- 1Department of Cardiology, Shenzhen Cardiovascular Minimally Invasive Medical Engineering Technology Research and Development Center, Shenzhen People's Hospital Second Clinical Medical College, Jinan University, Shenzhen, China
- 2First Affiliated Hospital, Southern University of Science and Technology, Shenzhen, China
Background: An accurate biomarker at hospital discharge is needed to identify patients with acute infective endocarditis (IE) who are at high risk of mortality. This prospective observational study evaluated the prognostic value of C-reactive protein (CRP).
Methods: Patients with acute IE (n = 343) and hospitalized at 2 university-affiliated medical centers from January 2014 to December 2019 were enrolled. Patients were categorized as having low or high CRP (n = 217 and 126, respectively) at hospital discharge according to the optimal cutoff (CRP = 6.5 mg/L) determined via receiver-operating characteristic curve analysis. The primary endpoint was all-cause death, from hospital discharge to 1 year. The secondary endpoint was the cumulative rate of rehospitalization or paravalvular abscess at 1 year.
Results: At the 12-month follow-up, the mortality rate of the high-CRP group (21.43%) was significantly higher than that of the low-CRP group (2.76%, log-rank P < 0.0001). The multivariate regression analysis indicated that the high-CRP group had a higher excess mortality hazard risk (HR = 4.182; 95% CI: 2.120, 5.211; P < 0.001). The cumulative 1-year incidence of paravalvular abscess of the high-CRP group (11.90%) was significantly higher than that of the low-CRP (5.07%; P = 0.022). The cumulative rate of heart rehospitalizations of the 2 groups were similar (18.25% cf. 14.29%, P = 0.273).
Conclusion: For hospitalized patients with acute IE, a high CRP at discharge suggests a poor prognosis for 1-year mortality and paravalvular abscess.
Introduction
IE is an important source of mortality and morbidity, entailing expensive treatments, long-term hospitalization and challenging interventions. Despite standardized medical therapy or surgical treatments, its poor prognosis is reflected by 8–24% in-hospital mortality that rises up to 18–37% during the first year after the IE episode (1). Moreover, temporal trend analyses describe an overall increasing incidence of IE over the past 20 years (2). The lack of prognosis biomarkers represents a challenge for both short and long-term risk stratification in IE. Accordingly, there should be a means to identify those patients at discharge who have an elevated risk of mortality.
Despite being a non-specific biomarker, CRP has proven to improve risk stratification in different forms of cardiovascular disease. Importantly, CRP at discharge was associated with an excess of 1-year mortality after acute decompensated heart failure hospitalization (3) and might be a long-term prognosis marker in IE. At hospital admission, C-reactive protein (CRP) is a putative predictor of adverse prognosis in patients with IE (4, 5), but data on its prognostic value after at discharge post-treatment is limited. Heiro et al. (5) reported that a rise in CRP of 50 mg/L was associated with a higher risk of death, both at 2 weeks (OR = 1.5, 95% CI, 0.97–2.3; P = 0.068) and 4 weeks after admission (OR = 2.3, 95% CI, 1.2–4.4; P = 0.009). As a response to chronic inflammation, the CRP level thus may be a useful indicator for patients with IE leaving the hospital.
To assist in determining a prognosis for patients with IE leaving the hospital, the present observational study is the first to assess an association between CRP level at discharge and clinical outcomes at 1 year, for patients with acute IE.
Materials and Methods
Patient Enrollment
The study was designed as a prospective observational trial and conducted at 2 medical centers (Shenzhen People's Hospital and Guangdong General Hospital) in China. The minimum sample size of each group (n = 69, alpha = 0.05, beta = 0.20, based on a frequency of 1-year mortality of 15% in the high-CRP group and 0.2% in the low-CRP group, with 1:1 sampling), was calculated using the chi-squared test in PASS 15 software (6). The Research Ethics Committee of Shenzhen People's Hospital approved the protocol (LL-KY-2014009). All patients provided informed consent before their enrollment.
