
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 15 July 2021
Sec. Cardiovascular Metabolism
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.704145
This article is part of the Research TopicInsulin Resistance, Metabolic Syndrome and Cardiovascular DiseaseView all 36 articles
 Xiao Li1,2†
Xiao Li1,2† Yajing Zhai3†
Yajing Zhai3† Jiaguo Zhao4
Jiaguo Zhao4 Hairong He5
Hairong He5 Yuanjie Li6
Yuanjie Li6 Yue Liu7
Yue Liu7 Aozi Feng1
Aozi Feng1 Li Li1
Li Li1 Tao Huang1
Tao Huang1 Anding Xu8*
Anding Xu8* Jun Lyu1*
Jun Lyu1*Background: Patients with metabolic syndrome (MetS) have a higher risk of developing cardiovascular diseases (CVD). However, controversy exists about the impact of MetS on the prognosis of patients with CVD.
Methods: Pubmed, Cochrane library, and EMBASE databases were searched. Cohort Studies and randomized controlled trials post hoc analyses that evaluated the impact of MetS on prognosis in patients (≥18 years) with CVD were included. Relative risk (RR), hazard rate (HR) and 95% confidence intervals (CIs) were calculated for each individual study by random-effect model. Subgroup analysis and meta-regression analysis was performed to explore the heterogeneity.
Results: 55 studies with 16,2450 patients were included. Compared to patients without MetS, the MetS was associated with higher all-cause death [RR, 1.220, 95% CI (1.103 to 1.349), P, 0.000], CV death [RR, 1.360, 95% CI (1.152 to 1.606), P, 0.000], Myocardial Infarction [RR, 1.460, 95% CI (1.242 to 1.716), P, 0.000], stroke [RR, 1.435, 95% CI (1.131 to 1.820), P, 0.000]. Lower high-density lipoproteins (40/50) significantly increased the risk of all-cause death and CV death. Elevated fasting plasma glucose (FPG) (>100 mg/dl) was associated with an increased risk of all-cause death, while a higher body mass index (BMI>25 kg/m2) was related to a reduced risk of all-cause death.
Conclusions: MetS increased the risk of cardiovascular-related adverse events among patients with CVD. For MetS components, there was an increased risk in people with low HDL-C and FPG>100 mg/dl. Positive measures should be implemented timely for patients with CVD after the diagnosis of MetS, strengthen the prevention and treatment of hyperglycemia and hyperlipidemia.
Cardiovascular disease (CVD) has attracted worldwide attention and accounts for 46.2% of deaths from non-communicable diseases (1). CVD is one of the main causes of premature death and disability. Metabolic syndrome (MetS), including dysglycemia, obesity (especially central obesity), high blood pressure, low high-density lipoprotein cholesterol (HDL-C), and elevated triglyceride levels, is a complex of risk factors for type 2 diabetes and CVD (2). Patients with MetS have a higher risk of developing CVD compared with those without MetS in the next 5–10 years, and the long-term risk is even higher (3). The National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria also considered MetS as the second major target for CVD prevention (4).
The prevalence of MetS is higher in patients with CVD than in patients without MetS. The prevalence of MetS in hospitalized patients with acute myocardial infarction (AMI) is 46%, similar to that of the acute coronary syndrome (43.4%) (5); This finding indicates that MetS is associated with CVD. Boulon et al. (6) suggested that despite active management, patients with MetS have a higher long-term risk of cardiovascular events (6). However, Selcuk et al. (7) suggested that the main determinant of long-term prognosis of AMI is heart failure rather than metabolic disorder (7). But some researchers suggested that MetS does not increase the mortality among patients with CVD (8). Therefore, controversy exists about the impact of MetS on patients with CVD.
MetS is a disease associated with multiple factors, and the main diagnostic indicators (components) include blood pressure, overweight and obesity, HDL-C, and fasting blood glucose (9, 10). Most studies have focused on the overall effect of MetS on the prognosis of CVD. However, whether a correlation exists between each component and prognosis and which factor is more important have not been elucidated. Considering these inconsistencies, we performed a meta-analysis of cohort studies and RCT post-hoc analysis from CVD patients to evaluate associations between different definitions of MetS and the risk of all cause death, CV death and cardiovascular events.
The study was registered with PROSPERO (CRD42021147609), and reported in accordance with the PRISMA statement (11).
(1) Influencing factors and study types: Studies that evaluated the influence of MetS and its components on patients with CVD were included. We included cohort and randomized controlled trials post hoc analyses and excluded single-group observational studies. (2) Types of patients: Patients with CVD were aged ≥18. (3) Outcomes: Primary outcomes were all-cause death, cardiovascular (CV) death, incidence of MI and stroke. Secondary outcomes were TVR, heart failure, cardiac arrest, angina pectoris, cardiogenic shock. All-cause death of high TG, low HDL-C, high BP, FPG>100 mg/dl, BMI>25kg/m2, high WC. CV death of high TG, low HDL-C, high BP, FPG>100 mg/dl, BMI>25kg/m2.
The definition of cardiovascular disease in this meta-analysis was history (comorbidity) of cardiovascular or cardiac disease. Hypertension/Cardiovascular Infections/Cardiovascular Abnormalities/Pregnancy Cardiovascular Complications/cardiomyopathy in specific terms was excluded because these diseases often overlap and potentially result in overestimation of cases.
(1) Studies that had incomplete or unavailable original data. (2) The diagnostic criteria for MetS were not specified. (3) Repeated published data. (4) Studies that evaluated the relationship between MetS and congenital heart disease.
We searched Pubmed, EMBASE, and Cochrane library from inception to October 18, 2020. The following subject and keywords were used in search: “cardiovascular disease,” “cardiovascular event,” “cardiocerebrovascular disease,” “cerebrovascular disease,” “cerebrovascular disorder,” “cerebrovascular attack,” “stroke,” “cerebral infarction,” “coronary artery disease,” “coronary heart disease,” “ischemic heart disease,” “myocardial infarction;” “metabolic syndrome,” “metabolic syndrome x,” “Metabolic X Syndrome;” “Randomized controlled trial,” “RCT,” “Clinical Trials, Randomized,” “Cohort Studies,” “Follow-Up Studies,” “Longitudinal Studies,” “Prospective Studies,” and “Retrospective Studies”. Supplementary Table 1 presents the search strategy. No date, language, or other restriction were incorporated into the searches. Two researchers (XL and YJZ) performed the data search.
