
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 10 August 2021
Sec. Coronary Artery Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.706979
 Jun-feng Li1†
Jun-feng Li1† Zhi-wei Lin1†
Zhi-wei Lin1† Chang-xi Chen1
Chang-xi Chen1 Shi-qi Liang1
Shi-qi Liang1 Lei-lei Du1
Lei-lei Du1 Xiang Qu1
Xiang Qu1 Zhan Gao1
Zhan Gao1 Yu-heng Huang1
Yu-heng Huang1 Shu-ting Kong1
Shu-ting Kong1 Jin-xin Chen1
Jin-xin Chen1 Ling-yue Sun2*
Ling-yue Sun2* Hao Zhou1*
Hao Zhou1*Objectives: To evaluate the effect of thrombus aspiration (TA) strategy on the outcomes and its interaction with D-dimer levels in patients with ST-segment elevation myocardial infarction (STEMI) during primary percutaneous coronary intervention (PCI) in “real-world” settings.
Materials and Methods: This study included 1,295 patients with STEMI who had undergone primary PCI with or without TA between January 2013 and June 2017. Patients were first divided into a TA+PCI group and a PCI-only group, and the baseline characteristics and long-term mortality between the two groups were analyzed. Furthermore, we studied the effect of TA on the clinical outcomes of patients grouped according to quartiles of respective D-dimer levels. The primary outcome was all-cause mortality, and the secondary outcomes were new-onset heart failure (HF), rehospitalization, re-PCI, and stroke.
Results: In the original cohort, there were no significant differences in all-cause mortality between the TA+PCI and PCI-only groups (hazard ratio, 0.789; 95% confidence interval, 0.556–1.120; p = 0.185). After a mean follow-up of 2.5 years, the all-cause mortality rates of patients in the TA + PCI and PCI-only groups were 8.5 and 16.2%, respectively. Additionally, differences between the two groups in terms of the risk of HF, re-PCI, rehospitalization, and stroke were non-significant. However, after dividing into quartiles, as the D-dimer levels increased, the all-cause mortality rate in the PCI group gradually increased (4.3 vs. 6.0 vs. 7.0 vs. 14.7%, p < 0.001), while the death rate in the TA+PCI group did not significantly differ (4.6 vs. 5.0 vs. 4.0 vs. 3.75%, p = 0.85). Besides, in the quartile 3 (Q3) and quartile 4 (Q4) groups, the PCI-only group was associated with a higher risk of all-cause mortality than that of the TA+PCI group (Q3: 4.0 vs. 7.0%, p = 0.029; Q4: 3.75 vs. 14.7%, p < 0.001). Moreover, the multivariate logistic regression analysis demonstrated that TA is inversely associated with the primary outcome in the Q4 group [odds ratio (OR), 0.395; 95% CI, 0.164–0.949; p = 0.038].
Conclusions: The findings of our real-world study express that routine manual TA during PCI in STEMI did not improve clinical outcomes overall. However, patients with STEMI with a higher concentration of D-dimer might benefit from the use of TA during primary PCI. Large-scale studies are recommended to confirm the efficacy of TA.
Prompt reperfusion therapy has been recommended as the first and best treatment in patients with acute ST-segment elevation myocardial infarction (STEMI) (1, 2). Primary percutaneous coronary intervention (PCI) is the most optimal and effective treatment in patients with STEMI. Coronary thrombosis is a hallmark of acute STEMI. Thrombus burden and reduced myocardial perfusion are essential predictors of poor clinical outcomes (3). Although thrombus aspiration (TA) has been considered one of the reperfusion strategies and is widely implemented in primary PCI, its benefits are debatable (1, 4). The benefits of TA have not been consistently demonstrated in previous clinical trials. Preliminary studies have confirmed that TA during primary PCI has clinical relevance compared with conventional primary PCI (5–8). However, recent major clinical randomized controlled trials (TASTE and TOTAL trials) have challenged the clinical benefits of TA and do not support the routine use of manual TA in patients with STEMI requiring primary PCI (9, 10). Due to such conflicting results, the routine use of TA during primary PCI is not recommended (1).
Given the pathophysiology of acute STEMI involving interactions among ruptured plaque-induced coronary thrombi and platelets and clotting factors (11, 12), the D-dimer level, a useful and direct biomarker of crosslinked procoagulant activity and ongoing fibrinolysis, has been associated with acute STEMI (13, 14). In these patients, elevated D-dimer levels at the time of admission were associated with increased cardiovascular mortality and all-cause mortality following primary PCI. Even in healthy individuals, the D-dimer level has been proven to be a predictor of cardiovascular events (15). To the best of our knowledge, the association between D-dimer levels and long-term outcomes in patients with STEMI undergoing TA during primary PCI has not been evaluated. Further studies are required to clarify whether patients with high D-dimer levels stand to benefit the most from TA.
To date, real-world data regarding TA and its effect in patients with STEMI have been conflicting (4, 16). Our study aimed to investigate the effect of TA on the clinical outcomes and prognoses and its relationship with different D-dimer levels in patients with STEMI undergoing primary PCI.