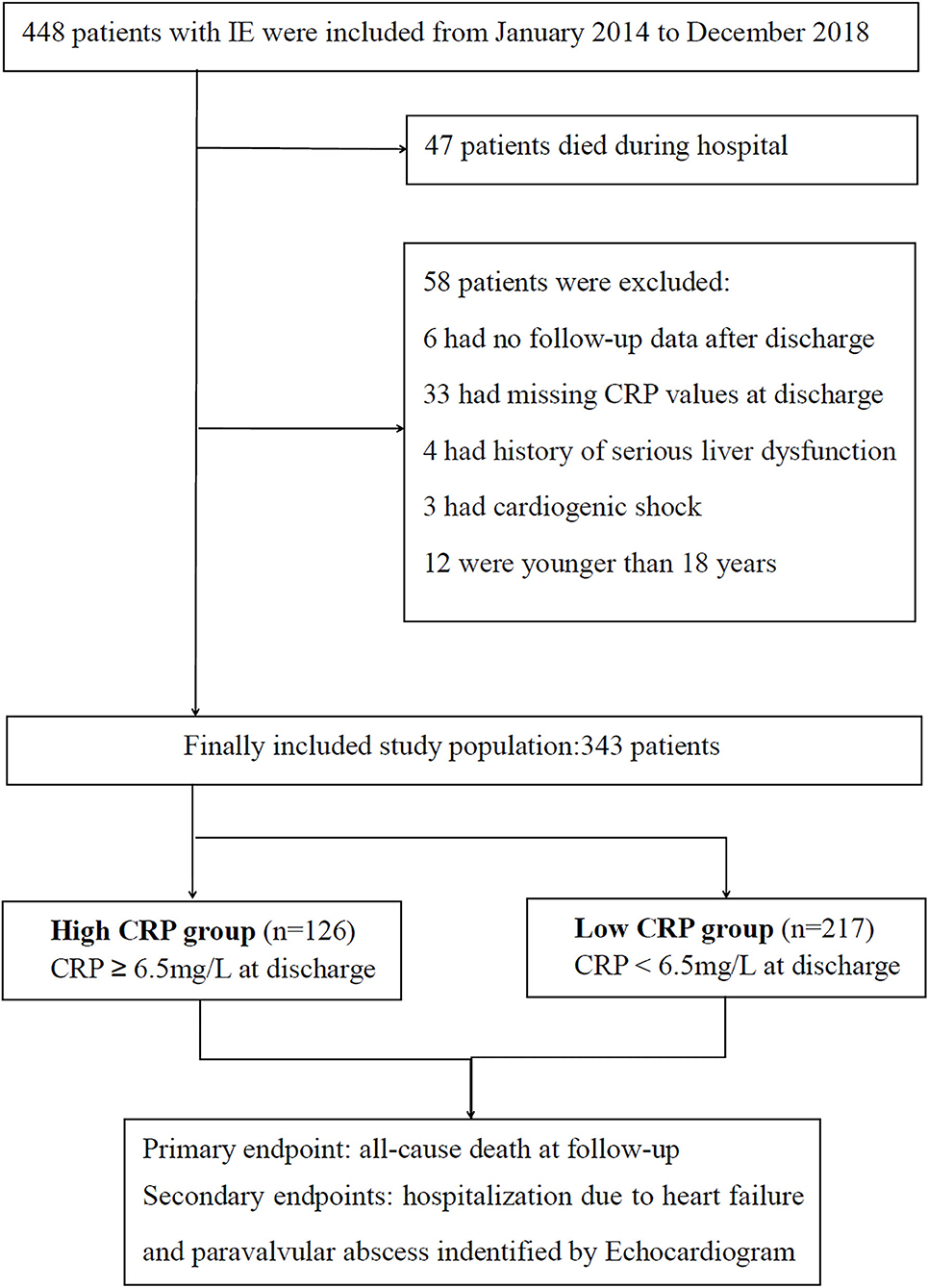
Based on the modified Duke criteria (7), 448 patients with acute IE were screened from January 2014 to December 2019 (Figure 1). Patients were excluded from this analysis for the following reasons: death during hospital stay (n = 47); missing CRP values at discharge (n = 33); younger than 18 years (n = 12); without follow-up data (n = 6); serious liver dysfunction, active neoplasm and other inflammatory diseases (n = 4); or cardiogenic shock (n = 3). Finally, 343 patients with acute IE were included in this study. Surgery was performed when the surgical criteria were met. Conservative drug therapy was initiated in circumstances of extremely poor health, or the patient could not afford the surgical fee.
Data Collection
The CRP value (normal reference value: 0–5 mg/L) was analyzed by immunoturbidimetry assay, on admission and at discharge. Transthoracic echocardiography was assessed within 24 h of admission. All-cause death was defined as subsequent readmission or mortality during the 1-year follow-up after discharge. Rehospitalization due to heart failure or relapse was defined as worsening heart failure or a repeat episode of IE caused by the same microorganism as the previous episode (within 6 months) (8), if either required intravenous drug therapy. Paravalvular abscess meant the presence of necrotic tissue in the valve annulus or remaining cavity after septic destruction of the valve annulus, as revealed by echocardiogram, transesophageal echocardiography, cardiac computed tomography, or surgery if needed during follow-up.
Study Endpoints
The primary study endpoint was the incidence of all-cause death at 1-year. The secondary endpoints included the cumulative rate of rehospitalization and paravalvular abscess at 1-year.
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software. Data was reported as mean ± standard deviation when normally distributed, quartile range when not normally distributed or count (percentage). Group comparisons of continuous data were conducted using Student's test, analysis of variance (ANOVA), or Kruskal-Wallis test; group comparisons of categorical data were calculated by chi-squared or Fisher's exact test. The optimal cutoff was determined via receiver operator characteristic curve (ROC) analysis. Univariate (crosstabs-relative risk) and multivariate (binary logistic regression) analyses were applied to evaluate the adjusted hazard ratio (HR) of mortality with a 1-year follow-up. The Kaplan-Meier method was applied to evaluate long-term survival. Enhanced predictive accuracy of the CRP-based model and the clinical model was evaluated by net reclassification improvement (NRI) and integrated discrimination improvement (IDI). P < 0.05 was considered statistically significant.
