- 1Department of Forensic Pathology, School of Forensic Medicine, China Medical University, Shenyang, China
- 2Department of Forensic Pathophysiology, School of Forensic Medicine, China Medical University, Shenyang, China
- 3Department of Forensic Medicine, School of Basic Medical Sciences, Fudan University, Shanghai, China
Biomarkers such as B-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP), cardiac troponin (cTn), and CK-MB contribute significantly to the diagnosis of cardiovascular disease (CVD). Recent studies have demonstrated that suppression of tumorigenicity 2 (ST2) is associated with CVD, but a meta-analysis of ST2 levels in different CVDs has yet to be conducted. Therefore, the present study aimed to investigate soluble ST2 (sST2) levels in patients with ischemic heart disease (IHD), myocardial infarction (MI), and heart failure (HF). A total of 1,425 studies were searched across four databases, of which 16 studies were included in the meta-analysis. The Newcastle–Ottawa Quality Assessment Scale (NOS) values of all 16 studies were ≥7. The meta-analysis results indicated that the sST2 level was not correlated with IHD (standard mean difference [SMD] = 0.58, 95% confidence interval [95% CI] = 0.00 to 1.16, p = 0.05) or MI (weighted mean difference [WMD] = 0.17, 95% CI = −0.22 to 0.55, p = 0.40) but was significantly associated with HF (WMD = 0.21, 95% CI = 0.04 to 0.38, p = 0.02; I2 = 99%, p < 0.00001). sST2 levels did not differ significantly between patients with IHD or MI and healthy individuals; however, we believe that ST2 could be used as an auxiliary diagnostic biomarker of HF.
Introduction
Cardiovascular disease (CVD) includes all pathologies of the heart or circulatory system, such as coronary artery disease (CAD), myocardial infarction (MI), heart failure (HF), and peripheral vascular disease. In 2019, the prevalence of CVD reached 523 million cases, resulting in 18.6 million deaths worldwide, and the number is still increasing, seriously affecting patient quality of life (1–4). In addition to successful rescue, early diagnosis and effective monitoring for patients with critical illness after disease onset are also needed.
Clinical biomarkers such as cardiac troponin (cTn), B-type natriuretic peptide (BNP), and N-terminal pro-BNP (NT-proBNP) are widely used in the diagnosis of MI and HF (5–7). Recent studies have demonstrated that suppression of tumorigenicity 2 (ST2) is also associated with CVD (8). ST2 is a member of the interleukin (IL)-1 receptor family and was first described in relation to inflammatory and autoimmune diseases in 1989 (9, 10). ST2 and its ligand interaction of IL-33 are involved in the immune response, and soluble ST2 (sST2) levels in plasma are elevated in patients with asthma, septic shock, trauma, and systemic lupus erythematosus (11). Blood sST2 levels are increased not only in inflammatory diseases but also in various heart diseases and are considered a valuable prognostic marker for CVD (12).
