
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Cardiovasc. Med. , 26 August 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.705972
This article is part of the Research Topic Advances in The Prevention and Rehabilitation of Cardiovascular Diseases via Aerobic Exercise View all 24 articles
 Ting Shen1†
Ting Shen1† Xiaoling Liu1†
Xiaoling Liu1† Bo Zhuang1
Bo Zhuang1 Qian Luo1
Qian Luo1 Yishan Jin1
Yishan Jin1 Guanghe Li1
Guanghe Li1 Yumei Jiang1
Yumei Jiang1 Dejie Li1
Dejie Li1 Xianchuan Chen2
Xianchuan Chen2 Nuo Tang3
Nuo Tang3 Zhimin Xu4
Zhimin Xu4 Lemin Wang1
Lemin Wang1 Liang Zheng5*
Liang Zheng5* Yuqin Shen1*
Yuqin Shen1*Background: Heart failure (HF) is one of the major causes of mortality worldwide, representing the terminal stage of several cardiovascular diseases. Exercise-based rehabilitation is a beneficial therapy for patients with chronic heart failure (CHF). However, there is a lack of specific guidance on clinical decision-making regarding optimal exercise intensity. It is necessary to optimize the clinical recommendations for HF exercises. We will evaluate the efficacy and safety of different aerobic exercise intensities in patients with heart failure with reduced ejection fraction (HFrEF): the HF-EI trial. This trial aims to assess the appropriate exercise intensity for patients with HFrEF.
Methods: After a baseline assessment to determine the safety of exercise, 180 patients will be randomly assigned to supervised high-intensity exercise training (ET) group, supervised moderate intensity training (MIT) group, and control group at a ratio of 1:1:1. Patients randomly receiving high intensity training (HIT) undergo supervised ET (3 times/week, 30 min) for aerobic endurance at 70% peak oxygen consumption (peak VO2) intensity for 12 weeks. The MIT patients will perform supervised aerobic ET (3 times/week, 35–42 min) at the anaerobic threshold (AT) intensity for 12 weeks. The control group will continue to maintain their daily activities and will not receive ET. During the baseline and follow-up period, physical examination, laboratory tests, cardiology diagnostic tests, cardiopulmonary exercise tests (CPET), 6-min walk distance (6MWD), scale scores, exercise steps, medications, and clinical events will be monitored. Throughout the research, sport bracelets and patient diaries will be used to monitor and record overall physical activity, training courses, and compliance.
Discussion: The HF-EI trial will evaluate the effects of different aerobic exercise intensities on peak VO2, quality of life (QoL), and clinical events among patients with HFrEF. The findings of this trial will provide a basis for formulating exercise prescriptions for patients with HFrEF.
Clinical Trial Registration: http://www.chictr.org.cn/, identifier: ChiCTR2000036381.
Chronic heart failure (CHF) is a severe and end-stage manifestation of several cardiovascular diseases. It is a syndrome with a high hospitalization rate, high disability rate, and high mortality rate, causing an economic and social burden on families (1). According to statistics, there are 64.3 million heart failure (HF) patients worldwide. In developed countries, the prevalence of adult HF is 1–2% (2). The China Hypertension Survey (CHS) showed that 1.3% of Chinese people aged ≥35 years, or an estimated 13.7 million individuals had HF (3). At present, there are many treatment option [s]s for HF, including general therapy, medication, and non-drug treatment (4). Despite active exploration of new medical and non-drug treatments for HF, the final effect is still unsatisfactory. The challenges facing HF intervention include optimizing HF treatment and reducing overall costs associated with long-term management.
Exercise training (ET) is a safe and effective treatment for HF. As shown in previous randomized controlled trials, it can significantly improve exercise capacity and quality of life (QoL) (5). Furthermore, ET is associated with reductions in mortality and hospitalization and recommended in the current guidelines on CHF management (6).
A massive amount of evidence suggests that ET is beneficial for patients with HF (7–16). A study by Belardinelli et al. in 2012 showed that exercise rehabilitation for up to 10 years could significantly reduce the readmission rate and the risk of cardiovascular death (17). A Cochrane review in 2019 found that exercise-based cardiac rehabilitation (CR) may have little effect on short-term all-cause mortality but may improve long-term all-cause mortality (>12 months follow up).CR probably reduces overall hospitalization rates in the short term (6).
However, data from the Exercise Training Meta-Analysis of Trials in Chronic Heart Failure (ExTraMATCH) II trial with at least 3 weeks of ET in HF showed that exercise-based CR had no significant effect on the risk of mortality and hospitalization in heart failure with reduced ejection fraction (HFrEF) (18).
Studies on the effects of exercise-based CR on the mortality and hospitalization rate of patients with HF show conflicting results, possibly attributed to differences in exercise pattern, exercise intensity, patient population selection, compliance, and follow-up time. All factors, especially exercise intensity, may affect the endpoint effect. Therefore, further research is needed to determine optimal exercise intensity to reduce the mortality and HF hospitalization rate.
