and longer duration of diabetes was 8–11 years according to the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) (24, 25). To preclude the influence of the duration of diabetes and glucose control status, which were introduced in the subgroup analysis, as shown in Table 2, we further performed a subgroup regression analysis stratified by HbA1c levels (<7 vs. ≥7%) and the duration of diabetes (<10 vs. ≥10 years). In multivariate logistic regression Model 2, the 10*MHR group had a significantly higher prevalence of DR regardless of the level of HbA1c (P < 0.05) and diabetic duration (P < 0.01).
Discussion
In the present study, the results provide evidence about the unique association between the MHR and DR. Elevated MHR levels were associated with increased odds of DR, independent of a variety of conventional DR risk factors. However, we did not detect a significant association between the MHR level and DR severity or macular edema in patients with T2DM.
Inflammation markers and monocytes play an important role in the development of diabetic complications (16, 26, 27). Retinal chronic inflammation plays a pivotal role in the development of DR (6, 7). The levels of monocytes are increased in the retinal vessels and differentiate into macrophages that secrete inflammatory cytokines and growth factors adhering to the outer surface of retinal capillaries, leading to the breakdown of the blood retinal barrier, increased retinal vascular permeability and capillary non-perfusion, which are considered characteristic pathologic features in early DR (28, 29). In the present study, neutrophil and monocyte counts were remarkably increased in patients with DR compared to healthy controls or patients without DR, which is consistent with the findings in previous studies. Hyperglycemia enhances the inflammatory status to release more neutrophils and monocytes from bone marrow and then recruit them into the retinal vessels, causing damage to these vessels (30).
Lipid disorders seem to contribute to the development and progression of DR (31, 32). Accumulated evidence indicates that poor control of triglycerides and LDL is associated with the incidence and progression of DR, while higher HDL-C levels and the use of lipid-lowering medication significantly reduce the risk of DR (33–35). Our results also showed higher levels of triglycerides and lower HDL in DR individuals.
Recently, the MHR has emerged as a novel and convenient marker with the integration of proinflammatory and anti-inflammatory factors (14–19). Emerging data suggest that higher MHR values are associated with various diseases or organ dysfunctions, such as endothelial dysfunction in Behçet disease, the presence and severity of metabolic syndrome, polycystic ovary syndrome, cardiac syndrome X, serum albumin level saphenous vein graft disease in coronary bypass, the high SYNTAX score in patients with stable coronary artery disease, asymptomatic organ damage in patients with primary hypertension, left atrial remodeling in atrial fibrillation, abdominal aortic aneurysm size, myocardial infarction, and CVD in patients with obstructive sleep apnea syndrome (14, 15, 36–43). It has also been shown that MHR is an independent predictor of in-hospital and long-term mortality and major adverse cardiac events in patients with acute coronary syndromes or a post-PCI status (44). Therefore, the MHR is a new prognostic marker in several CVDs, which are associated with inflammation. In addition, accumulated evidence has shown that the MHR is related to diabetes and diabetic complications (16, 45) and ocular disorders, including pseudoexfoliation syndrome (46), glaucoma, branch retinal vein occlusion (47), and central serous retinopathy (48). MHR values are increased in patients with diabetes compared to healthy controls, and an elevated MHR can predict diabetic nephropathy and diabetic axonal polyneuropathy (49). Moreover, there are a few studies with small sample size that evaluated the associations of the MHR with DR and got contradictory conclusions. Işil Çakir et al.' study showed that MHR was significantly higher in DR group than T2DM without DR group and found that specificity and sensitivity of MHR in detecting DR were relatively low (18); while it can be inferred that MHR is not affected by diabetes, but only by the proliferation process in Inhsan Solmaz et al.' study (19). Then, in the present study with larger sample size showed that diabetes, a chronic inflammatory disease, yields an elevated MHR level, and an elevated MHR can be an useful biomarker for DR independent of conventional risk factors, but not predict the severity stage of DR, which is not consistent with the previous study (18, 19). To date, several studies in addition to our study have consistently demonstrated that the MHR is a reliable factor for inflammation and is associated with diabetic micro- and macrovascular complications.