Endnote X9 was used to manage and screen the literature. Title, abstract, and full texts were selected based on inclusion/exclusion criteria. We designed a standardized form to extract data including study characteristics, diagnostic criteria, characteristics of the study population, risk of bias, and outcome measures.
We used the Newcastle–Ottawa Scale (NOS) to assess the quality of the cohort studies (12). To be specific, studies with scores >7 were treated as high quality, 4–6 as medium quality, and below 4 as low quality (13). Cochrane Collaboration's tool for assessing the risk of bias was applied to determine the quality of the included RCT post-hoc studies (12, 14). Two researchers (X Li, YJ Zhai) independently screened and extracted the data, and a third researcher (J Lyu) resolved any disagreements. Quality evaluation results are reported in Supplementary Table 2.
The diagnostic criteria for MetS vary among different regions and institutions, but the majority of them included central obesity, hypertension, low HDL-C, and high TG and fasting blood glucose (FBG) levels. Other diagnostic criteria also included dyslipidemia, chronic mild inflammation, endothelial dysfunction, insulin resistance, increased oxidative stress. The diagnostic criteria used in the included studies were NCEP2001 criteria (9), NCEP2005 criteria (4), and The International Diabetes Federation (IDF) criteria (10) (details reported in Supplementary Table 3). For specific diagnostic criteria, we compared the above criteria and divide into subgroups based on the comparison results.
Statistical analysis was performed using STATA 13 and R software. For dichotomous outcomes (all-cause death, CV-death, the incidence of MI, stroke, TVR, heart failure, cardiac arrest, angina pectoris, and cardiogenic shock), relative risk (RR) and 95% confidence intervals (CIs) were calculated for each individual study. For the impact of MetS components on patients with CVD (all-cause death and CV death), hazard rate (HR) and 95% confidence intervals (CIs) were determined for each study. The heterogeneity across studies was examined using the Chi-square test and I-square statistics. The results were pooled by the D-L random-effect model due to the large statistical heterogeneity among the studies.
To explore the sources of clinical heterogeneity and methodological heterogeneity, we performed subgroup analysis based on the following: (1) diagnostic criteria, studies were divided into four subgroups (NCEP2001, NCEP2005, IDF and “others”) and (2) study type, studies were divided into three subgroups (prospective cohort study, retrospective cohort study, and RCT post-hoc study). Meta-regression analysis of three covariates (follow-up time, male proportion, and patient age) was performed to explore the size and source of heterogeneity.
Effect measures [risk ratio (RR) vs. odds ratio (OR) vs. risk difference (RD)] and statistical models (D-L random-effects model vs. M-H fix-effects model) were used to examine the robustness of the results. We evaluated publication bias by Begg's tests and drew contour-enhanced funnel plots to assess whether the asymmetry of the funnel plots was caused by publication bias or other biases.
A total of 5,028 unique records were identified from the literature search. After excluding 226 duplicate articles, 125 studies were initially included by reading the title and abstract. Fifty-five studies were finally included after further reading the full text, including six RCT post-hoc studies (15–20) and 49 cohort studies (3, 5–8, 21–64) (Figure 1).
A total of 162,450 patients from 25 countries and regions were included, the sample size for each individual study varies from 57 to 44 548. Forty-one studies (145,390 patients) evaluated the risk of all-cause death among patients with CVD and MetS. Twenty-one studies with 95,049 patients reported CV death, 23 studies with 77,618 patients reported the incidence of MI, and 11 studies with 59,770 patients reported the incidence of stroke.
Twenty-six studies adopted NCEP-ATPIII (2005) criteria, 21 studies mainly adopted NCEP-ATPIII (2001) criteria, and 7 studies adopted IDF (2005). Baseline characteristics are listed in Table 1. Risk of bias was assessed in all of the 55 studies (Supplementary Table 2). The cohort studies comprised 16 medium-quality studies, and 33 high-quality studies. For RCT post-hoc studies, the risk of bias was deemed low in 2 studies and moderate in 4 studies.
Forty-one studies (145,897 patients) reported all-cause death. MetS was associated with higher all-cause death [RR = 1.220, 95% CI (1.103, 1.349), P = 0.000] according to the heterogeneity test I2 = 89% (Table 2, Figure 2). Subgroup analysis showed that among different diagnostic criteria of MetS, the results from NCEP-ATPIII (2001) and NCEP-ATPIII (2005) subgroups were consistent with the overall result (Table 3). Among different study types, the cohort study subgroup was in the same direction with the overall results. No statistically significant difference was found in the RCT post-hoc studies. Diagnostic criteria and study type were the factors that affected heterogeneity. Meta-regression showed that the follow-up time and male proportion were not the sources of heterogeneity (P > 0.05), and age only explained 1.6% of the heterogeneity (P = 0.022). The Begg's test result showed bias (P = 0.012), and the contour-enhanced funnel plots showed that the bias may be due to other reasons rather than publication bias.