This single-center study compared the effect of manual TA before PCI with PCI only on clinical outcomes and prognoses in patients with STEMI. A total of 1,295 consecutive patients were enrolled between January 2013 and June 2016 and were followed up to June 2017. The patients were divided into two groups based on treatment with manual TA before PCI (TA+PCI group) or PCI alone (PCI-only group). Patient inclusion was based on ≥18 years of age, STEMI diagnosis in ≤ 12 h of symptom onset, and angiographically determined Thrombolysis in Myocardial Infarction flow grade of 0–1. According to the European Society of Cardiology (ESC) guidelines, STEMI was defined as chest pain of more than 30 min suggestive of acute myocardial ischemia prior to hospital admission and an electrocardiogram reading ≥0.1 mV for the ST-segment elevation in two or more contiguous leads, new left bundle branch block (LBBB), or new-onset Q waves (1). Patients were excluded in case of the following: (1) contraindications to contrast agents and therapeutic medications, (2) history of coronary artery bypass surgery, (3) preoperative cardiogenic shock, (4) received fibrinolytic therapy, (5) life expectancy <6 months, (6) recent major surgery or trauma, and (7) unwilling or unable to provide informed consent.
Before primary PCI, pharmacological pretreatment comprised the administration of aspirin and clopidogrel or ticagrelor. During the primary PCI procedure, unless contraindicated, patients received a glycoprotein IIb/IIIa inhibitor (tirofiban) according to their weight and additional unfractionated heparin to achieve an activated clotting time of 250–300 s. Following primary PCI, the standard dual antiplatelet therapy comprised aspirin, clopidogrel, or ticagrelor for at least 12 months after stent implantation. Other pharmacological treatments, including β-blockers, statins, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), and diuretics, if necessary, were recommended based on current guidelines.
In this cohort study, the decision for manual TA was based on the operator's discretion, and it was performed according to the standard protocol. Thrombus grade was classified according to Gibson et al. (17): grade 0, no angiographic characteristics of thrombus are present; grade 1, possible thrombus, with angiographic characteristics such as reduced contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion suggestive but not diagnostic of thrombus; grade 2, definite thrombus, with the greatest dimension less than or equal to half of the vessel diameter; grade 3, definite thrombus but with the greatest linear dimension greater than half but less than twice the vessel diameter; grade 4, definite thrombus, with the largest dimension greater than or equal to twice the vessel diameter; and grade 5, total occlusion. The procedure was performed by investigators who were experienced interventional doctors. Investigators guided the catheter in place, sent the guidewire through the lesion, and then pushed the thrombectomy catheter. When the thrombectomy catheter reached the proximal part of the thrombus along the guidewire, suction was performed under negative pressure. Following aspiration, the guide catheter was confirmed to have entered the coronary ostium and the thrombectomy catheter was withdrawn under negative pressure.
Baseline demographics, clinical presentations, laboratory data, and echocardiography findings were collected from the medical records. Laboratory data during hospitalization, including peak troponin I, peak creatine kinase isoenzyme MB (CK-MB), brain natriuretic peptide (BNP), creatinine (Cr), low-density lipoprotein cholesterol, lactic acid, glucose, C-reactive protein, and D-dimer levels, were recorded before primary PCI, and laboratory data, including D-dimer levels, were collected as soon as emergency patients were admitted to the hospital, which was strictly controlled and recorded. This study was approved by the local ethics committee. All participants provided written informed consent. The study protocol was approved by the local research ethics board.
All enrolled participants were asked to return for a regular outpatient follow-up at 1 month and then each year after discharge. Clinical follow-up was conducted for all enrolled patients to evaluate the occurrence of the following major adverse cardiac events: death, non-fatal reinfarction, target vessel revascularization, and heart failure (HF). The primary endpoint was all-cause mortality during long-term follow-up, and secondary endpoints were new-onset HF, rehospitalization for coronary heart disease (including reinfarction, stent thrombosis, and target vessel revascularization), re-PCI, and stroke. Major adverse events were assessed up to June 30, 2017, and patient follow-up was censored at the time of death. Follow-up data were collected from outpatient visit or telephone call. The median follow-up duration was 2.5 years.
Baseline characteristics of patients with and without TA are described as mean ± standard deviation (SD) for all continuous variables, and non-normally distributed parameters are shown as median (25th and 75th percentile). Data for categorical variables are reported as values and percentages and were compared using the chi-square test or Fisher's exact test. Comparison of continuous variables between the two groups was performed using Student's t-test and analysis of variance for multiple comparisons. The Mann–Whitney U-test was used for non-normally distributed variables. All analyses were based on the intention-to-treat principle.
First, univariable analyses were performed to determine the difference between the two groups in the primary and secondary outcomes and its components (HF, rehospitalization, re-PCI, and stroke). Kaplan–Meier curves were generated to create event-free survival curves, and the log-rank test was used to compare the distributions of time-to-event during the follow-up. Second, multivariable Cox proportional hazards analyses were performed to examine the association between TA and all-cause mortality in each group. For statistical adjustment, we included the following variables: age; sex; stent; TA; multivessel disease; medical therapy with aspirin, ACEIs, metoprolol, spironolactone; laboratory data regarding lactic acid, BNP, and Cr; left ventricular ejection fraction (LVEF); and history of smoking and diabetes mellitus. Non-normally distributed variables were included in the multivariate regression model after logarithmic transformation. Hazard ratios (HRs), 95% confidence intervals (CIs), and p-values were calculated.