Furthermore, compared to other expensive inflammatory markers, such as interleukin factor (IL)-1, IL-6, tumor necrosis factor-α, and monocyte chemo-attractant protein-1, the MHR can be easily calculated from a simple blood analysis, making the use of this index more practical, cost-effective, and useful to predict DR (8).
It is important to note the limitations of our investigation. First, it was a single-center cross-sectional study; thus, causal relationships between MHR and DR cannot be confirmed. These findings should be cautiously interpreted, and further prospective studies are needed. Second, we did not exclude subjects with macrovascular complications, such as coronary artery disease, because the percentage of subjects with these complications did not vary significantly between the DR and non-DR groups. Finally, additional inflammation markers, such as CRP, interleukin factor (IL)-6, and tumor necrosis factor-α, were not evaluated herein.
In summary, the present study suggests that elevated MHR is a convenient and effective measurement for predicting the presence of DR in patients with T2DM.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics approval was obtained from the Third Affiliated Hospital of Sun Yat-sen University Network Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XT, YT, and YC contributed to the study design, formal analysis, and writing—original draft. XT, YT, YY, XH, ML, and HL contributed to the data acquisition and curation. YZ and YN contributed to the literature research. YL, GS, and PM revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by National Key R&D Program of China (2017YFA0105803), the National Natural Science Foundation of China (81770826, 82000278), the 5010 Clinical Research Projects of Sun Yat-sen University (2015015), the Key Area R&D Program of Guangdong Province (2019B020227003), the Science and Technology Plan Project of Guangzhou City (202007040003), the Guangdong Basic and Applied Basic Research Foundation (2020A1515010599), and the fostering special funding projects of the National Natural Science Foundation of China in the third affiliated hospital of SYSU (2020GZRPYQN04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. (2020) 16:321–31. doi: 10.1038/s41574-020-0334-z
2. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. (2017) 317:2515–23. doi: 10.1001/jama.2017.7596
3. Mai L, Wen W, Qiu M, Liu X, Sun L, Zheng H, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. (2021) 1–8. doi: 10.1111/dom.14490
4. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. (2021) 23:1746–53. doi: 10.1111/dom.14388
5. Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. (2020) 370:m2297. doi: 10.1136/bmj.m2297
6. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. (2010) 376:124–36. doi: 10.1016/S0140-6736(09)62124-3
7. Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. (2018) 19:942. doi: 10.3390/ijms19040942
8. Sasongko MB, Wong TY, Jenkins AJ, Nguyen TT, Shaw JE, Wang JJ. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet Med. (2015) 32:686–91. doi: 10.1111/dme.12640
9. Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. (2010) 17:53–9. doi: 10.1097/MOH.0b013e3283324f80
10. Klein BE, Myers CE, Howard KP, Klein R. Serum lipids and proliferative diabetic retinopathy and macular edema in persons with long-term type 1 diabetes mellitus: the wisconsin epidemiologic study of diabetic retinopathy. JAMA Ophthalmol. (2015) 133:503–10. doi: 10.1001/jamaophthalmol.2014.5108
11. Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, et al. High HDL cholesterol: a risk factor for diabetic retinopathy? Findings from NO BLIND study. Diabetes Res Clin Pract. (2019) 150:236–44. doi: 10.1016/j.diabres.2019.03.028
12. Sugano M, Tsuchida K, Makino N. High-density lipoproteins protect endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Biochem Biophys Res Commun. (2000) 272:872–6. doi: 10.1006/bbrc.2000.2877
13. Takaeko Y, Matsui S, Kajikawa M, Maruhashi T, Kishimoto S, Hashimoto H, et al. Association of extremely high levels of high-density lipoprotein cholesterol with endothelial dysfunction in men. J Clin Lipidol. (2019) 13:664–72.e1. doi: 10.1016/j.jacl.2019.06.004
14. Ganjali S, Gotto AM Jr, Ruscica M, Atkin SL, Butler AE, Banach M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. (2018) 233:9237–46. doi: 10.1002/jcp.27028
15. Zhang Y, Li S, Guo YL, Wu NQ, Zhu CG, Gao Y, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med. (2016) 48:305–12. doi: 10.3109/07853890.2016.1168935
16. Karatas A, Turkmen E, Erdem E, Dugeroglu H, Kaya Y. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomarkers Med. (2018) 12:953–9. doi: 10.2217/bmm-2018-0048
17. Gökçay Canpolat A, Emral R, Keskin Ç, Canlar S, Sahin M, Çorapçioglu D. Association of monocyte-to-high density lipoprotein-cholesterol ratio with peripheral neuropathy in patients with Type II diabetes mellitus. Biomarkers Med. (2019) 13:907–15. doi: 10.2217/bmm-2018-0451
18. Işil Ç, Hasan Basri A, Nahide Ekici G, Emine P, Derya S, Gökçen Alici S, et al. Monocyte to high-density lipoprotein ratio: a novel inflammation marker related to diabetic retinopathy. Erciyes Med J. (2020) 42:190–4. doi: 10.14744/etd.2020.32549
19. Ihsan S, Mine K. Monocyte count / HDL cholesterol ratio: A new marker in diabetic retinopathy. Ann Med Res. (2021) 28:258–60. doi: 10.5455/annalsmedres.2020.02.173
20. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53.