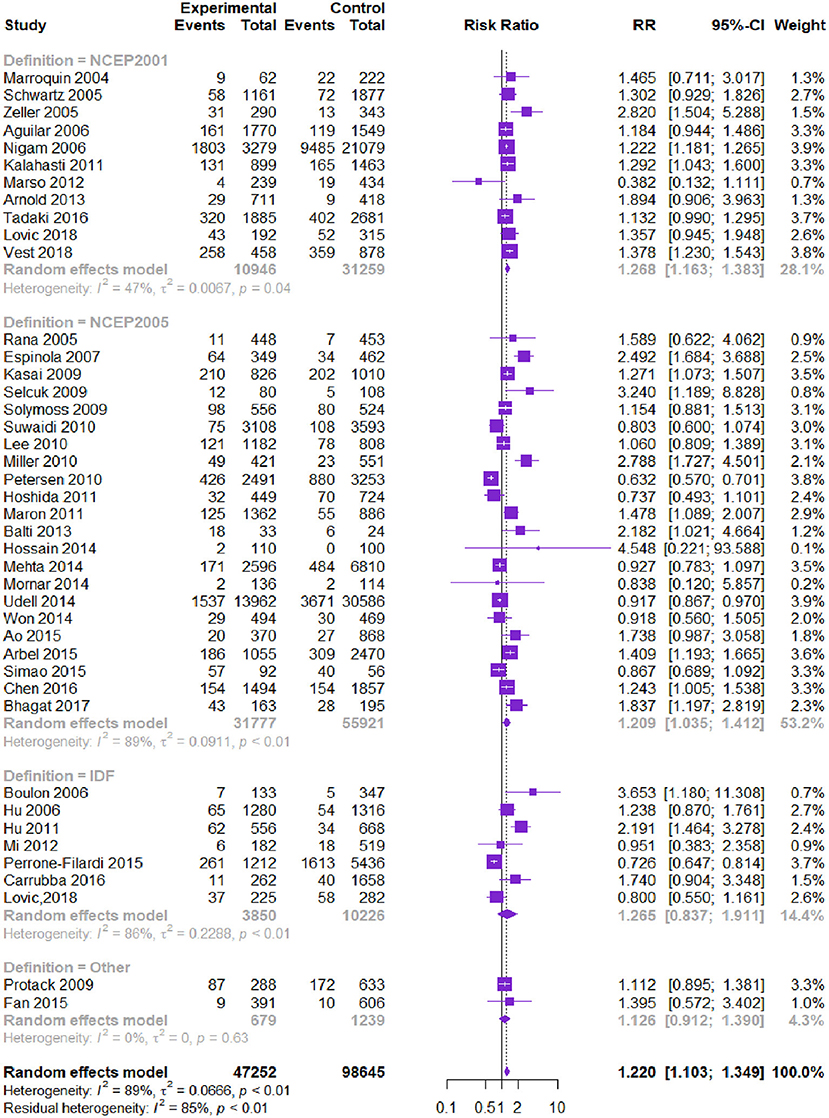
Figure 2. Meta-analysis of the risk of all-cause death in patients with CVD and MetS compared with that of patient without MetS.
Twenty-one studies with 94,542 patients reported CV-related death. The MetS group had higher CV death than the non-MetS group [RR = 1.360, 95% CI (1.152, 1.606), P = 0.000] according to the heterogeneity test I2=87.0% (Table 2, Figure 3). Subgroup analysis showed that among different diagnostic criteria of MetS, NCEP-ATPIII (2001) and NCEP-ATPIII (2005) subgroups were consistent with the overall result (Table 3). Among different study types, the subgroups were consistent with the overall results. Diagnostic criteria affected the heterogeneity. Meta-regression showed that follow-up time, age, and male proportion were not the source of heterogeneity (P > 0.05). The Begg's test and the contour-enhanced funnel plots showed that bias may be caused by publication bias and other reasons.
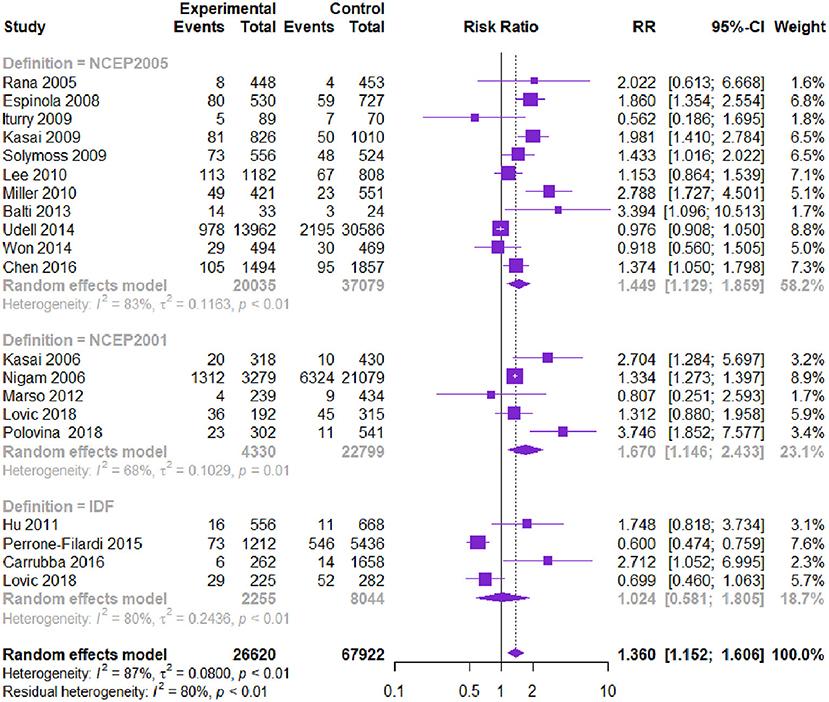
Figure 3. Meta-analysis of the risk of CV death in patients with CVD and MetS compared with that of patient without MetS.
Twenty-three studies with 77,125 patients reported the risk of MI. Patients with CVD and MetS had a higher risk of MI [RR = 1.460, 95% CI (1.242, 1.716), P = 0.000] according to the heterogeneity test I2 = 72% (Table 2, Figure 4). Subgroup analysis showed that among the diagnostic criteria of MetS, the results of NCEP-ATPIII (2001) and NCEP-ATPIII (2005) were consistent with the overall results (Table 3). Other subgroups had no statistically significant difference. Among the study types, the subgroup results were in the same direction as the overall results. Meta-regression showed that follow-up time, age, and male proportion were not the source of heterogeneity (P > 0.05). The Begg's test and the contour-enhanced funnel plots reported no publication bias (P = 0.125).
