
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 18 August 2021
Sec. Thrombosis and Haemostasis
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.698336
Aims: The presence of transvenous leads for cardiac device therapy may increase the risk of venous thromboembolisms. The epidemiology of these complications has not yet been determined systematically. Therefore, this study aims to determine (I) the incidence of symptomatic upper extremity deep vein thrombosis (UEDVT) and (II) the prevalence of asymptomatic upper extremity vein occlusion in patients with transvenous leads, both after the initial 2 months following lead implantation.
Methods: PubMed, EMBASE, and Cochrane Library were searched until March 31, 2020 to identify studies reporting incidence of UEDVT and prevalence of asymptomatic vein occlusion after the initial 2 months after implantation in adult patients with transvenous leads. Incidence per 100 patient years of follow-up (PY) and proportions (%) were calculated to derive pooled estimates of incidence and prevalence.
Results: Search and selection yielded 20 and 24 studies reporting on UEDVT and asymptomatic vein occlusion, respectively. The overall pooled incidence of UEDVT was 0.9 (95% CI 0.5–1.4) per 100PY after 2 months after lead implantation. High statistical heterogeneity was present among studies (I2 = 82.4%; P = < 0.001) and only three studies considered to be at low risk of bias. The overall pooled prevalence of asymptomatic upper extremity vein occlusion was 8.6% (95% CI 6.0–11.5) with high heterogeneity (I2 = 81.4%; P = <0.001). Meta-regression analysis showed more leads to be associated with a higher risk of UEDVT.
Conclusion: Transvenous leads are an important risk factor for symptomatic UEDVT, which may occur up to multiple years after initial lead implantation. Existing data on UEDVT after lead implantation is mostly of poor quality, which emphasizes the need for high quality prospective research. Asymptomatic vein occlusion is present in a substantial proportion of patients and may complicate any future lead addition.
Clinical Trial Registration: (URL: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020178136, Identifier: PROSPERO 2020 CRD42020178136).
Pacemakers and implantable cardioverter-defibrillators (ICD) are commonly used to respectively, control and restore heart rhythm. The European Society of Cardiology reported over 500,000 pacemakers and ~85,000 ICD implantations in 2013 and the prevalence of cardiac devices is likely to increase (1–3). For over 60 years, transvenous leads have been used in cardiac device therapy. Although, other manners of internal pacing and defibrillation have been developed, cardiac device therapy involving transvenous leads remains common practice.
The intravascular presence of leads comes with the risk of various complications. A long-known complication is the stenosis of the deep veins of the upper extremity, in which venous stasis and activation of the coagulation cascade–and the subsequent formation of a thrombus–play significant roles (4). Acute thrombosis in these veins may cause signs and symptoms of venous congestion–including oedema, pain, and fatigue–in the arm; a condition which is referred to as upper extremity deep vein thrombosis (UEDVT). In addition, venous thrombosis comes with the short term risk of an acute pulmonary embolism (5). On the long term, a post-thrombotic syndrome of the arm might develop (6). However, stenosis and even total occlusion of these deep veins often remains asymptomatic when a sufficient collateral vein system is present. Yet, an asymptomatic occlusion becomes clinically relevant when leads have to be added, complicating the procedure.
Previous studies found that UEDVT occurred in 0.2–0.7% after ICD implantation and replacement in the early postoperative period (7) and that venous thromboembolism (VTE) occurred in 0.5% of the patients within the first month after pacemaker implantation (8). Thrombotic complications occurring in the first months after lead implantation may be attributed to the surgical intervention, whereas, those occurring after the postoperative period might be provoked by the transvenous leads themselves. Since the pathogenesis of the thrombotic complications during and after the first postoperative months is likely to be different, the epidemiology might diverge with increasing time after lead implantation.
The precise epidemiology of non-surgery related thrombotic complications in patients with transvenous leads have to date not been reviewed systematically (4, 9–11). Determination of incidences and prevalence will give insight into the burden of thrombotic complications and helps to understand the association between transvenous leads and UEDVT and asymptomatic vein occlusion. Therefore, we aim to determine the incidence of UEDVT and prevalence of asymptomatic upper extremity vein occlusion in patients with transvenous leads for cardiac device therapy after the postoperative period by performing a systematic review and meta-analysis.
This study was conducted in accordance with PRISMA and MOOSE-guidelines (12, 13). A protocol was registered in PROSPERO prior to conducting the study (registration ID: CRD42020178136).
An electronic search in PubMed, EMBASE and Cochrane Library was performed from inception until 31 March 2020. The search strategy consisted of free and controlled terms for cardiac device therapy and thromboembolic complications (Supplementary Data 1) and was constructed in cooperation with a librarian. The search was limited to publications in Dutch, English, French, and German. Reference lists of previous reviews of the subject as well as the included articles were hand-searched to identify additional eligible studies. Clinicaltrials.gov was searched for relevant ongoing studies.
Following retrieval of the search results, duplicates were removed (Mendeley version 1.19.4) and two reviewers (DD, MW) screened the title and abstract of the remaining records independently (Rayyan QCRI) (14). Relevant articles and articles of which eligibility could not be assessed properly were selected for full-text assessment. Disagreement whether or not to include a study after full-text assessment was resolved through debate with a third reviewer (JW) in order to reach consensus.