21. Wilkinson CP, Ferris FL III, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. (2003) 110:1677–82. doi: 10.1016/S0161-6420(03)00475-5
22. Thomas RL, Dunstan F, Luzio SD, Roy Chowdury S, Hale SL, North RV, et al. Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: retrospective analysis. BMJ. (2012) 344:e874. doi: 10.1136/bmj.e874
23. Klein BE. Reduction in risk of progression of diabetic retinopathy. N Engl J Med. (2010) 363:287–8. doi: 10.1056/NEJMe1005667
24. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care. (2021) 44(Suppl. 1):S73–84. doi: 10.2337/dc21-S006
25. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2018) 41:2669–701. doi: 10.2337/dci18-0033
26. Wu J, Zheng H, Liu X, Chen P, Zhang Y, Luo J, et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ Heart Fail. (2020) 13:e007054. doi: 10.1161/CIRCHEARTFAILURE.120.007054
27. Kanbay M, Solak Y, Unal HU, Kurt YG, Gok M, Cetinkaya H, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. (2014) 46:1619–25. doi: 10.1007/s11255-014-0730-1
28. Schröder S, Palinski W, Schmid-Schönbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. (1991) 139:81–100. PubMed PMID: 1713023
29. Benhar I, Reemst K, Kalchenko V, Schwartz M. The retinal pigment epithelium as a gateway for monocyte trafficking into the eye. EMBO J. (2016) 35:1219–35. doi: 10.15252/embj.201694202
30. Kaplar M, Kappelmayer J, Veszpremi A, Szabo K, Udvardy M. The possible association of in vivo leukocyte-platelet heterophilic aggregate formation and the development of diabetic angiopathy. Platelets. (2001) 12:419–22. doi: 10.1080/09537100120078368
31. van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care. (2002) 25:1320–5. doi: 10.2337/diacare.25.8.1320
32. Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. (2004) 53:2883–92. doi: 10.2337/diabetes.53.11.2883
33. Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. (2002) 56:1–11. doi: 10.1016/S0168-8227(01)00341-2
34. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. (2010) 363:233–44. doi: 10.1056/NEJMoa1001288
35. Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. (2007) 370:1687–97. doi: 10.1016/S0140-6736(07)61607-9
36. Acikgoz N, Kurtoglu E, Yagmur J, Kapicioglu Y, Cansel M, Ermis N. Elevated monocyte to high-density lipoprotein cholesterol ratio and endothelial dysfunction in Behçet disease. Angiology. (2018) 69:65–70. doi: 10.1177/0003319717704748
37. Uslu AU, Sekin Y, Tarhan G, Canakci N, Gunduz M, Karagulle M. Evaluation of monocyte to high-density lipoprotein cholesterol ratio in the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost. (2018) 24:828–33. doi: 10.1177/1076029617741362
38. Akboga MK, Balci KG, Maden O, Ertem AG, Kirbas O, Yayla C, et al. Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomarkers Med. (2016) 10:375–83. doi: 10.2217/bmm-2015-0050
39. Li N, Ren L, Wang JH, Yan YR, Lin YN, Li QY. Relationship between monocyte to HDL cholesterol ratio and concomitant cardiovascular disease in Chinese Han patients with obstructive sleep apnea. Cardiovasc Diagn Ther. (2019) 9:362–70. doi: 10.21037/cdt.2019.08.02
40. Yayla KG, Canpolat U, Yayla Ç, Akboga MK, Akyel A, Akdi A, et al. A novel marker of impaired aortic elasticity in never treated hypertensive patients: monocyte/high-density lipoprotein cholesterol ratio. Acta Cardiol Sin. (2017) 33:41–9. doi: 10.6515/ACS20160427A