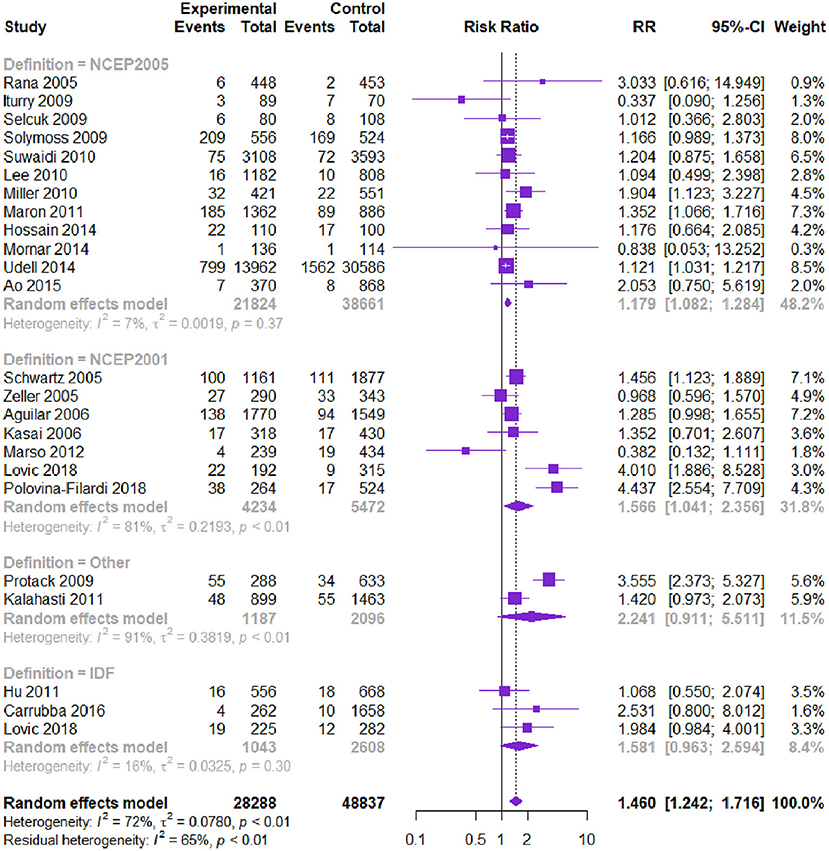
Figure 4. Meta-analysis of the risk of MI in patients with CVD and MetS compared with that of patients without MetS.
Eleven studies with 60,297 patients reported the risk of stroke. Patients with CVD and MetS had a higher risk of stroke [RR = 1.435, 95% CI (1.131, 1.820), P = 0.000] according to the heterogeneity test I2 = 75% (Table 3, Figure 5). The Begg's test and the contour-enhanced funnel plots showed that the bias may be caused by other reasons rather than publication bias.
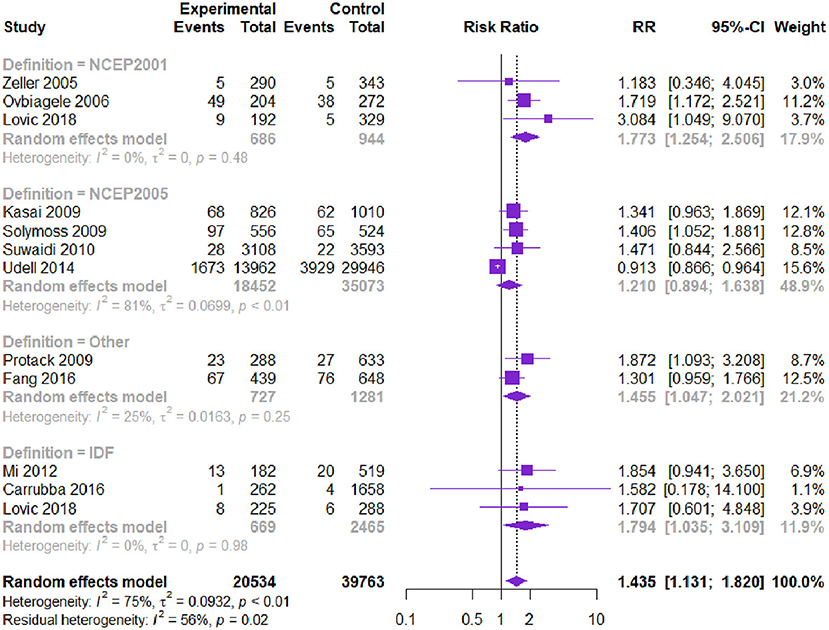
Figure 5. Meta-analysis of the risk of stroke in patients with CVD and MetS compared with that of patients without MetS.
The results of the TVR (13 studies) reported that patients with CVD and MetS had a higher risk to develop TVR [RR = 1.241, 95% CI (1.063, 1.448), P = 0.000]. Subgroup analysis showed that diagnostic criteria and study type explained the partial heterogeneity. The risk of heart failure was evaluated in eight studies. Patients with CVD and MetS were more likely to have heart failure [RR = 1.497, 95% CI (1.116, 2.007), P = 0.000]. Subgroup analysis showed that diagnostic criteria partly explained the heterogeneity (Table 2).
Other indicators include risk of cardiac arrest (4 studies), angina pectoris (3 studies), and cardiogenic shock (3 studies). We found no statistically significant difference in the risk of cardiac arrest [RR = 1.457, 95% CI (0.875, 2.429), P = 0.518], angina pectoris [RR = 1.280, 95% CI (0.967, 1.694), P = 0.030], and cardiogenic shock [RR = 0.923, 95% CI (0.752 1.132), P = 0.764].
Among MetS components, low HDL (40/50) was significantly associated with increased risks of all-cause death and CV death. Elevated FPG (>100 mg/dl) was significantly associated with an increased risk of all-cause death, whereas body mass index (BMI) > 25 kg/m2 was related to a reduced risk of all-cause death (Table 4).
We examined the robustness of our results. The sensitivity analysis of the effect measures showed that the OR increased the effect size and did not change the direction of the results, except for angina pectoris. The RD did not change the direction of the results. The sensitivity analysis of the statistical models did not change the direction of the results. Hence, the results of this meta-analysis were robust.
Fifty-five studies with 162,450 patients from 25 countries or regions were included. Most studies defined MetS using NCEP2001, NCEP2005, and IDF criteria, and other works adopted specific diagnostic criteria. Our results suggested that patients with CVD and MetS had an increased risk of all-cause death, CV-related death, MI, stroke, TVR, and heart failure. In the analysis of MS components, BMI>25 kg/m2 was negatively correlated with the prognosis of patients with CVD. Dyslipidemia and abnormal glucose metabolism were the main risk factors for the prognosis of CVD. Different spectrum within patients with cardiovascular diseases may be the sources of heterogeneity.