Since the 1980s, numerous studies have demonstrated the safety and effectiveness of moderate intensity training (MIT) (19, 20). However, recent data suggest that high intensity training (HIT) may offer some advantages over MIT (21–32). Within a specific intensity range, exercise intensity is proportional to the effect. However, higher exercise intensity is associated with higher risk, and a higher need for adjusted to ensure safety and effectiveness in patients with HF. The mainstream sports rehabilitation model in developed countries of Europe and the United States is based on rehabilitation centers and ET under electrocardiogram (ECG) monitoring. A 12-week, three times a week treadmill exercise is used as a classic exercise prescription (33). The advantage is that it considers the efficacy and safety of ET, providing tremendous benefits to patients with HF in the short term. Keteyian et al. found that a moderate amount of exercise (3–7 metabolic equivalent hours per week) in patients with HF reduces the risk of clinical events, and within this range, an increase in the amount of exercise is associated with increased benefits (34). Swank et al. also obtained similar results. After exercise rehabilitation in patients with HF, increased peak oxygen consumption (peak VO2) was associated with improved prognosis (35).
According to the grading standards for exercise intensity proposed by the 2020 European Society of Cardiology (ESC) Guidelines on sports cardiology and exercise in patients with cardiovascular disease, the anaerobic threshold (AT) intensity is moderate, 70% PeakVO2 intensity is high intensity (36). We will design a three-arm randomized controlled experiment to explore the effectiveness and safety of moderate exercise intensity (AT intensity) in the treatment of HFrEF. The HIT (70% PeakVO2 intensity) group served as the active control group, and the daily activity group served as the placebo control group. Mainly compare MIT and HIT, or MIT and daily activities, and explore the dose-effect relationship of exercise intensity on changes in clinical outcomes through regression analysis. We assume that MIT has a greater impact in the main results than HIT and daily activities.
We will evaluate the efficacy and safety of different aerobic exercise intensities in patients with HFrEF: the HF-EI trial. This study is the first multicenter randomized controlled trial (RCT) on the effects of different aerobic exercise intensities in patients with HFrEF in China. By collecting data on exercise capacity and exercise rehabilitation, we will evaluate the clinical efficacy of exercise-based CR in Chinese patients with HFrEF. Finally, suitable exercise intensity will be recommended for the popularized HFrEF aerobic exercise program for Chinese patients with HFrEF.
The primary objective of the HF-EI trial is to investigate the effects of different exercise intensities on exercise capacity and quality of life in patients with HFrEF. As secondary objectives, the HF-EI trial will evaluate AT oxygen uptake, 6-min walk distance (6MWD), left ventricle ejection fraction (LVEF), N-terminal prohormone of brain natriuretic peptide (NT pro-BNP), the Patient Health Questionnaire-9 (PHQ-9), General Anxiety Disorder-7(GAD-7), all-cause mortality, HF hospitalization, major cardiovascular events, and adverse events.
This will be a multi-center, parallel, three-group RCT to evaluate the efficacy and safety of MIT for HFrEF. Eligible participants will be randomly assigned to the HIT intervention group receiving a 12-week supervised training sessions (70% PeakVO2 intensity) plus usual medications, MIT intervention group receiving a 12-week supervised training sessions (AT intensity) plus usual medications, or a control group receiving only the usual medication and maintain daily activity. This study has a 12-week intervention and a 12-month follow-up period. Figure 1 shows the study design flow chart. This protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines and fulfills the SPIRIT checklist (Supplemental File 1); A SPIRIT checklist is provided in Figure 2 (37).
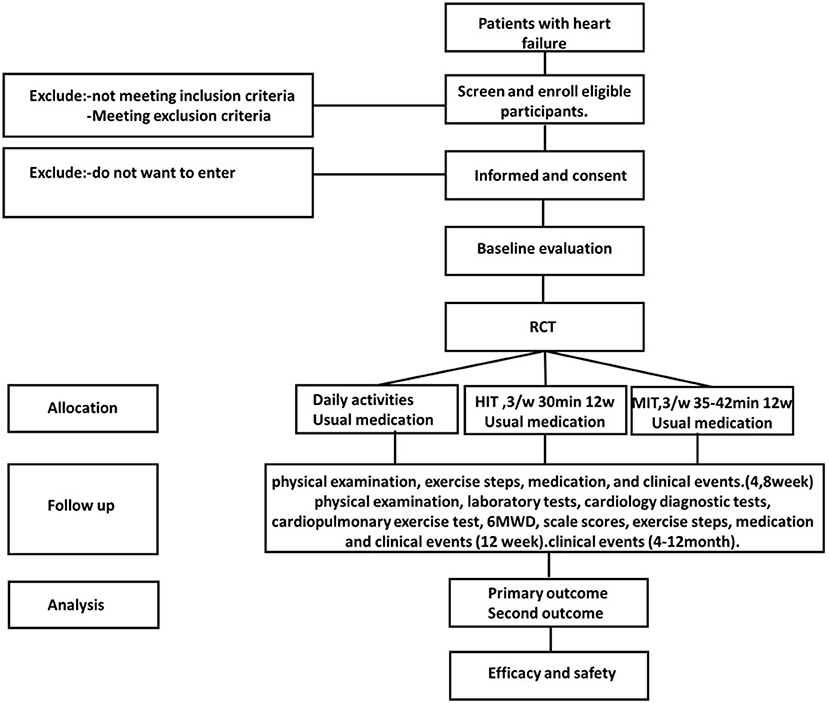
Figure 1. Flow chart of the study. RCT, randomised controlled trial; HIT, high intensity training; MIT, moderate intensity training.