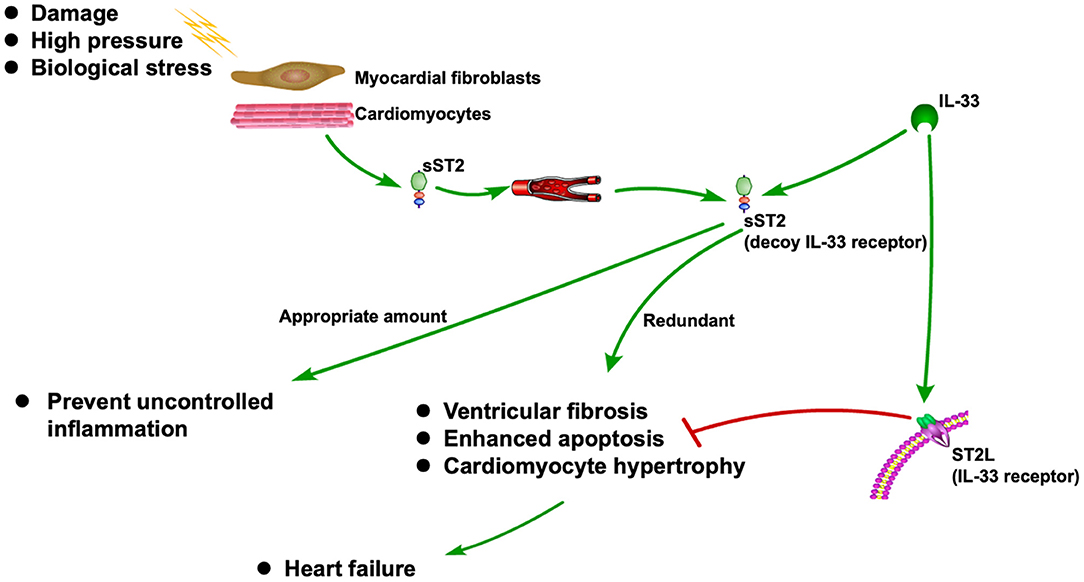
sST2 is released when cardiomyocytes stretch, neutralizing its ligand IL-33 (13, 14). It is also associated with inflammation during the MI and HF (15). As a decoy receptor of IL-33 receptor (ST2L), an appropriate amount of sST2 can prevent uncontrolled inflammation. However, redundant sST2 could also block the advantageous biological effect of IL-33 due to its competitive role against ST2L, which will eventually cause HF (15). Recently, sST2 is frequently reported to be associated with CVDs, especially HF (16–18). The ST2 pathway in CVD is shown in Figure 1.
However, a meta-analysis of sST2 levels in different CVDs has not yet been conducted. Therefore, the present study aimed to investigate sST2 levels in patients with ischemic heart disease (IHD), MI, and HF and to explore the value of sST2 in the diagnosis and prognosis of CVD.
Materials and Methods
Literature Search Strategy
Two independent researchers comprehensively searched four online databases (PubMed, Embase, Cochrane, and Web of Science), with the latest search conducted on October 1, 2020. The keywords searched in PubMed included “IL1RL1 protein, human” combined with “myocardial ischemia”, “myocardial infarction”, and “heart failure”. The search strategy for PubMed was refined by using the following PubMed/MeSH terms: “IL1RL1 protein, human” [MeSH terms] or “ST2 protein, human” [abstract/title], and “Myocardial Ischemia” [MeSH terms], “Ischemic Heart Diseases” [abstract/title], “Myocardial Infarction” [MeSH terms], “Cardiovascular Stroke” [abstract/title], “Heart Failure” [MeSH terms], or “Cardiac Failure” [abstract/title]. In an effort to obtain maximum search results, we did not impose language restrictions during the search process.
Inclusion Criteria
The inclusion criteria for studies in the systematic review and meta-analysis included (1) case–control studies; (2) studies comparing ST2 serum concentrations between healthy humans and patients with HF or cardiac diseases; (3) studies with sufficient data provided to obtain the weighted mean difference (WMD), 95% confidence interval (95% CI), or p-values for outcomes; and (4) studies with no significant differences in sex, age, diabetes, and hypertension observed between the control and disease groups.
Exclusion Criteria
Studies were excluded if they contained any of the following exclusion criteria: (1) duplicated studies; (2) poor-quality studies; (3) uncontrolled studies or studies with control groups containing unhealthy samples; (4) studies not focusing on the diagnostic role of ST2 in HF or cardiac diseases; and (5) studies with insufficient data provided to obtain a WMD or 95% CI.
Data Abstraction and Quality Assessment
Data from the included studies included the first author's name, publication year, sample characteristics (unit, size, method, and age), mean value, and standard deviation (SD). If the study data were not reported as mean value ± SD, researchers would independently estimate the mean and SD based on the sample size, medium, maximum or minimum values, range, and interquartile ranges, based on studies by Luo et al. (19) and Wan et al. (20).