MetS and its components are a complex of risk factors for CVD and diabetes (21). Ford (65) reported that the population attributable fractions for CVD, diabetes, and all-cause death among patients with MS were 12–17%, 30–52%, and 6–7%, respectively (65). However, for patients with CVD, whether MetS and its components is associated with the risk of CV events remains controversial.
Obesity is an independent risk factor for hypertension, CVD, and diabetes (66). Given the known association between obesity and CVD, the adverse consequences of obesity may persist after the onset of CVD. However, previous studies suggest a contradictory U-shaped relationship between obesity and CVD-related death; hence, overweight and mild obesity are related to lower short-term and long-term mortality (67–69) based on the concept of “Obesity paradox” or “reverse epidemiology” (66). Although the setting of obesity indicators was involved in different MetS diagnostic criteria, the core of the diagnosis was consistent. In NCEP-ATP III (2001) and NCEP-ATP III (2005) criteria, obesity is one of the five elements and is not a necessary condition; however, in IDF (2005), obesity is the first prerequisite. Interestingly, our result discovered that the diagnosis of MetS under different standards has a distinct prognosis of CVD. The result of the subgroup analysis of all-cause mortality and cardiovascular mortality as two core factors demonstrated that IDF (2005) standards were consistently different from the final result. However, under the standards of NCEP-ATP III (2001) and NCEP-ATP III (2005) who didn't consider obesity as a necessary condition, MetS is a significant risk factor of prognosis. Hence, we need to reconsider which diagnostic criteria can predict the prognosis of MetS among patients with CVD more accurately. The heterogeneity in this study may be associated with the proportion of obese patients included.
AHA/NHLBI 2009 diagnostic criteria were not adopted in all of the included studies, which may be related to the fact that the indicators and numerical intervals of abdominal obesity were not clearly given in the criteria. BMI was used in most of the studies as a proxy for waist circumference, but the cutoffs for the inclusion criteria in each study were different. This phenomenon may be related to two factors: (1) BMI is easier to obtain than waist circumference, and (2) BMI can be effectively docked with the WHO's definition of obesity. However, existing evidence suggests that MetS might be caused by excessive central obesity (70). Therefore, in future research on MetS, we suggest that BMI and waist circumference data should be collected at the same time for strict implementation of MetS diagnostic criteria.
The span of follow-up time included was very large, ranging from 0.33 years to 12.6 years. A 32-year prospective cohort study of male residents without MI or stroke in the community showed that the CV-related mortality curves among patients with MetS varied at 10–15 years of follow-up (70). The findings of Kasai et al. (26) and Nigam et al. (46) show that MetS and its components had a significantly positive association with all-cause death of patients with CVD during 4–5 years of follow-up (20, 26). However, the impact of MetS on patients with CVD might be underestimated in these studies.
In this study, the potential influences of the five components of MetS [TG, HDL, BP, FPG, BMI/Waist circumference (WC)] on CVD prognosis was analyzed. We found that abnormal blood glucose and lipid metabolism are important factors that could lead to poor prognosis of CVD. As such, these factors should be considered as intervention targets for predicting the prognosis of patients with CVD. BMI was negatively correlated, which was manifested as the obesity paradox. Waist circumference was included in only two studies with relatively small sample sizes and conducted among Chinese patients only. Further studies are needed to explore the rationality, applicability, and the risk prognosis of BMI and waist circumference.
Prediabetes is an intermediate metabolic state between normoglycemia and diabetes, includes impaired glucose tolerance and impaired fasting glucose (71). Compared with NCEP-ATP III (2001) criteria, the NCEP-ATP III (2005) reduced the fasting plasma glucose from 6.1 mmol/L to 5.6 mmol/L. Our results showed that the two diagnostic criteria had the same contribution in predicting the prognosis of patients with CVD. The results of Huang 2016 also found that prediabetes with impaired fasting glucose or impaired glucose tolerance is associated with an increased risk of composite cardiovascular events, coronary heart disease, stroke, and all-cause mortality (71). Our findings indirectly supported the modification of the American ADA guidelines to reduce the standard of pre-diabetes from 6.1 mmol/L (72) to 5.6 mmol/L (73). In response to this result, lifestyle intervention is the fundamental management approach for prediabetes (73, 74).
The span of follow-up time included was very large, ranging from 0.33 to 12.6 years, most studies were followed up for <5 years, the impact of MetS on patients with CVD in this study might be underestimated. As one of the diagnostic indicators of MetS, WC was only included in two studies, reflected the problems in the implementation of MetS diagnostic criteria and possibly underestimated the impact of central obesity on patients with CVD.
This meta-analysis was conducted using cohort studies and RCT post-hoc studies. MetS was found to be associated with an increased risk of CV-related adverse events among patients with CVD. For MetS components, there was an increased risk in people with low HDL-C and FPG>100 mg/dl. Positive measures should be implemented timely for patients with CVD after the diagnosis of MetS to reduce risk factors and strengthen the prevention and treatment of hyperglycemia and hyperlipidemia. Further studies need to clarify the selection of MetS diagnostic indicators (particularly the BMI or waist circumference).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
JL, AX, XL, and YZ conceived the study. YZ and HH designed the search strategy and XL performed the literature search. JZ and YZ screened studies for eligibility. HH, YLi, YLiu, and AF performed data extraction. XL, YZ, LL, and TH assessed the risk of bias. XL, YZ, HH, and JZ performed data analysis. JL interpreted the data analysis and assessed the certainty of evidence. XL and YZ wrote the first draft of the manuscript and all other authors revised the manuscript. All authors contributed to the article and approved the submitted version.