Results
Characteristics of Patients in the High- and Low-CRP Groups
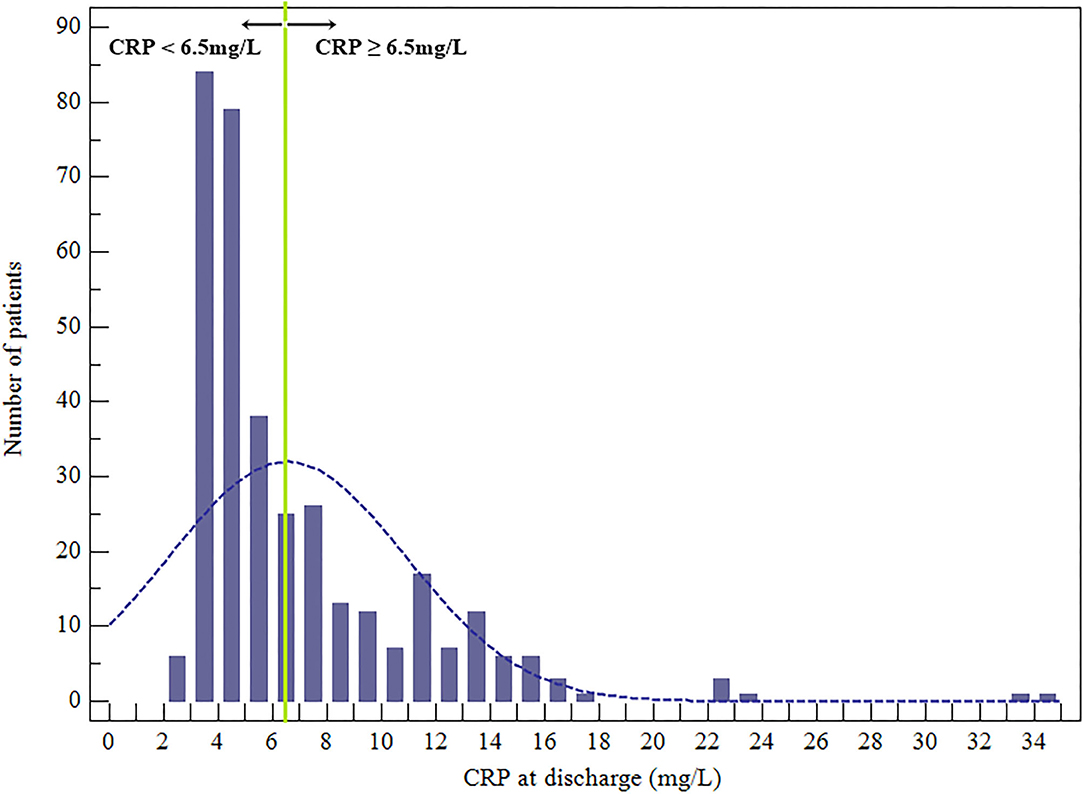
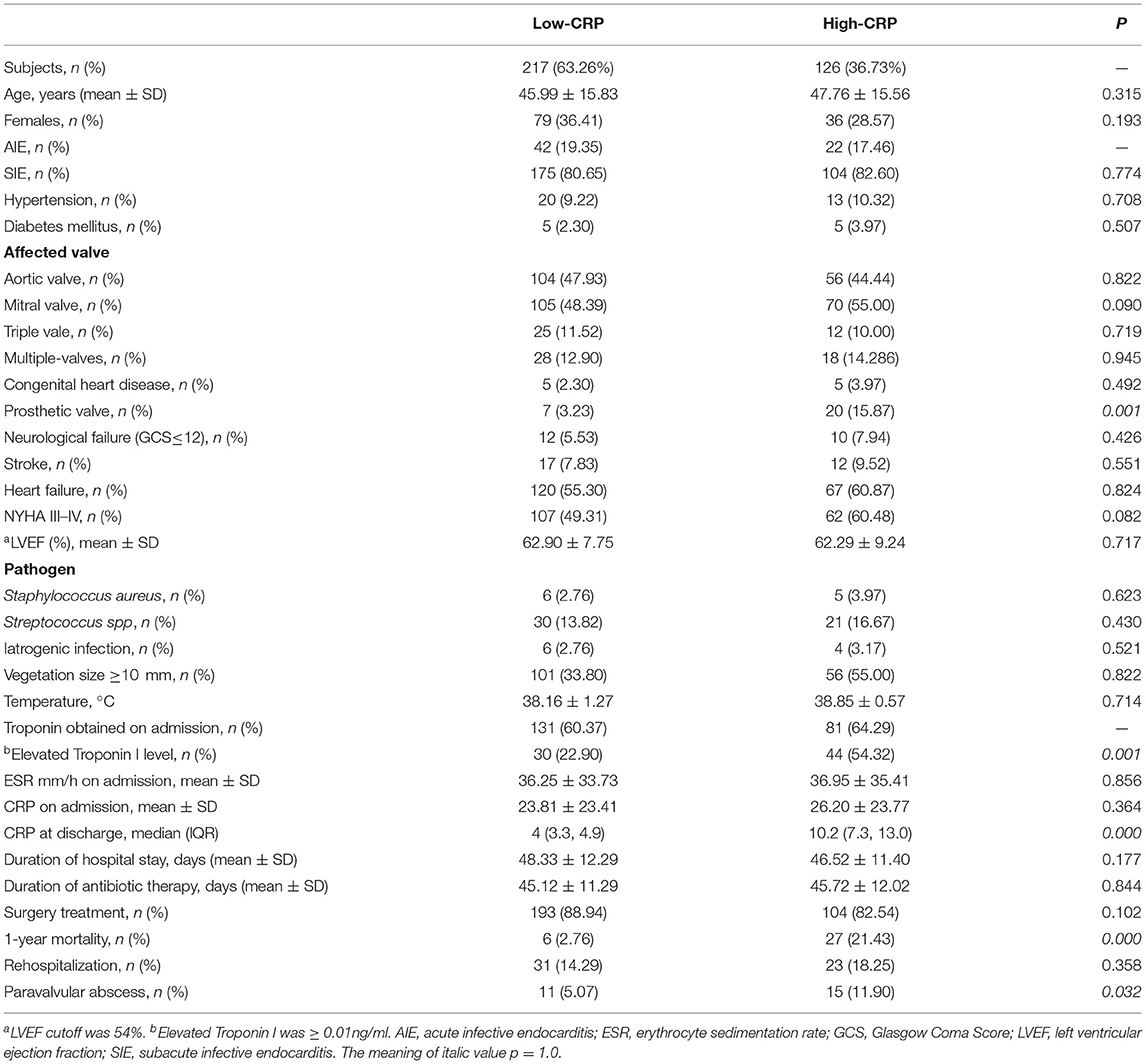
Overall, there were 343 patient participants (115 women), among whom 33 (9.62%) died during the 1-year follow-up. The CRP levels at discharge were abnormally distributed (Figure 2). According to the CRP value at the time of hospital discharge, according to the optimal cutoff patients were classified as low-CRP (<6.5 mg/L, n = 217) or high-CRP (≥6.5 mg/L, n = 126). The ages of the low- and high-CRP groups were comparable (45.99 ± 15.83 and 47.76 ± 15.56 y, respectively; P = 0.315). Upon admission, troponin I levels were measured in 212 patients (61.81%) of which 81 (64.29%) and 131 (60.37%) were apportioned to high-CRP or low-CRP groups, respectively. Importantly, 44 (54.32%) patients in the high-CRP group and 30 (22.90%) patients in the low-CRP group had