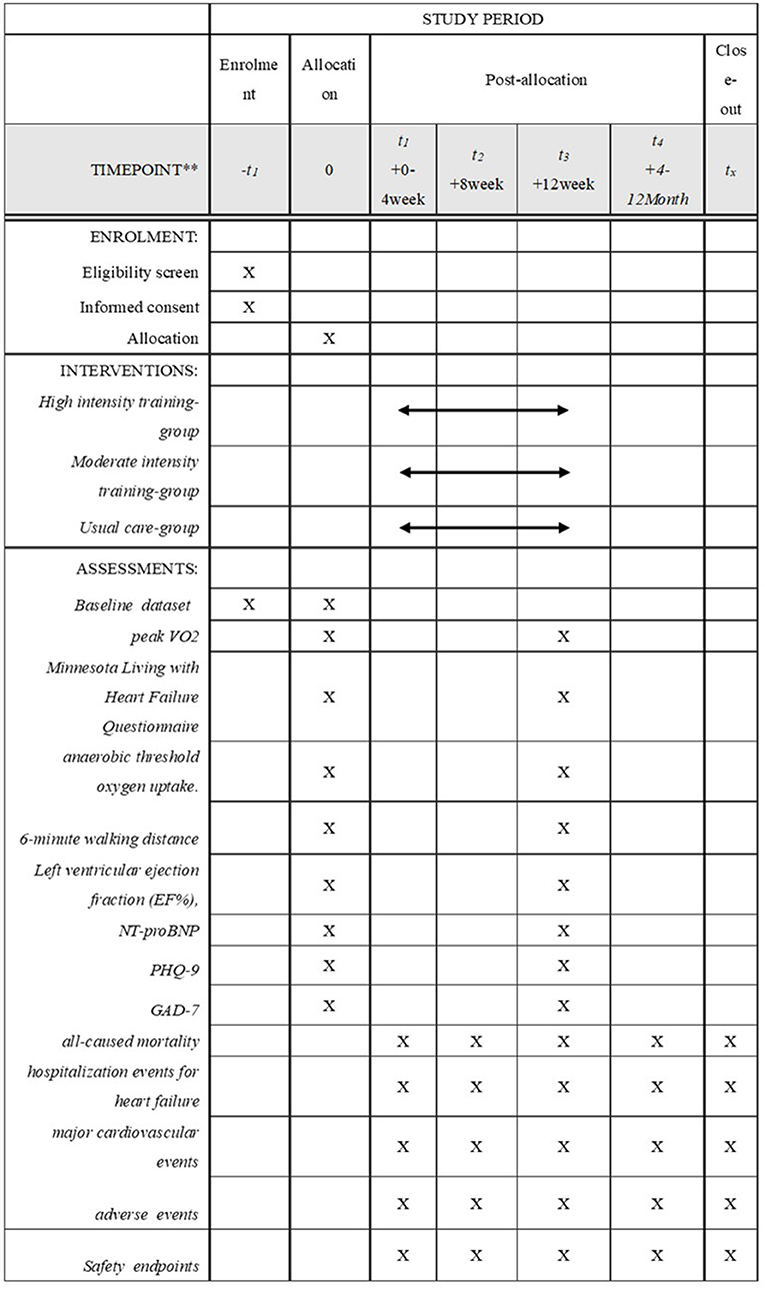
Figure 2. Standard protocol items. Peak VO2, peak oxygen consumption; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; PHQ-9, Patient Health Questionnaire-9;GAD-7, General Anxiety Disorder-7.
The HF-EI trial aims to recruit 180 stable HFrEF patients in three study sites (Tongji Hospital Affiliated to Tongji University, Yueyang Integrated Traditional Chinese Medicine and Western Medicine Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, and Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine) in China and apply standard therapy in accordance with the ESC HF guidelines. We have predefined a set of inclusion and exclusion criteria (Table 1) to ensure the feasibility and safety of ET. The main method of recruiting patients is to screen the patients on-site when visiting a doctor.