This systematic review was supported by the National Social Science Foundation of China (No.16BGL183).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.704145/full#supplementary-material
1. World Health Organization. Global Status Report on Noncommunicable Diseases 2014. World Health Organization. (2014). Available online at: https://apps.who.int/iris/handle/10665
2. Alberti KG, Eckel RH, rundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
3. Suwaidi JA, Zubaid M, El-Menyar AA, Singh R, Rashed W, Ridha M, et al. Prevalence of the metabolic syndrome in patients with acute coronary syndrome in six middle eastern countries. J Clin Hypertens. (2010) 12:890–9. doi: 10.1111/j.1751-7176.2010.00371.x
4. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
5. Zeller M, Steg PG, Ravisy J, Laurent Y, Janin-Manificat L, L'Huillier I, et al. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch Intern Med. (2005) 165:1192–8. doi: 10.1001/archinte.165.10.1192
6. Boulon C, Lafitte M, Richeboeuf V, Paviot B, Pradeau V, Coste P, et al. Prevalence of metabolic syndrome after acute coronary syndrome and its prognostic significance. Am J Cardiol. (2006) 98:1429–34. doi: 10.1016/j.amjcard.2006.07.025
7. Selcuk H, Temizhan A, Selcuk MT, Sen T, Maden O, Tekeli S, et al. Impact of metabolic syndrome on future cardiovascular events in patients with first acute myocardial infarction. Coron Artery Dis. (2009) 20:370–5. doi: 10.1097/MCA.0b013e32832ed31e
8. van Kuijk JP, Flu WJ, Chonchol M, Bax JJ, JM Verhagen H, Poldermans D. Metabolic syndrome is an independent predictor of cardiovascular events in high-risk patients with occlusive and aneurysmatic peripheral arterial disease. Atherosclerosis. (2010) 210:596–601. doi: 10.1016/j.atherosclerosis.2009.12.018
9. Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
10. Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The metabolic syndrome–a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8
11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
12. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
13. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) For Assessing the Quality of Nonrandomised Studies in Meta-Analyses [online]. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed December 21, 2020).
14. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane. (2020). Available online at: www.training.cochrane.org/handbook (accessed December 21, 2020).
15. Aguilar D, Fisher MR, O'Connor CM, Dunne MW, Muhlestein JB, Yao L, et al. Metabolic syndrome, C-reactive protein, and prognosis in patients with established coronary artery disease. Am Heart J. (2006) 152:298–304. doi: 10.1016/j.ahj.2005.11.011
16. Schwartz GG, Olsson AG, Szarek M, Sasiela WJ. Relation of characteristics of metabolic syndrome to short-term prognosis and effects of intensive statin therapy after acute coronary syndrome: an analysis of the myocardial ischemia reduction with aggressive cholesterol lowering (MIRACL) trial. Diabetes Care. (2005) 28:2508–13. doi: 10.2337/diacare.28.10.2508
17. Mehta RH, Westerhout CM, Zheng Y, Giugliano RP, Huber K, Prabhakaran D, et al. Association of metabolic syndrome and its individual components with outcomes among patients with high-risk non-ST-segment elevation acute coronary syndromes. Am Heart J. (2014) 16:182–8. doi: 10.1016/j.ahj.2014.04.009
18. Perrone-Filardi P, Savarese G, Scarano M, Cavazzina R, Trimarco B, Minneci S, et al. Prognostic impact of metabolic syndrome in patients with chronic heart failure: data from GISSI-HF trial. Int J Cardiol. (2015) 178:85–90. doi: 10.1016/j.ijcard.2014.10.094
19. Maron DJ, Boden WE, Spertus JA, Hartigan PM, Mancini GBJ, Sedlis SP, et al. Impact of metabolic syndrome and diabetes on prognosis and outcomes with early percutaneous coronary intervention in the COURAGE (clinical outcomes utilizing revascularization and aggressive drug evaluation) trial. J Am Coll Cardiol. (2011) 58:131–7. doi: 10.1016/j.jacc.2011.02.046
20. Ovbiagele B, Saver JL, Lynn MJ, Chimowitz M, WASID Study Group. Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology. (2006) 66:1344–9. doi: 10.1212/01.wnl.0000210530.46058.5c
21. Anderson JL, Horne BD, Jones HU, Reyna SP, Carlquist JF, Bair TL, et al. Which features of the metabolic syndrome predict the prevalence and clinical outcomes of angiographic coronary artery disease? Cardiology. (2004) 101:185–93. doi: 10.1159/000076695
22. Mornar JM, Babić Z, Pintarić H. The role of metabolic syndrome in patients with acute myocardial infarction. Diabetol Croatica. (2014) 43:11–9.
23. Arnold SV, Lipska KJ, Li Y, Goyal A, Maddox TM, McGuire DK, et al. The reliability and prognosis of in-hospital diagnosis of metabolic syndrome in the setting of acute myocardial infarction. J Am Coll Cardiol. (2013) 62:704–8. doi: 10.1016/j.jacc.2013.02.062
24. Kasai T, Miyauchi K, Kajimoto K, Kubota N, Yanagisawa N, Amano A, et al. Relationship between the metabolic syndrome and the incidence of stroke after complete coronary revascularization over a 10-year follow-up period. Atherosclerosis. (2009) 207:195–9. doi: 10.1016/j.atherosclerosis.2009.04.028
25. Arbel Y, Havakuk O, Halkin A, Revivo M, Berliner S, Herz I, et al. Relation of metabolic syndrome with long-term mortality in acute and stable coronary disease. Am J Cardiol. (2015) 115:283–7. doi: 10.1016/j.amjcard.2014.10.037
26. Kasai T, Miyauchi K, Kurata T, Ohta H, Okazaki S, Miyazaki T, et al. Prognostic value of the metabolic syndrome for long-term outcomes in patients undergoing percutaneous coronary intervention. Circ J. (2006) 70:1531–7. doi: 10.1253/circj.70.1531
27. Tadaki S, Sakata Y, Miura Y, Miyata S, Asakura M, Shimada K, et al. Prognostic impacts of metabolic syndrome in patients with chronic heart failure - a multicenter prospective cohort study. Circ J. (2016) 80:677–88. doi: 10.1253/circj.CJ-15-0942
28. La Carrubba D, Antonini-Canterin F, Fabiani I, Colonna P, Pugliese NR, Caso P, et al. Prevalence and prognostic impact of metabolic syndrome in asymptomatic (stage A and B heart failure) patients. Metab Syndr Relat Disord. (2016) 14:187–94. doi: 10.1089/met.2015.0143
29. Lovic MB, Savic L, Matic D, Djordjevic D, Nedeljkovic I, Tasic I. Predictive value of metabolic syndrome definitions in patients with myocardial infarction with ST segment elevation - are they all the same? Acta Cardiol. (2018) 73:574–82. doi: 10.1080/00015385.2018.1424599
30. Marso SP, Mercado N, Maehara A, Weisz G, Mintz GS, McPherson J, et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging. (2012) 5:S42–52. doi: 10.1016/j.jcmg.2012.01.008
31. Hossain MS, Azad KA, Biswas PK, Hossain MA, Ahmed SU. Outcome of patients hospitalized with acute coronary syndrome fulfilling the criteria of metabolic syndrome. Bang Soc Med. (2014) 15:31–5. doi: 10.3329/jom.v15i1.19857
32. Miller AM, Ruiz AA, Sánchez GB, Gutiérrez EA, Guzmán RM, Aguilar RJ. Metabolic syndrome: clinical and angiographic impact on patients with acute coronary syndrome. Cir Cir. (2010) 78:113–20.