elevated troponin I levels (P < 0.001; Table 1). Other features that were similar between the groups were the baseline demographics, echocardiography, causative pathogens, laboratory indexes, duration of antibiotic therapy, and hospital stay.
CRP at Discharge Predicting Mortality at 1-Year and Clinical Outcomes
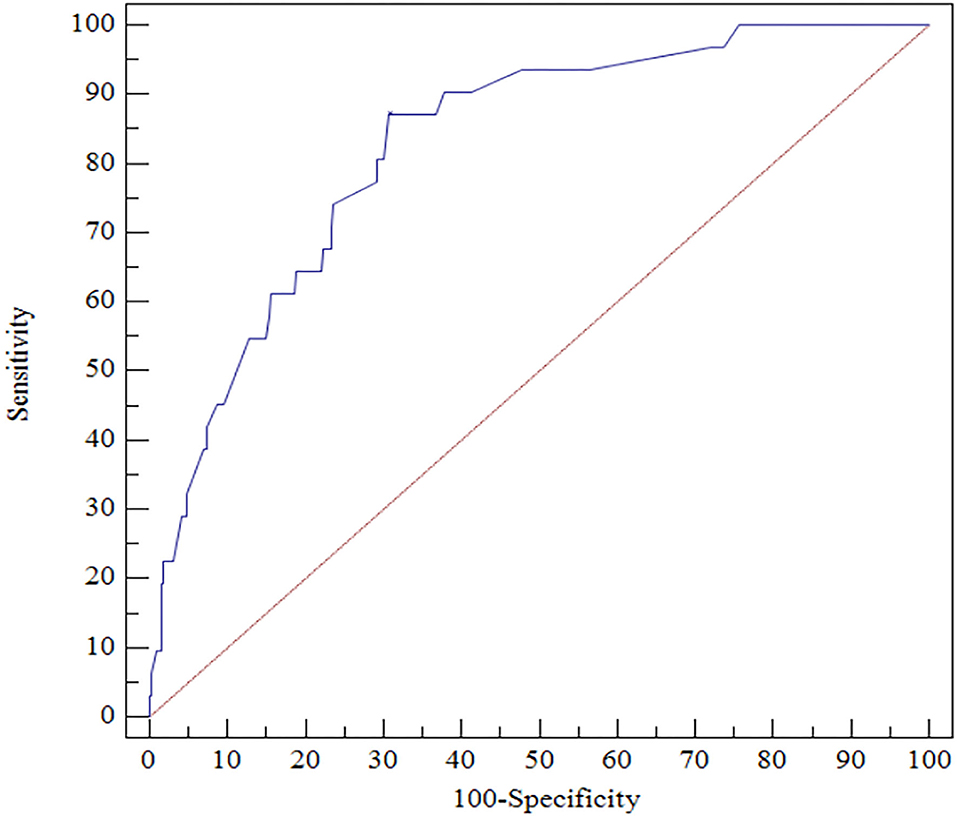
The ROC analysis indicated that CRP ≥ 6.5 mg/L at discharge could accurately predict 1-year mortality with a sensitivity of 87.10% and a specificity of 69.23% (area under the ROC curve = 0.827, 95% CI, 0.783–0.865, P < 0.0001; Figure 3).
Among the 343 patients, 33 (9.62%) died within the follow-up of 12 months, including 27 (27/126, 21.43%) in the high-CRP group and 6 (6/217, 2.76%) in the low-CRP group (P < 0.0001, chi-squared test). The Kaplan-Meier analysis showed a lower cumulative survival ratio in patients with high CRP ≥ 6.5 mg/L (P < 0.0001; Figure 4A).
