
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 13 August 2021
Sec. Cardiovascular Imaging
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.704762
This article is part of the Research Topic Advances in Cardiac Imaging and Heart Failure Management View all 42 articles
 Myriam Carpenito1*
Myriam Carpenito1* Diego Fanti2
Diego Fanti2 Simona Mega1
Simona Mega1 Giovanni Benfari2
Giovanni Benfari2 Maria Caterina Bono1
Maria Caterina Bono1 Andrea Rossi2
Andrea Rossi2 Flavio Luciano Ribichini2
Flavio Luciano Ribichini2 Francesco Grigioni1
Francesco Grigioni1In past cardiovascular medicine, the attention to the left ventricle-identified as the only indicator and determinant of healthy or unhealthy cardiac conditions- has systematically hidden the role of the left atrium (LA). The recent advances in cardiovascular imaging have provided a better understanding of LA anatomy, physiology, and pathology, making us realize that this functional structure is far from being an innocent spectator. We now know that the LA's mechanical and neuro-hormonal properties play a relevant part in several cardiovascular diseases, including atrial fibrillation, ischemic heart disease, valvular heart disease, and heart failure. The present review aims to describe the role of LA in the specific setting of heart failure. We provide currently available information on LA structure and function and summarize its role as a determinant of symptoms, prognosis, and potential therapeutic target in heart failure patients.
Heart failure (HF) with preserved ejection fraction (HFpEF) and reduced left ventricular (LV) ejection fraction (HFrEF) is associated with several structural and functional changes in the left atrium (LA). Until recently, the role of the LA in the development of HF was unclear. Traditionally, it was thought that this chamber modulated LV filling and cardiac output. New non-invasive imaging modalities have improved our understanding of the function and clinical impact of the LA (1, 2). Furthermore, the LA plays endocrine and regulatory roles closely related to its mechanical function (3), making it a potential treatment target and a predictor of cardiovascular events in a broad range of patient populations.
Measurement of LA size is a crucial element of a multiparametric assessment of patients with HF. LA size is measured with M-mode and two-dimensional transthoracic echocardiography (2DE) by evaluating the anteroposterior diameter (4). However, this has proven inaccurate, as the LA does not dilate uniformly. The maximal left atrial volume indexed to the body surface area (LAVi) is the method of choice as it is considered the most accurate. In fact, it is strongly associated with cardiac outcomes (5) and enables risk stratification. The predictive power of LAVi has been enhanced by the advent of three-dimensional echocardiography (3DE) (6, 7), which allows a more precise evaluation of the left atrial volume (LAV) without geometric assumptions and foreshortening (8) (Figure 1). Values from 3DE better correlate with the volume obtained with
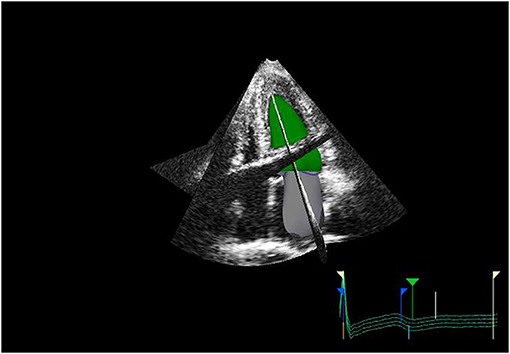
Figure 1. 3D echo reconstruction of the left atrium (LA) and left ventricle (LV). LA is shown at its end-diastolic phase in order to appreciate left maximal atrial volume.
cardiac magnetic resonance imaging (CMR) or cardiac computed tomography (CCT) (9). However, since CMR provides an adequate definition of the inner wall of the endocardium, and is able to detect pathological characteristics of the myocardial tissues, it is considered the gold standard in LAV measurement (10, 11).
The LA is a dynamic structure, and its mechanical function consists of three phases. It acts as a reservoir of oxygenated blood from the pulmonary veins, and its function depends on both the LV filling pressure and LV end-systolic volume; thus, any LV dysfunction will inevitably impact the atrial reservoir function (12). In early diastole, the LA acts as a conduit between the pulmonary veins and the left ventricle. The compliance of the atrial and ventricular chambers influences the conduit function of the LA, which is mutually related to the reservoir function (13). Usually, this phase makes a minor contribution to ventricular stroke volume but predominates in advanced stages of diastolic dysfunction, when the reservoir function upon atrial contraction is impaired (14, 15). Finally, the atrial booster pump function reflects atrial contractile function. It is dependent on intrinsic LA contractility, the degree of venous return, and LV end-diastolic pressures (16). Growing evidence suggests that the assessment of LA function provides more prognostic information than LA size in HF patients (17). The LA function can be evaluated through 3DE volumetric analysis by measuring all volumes from a single volume trace. Data relative to emptying volumes and fractions can be obtained by assessing the maximum, minimum, and pre-atrial contraction (immediately before) volumes. The transmitral spectral Doppler, pulmonary venous, and left atrial appendix flows also reflect phasic function but are currently rarely used (18). Alternatively, the phasic LA function can be derived from either tissue Doppler imaging (TDI) or 2D speckle-tracking echocardiography (2DSTE) (19, 20). Among these, 2DSTE is the most accurate method due to its ability to analyze myocardial deformation without angle dependency using frame-by-frame tracking of the speckles pattern generated by the interactions between ultrasound and myocardial tissue. The measurement of LA strain depends on whether the P wave (P-LASr) or the QRS (left atrial strain during reservoir phase, QRS-LASr) complex is used as the zero references. However, the recent European Association of Cardiovascular Imaging/American Society of Echocardiography recommends the use of QRS onset as the preferred method (21) mainly due to the impossibility of applying the P wave method to all patients, especially those with atrial fibrillation (AF) (22). The reservoir function is determined by the positive peak of atrial longitudinal strain (PALS), which indicates the LA's maximum elongation during LV systole (23). Hence, PALS also reflects the longitudinal contraction of the LV due to the interdependence between the LA and LV chambers (24). At the end of LA diastasis, there is a progressive shortening of the LA until the first negative peak of atrial contraction strain (PACS) or late diastolic strain. This event reflects the LA booster pump function (Figure 2).