33. Protack CD, Bakken AM, Xu J, Saad WA, Lumsden AB, Davies MG. Metabolic syndrome: a predictor of adverse outcomes after carotid revascularization. J Vasc Surg. (2009) 49:1172–80. doi: 10.1016/j.jvs.2008.12.011
34. Fang X, Liu H, Zhang X, Zhang H, Qin X, Ji X. Metabolic syndrome, its components, and diabetes on 5-year risk of recurrent stroke among mild-to-moderate ischemic stroke survivors: a multiclinic registry study. J Stroke Cerebrovasc Dis. (2016) 25:626–34. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.017
35. Saely CH, Aczel S, Marte T, Langer P, Hoefle G, Drexel H. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J Clin Endocrinol Metab. (2005) 90:5698–703. doi: 10.1210/jc.2005-0799
36. Udell JA, Steg PG, Scirica BM, Eagle KA, Ohman EM, Goto S, et al. Metabolic syndrome, diabetes mellitus, or both and cardiovascular risk in outpatients with or at risk for atherothrombosis. Eur J Prev Cardiol. (2014) 21:1531–40. doi: 10.1177/2047487313500541
37. Vest AR, Young JB, Cho L. The metabolic syndrome, cardiovascular fitness and survival in patients with advanced systolic heart failure. Am J Cardio. (2018) 122:1513–9. doi: 10.1016/j.amjcard.2018.07.024
38. Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Després JP, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. (2006) 47:2229–36. doi: 10.1016/j.jacc.2005.12.073
39. Marroquin OC, Kip KE, Kelley DE, Johnson BD, Shaw LJ, Merz CNB, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the women's ischemia syndrome evaluation. Circulation. (2004) 109:714–21. doi: 10.1161/01.CIR.0000115517.26897.A7
40. Petersen JL, Yow E, AlJaroudi W, Goyal A, McGuire DK, Peterson ED, et al. Metabolic syndrome is not associated with increased mortality or cardiovascular risk in nondiabetic patients with a new diagnosis of coronary artery disease. Circ Cardiovasc Qual Outcomes. (2010) 3:165–72. doi: 10.1161/CIRCOUTCOMES.109.864447
41. Simão AN, Lehmann MF, Alfieri DF, Meloni MZ, Flauzino T, Scavuzzi BM, et al. Metabolic syndrome increases oxidative stress but does not influence disability and short-time outcome in acute ischemic stroke patients. Metab Brain Dis. (2015) 30:1409–16. doi: 10.1007/s11011-015-9720-y
42. Canibus P, Faloia E, Piva T, Muçai A, Serenelli M, Perna GP, et al. Metabolic syndrome does not increase angiographic restenosis rates after drug-eluting stent implantation. Metabolism. (2008) 57:593–7. doi: 10.1016/j.metabol.2007.10.020
43. Won KB, Kim BK, Chang HJ, Shin DH, Kim JS, Ko YG, et al. Metabolic syndrome does not impact long-term survival in patients with acute myocardial infarction after successful percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv. (2014) 83:713–20. doi: 10.1002/ccd.25150
44. Rana JS, Monraats PS, Zwinderman AH, de Maat MPM, Kastelein JJP, Doevendans PAF, et al. Metabolic syndrome and risk of restenosis in patients undergoing percutaneous coronary intervention. Diabetes Care. (2005) 28:873–7. doi: 10.2337/diacare.28.4.873
45. Chen Q, Zhang Y, Ding D, Li D, Xia M, Li X, et al. Metabolic syndrome and its individual components with mortality among patients with coronary heart disease. Int J Cardiol. (2016) 224:8–14. doi: 10.1016/j.ijcard.2016.08.324
46. Nigam A, Bourassa MG, Fortier A, Guertin MC, Tardif JC. The metabolic syndrome and its components and the long-term risk of death in patients with coronary heart disease. Am Heart J. (2006) 151:514–21. doi: 10.1016/j.ahj.2005.03.050
47. Balti EV, Kengne AP, Fokou JV, Nouthé BE, Sobngwi E. Metabolic syndrome and fatal outcomes in the post-stroke event: a 5-year cohort study in Cameroon. PLoS ONE. (2013) 8:e60117. doi: 10.1371/annotation/8410c942-8d5a-44c7-83e0-dc6ffe6b7c85
48. Fan G, Fu K, Jin C, Wang X, Han L, Wang H, et al. A medical costs study of older patients with acute myocardial infarction and metabolic syndrome in hospital. Clin Interv Aging. (2015) 10:329–37. doi: 10.2147/CIA.S70372
49. Solymoss BC, Bourassa MG, Marcil M, Levesque S, Varga S, Campeau L. Long-term rates of cardiovascular events in patients with the metabolic syndrome according to severity of coronary-angiographic alterations. Coron Artery Dis. (2009) 20:1–8. doi: 10.1097/MCA.0b013e32831624a5
50. Hajer GR, Graaf Y, Olijhoek JK, Verhaar MC, Visseren FLJ, SMART Study Group. Levels of homocysteine are increased in metabolic syndrome patients but are not associated with an increased cardiovascular risk, in contrast to patients without the metabolic syndrome. Heart. (2007) 93:216–20. doi: 10.1136/hrt.2006.093971
51. Kalahasti V, Chew DP, Nambi V, Minor SG, Zuzek R, Ellis SG, et al. Influence of metabolic syndrome on outcome after percutaneous coronary intervention. Int Cardiol. (2011) 3:721–6. doi: 10.2217/ica.11.80
52. Lee MG, Jeong MH, Ahn Y, Chae SC, Hur SH, Hong TJ, et al. Impact of the metabolic syndrome on the clinical outcome of patients with acute ST-elevation myocardial infarction. J Korean Med Sci. (2010) 25:1456–61. doi: 10.3346/jkms.2010.25.10.1456
53. Mi D, Zhang L, Wang C, Liu L, Pu X, Zhao X, et al. Impact of metabolic syndrome on the prognosis of ischemic stroke secondary to symptomatic intracranial atherosclerosis in Chinese patients. PLoS ONE. (2012) 7:e51421. doi: 10.1371/journal.pone.0051421
54. Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. (2012) 60:216–23. doi: 10.1016/j.jacc.2012.03.052
55. Bhagat PR, Swami SV. Impact of metabolic syndrome on hospital outcomes in acute myocardial infarction patients. Med Pulse Int Med J. (2017) 4:399–403.