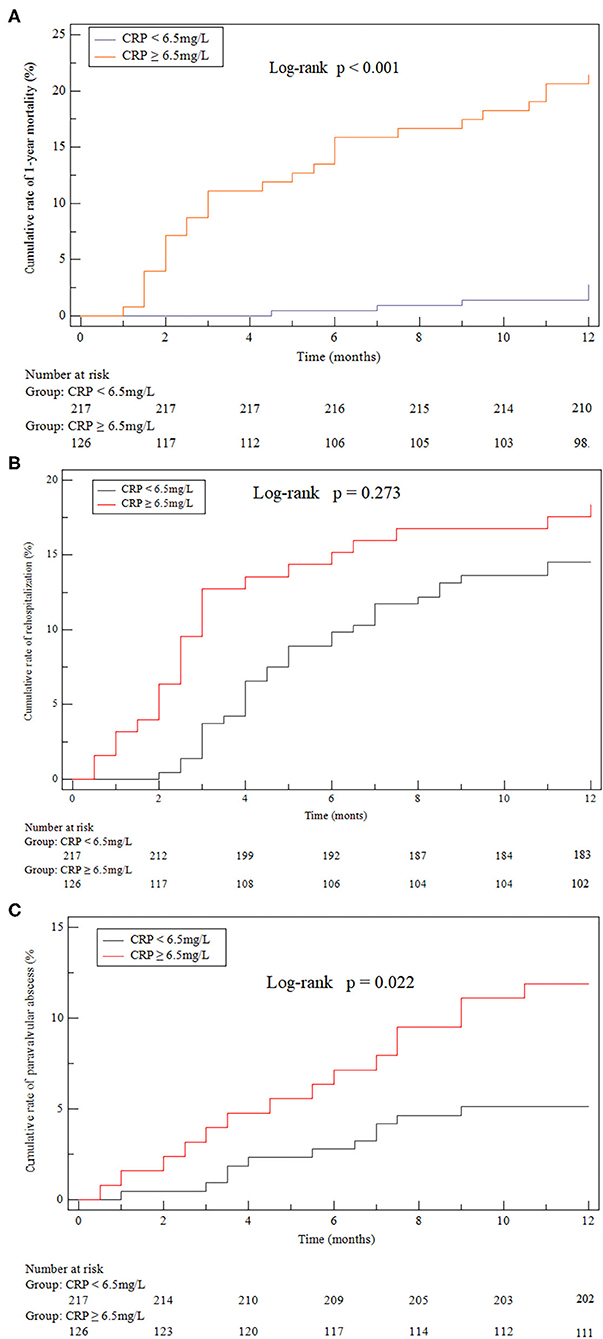
Figure 4. Kaplan-Meier curves. (A) all-cause death after discharge (21.43% cf. 2.76%, P < 0.001). (B) Heart failure hospitalizations after discharge (18.25% cf. 14.29%, P = 0.273). (C) Paravalvular (11.90% cf. 5.07%, P = 0.022) abscess identified by echocardiogram after discharge compared between the high-CRP and low-CRP groups.
According to the univariate logistic regression analysis (crosstabs-relative risk), additional significant indicators included age, diabetes mellitus, heart failure, New York Heart Association (NYHA) III–IV, endocarditis vegetation size ≥10 mm, Staphylococcus aureus, troponin, CRP, and surgery. After adjusting for the variables of significance shown by the univariate analysis, the multivariate regression analysis confirmed that CRP at discharge was an independent predictor of 1-year mortality (HR = 4.182; 95% CI: 2.120, 5.211, P < 0.000; concordance index = 0.81, 95% CI: 0.65–0.86; Table 2). Compared to CRP-based model, the clinical model did not showed the enhanced predictive accuracy with regard to NRI (0.002, p = 0.532) and IDI (0.047, p = 0.470).
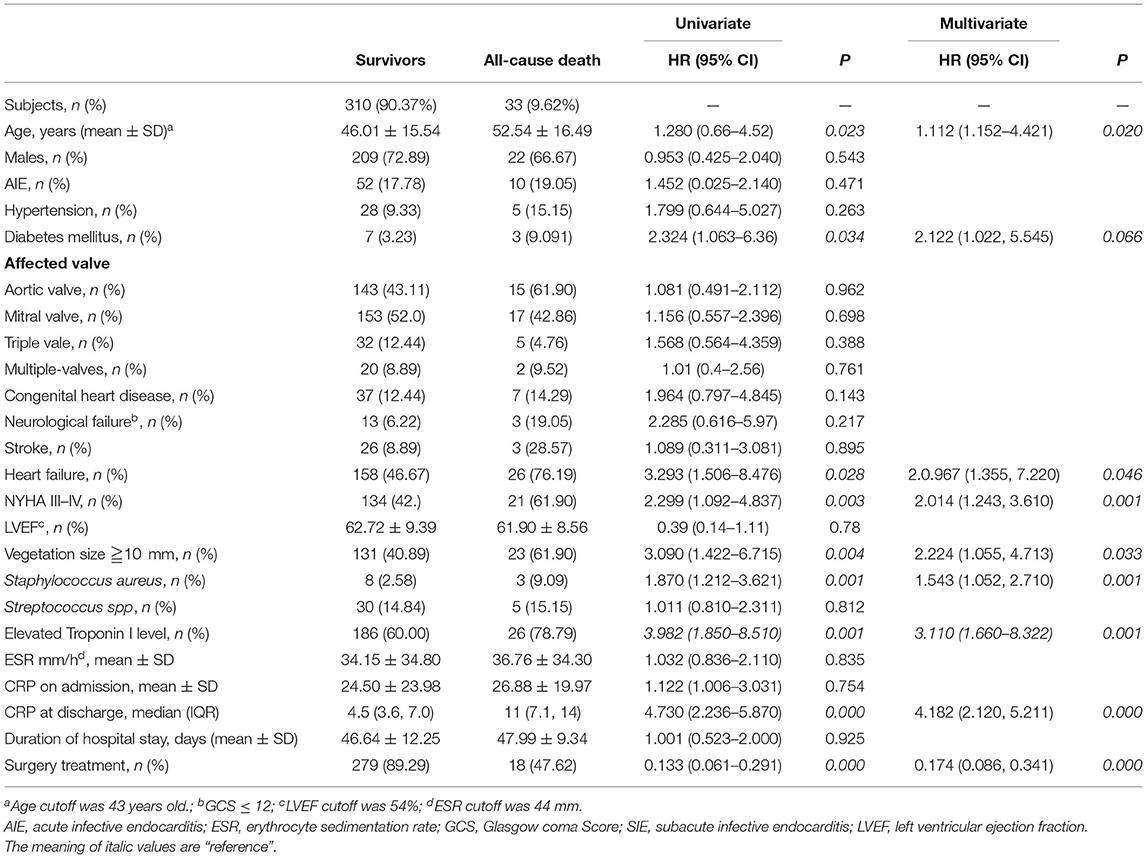
Table 2. Univariate analyses and multivariate Cox proportional hazard of factors associated with 1-year mortality.