Observational studies (cross-sectional, prospective, and retrospective cohort studies) and randomized controlled trials with a full-text article available were eligible for inclusion. Studies had to report on the incidence of UEDVT and/or prevalence of asymptomatic upper extremity vein occlusion in adult patients with active and/or abandoned leads for cardiac device therapy. Authors were contacted via email if additional information was required to assess eligibility. Studies were excluded if patients received temporary or transfemoral pacing, underwent haemodialysis, had a Fontan circulation; when the study focussed on perioperative management or lead extraction, and when postoperative follow-up of venous complications was restricted to 2 months or less. Finally, case reports, case series, post-mortem series as well as reviews were excluded.
One researcher (DD) extracted the data from the included studies using a predefined data extraction form (Supplementary Data 2). Adequate extraction of data was verified by a second researcher (MW) and disagreement was resolved by consensus through debate with a third reviewer (JW). In case of population duplicates, we included the most recent study or the one that reported most completely on the outcome of interest. Authors were contacted via email for additional data where necessary. Data was extracted on: first author; year of publication; study characteristics (country, design, aims, in- and exclusion criteria, sample size, follow-up duration, type of population); population characteristics (age, sex, predisposing factors for thrombosis, anticoagulant treatment, comorbidities, indication for and type of cardiac device, number of leads); definition and assessment of outcomes; incidence of UEDVT, follow-up duration at UEDVT and prevalence of asymptomatic vein occlusion.
Methodological quality and risk of bias of included studies was assessed by two reviewers (DD, MW) independently using the risk of bias tool of Hoy et al. (15), which is specifically developed for prevalence studies. With this tool, 10 items−4 on external and 6 on internal validity–are judged to be either at low or high risk of bias for each study. Item 9: Was the length of the shortest prevalence period for the parameter of interest appropriate? was considered inapplicable and was therefore omitted in the present study. A summary score of 0–1, 2–3, and 4–9 points represented low, moderate and high risk of bias, respectively. Complete risk of bias assessment of the studies included in the analysis can be found in Supplementary Data 3.
UEDVT was defined as any new thrombotic event with symptoms of venous congestion in the upper extremity occurring more than 2 months after transvenous lead implantation. The implantation procedure was considered a minor transient risk factor for VTE (16). Hence, all UEDVT occurring within 2 months after the implantation procedure were attributed to the procedure rather than the presence of transvenous leads and these cases were therefore excluded. Events of which timing in relation to the implantation could not be confirmed were included in the overall analysis and sensitivity analysis was conducted afterwards. Asymptomatic vein occlusion was defined as total occlusion of one or more deep veins of the upper extremity ascertained by venography or ultrasound without the presence of clinical symptoms of venous congestion. Visible superficial collateral vein formation by itself was not considered a symptom in both definitions. Primary outcomes were incidence rate per 100 patient years of follow-up (PY) for UEDVT and prevalence (%) for asymptomatic vein occlusion.
To compare the incidence of UEDVT across studies, we calculated incidence rates expressed as events per 100 PY, using the number of events and total follow-up time of the study population. Total PY for studies with cases confirmed to have occurred ≥2 months postoperatively or no cases were subtracted by 2 months per patient. For studies reporting median with interquartile range or total range, mean (μ) and standard deviation (SD) were estimated (17, 18). Prevalence of asymptomatic vein occlusion was expressed as proportion (%). Given the binary character of the data, the variance stabilizing double arcsine transformation was applied to calculate both incidence rate and prevalence (19).
Statistical heterogeneity was assessed using the Cochran's Q test (α < 0.1) and I2 statistic. The Cochran Q test tests whether there is significant heterogeneity among the reported effect sizes. The I2 statistic provides a quantitative estimate of the variability across studies; values of 0–25, 25–75, and 75–100% were considered to represent low, medium and high heterogeneity, respectively (20). Sensitivity analyses were conducted based on region; study design; risk of bias categories; assessed type of cardiac device; exclusion of patients with previous venous anomalies; whether number of events within 2 months postoperatively was unknown (UEDVT); patients with abandoned leads were subject of study (UEDVT); venography was used for ascertainment of outcome in all patients (asymptomatic vein occlusion).
To explore the source of heterogeneity, univariate random effects meta-regression analyses were conducted with incidence and prevalence as outcome in separate analyses and the following continuous variables as dependent variables: publication year; mean age; proportion of males; proportion of patients receiving anticoagulant therapy; mean number of leads per patient; and for the prevalence of asymptomatic vein occlusion mean follow-up time as well.
The DerSimonian and Laird random-effects model was applied because of the expected heterogeneity of studies included in the analysis (21). Potential publication bias was assessed by visual inspection of funnel plots and Egger's regression test (22). All statistical analyses were performed with the R Statistical Software (version 4.0.0; https://www.r-project.org/) using the metafor package (23). P-values <0.05 were considered statistically significant.