To further investigate the interaction of TA with D-dimer levels, patients were categorized into four groups (quartiles) according to the D-dimer levels. The primary and secondary outcomes were analyzed and compared based on whether TA was performed. Multivariable logistic regression models were used to determine independent predictors of all-cause mortality.
All analyses were conducted using SPSS version 25 (IBM Corporation, USA). Calculations were performed using MedCalc 12.9 statistical software (Mariakerke, Belgium). A value of p < 0.05 in the two-tailed test was considered statistically significant.
From January 2013 to July 2016, a total of 1,295 patients who met our inclusion criteria were prospectively enrolled to undergo primary PCI during the study period. Of these, 657 (50.7%) underwent PCI with TA and 638 (49.3%) underwent only PCI. Differences in the baseline characteristics were significant between the TA+PCI group and PCI-only group (Table 1). Patients in the TA + PCI group were younger; more likely to be smokers; had higher glucose, troponin I, and CK-MB levels; and had lower Cr levels and LVEF compared with those of the PCI-only group. Additionally, patients in the TA + PCI group were more often treated with aspirin, clopidogrel, statins, and β-blockers than those in the PCI-only group. In contrast, patients in the PCI-only group were more likely to have multivessel disease and less likely to have a stent implantation, and the culprit artery was more frequently the proximal left anterior descending artery compared with patients in the TA+PCI group.
After stratification by quartiles based on D-dimer levels, four groups of patients were obtained. Table 2 presents the baseline characteristics of patients stratified according to the D-dimer levels. The first quartile (Q1; D-dimer ≤ 0.54, n = 329), second quartile (Q2; 0.54 < D-dimer ≤ 0.91, n = 319), third quartile (Q3; 0.91 < D-dimer ≤ 1.50, n = 327), and fourth quartile (Q4; 1.50 < D-dimer, n = 320). With increasing D-dimer levels, patients were observed to be older, with a history of drinking, and with greater susceptibility to multivessel disease.
The primary and secondary endpoints in the TA + PCI and PCI-only groups are shown in Table 3 and Figure 1A. In the univariate analysis, the all-cause mortality in the TA + PCI group (8.5%) was lower than that in the PCI-only group (16.2%) (3.40 vs. 6.47% per year; HR, 0.502; 95% CI, 0.362–0.695; p < 0.001). However, the difference between the two groups was not significant in terms of the risk of HF (2.66 vs. 2.15% per year; HR, 1.369; 95% CI, 0.847–2.211; p = 0.200), re-PCI (1.86 vs. 1.93% per year; HR, 1.194; 95% CI, 0.687–2.075; p = 0.529), rehospitalization (4.27 vs. 3.64% per year; HR, 1.343; 95% CI, 0.918–1.965; p = 0.129), and stroke (0.13 vs. 0.15% per year; HR, 0.899; 95% CI, 0.126–6.401; p = 0.916). This seems to mean that emergency PCI patients can benefit from TA, thereby reducing long-term mortality. Table 4 presents the stepwise multivariate Cox regression analysis of the association between TA and all-cause mortality. Factors found to have predictive significance (p < 0.05) in the univariate analysis between the two groups were included in the multivariate regression model. In the multivariate analysis, no interaction with TA was noted with all-cause mortality (p = 0.185; HR, 0.789; 95% CI, 0.556–1.120), whereas sex, age, stent implantation, multiple vessels, administration of aspirin, BNP, and LVEF demonstrated a considerable interaction with the primary outcome.