Twenty-seven patient participants suffered from paravalvular abscess during the 1-year follow-up. Overall, paravalvular abscess occurred in 15 (55.56%) patients due to a relapse and in 9 (33.33%) patients due to a new IE. Three (11.11%) patients suffered from paravalvular abscess without a confirmed reason. The diagnosis of paravalvular abscess was made by transesophageal echocardiography in 17 (62.96%) patients and by cardiac computed tomography in 4 (14.81%) patients. In the remaining 6 (22.22%) patients the diagnosis was made at surgery. The cumulative 1-year incidence of paravalvular abscess of the high-CRP group (11.90%) was significantly higher than that of the low-CRP group (5.07%; P = 0.022, Figure 4C). The groups were statistically similar with regard to the cumulative rate of rehospitalizations due to heart failure and relapse (18.25% cf. 14.29%, P = 0.273, Figure 4B).
Discussion
This study is the first clinical trial to evaluate the CRP level at hospital discharge for predicting 1-year mortality in patients with acute IE. It was found that CRP ≥ 6.5 mg/L was strongly predictive of 1-year mortality at discharge from the index hospitalization, and in addition was associated with a high risk for paravalvular abscess by the 1-year follow-up. However, the groups above and below this CRP cutoff showed no significant difference in risk of hospitalization due to heart failure.
Despite the development of new antibiotics and modern surgical techniques in the past few decades, the mortality of patients with acute IE after discharge is still very high (9, 10). Previous studies have described elevated troponin as a surrogate for increased likelihood of mortality in patients with IE (11–13). A recent meta-analysis reported that patients with elevated troponin suffered a higher rate of in-hospital mortality (OR = 5.96, P < 0.0001), 1-year mortality (OR = 2.67, P = 0.002), surgery rates (OR = 2.34, P = 0.0008), and more frequent complications including central nervous system events (OR = 8.85, P < 0.0001) and cardiac abscesses (OR = 4.96, P = 0.0008) (14). Elevated troponin on admission was associated with 1-year mortality risk in this study. Other biochemical markers such as B-Type Natriuretic Peptide, D-dimer, and procalcitonin are reliable prognostic biomarkers associated with adverse outcomes (15–17). Recently, IL-5 and CCL4 were found to add prognostic value beyond CRP levels for in-hospital mortality in IE (18). Multimarker panels might be a useful tool to assess short and long-term prognosis in IE.
CRP is an acute-phase inflammatory serum protein that rapidly responds to infection, and has proved valuable for early risk stratification of sepsis-suspected mortality in patients (19–22). Mohanan et al. (4) found that, in patients with IE, CRP >40 mg/L in the first 3 days of admission was a strong predictor of in-hospital death (OR = 8.29, 95% CI: 1.39–49.41, P = 0.021); and 6-month mortality (OR = 5.55; 95% CI 1.65–18.72, P = 0.006). In an evaluation of left-sided native valve endocarditis, Verhagen et al. (23) reported that after 1 week of treatment, CRP >122 mg/L, or a slow percentage decline in CRP level, were indicators of serious infectious complications or death within 3 months (OR = 10.5, = 1.1, respectively). Wei et al. (24) showed that elevated CRP (>17.8 mg/L) was associated with in-hospital death in patients with blood culture-negative IE (OR = 2.41, 95% CI: 1.06–5.51, P = 0.037).
Unfortunately, these previous studies focused only on the efficacy of biochemical markers at admission or early hospitalization. However, a recent prospective cohort study suggested that serum CRP concentration fell significantly faster in patients with an uncomplicated recovery from IE compared with those with complications needing cardiac surgery (2.5% cf. 17.24%) or experiencing death (nil cf. 6.0%) within 3 months (5). Similarly, the present study showed that, for patients with IE, CRP ≥ 6.5 mg/L at discharge was a simple predictor of 1-year mortality caused by underlying inflammation that could not be controlled by antibiotic therapies. For most patients, escalating inflammation or uncontrolled infection caused hemodynamic collapse by increasing oxygen demand and surge (3, 25). Therefore, elevated CRP at discharge may reflect a persistent and chronic inflammatory response, even for patients who received optimal treatment or adequate antibiotic treatment.