We will use a block- randomization, stratified in groups for center to assign participants to the high- intensity exercise group, moderate- intensity exercise group and the control group in a 1:1:1 ratio. For the hierarchical random assignment operation, the statistical unit responsible adopted the SAS version 9.2 statistical software (procedure 'PROC PLAN') to complete program writing and randomization operations. The random distribution results will be released through the network of a central random distribution system. The results of random allocation will be managed by a person designated by the research group, and the results will be executed independently by clinicians. Due to the interventional study design, blinding is impossible for patients and clinical operators. However, the examiners, researchers collecting data on outcome indicators, data managers, and statisticians will be blinded.
The training program of intervention groups and the control group is shown in Figure 2. The HF-EI trial aims to ensure that patients receive appropriate and stable guide-based care before entering the trial. Throughout the trial, the three groups of patients will receive the usual medications, monthly follow-ups and the same number of visits. This study has a 12-week intervention and a 12-month follow-up period. All interventions and follow-ups will be completed by doctors (obtained a licensed physician qualification) in the Heart Rehabilitation Center.
Patients in the control group will continue to maintain their daily activities (daily activities monitor the number of exercise steps through a sport bracelet) and will not provide additional ET.
Patients randomized to receive HIT or MIT training receive 36 supervised training sessions, thrice weekly, in addition to usual care for 12 consecutive weeks of aerobic ET. Patients will be regularly encouraged to exercise on other days. The duration of each exercise session in the high intensity training group is 30 min, and the duration of each exercise session in the moderate intensity training group is 35–42 min. It is estimated that the high intensity training group and the moderate intensity training group have similar energy expenditure (38, 39). Table 2 summarizes the training program for the HF-EI trial. Participants who have serious adverse clinical events during the intervention will immediately stop the intervention measures and withdraw from this clinical study.
Adherence refers to the degree to which trial participants comply with the intervention plan. Patients need to complete at least 66% of possible supervised training courses before consideration for treatment. The HF-EI trial uses several methods to promote compliance. First, researchers and trainers should regularly encourage patients randomized to ET to participate in supervised training courses. Second, a diary is used to track patient attendance and training to monitor patient compliance throughout the trial.
The primary outcome for the HF-EI trial is the change in peak VO2 and Minnesota Living with Heart Failure Questionnaire scores (MLHFQ)after 12 weeks. We choose the common primary outcomes in order to obtain the comprehensive effect of different exercise intensities on HFrEF treatment. Aerobic exercise has a well-known positive effect on patients with HFrEF in exercise capacity and quality of life, and peakVO2 is an important indicator for evaluating the prognosis of HF (22, 40).
Secondary outcomes included, AT oxygen uptake, 6MWD, LVEF, NT pro-BNP, PHQ-9, GAD-7, all-cause- mortality, hospitalization events for HF, major cardiovascular events, and adverse events.
The test indicators require patients to be measured at the hospital rehabilitation center. The test details are as follows:
According to the recommendations of international guidelines, CPET will be performed before randomization to determine exercise safety in patients. We will evaluate patient exercise endurance using CPET peak power, exercise duration, peak oxygen uptake, and AT. The core laboratory trains and certifies all researchers performing CPET before recruiting personnel.
Perform 6MWT according to the guidelines of the American Thoracic Society (41). In the room, follow a long and straight corridor and instruct patients to walk back and forth at their chosen walking speed. The corridor is 30 m long, with a mark every 3 m, and turning points are marked with cones. During the test, all patients are guided by a unified standard record.
Echocardiographic images and loops will be digitally recorded and stored on-site for analysis in an assessor-blinded reference echocardiography core lab, using color Doppler ultrasound diagnostic equipment, probe frequency 2.5–3.5HZ. During the examination, the patient assumes the left lateral decubitus position to obtain, the left ventricular long-axis view and the left ventricular short-axis view. The sampling lines are collected at the papillary muscle levels, the level of the mitral valve, and the aortic root to acquire two-dimensional images and an M-shaped spectrum. Echocardiography is repeated 12 weeks after randomization.
After resting for at least 5 min, blood samples will be collected under standardized conditions. According to standard operating procedures, all samples will be centrifuged immediately, aliquoted, and stored at −80 °C. Blood tests will be performed by a locally accredited laboratory.
A validated standard questionnaire will be used to assess the quality of life at baseline with repeat assessments within 12 weeks of patient randomization.
The HF-EI research data collection process is shown in Figure 2.
We will monitor the demographic data, medical history, physical status, laboratory tests (NT pro-BNP), cardiology diagnostic tests (LVEF), cardiopulmonary exercise tests (peak VO2, AT oxygen uptake), 6MWD, scale score(MLHFQ, PHQ-9, and GAD-7)and medications during the baseline period. Baseline data will be collected through outpatient consultation and medical record review.