Out of 8,273 reports retrieved by the search strategy, 139 were in accordance with the eligibility criteria based on title and abstract. At full text assessment of these reports, 101 were excluded (Figure 1). Among the 38 included studies in the present systematic review, 14 studies report incidence of UEDVT (24–37), 12 report prevalence of asymptomatic vein occlusion (38–49) and 12 report both outcomes (50–61). No additional eligible reports were identified from a hand search of the included articles' references.
Out of 26 studies reporting incidence of UEDVT, 6 were not included in the meta-analysis due to unavailable total person years of follow-up (26–29, 52, 53). Of the remaining 20 studies (median year of publication 2007) (24, 25, 30–37, 50, 51, 54–61), one study reported on two separate populations of patients with transvenous leads; one population having leads in use and the other having abandoned leads in situ. Patient populations were recruited from Europe (n = 9), Asia (n = 5), the Middle East (n = 3), the USA (n = 2), and Brazil (n = 2). Two studies reported on a substantial larger cohort of patients (n = 903, PY = 3,695; n = 6,256, PY = 29,195) (30, 35) than the other studies, which described populations ranging from 21 to 202 patients (range of PY 17–1,323). Relevant characteristics of individual studies are presented in more detail in Supplementary Table 4.
A total of 72 cases of symptomatic UEDVT were reported in 8,671 patients followed for a total of 36,774 years, of which 35545 PY more than 2 months after lead implantation. Forty-two cases (58%) were confirmed to have occurred at 2 months or more post device implantation. The incidence rate of symptomatic UEDVT ranged from 0.0 to 10.0 cases per 100 years of follow-up across studies. The overall pooled incidence rate was 0.9 (95% CI 0.5–1.4) UEDVT per 100 PY, with high statistical heterogeneity among studies (I2 = 82.4%; P = <0.001) (Figure 2). Both the funnel plot, which was shaped asymmetrically (Figure 3), and Egger's test (P = <0.001) indicated publication bias.
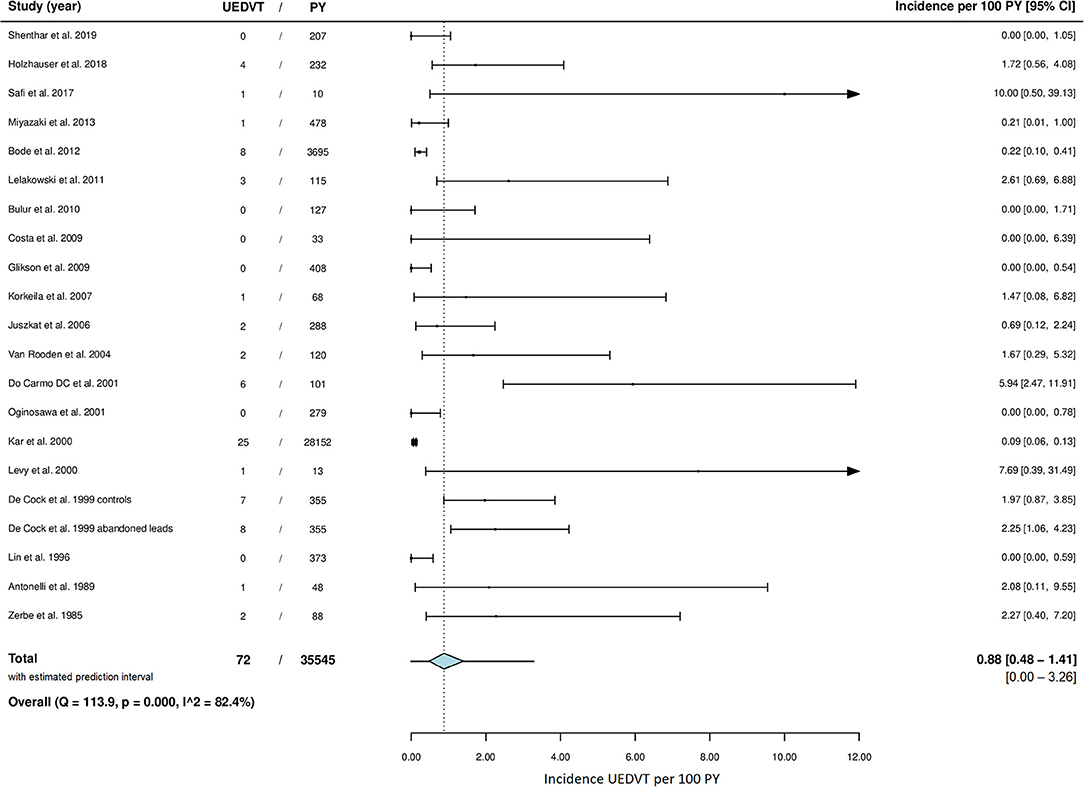
Figure 2. Meta-analysis of incidence rate of UEDVT. CI, confidence interval; PY, patient years; UEDVT, upper extremity deep vein thrombosis.