The present study determined that CRP ≥ 6.5 mg/L at discharge was associated with an excess risk for paravalvular abscess within 1 year, but not with risk of heart failure hospitalization. The mortality and morbidity rates of patients with active IE complicated with paravalvular abscess remain high after surgery, with 1-year mortality rates of 25 to 50% in patients with aortic periannular abscess (26, 27). Infection often occurs in the annulus or tissue of the prosthetic valve, and extends to the myocardium, eventually leading to valve rupture and paravalvular abscess (28). Accordingly, CRP, a reflection of systemic inflammatory response, is a significant predictor of infective complications and paravalvular abscess (29). However, in the present study the risk of rehospitalization for heart failure at discharge was not significantly higher in the high-CRP (≥6.5 mg/L) relative to the low-CRP group. Additionally, the baseline left ventricular ejection fraction, or NYHA class ≥ 2, did not differ significantly between these groups (Table 1). These findings were similar to that of previous studies, and suggests that elevated CRP levels at discharge are not associated with the severity of cardiac dysfunction or symptoms in chronic heart failure (25, 30).
It should be noted that in the present study, no comparison was conducted between different treatments (surgery or not) and clinical outcomes. In addition, while we considered the CRP level only on the date of discharge, in individuals CRP varies over time. Our next study will monitor CRP concentrations at various timepoints during hospitalization. The incidence of pathogens reported in this study was low and additional information on the epidemiology was lacking. Finally, in the present study only a single CRP cutoff was considered, although a scoring system and the evaluation of additional predictive factors may be more informative.
Conclusion
CRP at discharge is a convenient prognostic tool for patients with acute IE. A CRP ≥ 6.5 mg/L at hospital discharge was significantly associated with mortality during the first year.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Research Ethics Committee of Shenzhen People's Hospital approved the protocol. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL, YW, and XS collected, analyzed, and wrote this manuscript. BL, WB, and JC assisted in conducting the study. JY and SD were the principal investigators. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cresti A, Chiavarelli M, Scalese M, Nencioni C, Valentini S, Guerrini F, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospectiv e study. Cardiovasc Diagn Ther. (2017) 7:27–35. doi: 10.21037/cdt.2016.08.09
2. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to (2011). J Am Coll Cardiol. (2015) 65:2070–6. doi: 10.1016/j.jacc.2015.03.518
3. Nishimoto Y, Kato T, Morimoto T, Yaku H, Inuzuka Y, Tamaki Y, et al. C-reactive protein at discharge and 1-year mortality in hospitalised patients with acute decompensated heart failure: an observational study. BMJ Open. (2020) 10:e041068. doi: 10.1136/bmjopen-2020-041068
4. Mohanan S, Gopalan Nair R, Vellani H, Sajeev CG, George B, Krishnan MN. Baseline C-reactive protein levels and prognosis in patients with infective endocarditis: a prospective cohort study. Indian Heart J. (2018). 70(Suppl. 3):S43–S49. doi: 10.1016/j.ihj.2018.05.001
5. Heiro M, Helenius H, Sundell J, Koskinen P, Engblom E, Nikoskelainen J, et al. Utility of serum C-reactive protein in assessing the outcome of infective endocarditis. Eur Heart J. (2005) 26:1873–81. doi: 10.1093/eurheartj/ehi277
6. Shein-Chung, Chow JS, Wang H, Lokhnygina Y. Sample Size Calculations in Clinical Research, 3rd Edn. Boca Raton, FL: Taylor & Francis Group (2018).
7. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. (2000) 30:633–8. doi: 10.1086/313753
8. Chu VH, Sexton DJ, Cabell CH, Reller LB, Pappas PA, Singh RK, et al. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis. (2005) 41:406–9. doi: 10.1086/431590
9. Botelho-Nevers E, Thuny F, Casalta JP, Raoult D. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. (2009) 169:1290–8. doi: 10.1001/archinternmed.2009.192
10. Shih CJ, Chu H, Chao PW, Lee YJ, Kuo SC, Li SY, et al. Long-term clinical outcome of major adverse cardiac events in survivors of infective endocarditis: a nationwide population-based study. Circulation. (2014) 130:1684–91. doi: 10.1161/CIRCULATIONAHA.114.012717
11. Tsenovoy P, Aronow WS, Joseph J, Kopacz MS. Patients with infective endocarditis and increased cardiac troponin I levels have a higher incidence of in-hospital mortality and valve replacement than those with normal cardiac troponin I levels. Cardiology. (2009) 112:202–4. doi: 10.1159/000149573
12. Postigo A, Bermejo J, Muñoz P, Valerio M, Marín M, Pinilla B, et al. Troponin elevation is very common in patients with infective endocarditis and is associated with a poor outcome. Int J Cardiol. (2020) 307:82–6. doi: 10.1016/j.ijcard.2020.02.029