Physical examination, exercise steps, medication use, and clinical events (all-cause-mortality, hospitalization events for HF, major cardiovascular events, and adverse events) will receive outpatient follow-up in the 4th and 8th weeks after random assignment. At 12 weeks after randomization, physical examination, laboratory tests, cardiology diagnostic tests, cardiopulmonary exercise test, 6MWD, scale scores, exercise steps, medication, and clinical events will receive outpatient follow-up visits. 4–12 months after randomization, clinical events will be followed up by telephone every month. Both the baseline survey and follow-up will be conducted by two physicians.
The calculation of the sample size will be based on the primary outcome of the RCT. The sample size calculation was performed using PASS V11.0 (NCSS Company of the United States) with the following calculation formula:
The parameters are set to double-sided α = 0.05, β = 0.1, Power = 1–β = 90%, the average difference in peak VO2 of the three groups were 0, 2, 4(ml/kg/min) and the average difference in MLHFQ of the three groups were 2, 12, 10. The corresponding standard difference values of peakVO2 were 6, 4, 6 (ml/kg/min) and MLHFQ were 5, 18, 16.
The parameter estimation is based on the results of previous researches (22, 42, 43).
A total of 180 cases were required for the three groups, 60 patients in each group, considering the 20% loss to follow-up rate and stratification factors. Because each of the primary endpoints can be individually reflected in clinical significance. Therefore, if one of the two primary endpoints of the intervention group improves significantly, the study is successful.
Blinded statistical analysis will be performed by qualified statisticians using PASW Statistics 18.0 (IBM SPSS Inc., Armonk, New York, USA). The measurement data will be expressed as mean ± standard deviation (x ± s), measurement data by t test, and count data by chi-square test. The clinical events of the treatment groups will be statistically compared, according to the principle of intention-to-treat. The Kaplan–Meier method will be used to calculate the cumulative event rate. The event time of all patients will be measured starting from the randomization time (time zero). We will collect all available information on clinical events until the last patient contact, including patients who withdraw consent or those lost to follow-up. Relative risk (RR) will be calculated using the Cox proportional hazard model. If a sufficient number of events occur, the Kaplan–Meier curve will be used for stratified analysis of the exercise group, and the log-rank test will be used to test the difference in survival. The significance level will be set at p < 0.05.
The management structure includes a trial management team, a data monitoring committee, and a trial steering committee.
The trial management team (including two physicians and three investigators) is responsible for the implementation of the trial and will meet weekly to discuss the progress of the trial. The data monitoring committee is composed of three clinicians and two biostatisticians, and independently evaluates the safety, scientific validity and completeness of clinical trials. The data monitoring committee will convene a meeting before the start of patient recruitment and once a month after the patient begins to intervene. The person in charge of the center monitors the intermediate results during the clinical trial. Once serious side effects occur, the trial should be stopped immediately. If there are significant differences in the efficacy of the three groups (HIT group, MIT group, and control group), the trial will be considered to be terminated. Responsibility of the test steering committee is to approve the main research plans and revisions, supervise the test, guide the test, and solve the problems of the test management team. The committee will be composed of four experts and will meet at least once every 6 months.
Before recruitment, the entire research team needs to participate in a training seminar. The data collected in this trial will include questionnaire information, medical chart review and test results. Professionals double-enter the data and store the data confidentially in the electronic database. The person in charge of the sub-center has the right to access the data set of the sub-center, and the person in charge of the project has the right to access the final data set of all the centers. Researchers and sponsors will inform participants and other relevant personnel of the results of the trial by publishing articles. The data quality will be regularly checked by the research assistant and supervised by the supervisor.
Before enrolling patients, the protocol will be approved by the relevant institutional review boards, research ethics boards, and ethics committees of all the participating centers and the coordination center.
The clinical trial will be conducted in accordance with local laws. The study has been approved by Regional Committees for Medical Research Ethics of all participating centers (2020-KYSB-177). The study is registered at http://www.chictr.org.cn/ and the registration number is ChiCTR2000036381. The trial started in December 2020 and is currently recruiting.
The exercise-based CR is recommended in the guidelines for HFrEF but is still in infancy in China, where exercise-based CR services are rare in most regions, and the most suitable intensity ET for HFrEF patients remains unknown. Compared with global HFrEF exercise rehabilitation, HFrEF exercise rehabilitation in China started late and is still developing. Affected by factors, such as Chinese health and medical conditions and patients' exercise preferences, the overall level of CR in HFrEF still has some gaps compared with foreign countries. However, CR is a healthy behavior intervention affected by the social environment, cognition, and humanities. Simply introducing foreign experience cannot solve China's practical problems. The guidelines that are widely accepted abroad are referenced in China, but they need to be used in conjunction with China's actual adjustments. In this case, China currently lacks reference standards and effectiveness data on exercise intensity for HFrEF rehabilitation. The results of multi-center, randomized controlled registration studies from China are urgently needed to provide high-quality evidence and data from China.