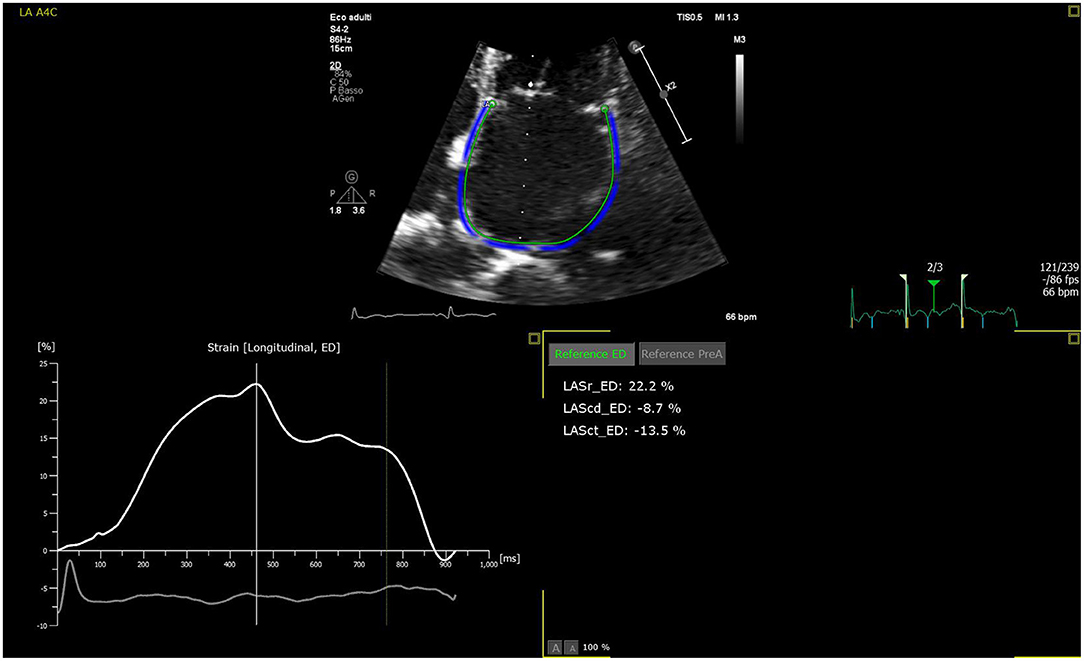
Figure 2. LA strain curve with R-R gating method in apical four-chamber view with zoom-in focus. The patient is an 83-year-old female with chronic heart failure. The patient is in sinus rhythm with reservoir function reduced. Zoomed image is used to increase the frame rate to enhance strain analysis accuracy.
LA dilatation is a compensatory mechanism required to maintain an average stroke volume, at least in the early stages of LV diastolic dysfunction (LVDD). LA dilatation reflects the chronicity and the severity of longstanding elevated LA pressure and LV high filling pressure (25, 26). It enhances conduit filling and initial improvement of its contractile function via the Frank-Starling effect. Modification of diastolic properties allows the LV to operate at a higher filling pressure during diastole; therefore, the relative contribution of the LA booster pump to LV filling increases until the LA preload reserve limits are reached. In this phase, LA behaves mainly like a conduit, and mechanical failure occurs (27). When the LA becomes dysfunctional, it loses its buffering effect, which leads to pulmonary congestion (28). Chronic exposure to elevated pressure leads to structural alterations of the LA (29), followed by myocyte hypertrophy, necrosis, apoptosis, and fibrosis. These events, along with altered ionic channel expression (30), contribute to electrical remodeling and the development of AF. LA dilatation is usually associated with annular dilatation and subsequent development of functional mitral regurgitation (MR), which even in the low range, contributes to the impairment of pulmonary hemodynamics (31) and the development of HF symptoms (32–34). LAV may also increase in some conditions characterized by normal diastolic function, such as in those with bradycardia, high-output states, atrial arrhythmias, and significant mitral valve disease, as well as trained athletes (35). The use of LA strain as a functional adaptive marker may provide valuable information on LA stiffness and indirectly estimate the LV end-diastolic pressure. It may identify atrial impairment at an early stage before dilatation occurs. Nevertheless, PALS needs to be employed with caution to identify isolated LA dysfunction (36) due to a close relationship between PALS and downward displacement of the LV base toward the apex and LV global longitudinal strain (GLS) (37).
HFpEF is the most prevalent type of HF in the outpatient setting, accounting for more than half of all hospitalizations with decompensated HF (38). It is associated with elevated morbidity and high cardiovascular and non-cardiovascular mortality (39). Additionally, in contrast to HFrEF, there are no definitive therapies that improve outcomes in HFpEF (40). For the diagnosis of HFpEF, signs or symptoms of HF must be present. These include preserved ejection fraction (EF), elevated biohumoral markers with concomitant structural changes in the myocardium, and/or increased filling pressure (40). The LA is responsible for ~30% of physiological stroke volume, and functional impairment is a significant contributor to HF (41). However, it is still unclear when the transition from LVDD to HFpEF occurs and whether an alteration in atrial function contributes to this step. Diagnosis can be challenging because not all patients with LVDD present with HFpEF. Guidelines recommend a multiparametric stepwise diagnostic process (42). Echocardiography plays a central role in the diagnosis of HFpEF. Some authors suggest that E/e' and tricuspidal regurgitation velocity during exercise test may detect HFpEF with increased sensitivity (43). LA dilatation, which indicates chronically elevated LA pressure (44), is a predictor of hospitalization and mortality in HFpEF, particularly when associated with increased pulmonary pressure (45). Recent studies have proposed that the minimum LAV might better reflect LV end-diastolic pressure (46), particularly in patients without valvular disease or AF. A recent study compared atrial function in HFpEF and HFrEF patients with similar, invasively measured mean LA pressures (47). Patients with HFrEF presented larger LAVs, while those with HFpEF had higher pressure pulsatility, with more significant wall stress variation. These results may explain the origin of AF in patients with HFpEF, where it is more commonly observed, despite smaller LAVs. Chronically elevated LV filling pressures cause eccentric hypertrophy of the LA with consequent atrial endocrine failure (48, 49). LA dysfunction, evaluated by measurement of the LA ejection fraction (LAEF), is also associated with HF mortality (47) and B-type natriuretic peptide (BNP) levels (50).