13. Lorson W, Veve MP, Heidel E, Shorman MA-O. Elevated troponin level as a predictor of inpatient mortality in patients with infective endocarditis in the Southeast United States. BMC Infect Dis. (2020) 20:24. doi: 10.1186/s12879-019-4755-z
14. Postigo AA-O, Vernooij RWM, Fernández-Avilés F, Martínez-Sellés M. Cardiac troponin and infective endocarditis prognosis: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. (2021) 10:356–66. doi: 10.1093/ehjacc/zuab008
15. Shiue AB, Stancoven AB, Purcell JB, Pinkston K, Wang A, Khera A, et al. Relation of level of B-type natriuretic peptide with outcomes in patients with infective endocarditis. Am J Cardiol. (2010) 106:1011–5. doi: 10.1016/j.amjcard.2010.05.034
16. Lin YW, Jiang M, Wei XB, Huang JL, Su Z, Wang Y, et al. Prognostic value of D-dimer for adverse outcomes in patients with infective endocarditis: an observational study. BMC Cardiovasc Disord. (2021) 21:279. doi: 10.1186/s12872-021-02078-3
17. Siciliano RF, Gualandro DM, Bittencourt MS, Paixão M, Marcondes-Braga F, Soeiro AM, et al. Biomarkers for prediction of mortality in left-sided infective endocarditis. Int J Infect Dis. (2020) 96:25–30. doi: 10.1016/j.ijid.2020.03.009
18. Ris T, Teixeira-Carvalho A, Coelho RMP, Brandao-de-Resende C, Gomes MS, Amaral LR, et al. Inflammatory biomarkers in infective endocarditis: machine learning to predict mortality. Clin Exp Immunol. (2019) 196:374–82. doi: 10.1111/cei.13266
19. Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperativ e patients with severe sepsis. J Crit Care. (2011) 26:54–64. doi: 10.1016/j.jcrc.2010.04.011
20. Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. (2003) 31:1737–41. doi: 10.1097/01.CCM.0000063440.19188.ED
21. Ruiz-Alvarez MJ, Garca-Valdecasas S, De Pablo R, Sanchez Garca M, Coca C, Groeneveld TW, et al. Diagnostic efficacy and prognostic value of serum procalcitonin concentration in patients with suspec ted sepsis. J Intensive Care Med. (2009) 24:63–71. doi: 10.1177/0885066608327095
22. Cappabianca G, Paparella D, Visicchio G, Capone G, Lionetti G, Numis F, et al. Preoperative C-reactive protein predicts mid-term outcome after cardiac surgery. Ann Thorac Surg. (2006) 82:2170–8. doi: 10.1016/j.athoracsur.2006.06.039
23. Verhagen DW, Hermanides J, Korevaar JC, Bossuyt PM, van den Brink RB, Speelman P, et al. Prognostic value of serial C-reactive protein measurements in left-sided native valve endocarditis. Arch Intern Med. (2008) 168:302–7. doi: 10.1001/archinternmed.2007.73
24. Wei XB, Liu YH, He PC, Zhou YL, Tan N, Chen JY, et al. Combined efficacy of C-reactive protein and red blood cell distribution width in prognosis of patient s with culture-negative infective endocarditis. Oncotarget. (2017) 8:71173–80. doi: 10.18632/oncotarget.16888
25. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi DJ, et al. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental G REAT registry. Eur J Heart Fail. (2017) 19:201–8. doi: 10.1002/ejhf.682
26. Ting M, Wang CH, Chi NH, Hsu RB, Chen YS, Yu HY. Outcome for surgical treatment of infective endocarditis with periannular abscess. J Formos Med Assoc. (2020) 119(1 Pt 1):113–24. doi: 10.1016/j.jfma.2019.02.009
27. Spiliopoulos K, Haschemi A, Fink G, Kemkes BM. Infective endocarditis complicated by paravalvular abscess: a surgical challenge. An 11-year single c enter experience. Heart Surg Forum. (2010) 13:E67–73. doi: 10.1532/HSF98.20081141
28. Subbaraju P, Rai S, Morakhia J, Midha G, Kamath A, Saravu K. Clinical-microbiological characterization and risk factors of mortality in infective endocarditis from a tertiary care academic hospital in Southern India. Indian Heart J. (2018) 70:259–65. doi: 10.1016/j.ihj.2017.08.007
29. Ramanathan ML, MacKay G, Platt J, Horgan PG, McMillan DC. The impact of open versus laparoscopic resection for colon cancer on C-reactive protein concentrations as a predictor of postoperative infective complications. Ann Surg Oncol. (2015) 22:938–43. doi: 10.1245/s10434-014-4065-z
Keywords: C-reactive protein, acute infective endocarditis, all-cause death, paravalvular abscess, rehospitalization
Citation: Lin Y, Chen J, Liao B, Bei W, Wang Y, Sun X, Yuan J and Dong S (2021) C-Reactive Protein at Hospital Discharge and 1-Year Mortality in Acute Infective Endocarditis: A Prospective Observational Study. Front. Cardiovasc. Med. 8:706684. doi: 10.3389/fcvm.2021.706684
Received: 07 May 2021; Accepted: 09 July 2021;
Published: 09 August 2021.
Edited by:
Eva Jover, Instituto de Investigación Sanitaria de Navarra (IdiSNA), SpainReviewed by:
Manuel Martínez-Sellés, Gregorio Marañón Hospital, SpainAlfredo Jose Mansur, Universidade de São Paulo, Brazil
Copyright © 2021 Lin, Chen, Liao, Bei, Wang, Sun, Yuan and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yuan, eG5reXVhbmppZUB5ZWFoLm5ldA==; Shaohong Dong, eG5rZHNoQHllYWgubmV0
 Yaowang Lin
Yaowang Lin Jie Chen1,2
Jie Chen1,2 Jie Yuan
Jie Yuan Shaohong Dong
Shaohong Dong