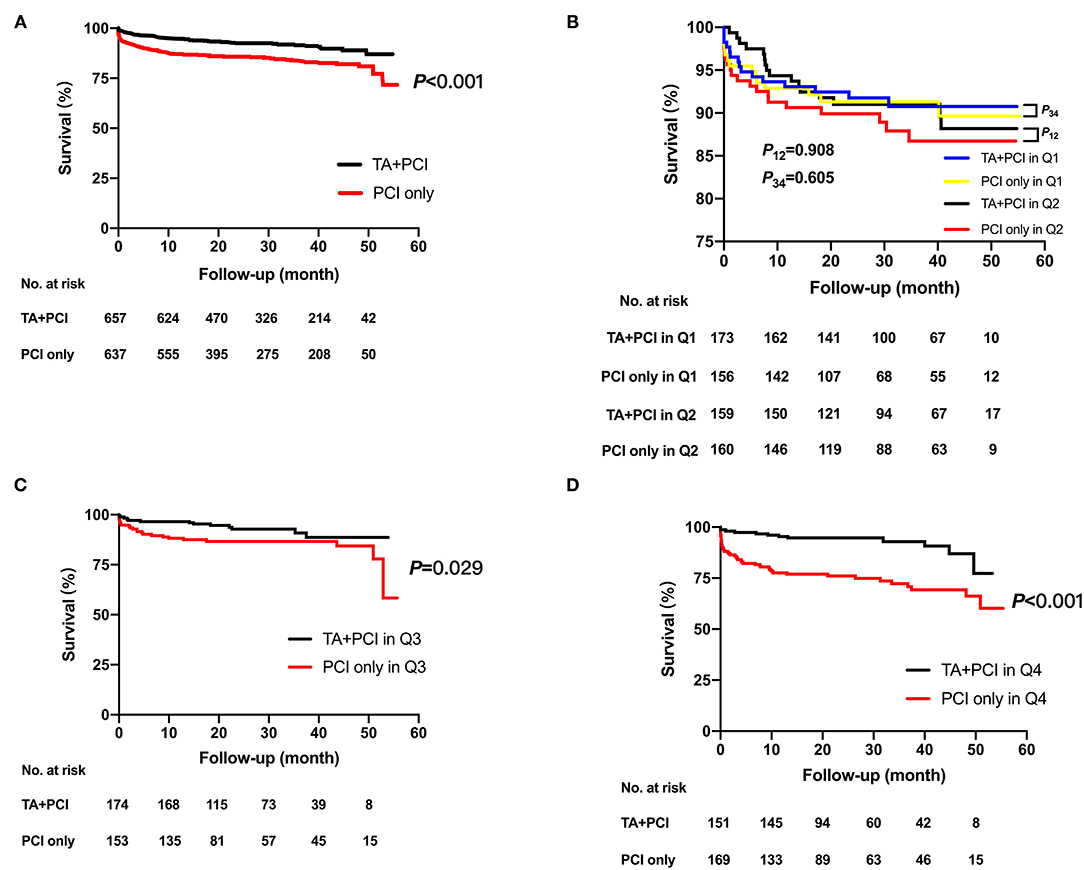
Figure 1. Kaplan–Meier time-to-event curves for all-cause death for the total duration of follow-up after the intervention. Kaplan–Meier estimates in TA + PCI group (red curve) and PCI-only group (black curve) for the rate of all-cause mortality in the whole population (A) and all-cause mortality of TA + PCI group (blue curve) and PCI-only group (yellow curve) in quartile 1; all-cause mortality of TA + PCI group (black curve) and PCI-only group (red curve) in quartile 2 (B); all-cause mortality of TA + PCI group (black curve) and PCI-only group (red curve) in quartile 3 (C); and all-cause mortality of TA + PCI group (black curve) and PCI-only group (red curve) in quartile 4 (D). TA, thrombus aspiration; PCI, percutaneous coronary intervention; P12, p-value between the TA + PCI and PCI-only in group Quartile 1; P34, p-value between the TA + PCI and PCI-only in group Quartile 2.
To determine the association between TA and D-dimer levels, the primary and secondary endpoints were calculated in patients stratified by quartiles of D-dimer levels. Multivariate logistic regression models were used to determine independent predictors of all-cause mortality. The results showed that as the levels of D-dimer increased, the all-cause mortality rates gradually increased in patients who had undergone only PCI (4.3 vs. 6.0 vs. 7.0 vs. 14.7%, p < 0.001). However, the mortality rates in patients who underwent TA + PCI did not change significantly (4.6 vs. 5.0 vs. 4.0 vs. 3.75%, p = 0.85). Specifically, patients in Q4 had the worst outcomes following PCI. At the same time, patients in the PCI-only group were associated with a higher all-cause mortality than those in the TA+PCI group in Q3 and Q4 (Q3: 4.0 vs. 7.0%, p = 0.029; Q4: 3.75 vs. 14.7%, p < 0.001) (Table 5; Figures 1B–D).
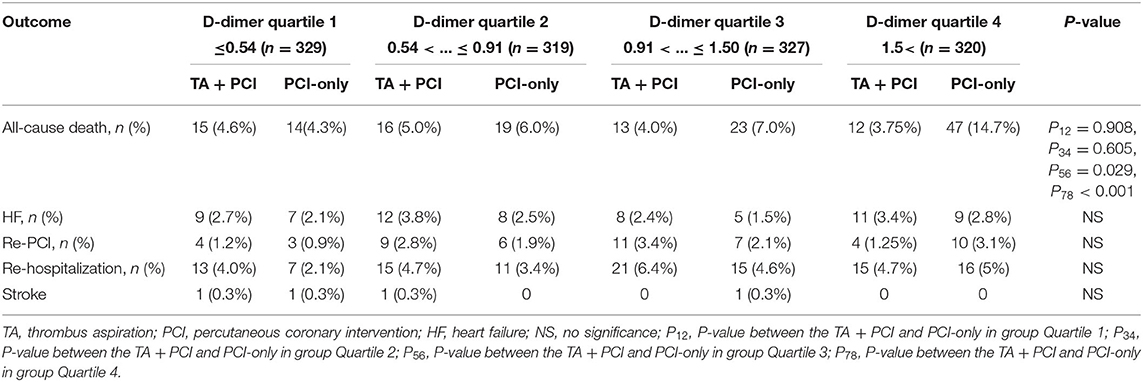
Table 5. Primary and secondary end points for the subgroup analysis with different levels of D-dimer.
Moreover, the results of multivariate logistic regression analysis for all-cause mortality, which was used to investigate the role of TA for the different D-dimer quartiles, are given in Table 6. In Q4 (D-dimer >1.5), TA was inversely associated with the primary outcome, which indicated that TA was an independent predictor for all-cause mortality in patients with high D-dimer levels (OR, 0.395; 95% CI, 0.164–0.949; p = 0.038). No significant association between TA and all-cause mortality was observed in the first three groups (Q1: OR, 1.446; 95% CI, 0.537–3.897; p = 0.466; Q2: OR, 1.304; 95% CI, 0.518–3.287; p = 0.573; and Q3: OR, 0.779; 95% CI, 0.314–1.932; p = 0.590).