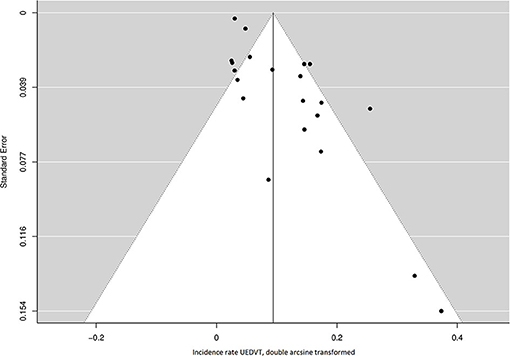
Figure 3. Funnel plot of the included studies for the incidence rate of UEDVT. UEDVT, upper extremity deep vein thrombosis.
UEDVT occurred at a median of 11 months (range 2–48) after lead implantation for the smaller studies (19 cases) and at a mean of 26 months postoperatively in the cohort described by Kar et al. (35).
Sensitivity analysis (Table 1) showed a pooled incidence rate of 0.6 (95% CI: 0.0–1.7) UEDVT per 100 PY for prospective studies and an incidence rate 0.2 (0.0–0.9) for retrospective studies. Including studies considered to be at low risk of bias only resulted in a pooled incidence rate of 1.3 (0.3–2.9) per 100 PY and addition of the studies regarded to be at moderate risk of bias resulted in an incidence rate of 0.1 (0.0–0.5) per 100 PY. Forming subgroups based on the included population resulted in an incidence rate of 0.7 (0.0–2.9) for patients with abandoned leads and 0.4 (0.0–1.4) for pacemaker only studies. The pooled incidence rate of the studies from which all cases of UEDVT were confirmed to have occurred later than 2 months postoperatively was 0.1 (0.0–0.5). A separate analysis with exclusion of the two largest study populations was performed (Supplementary Table 6), since these studies comprised 89% of the total included studies' PY (30, 35). A total of 39 cases of UEDVT remained in 3,698 PY of follow up with a pooled incidence rate of 1.2 (0.6–2.0) per 100 PY. Studies with inclusion of ICD patients only and studies from Asia had a significant lower incidence of UEDVT.
Univariate meta-regression analyses showed no association with publication year, age, sex, proportion of patients on anticoagulation. However, a higher mean number of leads per patient was significantly associated with a higher incidence rate of UEDVT (P 0.002) (Table 3).
Median publication year of the 24 studies included for prevalence of asymptomatic vein occlusion was 2002 (38–61). Studies were conducted in Europe (n = 8), Asia (n = 6), the Middle East (n = 5), the USA (n = 3), and Brazil (n = 2). The sample size of the studies ranged from 20 to 227 participants and patients were assessed at a median of 3.8 years after transvenous lead placement. Characteristics of individual studies are displayed in Supplementary Table 5.
Asymptomatic upper extremity vein occlusion was present in 219 of the 2,323 patients. The prevalence ranged from 0.0 to 34.0% across studies. The overall pooled prevalence of asymptomatic vein occlusion is 8.6% (95% CI 6.0–11.5) with high heterogeneity among studies (I2 = 81.4%; P = <0.001) (Figure 4). Both Egger's test (P = 0.494) and the funnel plot (Figure 5) did not suggest the presence of publication bias.
Sensitivity analysis for asymptomatic vein occlusion (Table 2) showed a pooled prevalence of 6.5% (4.5–8.8) for prospective studies, 9.3% (3.6–17.1) for retrospective, and 10.3% (0.9–26.7) for cross-sectional studies. A pooled prevalence of 7.8% (4.7–11.4) was found in studies with low risk of bias. Combining those studies considered to be at low and moderate risk of bias resulted in a prevalence of 8.0% (5.2–11.3). The pooled prevalence of studies obtaining venogram of each included patient was 8.1% (5.2–11.6); this was 6.4% (3.8–9.6) for studies which did not.
Meta-regression analyses showed no effect for publication year, age, sex, proportion of patients on anticoagulation therapy, mean number of leads per patient and mean follow-up duration on the prevalence of asymptomatic vein occlusion (Table 3).
The incidence rate of symptomatic UEDVT after the first 2 months following lead implantation ranged from 0.0 to 10.0 per 100 PY across individual studies and averaged ~0.9 UEDVT per 100 PY in the pooled analysis.
We report a substantial higher incidence of UEDVT among patients with transvenous leads when compared to data from the general population. An estimated incidence of 0.0036 primary and secondary UEDVT per 100 PY was found in the population of Malmö (Sweden) (62), whereas, an incidence of 0.025 UEDVT per 100 PY was reported in a French population aged between 60 and 74 years (63). The 35 to 244-fold higher incidence rate in patients with transvenous leads implies that the presence of these leads is an evident risk factor for symptomatic UEDVT.
The incidence we report underlines the difference in epidemiology between thrombotic complications immediately after lead implantation and those occurring after the initial postoperative period, suggesting a different etiology. Our results show that UEDVT is not reserved to the first months postoperatively but may occur up to multiple years after transvenous lead implantation. Given the large spread in timing of occurrence and low incidence, we do not advise routine screening on UEDVT in patients with transvenous leads.