To our knowledge, this is the first multicenter randomized controlled trial protocol to explore the appropriate exercise intensity for patients with HFrEF in China. The HF-EI trial will assess the impact of aerobic exercise intensity on the peak VO2 and QoL in patients with HFrEF. It also provides a rationale for improving functional capacity and cardiovascular prognosis in patients with HFrEF. We hope that this research fills the gap in the literature and offers high-quality evidence on the recommendations for the treatment of HFrEF.
AT relates to the point when the exercise load increases to a certain amount and the tissue demand for oxygen exceeds the amount that the circulation can provide, and the tissues undergo anaerobic metabolism to provide more energy. The critical point from aerobic metabolism to anaerobic metabolism is called AT, expressed as the threshold oxygen consumption of anaerobic metabolism (VO2 AT), equivalent to 50–60% of peak oxygen consumption, and directly detected by CPET (44). The advantages of using AT as the basis for formulating exercise prescriptions are sub-maximal exercise intensity, which can prevent the continuous increase of lactic acid levels, avoid hyperventilation and shortness of breath, avoid metabolic alkalosis, reduce the occurrence of overload on the heart and arrhythmia, and ensure high safety. Aerobic exercise with the intensity of the AT has protective effects on CHF, confirmed by the literature (45). For patients with HFrEF, exercise intensity is a range, not a point; AT belongs to sub-maximum medium intensity. Although 70% of peakVO2 is high- intensity, it is not ultra-high intensity. This study will explore the effects of these two different intensities.
AT intensity has not been widely accepted internationally. This study compares the effectiveness and safety of AT intensity and high intensity in patients with HF, and aims to reflect the superiority of AT intensity exercise. It has reference value for other colleagues in the world.
Limitations of the study should be recognized as follows: lost to follow-up, influencing factors including patient age, difficulties attending appointments, concurrent illnesses, and the nature of the intervention that made blinding impossible. We made every effort to ensure that evaluators, laboratory technicians and statisticians remain unaware of the treatment allocation.
Altogether, the results of the HF-EI trial may provide evidence on the effective delivery of a contextually adapted exercise-based CR program in China. At the same time, it provides evidence of AT exercise intensity for the world's CR field.
The HF-EI trial will assess the effects of different aerobic exercise intensities on peak VO2 and QoL in patients with HFrEF. It will provide a rationale for improving functional capacity and cardiovascular outcomes in patients with HFrEF.
The studies involving human participants were reviewed and approved by Shanghai Tongji Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
TS and XL prepared the manuscript and all the authors participated in the design of clinical and related research. All authors gave final approval and agreed to be accountable for the integrity and accuracy of all aspects of the work.
National Natural Science Foundation [81974359 and 81700316]. Advanced proper technology promotion project of Municipal Health and Health Commission in Shanghai, China [2019SY014].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all the authors who provided important contributions to the drafting of this protocol.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.705972/full#supplementary-material
6MWD, 6-min walk distance; 6MWT, 6-min walking test; AT, anaerobic threshold; BMI, body mass index; CHF, chronic heart failure; CHS, China hypertension survey; CPET, cardiopulmonary exercise testing; CR, cardiac rehabilitation; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; ET, Exercise training; ExTraMATCH, Exercise Training Meta-Analysis of Trials in Chronic Heart Failure; GAD-7, General Anxiety Disorder-7; HF, heart failure; HF-EI trial, the efficacy and safety of different aerobic exercise intensities in patients with heart failure with reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; HIT, high intensity training; ICD, implantable cardioverter-defibrillator; LVEF, left ventricle ejection fraction; MET, metabolic equivalent; MIT, moderate intensity training; MLHFQ, Minnesota Living with Heart Failure Questionnaire scores; NT pro-BNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; Peak VO2, peak oxygen consumption; PHQ-9, the Patient Health Questionnaire-9; QoL, quality of life; RCT, randomized controlled trial; RR, relative risk; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
1. Adams KF, Lindenfeld J, Arnold JMO, Baker DW, Barnard DH, Baughman KL, et al. Executive summary: HFSA 2006 comprehensive heart failure practice guideline. J Cardiac Failure. (2006) 12:10–38. doi: 10.1016/j.cardfail.2005.12.001
2. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22:1342–56. doi: 10.1002/ejhf.1858
3. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. (2019) 21:1329–37. doi: 10.1002/ejhf.1629
4. Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. (2009) 95:1727–31. doi: 10.1136/hrt.2008.150177
5. Aronow WS, Shamliyan TA. Exercise for preventing hospitalization and readmission in adults with congestive heart failure. Cardiol Rev. (2019) 27:41–48. doi: 10.1097/CRD.0000000000000210
6. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. (2019) 1: CD003331. doi: 10.1002/14651858.CD003331.pub5
7. Cops J Haesen S De Moor B Mullens W and Hansen D. Exercise intervention in hospitalized heart failure patients, with emphasis on congestion-related complications: a review. Heart Fail Rev. (2020) 25:257–68. doi: 10.1007/s10741-019-09833-x
8. Stolen T, Shi M, Wohlwend M, Hoydal MA, Bathen TF. Ellingsen O, Esmaeili M. Effect of exercise training on cardiac metabolism in rats with heart failure. Scand Cardiovasc J. (2020) 54:84–91. doi: 10.1080/14017431.2019.1658893
9. Schmidt C, Moreira-Goncalves D, Santos M, Leite-Moreira A, Oliveira J. Physical activity and exercise training in heart failure with preserved ejection fraction: gathering evidence from clinical and pre-clinical studies. Heart Fail Rev. (2020). doi: 10.1007/s10741-020-09973-5. [Epub ahead of print].