The introduction of strain imaging provided further understanding of the role of the LA. Kurt and colleagues (51) found significantly lower levels of LA systolic strain in patients with HFpEF than in patients with LVDD without HF. LA reservoir strain <23% was associated with worse New York Heart Association (NYHA) functional class and elevated estimated pulmonary capillary wedge pressure (PCWP), even in the absence of LA enlargement (52). Reservoir function correlates well with symptoms (53) and peak oxygen consumption at cardiopulmonary exercise testing, even after adjustment for LV and RV longitudinal strain (54). Indeed, a reduction in the reservoir function was found in patients with hypertension (55) and diabetes mellitus (56, 57), which are well-known conditions associated with diastolic dysfunction and HFpEF. A low PALS value is associated with higher disease burden in terms of a history of AF and prior HF hospitalizations (58). However, the interactions between the LV and LA are complex. Some authors question the ability of LA reservoir strain to predict the recurrence of hospitalization for HF after adjustment for LV GLS and E/e' (59). Few studies have explored HF with mid-range ejection fraction (HFmEF). This condition presents higher BNP levels than HFpEF, and worse LA reservoir, conduit, and pump function without a significant difference in the LA size and LV diastolic function (60). The study of LA function should lead to improved therapy management and should not be limited only to the diagnosis and prognosis stratification in patients with suspected or confirmed HFpEF. A better understanding of the reactivity of LA strain parameters to drug treatment may be an essential future endpoint for determining therapeutic efficacy.
In recent decades, the role of the LA in HFrEF patients has been ignored, while much effort has been dedicated to understanding impaired ventricular function and remodeling. The impact of an enlarged LAV on prognosis has been well-characterized (7, 61), with a prediction of increased mortality and the need for heart transplantation in more advanced phases of HF (62–64). In trials on left ventricular dysfunction (SOLVD) (61), with 1172 HFrEF patients enrolled, the LA dimension was a significant predictor of mortality and HF hospitalization. In particular, in a meta-analysis of 18 studies involving 1157 HFrEF patients, the atrial area was identified as a powerful predictor of death or hospitalization for HF, independent of age, NYHA functional class, LVEF, and restrictive filling pattern (65). Moreover, the maximum LAV was independently associated with death and transplantation in patients with dilated cardiomyopathy (66). Diastolic alteration quantified by E/e' was also associated with long-term mortality in patients with HFrEF (62). However, LV fibrosis and restricted mitral annular motion make this method unreliable for quantifying LV diastolic pressure (67). Tissue-Doppler velocity during atrial contraction provides information on atrial contractile function. When related to LAVi, it gives origin to the left atrial volumetric/mechanical coupling index, a useful predictor of death in HFrEF patients, and functional MR (68). The introduction of strain imaging has enabled a deeper understanding of atrial function. Some authors have proposed PALS as a better predictor of cardiovascular events than LAEF and LA function index (69). The reservoir strain is reduced in HFrEF and HFpEF patients, but HFrEF patients showed a more significant reduction in LA strain proportional to LV GLS (70). Moreover, lower PALS values were associated with adverse events and HF symptoms, and the outcomes remained significant after adjusting for BNP levels, LAVi, E/e' ratio, and LV GLS. Specifically, PALS value <12.9% was correlated with an augmented risk of 30% per year adverse event rate, which decreased to only 4.9% when PALS was above 18.6% per year (71). Cameli et al. (72) demonstrated, in 36 patients with HFrEF who underwent right heart catheterization, that LA systolic strain is best correlated to PCWP and provided the highest diagnostic accuracy in predicting elevated LV end-diastolic pressure. Interestingly, the lack of reserve in LA contractility in patients with HFrEF was associated with right ventricular-to-pulmonary uncoupling during exercise and recovery along with ventilation inefficiency (73). Furthermore, PALS is strongly correlated with functional capacity during exertion and is more depressed in the idiopathic form of dilated cardiomyopathy than in the ischemic one (74).
In acute HF, PALS is a strong predictor of prognosis regardless of HF phenotype, sex, age, ventricular function, or LAVi (75). Similar to a non-acute setting, PALS is associated with GLS at baseline but decreases disproportionately with congestion; decongestant therapy is correlated with a prompt reduction in LA pressure and immediate improvement in the function of the reservoir, independent of changes in LV GLS, LAV, or MR severity (76). The marked improvement in PALS is independently related to a reduced risk or all-cause or HF readmissions (76). Furthermore, the booster pump function recovers after 6 weeks. This demonstrates that increased atrial afterload is not the only factor that induces atrial dysfunction as concomitant contractile impairment occurs.
HF increases the risk of developing AF by 10–50% in several ways: dysregulation of intracellular calcium, interstitial fibrosis, and autonomic and endocrine dysfunction (77). Moreover, most HF patients (regardless of LVEF) have enlarged LA and mechanical dysfunction, which secondarily contribute to pulmonary hypertension (PH) and eventually AF development (49). Furthermore, worsening of diastolic function is correlated with a cumulative risk of developing AF, suggesting that the stretch imposed on the atrial cells by increased LV filling pressure may be proarrhythmic. MR is also an important predisposing factor for the onset of arrhythmia due to the pronounced dilatation of the atrial chamber (78, 79). Thus, once established, AF may either contribute to degenerative MR disease progression or unfavorably influence prognosis on its own, or both (80). Among the predisposing factors for the development of AF, advanced age and LA dilation play a predominant role. When patients with degenerative MR receive a surgical indication, the degree of enlargement of the atrial chamber must always be evaluated (81). Therefore, it is crucial to evaluate echocardiographic parameters to identify structural and functional alterations that could predict the onset of AF (82). A large study suggested that echocardiographic parameters associated with a diastolic function such as transmitral peak E wave velocity and A wave VTI and the dimension of LA may predict AF (83). A 43% increase in the risk of developing AF is associated with a 30% increase in maximum LAV, independent of clinical risk factors (84), LVEF, and the severity of diastolic dysfunction (26). Persistent AF forms are associated with higher fibrosis measured by CMR and more depressed LA function, as assessed by strain analysis (85). AF is also associated with an augmented risk of cardiovascular outcomes in HF regardless of EF baseline. The recent onset of AF in HFpEF patients is an adverse prognostic indicator that elevates the risk of adverse events, approaching that of HFrEF patients in sinus rhythm (86).