It is unclear whether patients with transvenous leads warrant prophylactic anticoagulation for primary prevention of UEDVT. Our results do not provide guidance on the use of thromboprophylaxis postoperatively after lead implantation. After the initial postoperative months, the increased risk of major bleedings with direct oral anticoagulants (2.0–3.9 per 100 PY) and vitamin K antagonists (3.6 per 100 PY) as established in other populations, does not seem to outweigh any decrease in the risk of a first UEDVT (0.9 per 100 PY) in patients with transvenous leads (64).
When a first UEDVT occurs, transvenous leads should be considered a major provoking risk factor since the risk of UEDVT increases more than 10-fold after lead implantation (16). Further research is needed to provide insight into the association between leads and UEDVT over time to elucidate whether transvenous leads should be regarded a transient or persistent risk factor. An answer to this question would provide clarity as to whether anticoagulation may be stopped after the initial 3 months of treatment for a first VTE or extended anticoagulant treatment should be considered.
Significantly different UEDVT incidence rates were present in some subgroups compared to the overall pooled incidence. The lower incidence of UEDVT among retrospective studies compared to prospective studies may be explained by underreport of cases, especially with increasing follow-up duration. The pooled incidence for studies with only ICD patients was based on merely two studies and should therefore be interpreted with caution. In addition, the incidence of VTE is suggested to be generally lower in Asian compared to Western populations which might have resulted in a lower incidence rate among Asian studies in our analysis as well (65).
Furthermore, the results of our meta-regression analysis show that a higher number of transvenous leads is related to the occurrence of UEDVT, which is in line with earlier studies (38, 39, 43, 44, 54). A higher number of leads implies a larger total diameter of foreign intravascular material and may induce an increasingly thrombogenic environment. This is in line with the proposed pathophysiology of thrombosis around transvenous leads which includes lead endothelialisation, endovascular damage and venous stasis (4). A clinical application might be that dysfunctional transvenous leads–often let abandoned to avoid acute complications of extraction–are extracted in patients with a high thrombosis risk and low bleeding risk to prevent UEDVT in the long term.
Whereas, UEDVT could virtually affect all patients with transvenous leads, asymptomatic upper extremity vein occlusion is only relevant to those admitted for lead revision. The pooled analysis showed asymptomatic upper extremity vein occlusion to be present in 8.6% of the patients after transvenous lead placement. A previous comprehensive review reported a comparable prevalence of 8.3% of asymptomatic vein occlusion across seven studies; all of them, except for one, included in the current analysis as well (9). A more recent review stated that asymptomatic vein occlusion is present in 2–25% of the patients after lead placement, which lies entirely within the range of 0 to 34% on which we report (4). Given a 1-year reintervention rate of 4.2% after pacemaker implantation and 6.3% after ICD or CRT implantation as found in the UK national audit 2017, upper extremity vein occlusion frequently causes clinical difficulties (66).
The results of the present analysis apply to all patients with transvenous leads; patients with both active as well as abandoned leads, patients from different continents and patients with all types of cardiac devices were included. In addition, we used well-defined outcomes which are clinically relevant to both patient and physician.
However, several limitations should be addressed when interpreting the results of the current meta-analysis, especially regarding UEDVT incidence. First, the incidence rate of UEDVT might have been underestimated as the studies did not use a uniform definition. Second, only 42 out of 72 UEDVT could be confirmed to have occurred at ≥2 months postoperatively. As a result, the reported UEDVT incidence may be an overestimation of the actual incidence. Third, only three studies were of low risk of bias which implies that the overall pooled incidence was derived from studies of poor quality predominantly. Lastly, a substantial amount of heterogeneity was present among the studies. Methodological heterogeneity arose from difference in study design; clinical heterogeneity followed from inclusion of both studies assessing patients with functional and non-functional (abandoned) leads.
Regarding asymptomatic vein occlusion, we determined the prevalence of total vein occlusion only. Sub-occlusive upper extremity vein stenoses may occur even more frequent but are clinically less relevant in case of lead revision. In addition, heterogeneity in the reported categories of venous obstruction across studies hampered pooled analyses.
In the meta-analysis of both outcomes, substantial statistical heterogeneity was present, and could not be explained sufficiently by sensitivity and meta-regression analyses.
The incidence of symptomatic UEDVT in patients with transvenous leads is 0.9 per 100 PY after the first 2 months following lead implantation. Given the much lower incidence of UEDVT in the general population, the presence of transvenous leads must be considered an important risk factor for UEDVT. In addition, the presence of more transvenous leads was identified as a potential risk factor for UEDVT. The prevalence of asymptomatic upper extremity vein occlusion after transvenous lead implantation is 8.6%. Although, this condition will remain subclinical in most cases, vein occlusion will complicate any future lead addition. Future research should assess clinically relevant outcomes–e.g., symptomatic UEDVT and asymptomatic vein occlusion–and focus on risk factors for thrombotic complications and the role of prophylactic anticoagulation therapy in patients with transvenous leads. In order to gain more high-quality data on the epidemiology of thrombotic complications after lead implantation, we advise to conduct dedicated prospective studies and we call registries to record UEDVT as a complication of lead implantation with a focus on time between implantation and diagnosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
DD and MW: conceived and designed the analysis, collected data, performed the analysis, and wrote the paper. MN and JW: conceived and designed the analysis and substantial contributions to the text of the paper. AT: substantial contributions to the text of the paper and feedback on the content of the presented data. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.698336/full#supplementary-material
CRT, Cardiac Resynchronization Therapy; ICD, Implantable Cardioverter-Defibrillator; PM, Pacemaker; PY, Patient Years; UEDVT, Upper Extremity Deep Vein Thrombosis.