The principal findings of this cohort study can be summarized as follows: (1) compared with only PCI, routine TA before PCI resulted in lower all-cause mortality in the univariate analysis. However, in the multivariate analysis, TA was not an independent predictive factor given the differences in the baseline characteristics and coexistence of confounders. Hence, we were unable to conclude whether TA was associated with a decrease in mortality in the entire population. (2) In the subgroup analysis according to quartiles of D-dimer levels, treatment with only PCI in patients with high D-dimer levels was associated with higher all-cause mortality than that in patients with high D-dimer levels treated with TA+PCI. Moreover, multivariate logistic regression analysis revealed a remarkable inverse relationship between TA and all-cause mortality in patients with D-dimer levels >1.5 mg/L, which proved that TA was an independent predictive factor for high D-dimer levels.
A crucial hallmark of acute STEMI is the occlusion of the culprit vessel with an intracoronary thrombus (1, 2, 11, 18). In several randomized controlled trials, TA has been shown to be effective in reducing the coronary thrombus burden and improving microvascular perfusion compared with only PCI for acute STEMI during primary PCI (19–24).
Elevated levels of specific inflammatory and oxidative stress markers have been observed in patients with a high thrombus burden, leading to impaired myocardial reperfusion in patients with STEMI (25). Especially, in patients with diabetes, the increased thrombogenicity observed in type 2 diabetes mellitus has been associated with higher glycoxidative stress, as quantified by plasma hemoglobin A1C levels and oxidative response (26). In our original cohort, the TA + PCI group had higher blood glucose levels, indicating that STEMI patients with hyperglycemia may have a higher thrombus burden, which is consistent with the results obtained by D'Onofrio et al. (27). Their study indicated that the miR33/SIRT1 pathway was involved in the increased pro-inflammatory and procoagulant status of coronary thrombosis in such patients (27). Furthermore, studies have pointed out that the mechanism of hyperglycemia leading to a high thrombus burden in patients with STEMI may include a pro-inflammatory burden. Patients in the hyperglycemic STEMI group had higher levels of pro-inflammatory cytokines [tumor necrosis factor-α (TNF-α)] (28). However, our study demonstrated that there was no significant difference in C-reactive protein levels in the patients at baseline. Whether TNF-α can be used as an indicator to verify the effect of the patients' inflammation levels on thrombosis requires further research. Therefore, it is essential to strictly treat patients with hyperglycemia; rigorous blood glucose control may increase the regenerative ability of ischemic myocardium (29), and optimal target glycemic levels and treatment regimens in patients with acute coronary syndrome (ACS) need further study (30). In patients with normal glucose tolerance undergoing PCI, there are also some factors related to disease progression. The AIRE study found that adiponectin and insulin resistance were associated with restenosis and independently related to ischemic heart disease (31). Although the findings were interesting, future studies are required due to the relatively small sample size. The TAPAS study (7) demonstrated improvement in long-term clinical outcomes with TA compared with those with PCI alone for acute STEMI during primary PCI. Two other small randomized trials showed that TA was associated with significantly less left ventricular remodeling and lower end-systolic and end-diastolic left ventricular volumes than PCI alone (22, 23). Rheolytic thrombectomy has also been reported to be safe and effective for treating proximal deep venous thrombosis (DVT) (32). To our knowledge, however, conflicting results have been reported by other trials of manual TA (33) and have largely been negative (9, 10, 34–36). Both the TASTE and TOTAL trials, two large randomized controlled trials, showed that routine TA did not lower long-term clinical outcomes following PCI for STEMI. Similar to the TASTE and TOTAL trials, during long-term follow-up, our study also showed no significant differences in all-cause mortality between the TA and PCI-only groups. Although not significant, in the subgroup of patients with a high thrombus burden, there was an increase in stroke or transient ischemic attack (TIA). Routine manual thrombectomy was related to an increased risk of stroke and no-reflow phenomenon (NRP) relative to that with PCI only (37, 38). However, in our study, only four patients experienced stroke, and there were no differences in the risk of stroke and NRP between the TA + PCI and PCI-only groups. Sex and age influenced the demographics and morbidity of hospitalized elderly patients (39). Currently, techniques can be used to differentiate unidentical frailty phenotypes and to help define the optimal nursing strategy according to the specific patient needs (40). Moreover, a study showed that TA may benefit patients with STEMI in high-risk groups, such as the frail elderly population (41). Our research circumvented the influence of age or other comorbidities on the results through a multivariate analysis. As TA may benefit elderly patients more, further research is required.