56. Hu B, Zhou Y, Liu Y, Shi D, Zhao Y, Jia D, et al. Impact of metabolic syndrome on clinical outcomes after drug-eluting stent implantation in patients with coronary artery disease. Angiology. (2011) 62:440–6. doi: 10.1177/0003319711398473
57. Espinola-Klein C, Rupprecht HJ, Bickel C, Post F, Genth-Zotz S, Lackner K, et al. Impact of metabolic syndrome on atherosclerotic burden and cardiovascular prognosis. Am J Cardiol. (2007) 99:1623–8. doi: 10.1016/j.amjcard.2007.01.049
58. Iturry-Yamamoto GR, Zago AC, Moriguchi EH, Camargo JL, Gross JL, Zago AJ. Impact of metabolic syndrome and C-reactive protein on outcome after coronary stenting. J Endocrinol Invest. (2009) 32:383–6. doi: 10.1007/BF03345730
59. Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Genth-Zotz S, Post F, et al. Impact of inflammatory markers on cardiovascular mortality in patients with metabolic syndrome. Eur J Cardiovasc Prev Rehabil. (2008) 15:278–84. doi: 10.1097/HJR.0b013e3282f37a6e
60. Ao H, Xu F, Wang X, Tang X, Zheng Z, Hu S. Effects of metabolic syndrome with or without obesity on outcomes after coronary artery bypass graft. A cohort and 5-year study. PLoS ONE. (2015) 10:e0117671. doi: 10.1371/journal.pone.0117671
61. Hu R, Ma C, Nie S, Lü Q, Kang J, Du X, et al. Effect of metabolic syndrome on prognosis and clinical characteristics of revascularization in patients with coronary artery disease. Chin Med J. (2006) 119:1871–6. doi: 10.1097/00029330-200611020-00005
62. Nakatani D, Sakata Y, Sato H, Mizuno H, Shimizu M, Suna S, et al. Clinical impact of metabolic syndrome and its additive effect with smoking on subsequent cardiac events after acute myocardial infarction. Am J Cardiol. (2007) 99:885–9. doi: 10.1016/j.amjcard.2006.11.033
63. Polovina M, Hindricks G, Maggioni A, Mizuno H, Shimizu M, Suna S, et al. Association of metabolic syndrome with non-thromboembolic adverse cardiac outcomes in patients with atrial fibrillation. Eur Heart J. (2018) 39:4030–9. doi: 10.1093/eurheartj/ehy446
64. Hoshida S, Teragaki M, Lim Y, Mishima M, Nakajima O, Kijima Y, et al. Admission with metabolic disorder is a useful predictor of the 1-year prognosis for patients with unstable angina, but not for patients with acute myocardial infarction: east-Osaka acute coronary syndrome registry. Coron Artery Disn. (2011) 22:416–20. doi: 10.1097/MCA.0b013e3283472a87
65. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. (2005) 28:1769–78. doi: 10.2337/diacare.28.7.1769
66. Ghoorah K, Campbell P, Kent A, Maznyczka A, Kunadian V. Obesity and cardiovascular outcomes: a review. Eur Heart J Acute Cardiovasc Care. (2016) 5:77–85. doi: 10.1177/2048872614523349
67. Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. (2012) 110:77–82. doi: 10.1016/j.amjcard.2012.02.050
68. Dhoot J, Tariq S, Erande A, Amin A, Patel P, Malik S. Effect of morbid obesity on in-hospital mortality and coronary revascularization outcomes after acute myocardial infarction in the United States. Am J Cardiol. (2013) 111:1104–10. doi: 10.1016/j.amjcard.2012.12.033
69. Wohlfahrt P, Lopez-Jimenez F, Krajcoviechova A, Jozifova M, Mayer O, Vanek J, et al. The obesity paradox and survivors of ischemic stroke. J Stroke Cerebrovasc Dis. (2015) 24:1443–50. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.008
70. Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2010) 375:181–3. doi: 10.1016/S0140-6736(09)61794-3
71. Yuli Huang, Xiaoyan Cai, Weiyi Mai, Meijun Li, Yunzhao Hu. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. (2016) 355:i5953. doi: 10.1136/bmj.i5953
72. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. (2004) 27:15–35.
73. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. (2003) 26:S33–50. doi: 10.2337/diacare.26.2007.s33
Keywords: cardiovascular disease, metabolic syndrome, all-cause death, prognosis, meta-analysis
Citation: Li X, Zhai Y, Zhao J, He H, Li Y, Liu Y, Feng A, Li L, Huang T, Xu A and Lyu J (2021) Impact of Metabolic Syndrome and It's Components on Prognosis in Patients With Cardiovascular Diseases: A Meta-Analysis. Front. Cardiovasc. Med. 8:704145. doi: 10.3389/fcvm.2021.704145
Received: 01 May 2021; Accepted: 21 June 2021;
Published: 15 July 2021.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
Walter Espeche, National University of La Plata, ArgentinaCopyright © 2021 Li, Zhai, Zhao, He, Li, Liu, Feng, Li, Huang, Xu and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Lyu, bHl1anVuMjAyMEBqbnUuZWR1LmNu; Anding Xu, dGxpbEBqbnUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.