1. Raatikainen MJP, Arnar DO, Zeppenfeld K, Merino JL, Levya F, Hindriks G, et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace. (2015) 17(Suppl. 1):i1–75. doi: 10.1093/europace/euu300
2. Bradshaw PJ, Stobie P, Knuiman MW, Briffa TG, Hobbs MST. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart. (2014) 1:e000177. doi: 10.1136/openhrt-2014-000177
3. Uslan DZ, Tleyjeh IM, Baddour LM, Friedman PA, Jenkins SM, St Sauver JL, et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J. (2008) 155:896–903. doi: 10.1016/j.ahj.2007.12.022
4. Donnelly J, Gabriels J, Galmer A, Willner J, Beldner S, Epstein LM, et al. Venous obstruction in cardiac rhythm device therapy. Curr Treat Options Cardiovasc Med. (2018) 20:64. doi: 10.1007/s11936-018-0664-5
5. Levy MM, Albuquerque F, Pfeifer JD. Low incidence of pulmonary embolism associated with upper-extremity deep venous thrombosis. Ann Vasc Surg. (2012) 7:964–72. doi: 10.1016/j.avsg.2011.12.016
6. Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res. (2006) 117:609–14. doi: 10.1016/j.thromres.2005.05.029
7. Prutkin JM, Poole JE. Complications of ICD generator change and implantations. Card Electrophysiol Clin. (2011) 3:389–401. doi: 10.1016/j.ccep.2011.05.005
8. Cantillon DJ, Exner DV, Badie N, Davis K, Gu NY, Nabutovsky Y, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. (2017) 3:1296–305. doi: 10.1016/j.jacep.2017.05.007
9. Rozmus G, Daubert JP, Huang DT, Rosero S, Hall B, Francis C. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Card Electrophysiol. (2005) 13:9–19. doi: 10.1007/s10840-005-1140-1
10. Spittell PC, Hayes DL. Venous complications after insertion of a transvenous pacemaker. Mayo Clin Proc. (1992) 67:258–65. doi: 10.1016/S0025-6196(12)60103-7
11. O'Leary B, Allaqaband S. Subclavian vein stenosis/occlusion following transvenous cardiac pacemaker and defibrillator implantation: incidence, pathophysiology and current management. J patient-centered Res Rev. (2015) 2:112–7. doi: 10.17294/2330-0698.1074
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. J Am Med Assoc. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
14. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
15. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. (2012) 65:934–9. doi: 10.1016/j.jclinepi.2011.11.014
16. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. (2016) 14:1480–3. doi: 10.1111/jth.13336
17. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
18. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. (1950) 21:607–11. doi: 10.1214/aoms/1177729756
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
22. Egger M, Davey G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw. (2010) 36:119295. doi: 10.18637/jss.v036.i03
24. Holzhauser L, Imamura T, Nayak HM, Sarswat N, Kim G, Raikhelkar J, et al. Consequences of retained defibrillator and pacemaker leads after heart transplantation-an underrecognized problem. J Card Fail. (2018) 24:101–8. doi: 10.1016/j.cardfail.2017.12.012
25. Miyazaki S, Uchiyama T, Komatsu Y, Taniguchi H, Kusa S, Nakamura H, et al. Long-term complications of implantable defibrillator therapy in brugada syndrome. Am J Cardiol. (2013) 111:1448–51. doi: 10.1016/j.amjcard.2013.01.295
26. Alvarez PA, Sperry BW, Perez AL, Varian K, Raymond T, Tong M, et al. Burden and consequences of retained cardiovascular implantable electronic device lead fragments after heart transplantation. Am J Transplant. (2018) 18:3021–8. doi: 10.1111/ajt.14755
27. Rahbi H, El-Din M, Salloum M, Shaukat N, Farooq M. Complex cardiac pacing in the setting of a district general hospital: procedural success and complications. Heart Asia. (2014) 6:94–9. doi: 10.1136/heartasia-2013-010421
28. Böhm A, Pintér A, Duray G, Lehoczky D, Dudás G, Tomcsányi I, et al. Complications due to abandoned noninfected pacemaker leads. PACE - Pacing Clin Electrophysiol. (2001) 24:1721–4. doi: 10.1046/j.1460-9592.2001.01721.x
29. Williams EH, Tyers GF, Shaffer CW. Symptomatic deep venous thrombosis of the arm associated with permanent transvenous pacing electrodes. Chest. (1978) 73:613–5. doi: 10.1378/chest.73.5.613
30. Bode F, Himmel F, Reppel M, Mortensen K, Schunkert H, Wiegand UK. Should all dysfunctional high-voltage leads be extracted? Results of a single-centre long-term registry. Europace. (2012) 14:1764–70. doi: 10.1093/europace/eus202
31. Lelakowski J, Domagała TB, Cieśla-Dul M, Rydlewska A, Majewski J, Piekarz J, et al. Association between selected risk factors and the incidence of venous obstruction after pacemaker implantation: demographic and clinical factors. Kardiol Pol. (2011) 69:1033–40.