D-dimer is the final product of crosslinked fibrin degradation by plasmin and indicates active thrombus formation and lysis and has been linked to thrombus burden. In addition to the diagnostic use of D-dimer for STEMI, this marker may have a potential prognosis (42). A significant increase in the level of D-dimer indicates thrombotic complications in patients with MI, which suggests that in addition to being used as a marker for early diagnosis, D-dimer is a risk factor in the development of MI complications (43–45). The prognostic information of D-dimer levels may be particularly useful in patients with STEMI. Plasma concentrations of D-dimer were shown to be significantly associated with thrombus burden in STEMI and were increased in ongoing or recent thrombosis (14, 46). In the present study, high D-dimer levels predicted larger myocardial infarcts, possibly due to the greater thrombosis and clot load leading to increased D-dimer levels. We hypothesize that TA during primary PCI can lower the risk of all-cause mortality in patients with STEMI with high D-dimer levels. To support this hypothesis, we conducted an analysis by dividing patients into quartile groups based on their D-dimer levels. Our study showed that the range of D-dimer levels did influence the effect of TA on all-cause mortality, and we found that TA could decrease the primary outcome in patients with D-dimer levels higher than 1.5 mg/L. However, in patients with D-dimer levels <1.5 mg/L, TA was not helpful in improving the prognoses. This indicated that TA might be an independent predictive factor in patients with STEMI with a high D-dimer level.
TA was mainly aimed at patients with heavy thrombus burden, and the connection between thrombi and D-dimer levels has also been mentioned. Therefore, when we grouped patients into quartiles of D-dimer levels, TA was linked with different results for patients with different thrombus burdens. This may explain the different outcomes between the overall population and the grouped population in the multivariate analysis. Q4 represented a group of patients with the highest thrombus burden, so they could benefit most from TA, while the first three groups may benefit less because of their lighter thrombus load.
Overall, in this study, we inferred that the effect of TA was cumulatively associated with D-dimer levels in patients with STEMI undergoing PCI. Accordingly, our data provide additional considerations for physicians in deciding if TA in patients undergoing primary PCI is beneficial.
Some limitations of our study should be considered. First, the trial was a single-center, non-randomized, observational study with 1,295 patients, which had limited power to investigate the interaction of TA and D-dimer levels with the clinical outcomes. We may not have been able to exclude the residual confounders despite performing extensive adjustments. Second, the findings of our study may be potentially limited by ascertainment biases related to unmeasured or hidden confounders. It was not clear in the Q3 group (0.91 < D-dimer ≤ 1.50) whether TA before PCI surgery would improve postoperative outcomes, which may be explained by the interference of other unknown confounders, and the effect of TA on patients with a D-dimer level within this range remains unknown. Third, some prespecified subgroup comparisons, such as patients with stroke, were based on a rather small number of individuals. Therefore, type II errors cannot be excluded. Our research also has several strengths. First, we assessed the effect of TA at different D-dimer levels through the D-dimer quartile level. According to this, we got a more comprehensive result instead of just setting a cutoff value to divide patients into high and low D-dimer groups, and this aspect could enrich the conclusions drawn by our research. Second, our study had a low loss ratio of follow-up rate, which would help us to obtain a more accurate judgment.
The findings of our real-world study were also consistent with the 2017 ESC recommendation, which reported that routine manual TA during PCI for STEMI did not improve clinical outcomes in the study population. However, patients with STEMI with a high D-dimer level may benefit from TA during primary PCI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HZ: the proposal of paper concept, funding acquisition, methodology, and responsible for the overall content as guarantor. L-yS: the proposal of paper concept and original draft writing. J-fL: methodology, original draft writing, and data analysis. Z-wL: follow up, validation, and data supervision. C-xC: data supervision and project administration. S-qL: follow up and draft editing. L-lD: data supervision. XQ: data supervision and analysis. ZG: project administration. Y-hH and J-xC: follow up. S-tK: software. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This research was supported by the National Natural Science Foundation of China (81800048 and 81873468) and the Science and Technology Planning Project of Wenzhou Science & Technology Bureau of Zhejiang Province of China (Y20160118).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
TA, thrombus aspiration; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; HF, heart failure; HR, hazard ratio; 95% CI, 95% confidence interval; TASTE, thrombus aspiration in myocardial infarction; TOTAL, A trial of routine aspiration thrombectomy with percutaneous coronary intervention (PCI) vs. PCI alone in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; CK-MB, creatine kinase isoenzyme MB; BNP, brain natriuretic peptide; Cr, creatinine; LVEF, left ventricular ejection fraction; SD, standard deviation; TAPAS, Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study.