10. Fukuta H. Effects of Exercise Training on Cardiac Function in Heart Failure with Preserved Ejection Fraction. Card Fail Rev. (2020) 6: e27. doi: 10.15420/cfr.2020.17
11. Leggio M, Fusco A, Loreti C, Limongelli G, Bendini MG, Mazza A, et al. Effects of exercise training in heart failure with preserved ejection fraction: an updated systematic literature review. Heart Fail Rev. (2020) 25:703–711. doi: 10.1007/s10741-019-09841-x
12. Bjarnason-Wehrens B, Nebel R, Jensen K, Hackbusch M, Grilli M, Gielen S, et al. Exercise-based cardiac rehabilitation in patients with reduced left ventricular ejection fraction: The Cardiac Rehabilitation Outcome Study in Heart Failure (CROS-HF): A systematic review and meta-analysis. Eur J Prev Cardiol. (2020) 27:929–952. doi: 10.1177/2047487319854140
13. Roh JD, Houstis N, Yu A, Chang B, Yeri A, Li H, et al. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell. (2020) 19: e13159. doi: 10.1111/acel.13159
14. Bean G, Mou J, Pflugeisen B, Olsen L, Hoag S, Silva A, et al. Exercise training in patients with heart failure with preserved ejection fraction: A community hospital pilot study. J Cardiovasc Nurs. (2021) 36:124–30. doi: 10.1097/JCN.0000000000000737
15. Tucker WJ, Angadi SS, Haykowsky MJ, Nelson MD, Sarma S, Tomczak CR. Pathophysiology of exercise intolerance and its treatment with exercise-based cardiac rehabilitation in heart failure with preserved ejection fraction. J Cardiopulm Rehabil Prev. (2020) 40:9–16. doi: 10.1097/HCR.0000000000000481
16. Redwine LS Pung MA Wilson K Bangen KJ Delano-Wood L and Hurwitz B. An exploratory randomized sub-study of light-to-moderate intensity exercise on cognitive function, depression symptoms and inflammation in older adults with heart failure. J Psychosom Res. (2020) 128:109883. doi: 10.1016/j.jpsychores.2019.109883
17. Belardinelli R Georgiou D Cianci G and Purcaro A. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol. (2012) 60:1521–8. doi: 10.1016/j.jacc.2012.06.036
18. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. (2018) 20:1735–43. doi: 10.1002/ejhf.1311
19. Abdelbasset WK, Alqahtani BA, Elshehawy AA, Tantawy SA, Elnegamy TE, Kamel DM. Examining the impacts of 12 weeks of low to moderate-intensity aerobic exercise on depression status in patients with systolic congestive heart failure - A randomized controlled study. Clinics (São Paulo). (2019) 74:e1017. doi: 10.6061/clinics/2019/e1017
20. Abdelbasset WK, Alqahtani BA, Alrawaili SM, Ahmed AS, Elnegamy TE, Ibrahim AA, et al. Similar effects of low to moderate-intensity exercise program vs moderate-intensity continuous exercise program on depressive disorder in heart failure patients: A 12-week randomized controlled trial. Medicine (Baltimore). (2019) 98:e16820. doi: 10.1097/MD.0000000000016820
21. Ding R. Exercise-Based Rehabilitation for Heart Failure: Clinical Evidence. Adv Exp Med Biol. (2017) 1000:31–49. doi: 10.1007/978-981-10-4304-8_3
22. Gomes Neto M, Duraes AR, Conceicao LSR, Saquetto MB, Ellingsen O, Carvalho VO. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis. Int J Cardiol. (2018) 261:134–41. doi: 10.1016/j.ijcard.2018.02.076
23. Dun Y, Smith JR, Liu S, Olson TP. High-intensity interval training in cardiac rehabilitation. Clin Geriatr Med. (2019) 35:469–87. doi: 10.1016/j.cger.2019.07.011
24. Giallauria F, Piccioli L, Vitale G, Sarullo FM. Exercise training in patients with chronic heart failure: A new challenge for Cardiac Rehabilitation Community. Monaldi Arch Chest Dis. (2018) 88:987. doi: 10.4081/monaldi.2018.987
25. Moreno-Suarez I, Scheer A, Lam K, Dembo L, Spence AL, Hayward C, et al. High-intensity interval training in patients with left ventricular assist devices: A pilot randomized controlled trial. J Heart Lung Transplant. (2020) 39:1380–8. doi: 10.1016/j.healun.2020.08.005
26. Keyhani D, Tartibian B, Dabiri A, Teixeira AMB. Effect of high-intensity interval training versus moderate-intensity aerobic continuous training on galectin-3 Gene expression in postmenopausal women: a randomized controlled trial. J Aging Phys Act. (2020) 1-9. doi: 10.1123/japa.2019-0213