41. Akboga MK, Yayla C, Balci KG, Ozeke O, Maden O, Kisacik H, et al. Relationship between serum albumin level and monocyte-to-high-density lipoprotein cholesterol ratio with saphenous vein graft disease in coronary bypass. Thor Cardiovasc Surg. (2017) 65:315–21. doi: 10.1055/s-0036-1582260
42. Sercelik A, Besnili AF. Increased monocyte to high-density lipoprotein cholesterol ratio is associated with TIMI risk score in patients with ST-segment elevation myocardial infarction. Rev Port Cardiol. (2018) 37:217–23. doi: 10.1016/j.repc.2017.06.021
43. Saskin H, Serhan Ozcan K, Yilmaz S. High preoperative monocyte count/high-density lipoprotein ratio is associated with postoperative atrial fibrillation and mortality in coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. (2017) 24:395–401. doi: 10.1093/icvts/ivw376
44. Zhang DP, Baituola G, Wu TT, Chen Y, Hou XG, Yang Y, et al. An elevated monocyte-to-high-density lipoprotein-cholesterol ratio is associated with mortality in patients with coronary artery disease who have undergone PCI. Biosci Rep. (2020) 40:BSR20201108. doi: 10.1042/BSR20201108
45. Ekizler FA, Cay S, Açar B, Tak BT, Kafes H, Ozeke O, et al. Monocyte to high-density lipoprotein cholesterol ratio predicts adverse cardiac events in patients with hypertrophic cardiomyopathy. Biomarkers Med. (2019) 13:1175–86. doi: 10.2217/bmm-2019-0089
46. Mirza E, Oltulu R, Katipoglu Z, Mirza GD, Özkagnici A. Monocyte/HDL ratio and lymphocyte/monocyte ratio in patients with pseudoexfoliation syndrome. Ocular Immunol Inflamm. (2020) 28:142–6. doi: 10.1080/09273948.2018.1545913
47. Satirtav G, Mirza E, Oltulu R, Mirza GD, Kerimoglu H. Assessment of monocyte/HDL ratio in branch retinal vein occlusion. Ocular Immunol Inflamma. (2020) 28:463–7. doi: 10.1080/09273948.2019.1569244
48. Sirakaya E, Duru Z, Kuçuk B, Duru N. Monocyte to high-density lipoprotein and neutrophil-to-lymphocyte ratios in patients with acute central serous chorioretinopathy. Indian J Ophthalmol. (2020) 68:854–8. doi: 10.4103/ijo.IJO_1327_19
Keywords: monocyte to high-density lipoprotein cholesterol ratio, type 2 diabetes, diabetic retinopathy, biomarker, inflammation
Citation: Tang X, Tan Y, Yang Y, Li M, He X, Lu Y, Shi G, Zhu Y, Nie Y, Li H, Mu P and Chen Y (2021) Association of the Monocyte–to–High-Density Lipoprotein Cholesterol Ratio With Diabetic Retinopathy. Front. Cardiovasc. Med. 8:707008. doi: 10.3389/fcvm.2021.707008
Received: 08 May 2021; Accepted: 27 August 2021;
Published: 21 September 2021.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
C. Roger White, University of Alabama at Birmingham, United StatesJingming Li, The First Affiliated Hospital of Xi'an Jiaotong University, China
Copyright © 2021 Tang, Tan, Yang, Li, He, Lu, Shi, Zhu, Nie, Li, Mu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Chen, Y2h5YW5tQG1haWwuc3lzdS5lZHUuY24=; Yanhua Zhu, emh1eWFuaDJAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xixiang Tang1,2†
Xixiang Tang1,2† Ying Tan
Ying Tan Mei Li
Mei Li Yan Lu
Yan Lu Guojun Shi
Guojun Shi Yanhua Zhu
Yanhua Zhu Haicheng Li
Haicheng Li Yanming Chen
Yanming Chen