1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
2. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. (2016) 67:1235–50. doi: 10.1016/j.jacc.2015.10.005
3. Stone GW, Peterson MA, Lansky AJ, Dangas GD, Mehran R, Leon MB. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J Am Coll Cardiol. (2002) 39:591–7. doi: 10.1016/S0735-1097(01)01779-X
4. Jolly SS, James S, Dzavik V, Cairns JA, Mahmoud KD, Zijlstra F, et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: thrombectomy trialists collaboration. Circulation. (2017) 135:143–52. doi: 10.1161/CIRCULATIONAHA.116.025371
5. Ikari Y, Sakurada M, Kozuma K, Kawano S, Katsuki T, Kimura K, et al. Upfront thrombus aspiration in primary coronary intervention for patients with ST-segment elevation acute myocardial infarction: report of the VAMPIRE (VAcuuM asPIration thrombus REmoval) trial. J Am Coll Cardiol Intv. (2008) 1:424–31. doi: 10.1016/j.jcin.2008.06.004
6. Burzotta F, De Vita M, Gu YL, Isshiki T, Lefevre T, Kaltoft A, et al. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur Heart J. (2009) 30:2193–203. doi: 10.1093/eurheartj/ehp348
7. Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, et al. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. (2008) 371:1915–20. doi: 10.1016/S0140-6736(08)60833-8
8. Noman A, Egred M, Bagnall A, Spyridopoulos I, Jamieson S, Ahmed J. Impact of thrombus aspiration during primary percutaneous coronary intervention on mortality in ST-segment elevation myocardial infarction. Eur Heart J. (2012) 33:3054–61. doi: 10.1093/eurheartj/ehs309
9. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. (2013) 369:1587–97. doi: 10.1056/NEJMoa1308789
10. Lagerqvist B, Frobert O, Olivecrona GK, Gudnason T, Maeng M, Alstrom P, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. (2014) 371:1111–20. doi: 10.1056/NEJMoa1405707
11. Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. (2000) 101:570–80. doi: 10.1161/01.CIR.101.5.570
12. Kotani J, Nanto S, Mintz GS, Kitakaze M, Ohara T, Morozumi T, et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation. (2002) 106:1672–7. doi: 10.1161/01.CIR.0000030189.27175.4E
13. Jonathan BK, Patrick JC, John JF, Ralph EK. Fibrin and fibrinogen-related antigens in patients with stable and unstable coronary artery disease. N Engl J Med. (1987) 317:1361–5. doi: 10.1056/NEJM198711263172201
14. Christersson C, Oldgren J, Bylock A, Siegbahn A, Wallentin L. Early decrease in coagulation activity after myocardial infarction is associated with lower risk of new ischaemic events: observations from the ESTEEM Trial. Eur Heart J. (2007) 28:692–8. doi: 10.1093/eurheartj/ehl564
15. Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. (1994) 90:2236–40. doi: 10.1161/01.CIR.90.5.2236
16. Mahmoud KD, Zijlstra F. Thrombus aspiration in acute myocardial infarction. Nat Rev Cardiol. (2016) 13:418–28. doi: 10.1038/nrcardio.2016.38
17. Gibson CM, de Lemos JA, Murphy SA, Marble SJ, McCabe CH, Cannon CP, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. (2001) 103:2550–4. doi: 10.1161/01.CIR.103.21.2550
18. Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. (2008) 359:938–49. doi: 10.1056/NEJMra0801082
19. Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. (2008) 358:557–67. doi: 10.1056/NEJMoa0706416
20. Burzotta F, Trani C, Romagnoli E, Mazzari MA, Rebuzzi AG, De Vita M, et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. (2005) 46:371–6. doi: 10.1016/j.jacc.2005.04.057
21. Silva-Orrego P, Colombo P, Bigi R, Gregori D, Delgado A, Salvade P, et al. Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (dethrombosis to enhance acute reperfusion in myocardial infarction) study. J Am Coll Cardiol. (2006) 48:1552–9. doi: 10.1016/j.jacc.2006.03.068
22. Galiuto L, Garramone B, Burzotta F, Lombardo A, Barchetta S, Rebuzzi AG, et al. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA Trial. J Am Coll Cardiol. (2006) 48:1355–60. doi: 10.1016/j.jacc.2006.05.059
23. De Luca L, Sardella G, Davidson CJ, De Persio G, Beraldi M, Tommasone T, et al. Impact of intracoronary aspiration thrombectomy during primary angioplasty on left ventricular remodelling in patients with anterior ST elevation myocardial infarction. Heart. (2006) 92:951–7. doi: 10.1136/hrt.2005.074716
24. Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. (2009) 53:309–15. doi: 10.1016/j.jacc.2008.10.017
25. Yunoki K, Naruko T, Sugioka K, Inaba M, Iwasa Y, Komatsu R, et al. Erythrocyte-rich thrombus aspirated from patients with ST-elevation myocardial infarction: association with oxidative stress and its impact on myocardial reperfusion. Eur Heart J. (2012) 33:1480–90. doi: 10.1093/eurheartj/ehr486
26. Sambola A, Ruiz-Meana M, Barba I, Del Blanco BG, Barrabés JA, Lip GY, et al. Glycative and oxidative stress are associated with altered thrombus composition in diabetic patients with ST-elevation myocardial infarction. Int J Cardiol. (2017) 243:9–14. doi: 10.1016/j.ijcard.2017.04.089
27. D'Onofrio N, Sardu C, Paolisso P, Minicucci F, Gragnano F, Ferraraccio F, et al. MicroRNA-33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J Cell Physiol. (2020) 235:1438–52. doi: 10.1002/jcp.29064