32. Glikson M, Suleiman M, Luria DM, Martin ML, Hodge DO, Shen WK, et al. Do abandoned leads pose risk to implantable cardioverter-defibrillator patients? Hear Rhythm. (2009) 6:65–8. doi: 10.1016/j.hrthm.2008.10.012
33. Juszkat R, Pukacki F, Zieliński M, Oszkinis G, Gabriel M, Kubicka A, et al. Ultrasound evaluation of vascular thrombotic complications following endovascular implantation of cardiac pacemaker electrodes. Acta Angiol. (2006) 12:69–79.
34. Levy T, Walker S, Rex S, Paul V. A comparison between passive and active fixation leads in the coronary sinus for biatrial pacing: initial experience. Europace. (2000) 2:228–32. doi: 10.1053/eupc.2000.0105
35. Kar AK, Ghosh S, Majumdar A, Mondal M, Dutta I. Venous obstruction after permanent pacing. Indian Heart J. (2000) 52:431–3.
36. de Cock CC, Vinkers M, Van Campe LC, Verhorst PM, Visser CA. Long-term outcome of patients with multiple (≥ 3) noninfected transvenous leads: a clinical and echocardiographic study. PACE - Pacing Clin Electrophysiol. (2000) 23:423–6. doi: 10.1111/j.1540-8159.2000.tb00821.x
37. Zerbe F, Ponizyński A, Dyszkiewicz W, Ziemiański A, Dziegielewski T, Krug H. Functionless retained pacing leads in the cardiovascular system. A complication of pacemaker treatment. Br Heart J. (1985) 54:76–9. doi: 10.1136/hrt.54.1.76
38. Morani G, Bolzan B, Valsecchi S, Morosato M, Ribichini FL. Chronic venous obstruction during cardiac device revision: incidence, predictors, and efficacy of percutaneous techniques to overcome the stenosis. Hear Rhythm. (2020) 17:258–64. doi: 10.1016/j.hrthm.2019.08.012
39. Abu-El-Haija B, Bhave PD, Campbell DN, Mazur A, Hodgson-Zingman DM, Cotarlan V, et al. Venous stenosis after transvenous lead placement: a study of outcomes and risk factors in 212 consecutive patients. J Am Heart Assoc. (2015) 4:e001878. doi: 10.1161/JAHA.115.001878
40. Chow WH, Yip AS, Tai YT, Cheung KL. Venous thrombosis after long-term transvenous pacing in the Chinese. Angiology. (1991) 42:522–6. doi: 10.1177/000331979104200702
41. Mitrović V, Thormann J, Schlepper M, Neuss H. Thrombotic complications with pacemakers. Int J Cardiol. (1983) 2:363–74. doi: 10.1016/0167-5273(83)90008-6
42. Santini M, Di Fusco SA, Santini A, Magris B, Pignalberi C, Aquilani S, et al. Prevalence and predictor factors of severe venous obstruction after cardiovascular electronic device implantation. Europace. (2016) 18:1220–6. doi: 10.1093/europace/euv391
43. Yeşil M, Bayata S, Arikan E, Ekinci S, Gürsul E, Postaci N. Central venous obstruction and clinical predictors in patients with permanent pacemaker. Anadolu Kardiyol Derg. (2012) 12:401–5. doi: 10.5152/akd.2012.122
44. Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. (2007) 9:328–32. doi: 10.1093/europace/eum019
45. Lickfett L, Bitzen A, Arepally A, Nasir K, Wolpert C, Jeong KM, et al. Incidence of venous obstruction following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace. (2004) 6:25–31. doi: 10.1016/j.eupc.2003.09.001
46. Sticherling C, Chough SP, Baker RL, Wasmer K, Oral H, Tada H, et al. Prevalence of central venous occlusion in patients with chronic defibrillator leads. Am Heart J. (2001) 141:813–6. doi: 10.1067/mhj.2001.114195
47. Zuber M, Huber P, Fricker U, Buser P, Jäger K. Assessment of the subclavian vein in patients with transvenous pacemaker leads. Pacing Clin Electrophysiol. (1998) 21:2621–30. doi: 10.1111/j.1540-8159.1998.tb00039.x
48. Goto Y, Abe T, Sekine S, Sakurada T. Long-term thrombosis after transvenous permanent pacemaker implantation. Pacing Clin Electrophysiol. (1998) 21:1192–5. doi: 10.1111/j.1540-8159.1998.tb00177.x
49. Nishino M, Tanouchi J, Ito T, Tanaka K, Aoyama T, Kitamura M, et al. Echographic detection of latent severe thrombotic stenosis of the superior vena cava and innominate vein in patients with a pacemaker: integrated diagnosis using sonography, pulse Doppler, and color flow. PACE - Pacing Clin Electrophysiol. (1997) 20:946–52. doi: 10.1111/j.1540-8159.1997.tb05498.x
50. Shenthar J, Padmanabhan D, Banavalikar B, Parvez J, Vallapil SP, Singha I, et al. Incidence, predictors, and gradation of upper extremity venous obstruction after transvenous pacemaker implantation. Indian Heart J. (2019) 71:123–5. doi: 10.1016/j.ihj.2019.02.002