27. Besnier F, Labrunee M, Richard L, Faggianelli F, Kerros H, Soukarie L, et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann Phys Rehabil Med. (2019) 62:321–8. doi: 10.1016/j.rehab.2019.06.013
28. Angadi SS, Jarrett CL, Sherif M, Gaesser GA, Mookadam F. The effect of exercise training on biventricular myocardial strain in heart failure with preserved ejection fraction. ESC Heart Fail. (2017) 4:356–9. doi: 10.1002/ehf2.12149
29. Wu LH, Chang SC, Fu TC, Huang CH, Wang JS. High-intensity interval training improves mitochondrial function and suppresses thrombin generation in platelets undergoing hypoxic stress. Sci Rep. (2017) 7:4191. doi: 10.1038/s41598-017-04035-7
30. Giallauria F Smart NA Cittadini A and Vigorito C. Exercise training modalities in chronic heart failure: does high intensity aerobic interval training make the difference? Monaldi Arch Chest Dis. (2016) 86:754. doi: 10.4081/monaldi.2016.754
31. Fleg JL. Salutary effects of high-intensity interval training in persons with elevated cardiovascular risk. F1000Res. (2016) 5:F1000 Faculty Rev-2254. doi: 10.12688/f1000research.8778.1
32. Ellingsen O, Halle M, Conraads V, Stoylen A, Dalen H, Delagardelle C, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. (2017) 135:839–49. doi: 10.1161/CIRCULATIONAHA.116.022924
33. Piepoli MF, Conraads V, Corra U, Dickstein K, Francis DP, Jaarsma T, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. (2011) 13:347–57. doi: 10.1093/eurjhf/hfr017
34. Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, O'Connor CM, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. (2012) 60:1899–905. doi: 10.1016/j.jacc.2012.08.958
35. Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. (2012) 5:579–85. doi: 10.1161/CIRCHEARTFAILURE.111.965186
36. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. (2021) 42:17–96. doi: 10.1093/eurheartj/ehaa605
37. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartssonet al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583
38. Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond). (2007) 31:1786–97. doi: 10.1038/sj.ijo.0803683
39. Rognmo O Hetland E Helgerud J Hoff J and Slordahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. (2004) 11:216–222. doi: 10.1097/01.hjr.0000131677.96762.0c
40. Paolillo S, Veglia F, Salvioni E, Corra U, Piepoli M, Lagioia R, et al. Heart failure prognosis over time: how the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur J Heart Fail. (2019) 21:208–17. doi: 10.1002/ejhf.1364
41. Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102
42. Chen YW, Wang CY, Lai YH, Liao YC, Wen YK, Chang ST, et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure. Medicine (Baltimore). (2018) 97: e9629. doi: 10.1097/MD.0000000000009629
43. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. (2007) 115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041
44. Tanisho K, Hirakawa K. Training effects on endurance capacity in maximal intermittent exercise: comparison between continuous and interval training. J Strength Cond Res. (2009) 23:2405–10. doi: 10.1519/JSC.0b013e3181bac790
Keywords: exercise intensities, HFREF, randomized controlled trial, anaerobic threshold, exercise training
Citation: Shen T, Liu X, Zhuang B, Luo Q, Jin Y, Li G, Jiang Y, Li D, Chen X, Tang N, Xu Z, Wang L, Zheng L and Shen Y (2021) Efficacy and Safety of Different Aerobic Exercise Intensities in Patients With Heart Failure With Reduced Ejection Fraction: Design of a Multicenter Randomized Controlled Trial (HF-EI Trial). Front. Cardiovasc. Med. 8:705972. doi: 10.3389/fcvm.2021.705972
Received: 06 May 2021; Accepted: 04 August 2021;
Published: 26 August 2021.
Edited by:
Richard Yang Cao, Shanghai Xuhui Central Hospital, ChinaReviewed by:
Xiankun Chen, Karolinska Institutet (KI), SwedenCopyright © 2021 Shen, Liu, Zhuang, Luo, Jin, Li, Jiang, Li, Chen, Tang, Xu, Wang, Zheng and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Zheng, emhlbmdsaWFuZ0B0b25namkuZWR1LmNu; Yuqin Shen, c3lfMTk2M0AxMjYuY29t
†These authors have contributed equally to this work and share co-first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.