28. Sardu C, D'Onofrio N, Mauro C, Balestrieri ML, Marfella R. Thrombus aspiration in hyperglycemic patients with high inflammation levels in coronary thrombus. J Am Coll Cardiol. (2019) 73:530–1. doi: 10.1016/j.jacc.2018.10.074
29. Marfella R, Sasso FC, Cacciapuoti F, Portoghese M, Rizzo MR, Siniscalchi M, et al. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J Clin Endocrinol Metab. (2012) 97:933–42. doi: 10.1210/jc.2011-2037
30. Sasso FC, Rinaldi L, Lascar N, Marrone A, Pafundi PC, Adinolfi LE, et al. Role of tight glycemic control during acute coronary syndrome on CV outcome in type 2 diabetes. J Diabetes Res. (2018) 2018:1–8. doi: 10.1155/2018/3106056
31. Sasso FC, Pafundi PC, Marfella R, Calabrò P, Piscione F, Furbatto F, et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: the prospective AIRE Study. Cardiovasc Diabetol. (2019) 18:24. doi: 10.1186/s12933-019-0826-0
32. Tsuji A, Yamada N, Ota S, Ishikura K, Nakamura M, Ito M. Early results of rheolytic thrombectomy in patients with proximal deep vein thrombosis. Circ J. (2011) 75:1742–6. doi: 10.1253/circj.CJ-10-0617
33. Hugelshofer S, Roffi M, Witassek F, Eberli FR, Pilgrim T, Pedrazzini G, et al. Impact of total ischemic time on manual thrombus aspiration benefit during primary percutaneous coronary intervention. Am Heart J. (2018) 204:34–42. doi: 10.1016/j.ahj.2018.05.019
34. Jolly SS, Cairns JA, Yusuf S, Rokoss MJ, Gao P, Meeks B, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. (2016) 387:127–35. doi: 10.1016/S0140-6736(15)00448-1
35. Mahmoud KD, Jolly SS, James S, Dzavik V, Cairns JA, Olivecrona GK, et al. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: Thrombectomy Trialists Collaboration. Eur Heart J. (2018) 39:2472–9. doi: 10.1093/eurheartj/ehy219
36. Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, et al. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation. (2006) 114:40–7. doi: 10.1161/CIRCULATIONAHA.105.595660
37. Jolly SS, Cairns JA, Yusuf S, Meeks B, Gao P, Hart RG, et al. Stroke in the TOTAL trial: a randomized trial of routine thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction. Eur Heart J. (2015) 36:2364–72. doi: 10.1093/eurheartj/ehv296
38. Januszek R, Siudak Z, Malinowski KP, Wojdyła R, Mika P, Wańha W, et al. Aspiration thrombectomy in patients with acute myocardial infarction-5-year analysis based on a Large National Registry (ORPKI). J Clin Med. (2020) 9:3610. doi: 10.3390/jcm9113610
39. Corrao S, Santalucia P, Argano C, Djade CD, Barone E, Tettamanti M, et al. Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med. (2014) 25:617–23. doi: 10.1016/j.ejim.2014.06.027
40. Marcucci M, Franchi C, Nobili A, Mannucci PM, Ardoino I. Defining aging phenotypes and related outcomes: clues to recognize frailty in hospitalized older patients. J Gerontol A Biol Sci Med Sci. (2017) 72:395–402.
41. Mone P, Gambardella J, Pansini A, Rizzo M, Mauro C, Minicucci F, et al. Impact of thrombus aspiration in frail STEMI patients. Aging Clin Exp Res. (2021). doi: 10.1007/s40520-021-01848-5. [Epub ahead of print].
42. Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, et al. Thrombogenic factors and recurrent coronary events. Circulation. (1999) 99:2517–22. doi: 10.1161/01.CIR.99.19.2517
43. Kikkert WJ, Claessen BE, Stone GW, Mehran R, Witzenbichler B, Brodie BR, et al. D-dimer levels predict ischemic and hemorrhagic outcomes after acute myocardial infarction: a HORIZONS-AMI biomarker substudy. J Thromb Thrombolysis. (2014) 37:155–64. doi: 10.1007/s11239-013-0953-5
44. Erkol A, Oduncu V, Turan B, Kilicgedik A, Sirma D, Gozubuyuk G, et al. The value of plasma D-dimer level on admission in predicting no-reflow after primary percutaneous coronary intervention and long-term prognosis in patients with acute ST segment elevation myocardial infarction. J Thromb Thrombolysis. (2014) 38:339–47. doi: 10.1007/s11239-013-1044-3
45. Tataru MC, Heinrich J, Junker R, Schulte H, von Eckardstein A, Assmann G, et al. D-dimers in relation to the severity of arteriosclerosis in patients with stable angina pectoris after myocardial infarction. Eur Heart J. (1999) 20:1493–502. doi: 10.1053/euhj.1999.1519
Keywords: thrombus aspiration, D-D, percutaneous coronary intervention, STEMI, coronary artery disease
Citation: Li J-f, Lin Z-w, Chen C-x, Liang S-q, Du L-l, Qu X, Gao Z, Huang Y-h, Kong S-t, Chen J-x, Sun L-y and Zhou H (2021) Clinical Impact of Thrombus Aspiration and Interaction With D-Dimer Levels in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 8:706979. doi: 10.3389/fcvm.2021.706979
Received: 08 May 2021; Accepted: 08 July 2021;
Published: 10 August 2021.
Edited by:
Celestino Sardu, Second University of Naples, ItalyReviewed by:
Ferdinando Carlo Sasso, University of Campania Luigi Vanvitelli, ItalyCopyright © 2021 Li, Lin, Chen, Liang, Du, Qu, Gao, Huang, Kong, Chen, Sun and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zhou, d3l6aDY2QDEyNi5jb20=; Ling-yue Sun, bGluZ3l1ZXN1bkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.