The assessment of LA function in patients with aortic stenosis (AS) is of increasing interest. All three LA phasic functions in AS are significantly lower in patients with severe symptomatic forms of valvular disease than in asymptomatic ones (87). LA booster pump function is strictly correlated to the severity of AS and LVDD (88). Marques-Alves and colleagues (89) demonstrated that LA reservoir strain was closely associated with the aortic valve area and mean transvalvular aortic gradient, whereas, LV GLS was not. This may indicate poor LA compliance, even before LV dysfunction occurs. LA reservoir function is a recognized marker of poor prognosis in patients with AS (90). PALS values of ≤ 21% are associated with a significant risk of cardiac hospitalization, worsening HF, and cardiac death (91). Calin et al. (92) found that LA dysfunction and dilatation were significantly related to PH in patients with severe AS and preserved ventricular systolic performance. In particular, LA booster pump function correlated independently with PH in multivariable analysis. LA systolic strain, as an indicator of LA reverse remodeling, predicts postoperative development of AF (93). It also improves after aortic valve replacement (88), along with reduced LA size. Interestingly, the most remarkable changes have been reported during the first 40 days following intervention (94), with a residual postoperative aortic mean gradient significantly affecting recovery of atrial function (95).
LA dilatation resulting from volume overload is common in chronic MR. It reflects the duration of regurgitation and the severity of valvular disease (96) and has long been considered an essential predictor of adverse cardiovascular outcomes (97, 98). The characterization of atrial function through 2DSTE can offer insights into atrial adaptation to chronic MR. Cameli et al. (99) demonstrated that in a heterogeneous group of patients with asymptomatic mitral valvular prolapse (MVP), global PALS was raised in mild MR due to an increase in LA compliance but decreased linearly with increasing severity. In contrast, these decreases in patients with moderate and severe regurgitation may reflect ultrastructural abnormalities and adverse remodeling. Evaluation of LA function compared to LA size may provide further information on the optimal surgery timing and predict postoperative outcomes. In 87 subjects with degenerative MR enrolled in the randomized EVEREST II trial (100), LA strain modification was correlated with baseline LV and LA function. Changes in LA strain after reduction in regurgitation may reflect a decrease in LA enlargement but may also be affected by the degree of pre-existing LA dysfunction (101). Although, LA volume and function are strongly correlated, modification of LA function appears earlier than cavity remodeling and can be present even if LAV is normal (57). This dissociation between atrial volume and pressure may be explained in the early phases of acute MR by the lack of extracellular LA fibrosis. In this phase, the LA is still compliant and can compensate for atrial volume overload. When compliance is lost, there is an increase in the pulmonary pressure, reflecting the complex underlying interaction of right ventricle–pulmonary circulation uncoupling.
The LA can undergo inverse remodeling after reducing LA pressure and/or LAV overload (49), leading to subsequent improvements in ventricular compliance and function due to LA–LV interdependency (84). Reverse remodeling is possible after treatment for different diseases, such as MR, AF, and hypertension, or following cardiac resynchronization therapy (CRT) (26, 102, 103). In HF patients, administration of angiotensin receptor inhibitors reduces atrial fibrosis, electrical remodeling, and the occurrence of the first episode of AF (104). The secretion of atrial natriuretic peptide (ANP) is also impaired in HF and is correlated with fibrotic alterations in the LA, leading to sodium and fluid retention. However, restoration of endocrine function occurs upon administration of sacubitril/valsartan. In patients with HFrEF, this treatment may promote LA reverse remodeling within 9 months, improving reservoir function and LAEF (105). The degree of recovery is correlated with improvements in atrial mechanical function and reduced LAV. Interestingly, a rapid and significant rise in ANP levels is associated with greater gains in LVEF after therapy, suggesting that the LA may be an indicator of responsiveness to HF therapy (106). Reverse remodeling has been observed even in patients with HFpEF, as demonstrated by a reduction in LAV and BNP levels during therapy with carvedilol, angiotensin receptor, and neprilysin inhibitor (3, 107). Although, CRT improves outcomes and overall survival and is recommended in patients with HFrEF, its efficacy is limited by a high percentage of non-responders. Those patients with maximum LAV > 59.4 ml/m2 continue to have increased mortality despite CRT (108). However, patients with lower LAV and mild MR show a more significant response (109).
As shown in the literature, the LA plays a crucial role in HF. Global LA failure is associated with an increased risk of incident AF, poor exercise tolerance, and increased morbidity and mortality. 2DSTE is a promising technique that allows for the quantification of myocardial deformation and provides additive information about cardiac function. It can detect subtle myocardial damage and has excellent clinical diagnostic and prognostic value for HF evaluation, heart valve disease, and AF. Furthermore, due to its evident accuracy in predicting results and the recent standardization of myocardial deformation imaging, it could be considered as part of risk stratification protocols. Therefore, the assessment of the LA chamber becomes of crucial importance for tailored therapies in different clinical scenarios.