51. Safi M, Akbarzadeh MA, Azinfar A, Namazi MH, Khaheshi I. Upper extremity deep venous thrombosis and stenosis after implantation of pacemakers and defibrillators; a prospective study. Rom J Intern Med. (2017) 55:139–44. doi: 10.1515/rjim-2017-0018
52. Crook BR, Gishen P, Robinson CR, Oram S. Occlusion of the subclavian vein associated with cephalic vein pacemaker electrodes. Br J Surg. (1977) 64:329–31. doi: 10.1002/bjs.1800640508
53. Stoney WS, Addlestone RB, Alford WC Jr, Burrus GR, Frist RA, Thomas CS Jr. The incidence of venous thrombosis following long-term transvenous pacing. Ann Thorac Surg. (1976) 22:166–70. doi: 10.1016/S0003-4975(10)63980-X
54. Bulur S, Vural A, Yazici M, Ertaş G, Özhan H, Ural D. Incidence and predictors of subclavian vein obstruction following biventricular device implantation. J Interv Card Electrophysiol. (2010) 29:199–202. doi: 10.1007/s10840-010-9516-2
55. Costa R, Da Silva KR, Rached R, Martinelli Filho M, Carnevale FC, Moreira LF, et al. Prevention of venous thrombosis by warfarin after permanent transvenous leads implantation in high-risk patients. PACE - Pacing Clin Electrophysiol. (2009) 32:S247–51. doi: 10.1111/j.1540-8159.2008.02295.x
56. Korkeila P, Nyman K, Ylitalo A, Koistinen J, Karjalainen P, Lund J, et al. Venous obstruction after pacemaker implantation. PACE - Pacing Clin Electrophysiol. (2007) 30:199–206. doi: 10.1111/j.1540-8159.2007.00650.x
57. van Rooden CJ, Molhoek SG, Rosendaal FR, Schalij MJ, Meinders AE, Huisman MV. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol. (2004) 15:1258–62. doi: 10.1046/j.1540-8167.2004.04081.x
58. Da Costa SS, Scalabrini Neto A, Costa R, Caldas JG, Martinelli Filho M. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: A 6-month follow-up prospective study. PACE - Pacing Clin Electrophysiol. (2002) 25:1301–6. doi: 10.1046/j.1460-9592.2002.01301.x
59. Oginosawa Y, Abe H, Nakashima Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. PACE - Pacing Clin Electrophysiol. (2002) 25:1605–11. doi: 10.1046/j.1460-9592.2002.01605.x
60. Lin LJ, Lin JL, Tsai WC, Teng JK, Tsai LM, Chen JH. Venous access thrombosis detected by transcutaneous vascular ultrasound in patients with single-polyurethane-lead permanent pacemaker. PACE - Pacing Clin Electrophysiol. (1998) 21:396–400. doi: 10.1111/j.1540-8159.1998.tb00063.x
61. Antonelli D, Turgeman Y, Kaveh Z, Artoul S, Rosenfeld T. Short-term thrombosis after transvenous permanent pacemaker insertion. Pacing Clin Electrophysiol. (1989) 12:280–282. doi: 10.1111/j.1540-8159.1989.tb02660.x
62. Isma N, Svensson PJ, Gottsäter A, Lindblad B. Upper extremity deep venous thrombosis in the population-based Malmö thrombophilia study (MATS). Epidemiology, risk factors, recurrence risk, and mortality. Thromb Res. (2010) 125:e335–8. doi: 10.1016/j.thromres.2010.03.005
63. Delluc A, Le Mao R, Tromeur C, Chambry N, Rault-Nagel H, Bressollette L, et al. Incidence of upper-extremity deep vein thrombosis in western France: a community-based study. Haematologica. (2019) 104:e29–31. doi: 10.3324/haematol.2018.194951
64. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-Vitamin K antagonist oral anticoagulants and warfarin in patients with Atrial fibrillation: propensity weighted nationwide cohort study. BMJ. (2016) 353:i3189. doi: 10.1136/bmj.i3189
Keywords: deep vein thrombosis, cardiac device therapy, transvenous leads, epidemiology, systematic review and meta-analysis
Citation: Duijzer D, de Winter MA, Nijkeuter M, Tuinenburg AE and Westerink J (2021) Upper Extremity Deep Vein Thrombosis and Asymptomatic Vein Occlusion in Patients With Transvenous Leads: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:698336. doi: 10.3389/fcvm.2021.698336
Received: 21 April 2021; Accepted: 15 July 2021;
Published: 18 August 2021.
Edited by:
Avi Leader, Rabin Medical Center, IsraelReviewed by:
Eyal Ben-Assa, Assuta Ashdod University Hospital, IsraelCopyright © 2021 Duijzer, de Winter, Nijkeuter, Tuinenburg and Westerink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Westerink, ai53ZXN0ZXJpbmstM0B1bWN1dHJlY2h0Lm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.