MC: conceptualization, review of the literature, writing and review, and editing of the manuscript. DF, SM, and MB: review of the literature and writing. GB and AR: critical revision to scientific content. FR and FG: conceptual guidance, critical revision, and editing of the manuscript. All authors read, commented, and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AF, atrial fibrillationcANP, atrial natriuretic peptide; AS, aortic stenosis; ASE, American Society of Echocardiography; BNP, B-type natriuretic peptide; CCT-cardiac computed tomography; CMR, cardiac magnetic resonance; CRT, cardiac resynchronization therapy; EACVI, European Association of Cardiovascular Imaging; EF, ejection fraction; GLS, global longitudinal strain; HF, heart failure; HFmEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrium; LAEF, left atrial ejection fraction; LAV, left atrial volume; LAVi, left atrial volume indexed to body surface area; LV, left ventricle/ventricular; LVDD, left ventricle diastolic dysfunction; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MVP, mitral valve prolapse; NYHA, New York Heart Association; PACS, peak of atrial contraction strain; PALS, peak of atrial longitudinal strain; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; TDI, tissue Doppler imaging; 2DSTE, 2D speckle-tracking echocardiography; 2DE, two dimensional echocardiography; 3DE, three dimensional echocardiography.
1. Rossi A, Carluccio E, Cameli M, Inciardi R, Mandoli GE, D'Agostino A, et al. Left atrial remodeling in heart failure: coexistence and additive prognostic power of atrial dilation and dysfunction. Eur Heart J. (2020) 41(Suppl. 2):ehaa946.1023. doi: 10.1093/ehjci/ehaa946.1023
2. Tan YT, Wenzelburger F, Lee E, Nightingale P, Heatlie G, Leyva F, et al. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Heart. (2010) 96:1017–23. doi: 10.1136/hrt.2009.189118
3. Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. (2014) 7:1042–9. doi: 10.1161/CIRCHEARTFAILURE.114.001276
4. Rosca M, Lancellotti P, Popescu BA, Piérard LA. Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications. Heart. (2011) 97:1982–9. doi: 10.1136/heartjnl-2011-300069
5. Tsang TSM, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. (2006) 47:1018–23. doi: 10.1016/j.jacc.2005.08.077
6. Caselli S, Canali E, Foschi ML, Santini D, Di Angelantonio E, Pandian NG, et al. Long-term prognostic significance of three-dimensional echocardiographic parameters of the left ventricle and left atrium. Eur J Echocardiogr. (2010) 11:250–6. doi: 10.1093/ejechocard/jep198
7. Suh I-W, Song J-M, Lee E-Y, Kang S-H, Kim M-J, Kim J-J, et al. Left atrial volume measured by real-time 3-dimensional echocardiography predicts clinical outcomes in patients with severe left ventricular dysfunction and in sinus rhythm. J Am Soc Echocardiogr. (2008) 21:439–45. doi: 10.1016/j.echo.2007.09.002
8. Mor-Avi V, Yodwut C, Jenkins C, Kühl H, Nesser H-J, Marwick TH, et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. (2012) 5:769–77. doi: 10.1016/j.jcmg.2012.05.011
9. Kühl JT, Lønborg J, Fuchs A, Andersen MJ, Vejlstrup N, Kelbæk H, et al. Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int J Cardiovasc Imaging. (2012) 28:1061–71. doi: 10.1007/s10554-011-9930-2
10. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. (2009) 119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877
11. Benfari G, Vinco G, Sayegh K, Friedrich M, Rossi A. A new method to evaluate atrial hemodynamic and quantify mitral regurgitation using cardiovascular magnetic resonance: the pulmonary venous flow approach. J Heart Valve Dis. (2017) 26:456–9.
12. Appleton CP, Kovács SJ. The role of left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. (2009) 2:6–9. doi: 10.1161/CIRCIMAGING.108.845503
13. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. (2014) 63:493–505. doi: 10.1016/j.jacc.2013.10.055
14. Schweitzer A, Agmon Y, Aronson D, Abadi S, Mutlak D, Carasso S, et al. Assessment of left sided filling dynamics in diastolic dysfunction using cardiac computed tomography. Eur J Radiol. (2015) 84:1930–7. doi: 10.1016/j.ejrad.2015.07.006
15. Boogers MJ, van Werkhoven JM, Schuijf JD, Delgado V, El-Naggar HM, Boersma E, et al. Feasibility of diastolic function assessment with cardiac CT: feasibility study in comparison with tissue Doppler imaging. JACC Cardiovasc Imaging. (2011) 4:246–56. doi: 10.1016/j.jcmg.2010.11.017
16. Vieira MJ, Teixeira R, Gonçalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. (2014) 27:463–78. doi: 10.1016/j.echo.2014.01.021
17. Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, Iwama M, et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging. (2012) 13:227–34. doi: 10.1093/ejechocard/jer281
18. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1961–77. doi: 10.1016/j.jacc.2019.01.059
19. Sirbu C, Herbots L, D'hooge J, Claus P, Marciniak A, Langeland T, et al. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr. (2006) 7:199–208. doi: 10.1016/j.euje.2005.06.001
20. Cameli M, Lisi M, Righini FM, Mondillo S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound. (2012) 10:4. doi: 10.1186/1476-7120-10-4
21. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19:591–600. doi: 10.1093/ehjci/jey042
22. Cameli M, Miglioranza MH, Magne J, Mandoli GE, Benfari G, Ancona R, et al. Multicentric atrial strain comparison between two different modalities: MASCOT HIT study. Diagnostics. (2020) 10:946. doi: 10.3390/diagnostics10110946
23. Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr. (2017) 30:59–70.e8. doi: 10.1016/j.echo.2016.09.007
24. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. (2011) 24:277–313. doi: 10.1016/j.echo.2011.01.015
25. Wu VC-C, Takeuchi M, Kuwaki H, Iwataki M, Nagata Y, Otani K, et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. (2013) 6:1025–35. doi: 10.1016/j.jcmg.2013.08.002
26. Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. (2009) 10:282–6. doi: 10.1093/ejechocard/jen235
27. Dernellis JM, Stefanadis CI, Zacharoulis AA, Toutouzas PK. Left atrial mechanical adaptation to long-standing hemodynamic loads based on pressure-volume relations. Am J Cardiol. (1998) 81:1138–43. doi: 10.1016/S0002-9149(98)00134-9
28. Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. (2012) 126:975–90. doi: 10.1161/CIRCULATIONAHA.111.085761
29. Casaclang-Verzosa G, Gersh BJ, Tsang TSM. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. (2008) 51:1–11. doi: 10.1016/j.jacc.2007.09.026
30. Dzeshka MS, Lip GYH, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. (2015) 66:943–59. doi: 10.1016/j.jacc.2015.06.1313
31. Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, et al. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail. (2020) 22:499–506. doi: 10.1002/ejhf.1677
32. Benfari G, Setti M, Nistri S, Fanti D, Maffeis C, Tafciu E, et al. Relevance of functional mitral regurgitation in aortic valve stenosis. Am J Cardiol. (2020) 136:115–21. doi: 10.1016/j.amjcard.2020.09.016
33. Benfari G, Nistri S, Faggiano P, Clavel M-A, Maffeis C, Enriquez-Sarano M, et al. Mitral effective regurgitant orifice area predicts pulmonary artery pressure level in patients with aortic valve stenosis. J Am Soc Echocardiogr. (2018) 31:570–7.e1. doi: 10.1016/j.echo.2017.12.004
34. Grigioni F, Russo A, Pasquale F, Biagini E, Barberini F, Ferlito M, et al. Clinical use of doppler echocardiography in organic mitral regurgitation: from diagnosis to patients' management. J Cardiovasc Ultrasound. (2015) 23:121–33. doi: 10.4250/jcu.2015.23.3.121
35. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2016) 17:1321–60. doi: 10.1093/ehjci/jew082
36. Bowman AW, Kovács SJ. Assessment and consequences of the constant-volume attribute of the four-chambered heart. Am J Physiol Heart Circulat Physiol. (2003) 285:H2027–33. doi: 10.1152/ajpheart.00249.2003
37. Ersbøll M, Andersen MJ, Valeur N, Mogensen UM, Waziri H, Møller JE, et al. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging. (2013) 6:26–33. doi: 10.1161/CIRCIMAGING.112.978296
38. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. (2007) 50:768–77. doi: 10.1016/j.jacc.2007.04.064
39. Vergaro G, Ghionzoli N, Innocenti L, Taddei C, Giannoni A, Valleggi A, et al. Non-cardiac versus cardiac mortality in heart failure with preserved, midrange, and reduced ejection fraction. J Am Heart Assoc. (2019) 8:e013441. doi: 10.1161/JAHA.119.013441
40. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis treatment of acute chronic heart failure: the Task Force for the diagnosis treatment of acute chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.592
41. Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. (2012) 59:673–80. doi: 10.1016/j.jacc.2011.11.012
42. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40:3297–17. doi: 10.1093/eurheartj/ehz641
43. Belyavskiy E, Morris DA, Url-Michitsch M, Verheyen N, Meinitzer A, Radhakrishnan A-K, et al. Diastolic stress test echocardiography in patients with suspected heart failure with preserved ejection fraction: a pilot study: diastolic stress test in HFpEF. ESC Heart Failure. (2019) 6:146–53. doi: 10.1002/ehf2.12375
44. Rossi A, Cicoira M, Florea VG, Golia G, Florea ND, Khan AA, et al. Chronic heart failure with preserved left ventricular ejection fraction: diagnostic and prognostic value of left atrial size. Int J Cardiol. (2006) 110:386–92. doi: 10.1016/j.ijcard.2005.08.049
45. Donal E, Lund LH, Oger E, Bosseau C, Reynaud A, Hage C, et al. Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. (2017) 18:629–35. doi: 10.1093/ehjci/jex005
46. Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, et al. LA phasic volumes and reservoir function in the elderly by real-time 3D echocardiography: normal values, prognostic significance, and clinical correlates. JACC Cardiovasc Imaging. (2017) 10:976–85. doi: 10.1016/j.jcmg.2016.07.015
47. Melenovsky V, Hwang S-J, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. (2015) 8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667
48. Hohendanner F, Messroghli D, Bode D, Blaschke F, Parwani A, Boldt L-H, et al. Atrial remodelling in heart failure: recent developments and relevance for heart failure with preserved ejection fraction. ESC Heart Fail. (2018) 5:211–21. doi: 10.1002/ehf2.12260
49. Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, et al. Global left atrial failure in heart failure. Eur J Heart Fail. (2016) 18:1307–20. doi: 10.1002/ejhf.645
50. Kanagala P, Arnold JR, Cheng ASH, Singh A, Khan JN, Gulsin GS, et al. Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. (2020) 36:101–10. doi: 10.1007/s10554-019-01684-9
51. Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. (2009) 2:10–5. doi: 10.1161/CIRCIMAGING.108.813071
52. Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. (2018) 11:1405–15. doi: 10.1016/j.jcmg.2017.07.029
53. Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, et al. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. (2011) 24:651–62. doi: 10.1016/j.echo.2011.02.004
54. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. (2016) 9:10. doi: 10.1161/CIRCIMAGING.115.003754
55. Leite L, Mendes SL, Baptista R, Teixeira R, Oliveira-Santos M, Ribeiro N, et al. Left atrial mechanics strongly predict functional capacity assessed by cardiopulmonary exercise testing in subjects without structural heart disease. Int J Cardiovasc Imaging. (2017) 33:635–42. doi: 10.1007/s10554-016-1045-3
56. Vukomanovic V, Suzic-Lazic J, Celic V, Cuspidi C, Grassi G, Galderisi M, et al. Is there association between left atrial function and functional capacity in patients with uncomplicated type 2 diabetes? Int J Cardiovasc Imaging. (2020) 36:15–22. doi: 10.1007/s10554-019-01680-z
57. Cameli M, Mandoli GE, Lisi E, Ibrahim A, Incampo E, Buccoliero G, et al. Left atrial, ventricular and atrio-ventricular strain in patients with subclinical heart dysfunction. Int J Cardiovasc Imaging. (2019) 35:249–58. doi: 10.1007/s10554-018-1461-7
58. Freed BH, Shah SJ. Stepping out of the left ventricle's shadow: time to focus on the left atrium in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. (2017) 10:e006267. doi: 10.1161/CIRCIMAGING.117.006267
59. Santos ABS Roca GQ Claggett B Sweitzer NK Shah SJ Anand IS . Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. (2016) 9:e002763. doi: 10.1161/CIRCHEARTFAILURE.115.002763
60. Al Saikhan L, Hughes AD, Chung W-S, Alsharqi M, Nihoyannopoulos P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging. (2019) 20:279–90. doi: 10.1093/ehjci/jey171
61. Quiñones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM, et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. (2000) 35:1237–44. doi: 10.1016/S0735-1097(00)00511-8
62. Benfari G, Miller WL, Antoine C, Rossi A, Lin G, Oh JK, et al. Diastolic determinants of excess mortality in heart failure with reduced ejection fraction. JACC Heart Fail. (2019) 7:808–17. doi: 10.1016/j.jchf.2019.04.024
63. Carluccio E, Dini FL, Biagioli P, Lauciello R, Simioniuc A, Zuchi C, et al. The ‘Echo Heart Failure Score': an echocardiographic risk prediction score of mortality in systolic heart failure. Eur J Heart Fail. (2013) 15:868–76. doi: 10.1093/eurjhf/hft038
64. Bosch L, Carluccio E, Coiro S, Gong L, Sim D, Yeo D, et al. Risk stratification of Asian patients with heart failure and reduced ejection fraction: the effectiveness of the Echo Heart Failure Score: Risk stratification of Asian patients with heart failure and reduced ejection fraction. Eur J Heart Fail. (2017) 19:1732–5. doi: 10.1002/ejhf.922
65. Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail. (2009) 11:929–36. doi: 10.1093/eurjhf/hfp112
66. Rossi A, Cicoira M, Zanolla L, Sandrini R, Golia G, Zardini P, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. (2002) 40:1425. doi: 10.1016/S0735-1097(02)02305-7
67. Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, et al. Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. (2016) 33:398–405. doi: 10.1111/echo.13094
68. Benfari G, Essayagh B, Nistri S, Maalouf J, Rossi A, Thapa P, et al. Left atrial volumetric/mechanical coupling index: a novel predictor of outcome in heart failure with reduced ejection fraction. Circ Cardiovasc Imaging. (2021) 14:e011608. doi: 10.1016/j.jcmg.2021.05.017
69. Sargento L, Vicente Simões A, Longo S, Lousada N, Palma Dos Reis R. Left atrial function index predicts long-term survival in stable outpatients with systolic heart failure. Eur Heart J Cardiovasc Imaging. (2017) 18:119–27. doi: 10.1093/ehjci/jew196
70. Frydas A, Morris DA, Belyavskiy E, Radhakrishnan A, Kropf M, Tadic M, et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Failure. (2020) 7:1956–65. doi: 10.1002/ehf2.12820
71. Carluccio E, Biagioli P, Mengoni A, Francesca Cerasa M, Lauciello R, Zuchi C, et al. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction: the importance of atrial strain by speckle tracking echocardiography. Circ Cardiovascular Imaging. (2018) 11:e007696. doi: 10.1161/CIRCIMAGING.118.007696
72. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. (2010) 8:14. doi: 10.1186/1476-7120-8-14
73. Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure: pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc Imaging. (2017) 10(10 Pt. B):1253–64. doi: 10.1016/j.jcmg.2016.09.021
74. D'Andrea A, Caso P, Romano S, Scarafile R, Cuomo S, Salerno G, et al. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two-dimensional speckle strain study. Int J Cardiol. (2009) 132:354–63. doi: 10.1016/j.ijcard.2007.11.102
75. Park J-H, Hwang I-C, Park JJ, Park J-B, Cho G-Y. Prognostic power of left atrial strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging. (2021) 22:210–9. doi: 10.1093/ehjci/jeaa013
76. Deferm S, Martens P, Verbrugge FH, Bertrand PB, Dauw J, Verhaert D, et al. LA mechanics in decompensated heart failure: insights from strain echocardiography with invasive hemodynamics. JACC Cardiovasc Imaging. (2020) 13:1107–15. doi: 10.1016/j.jcmg.2019.12.008
77. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. (2009) 119:2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306
78. Verheule S, Wilson E, Everett T, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. (2003) 107:2615–22. doi: 10.1161/01.CIR.0000066915.15187.51
79. Anné W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans P, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. (2005) 67:655–66. doi: 10.1016/j.cardiores.2005.04.016
80. Grigioni F, Benfari G, Vanoverschelde J-L, Tribouilloy C, Avierinos J-F, Bursi F, et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol. (2019) 73:264–74. doi: 10.1016/j.jacc.2018.10.067
81. Grigioni F, Avierinos J-F, Ling LH, Scott CG, Bailey KR, Tajik AJ, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. (2002) 40:84–92. doi: 10.1016/S0735-1097(02)01922-8
82. Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: a useful index in atrial fibrillation. Int J Cardiol. (2016) 220:208–13. doi: 10.1016/j.ijcard.2016.06.197
83. Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ. Echocardiographic diastolic parameters and risk of atrial fibrillation: the cardiovascular health study. Eur Heart J. (2012) 33:904–12. doi: 10.1093/eurheartj/ehr378
84. Inciardi RM, Rossi A. Left atrium: a forgotten biomarker and a potential target in cardiovascular medicine. J Cardiovasc Med. (2019) 20:797–808. doi: 10.2459/JCM.0000000000000886
85. Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. (2010) 3:231–9. doi: 10.1161/CIRCIMAGING.109.865683
86. Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJV, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. (2006) 47:1997–2004. doi: 10.1016/j.jacc.2006.01.060
87. Imanishi J, Tanaka H, Sawa T, Motoji Y, Miyoshi T, Mochizuki Y, et al. Association of left atrial booster-pump function with heart failure symptoms in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Echocardiography. (2015) 32:758–67. doi: 10.1111/echo.12733
88. O'Connor K, Magne J, Rosca M, Piérard LA, Lancellotti P. Impact of aortic valve stenosis on left atrial phasic function. Am J Cardiol. (2010) 106:1157–62. doi: 10.1016/j.amjcard.2010.06.029
89. Marques-Alves P, Marinho AV, Teixeira R, Baptista R, Castro G, Martins R, et al. Going beyond classic echo in aortic stenosis: left atrial mechanics, a new marker of severity. BMC Cardiovasc Disord. (2019) 19:215. doi: 10.1186/s12872-019-1204-2
90. Pastore MC, De Carli G, Mandoli GE, D'Ascenzi F, Focardi M, Contorni F, et al. The prognostic role of speckle tracking echocardiography in clinical practice: evidence and reference values from the literature. Heart Fail Rev. (2020) doi: 10.1007/s10741-020-09945-9
91. Galli E, Fournet M, Chabanne C, Lelong B, Leguerrier A, Flecher E, et al. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging. (2016) 17:533–41. doi: 10.1093/ehjci/jev230
92. Calin A, Mateescu AD, Rosca M, Beladan CC, Enache R, Botezatu S, et al. Left atrial dysfunction as a determinant of pulmonary hypertension in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Int J Cardiovasc Imaging. (2017) 33:1939–47. doi: 10.1007/s10554-017-1211-2
93. Cameli M, Lisi M, Reccia R, Bennati E, Malandrino A, Solari M, et al. Pre-operative left atrial strain predicts post-operative atrial fibrillation in patients undergoing aortic valve replacement for aortic stenosis. Int J Cardiovasc Imaging. (2014) 30:279–86. doi: 10.1007/s10554-013-0323-6
94. Garg V, Ho JK, Vorobiof G. Changes in myocardial deformation after transcatheter and surgical aortic valve replacement. Echocardiography. (2017) 34:603–13. doi: 10.1111/echo.13485
95. Lisi M, Henein MY, Cameli M, Ballo P, Reccia R, Bennati E, et al. Severity of aortic stenosis predicts early post-operative normalization of left atrial size and function detected by myocardial strain. Int J Cardiol. (2013) 167:1450–5. doi: 10.1016/j.ijcard.2012.04.057
96. Kennedy JW, Yarnall SR, Murray JA, Figley MM. Quantitative angiocardiography. IV. relationships of left atrial and ventricular pressure and volume in mitral valve disease. Circulation. (1970) 41:817–24. doi: 10.1161/01.CIR.41.5.817
97. Le Tourneau T, Messika-Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. (2010) 56:570–8. doi: 10.1016/j.jacc.2010.02.059
98. Boudoulas H, Boudoulas D, Sparks EA, Pearson AC, Nagaraja HN, Wooley CF. Left atrial performance indices in chronic mitral valve disease. J Heart Valve Dis. (1995) 4(Suppl. 2):S242–7; discussion S248.
99. Cameli M, Lisi M, Giacomin E, Caputo M, Navarri R, Malandrino A, et al. Chronic mitral regurgitation: left atrial deformation analysis by two-dimensional speckle tracking echocardiography. Echocardiography. (2011) 28:327–34. doi: 10.1111/j.1540-8175.2010.01329.x
100. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. (2011) 364:1395–406. doi: 10.1056/NEJMoa1009355
101. Gucuk Ipek E, Singh S, Viloria E, Feldman T, Grayburn P, Foster E, et al. Impact of the mitraclip procedure on left atrial strain and strain rate. Circ Cardiovasc Imaging. (2018) 11:e006553. doi: 10.1161/CIRCIMAGING.117.006553
102. Fung JWH, Yip GWK, Zhang Q, Fang F, Chan JYS, Li CM, et al. Improvement of left atrial function is associated with lower incidence of atrial fibrillation and mortality after cardiac resynchronization therapy. Heart Rhythm. (2008) 5:780–6. doi: 10.1016/j.hrthm.2008.02.043
103. Troughton RW, Prior DL, Pereira JJ, Martin M, Fogarty A, Morehead A, et al. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. (2004) 43:416–22. doi: 10.1016/j.jacc.2003.08.046
104. Vermes E, Tardif J-C, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. (2003) 107:2926–31. doi: 10.1161/01.CIR.0000072793.81076.D4
105. Castrichini M, Manca P, Nuzzi V, Barbati G, De Luca A, Korcova R, et al. Sacubitril/valsartan induces global cardiac reverse remodeling in long-lasting heart failure with reduced ejection fraction: standard and advanced echocardiographic evidences. J Clin Med. (2020) 9:906. doi: 10.3390/jcm9040906
106. Murphy SP, Prescott MF, Camacho A, Iyer SR, Maisel AS, Felker GM, et al. Atrial natriuretic peptide and treatment with sacubitril/valsartan in heart failure with reduced ejection fraction. JACC Heart Fail. (2021) 9:127–36. doi: 10.1016/j.jchf.2020.09.013
107. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. (2012) 380:1387–95. doi: 10.1016/S0140-6736(12)61227-6
108. Shen X, Nair CK, Holmberg MJ, Mooss AN, Koruth J, Wang F, et al. Impact of left atrial volume in prediction of outcome after cardiac resynchronization therapy. Int J Cardiol. (2011) 152:13–7. doi: 10.1016/j.ijcard.2010.06.016
Keywords: left atrium, heart failure, speckle-tracking echocardiography, left atrial strain, cardiovascular disease, left atrial function
Citation: Carpenito M, Fanti D, Mega S, Benfari G, Bono MC, Rossi A, Ribichini FL and Grigioni F (2021) The Central Role of Left Atrium in Heart Failure. Front. Cardiovasc. Med. 8:704762. doi: 10.3389/fcvm.2021.704762
Received: 03 May 2021; Accepted: 19 July 2021;
Published: 13 August 2021.
Edited by:
Giulia Elena Mandoli, University of Siena, ItalyReviewed by:
Andreea Motoc, University Hospital Brussels, BelgiumCopyright © 2021 Carpenito, Fanti, Mega, Benfari, Bono, Rossi, Ribichini and Grigioni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myriam Carpenito, bS5jYXJwZW5pdG9AdW5pY2FtcHVzLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.