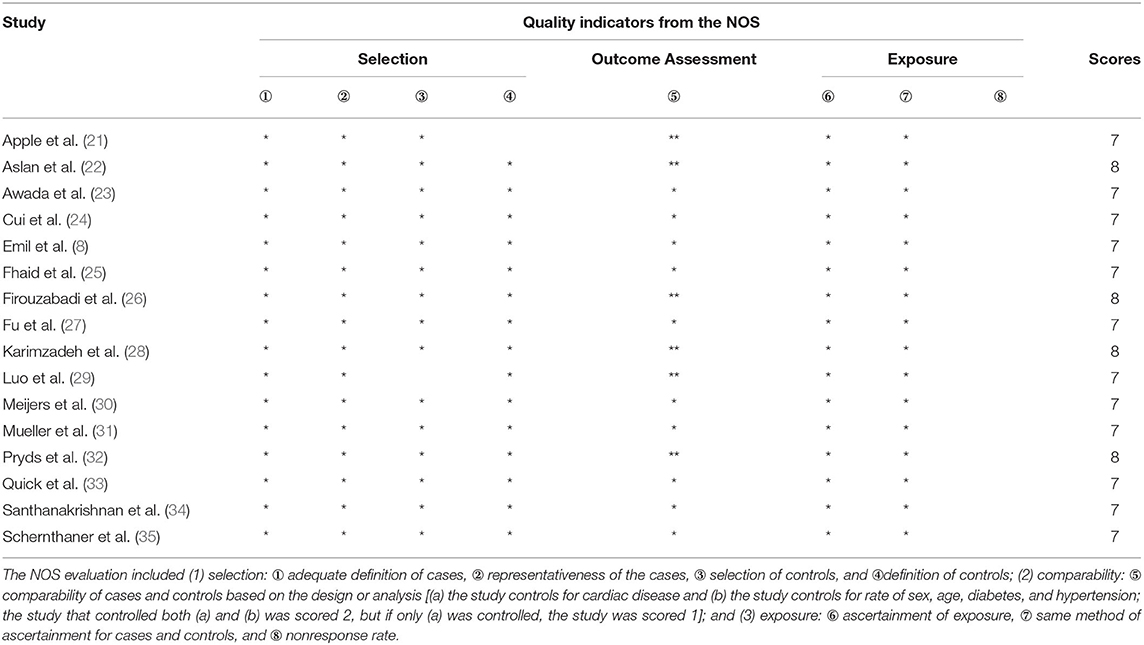
Quality assessment of the included studies was conducted using the Newcastle–Ottawa Quality Assessment Scale (NOS), and studies with NOS scores ≥7 were considered of high quality. The assessment criteria were as follows:
(1) selection: ① adequate definition of cases, ② representativeness of the cases, ③ selection of controls, and ④ definition of controls;
(2) comparability: ⑤ comparability of cases and controls based on the design or analysis; and
(3) exposure: ⑥ ascertainment of exposure, ⑦ same method of ascertainment for cases and controls, and ⑧ nonresponse rate.
Statistical Analysis
The data were divided into three groups for statistical analysis: serum sST2 levels in IHD (group 1); serum sST2 levels in MI (group 2); and serum sST2 levels in HF (group 3). Within group 3, two subgroups were identified: serum sST2 levels in HF measured by ELISA (subgroup 3.1) and serum sST2 levels in HF measured using the Presage® ST2 assay kit or commercially available immunoassays (subgroup 3.2).
Review Manager (RevMan) software version 5.3 (The Cochrane Collaboration, 2014) was used to perform the meta-analysis. WMD and 95% CI were used between groups. Heterogeneity was evaluated using Cochran's Q test and Higgins I2, and significant heterogeneity was shown when p < 0.05 and/or I2 > 50%. A random-effect model was applied if significant heterogeneity was determined; if not, a fixed-effect model was used.
Receiver Operating Characteristic Curve
The mean sST2 concentration values for each study in group 3 and subgroup 3.2 were used to plot ROC curves using IBM SPSS Statistics version 26.0 software for Mac (IBM Corp., Armonk, NY, USA).
Results
Included Studies
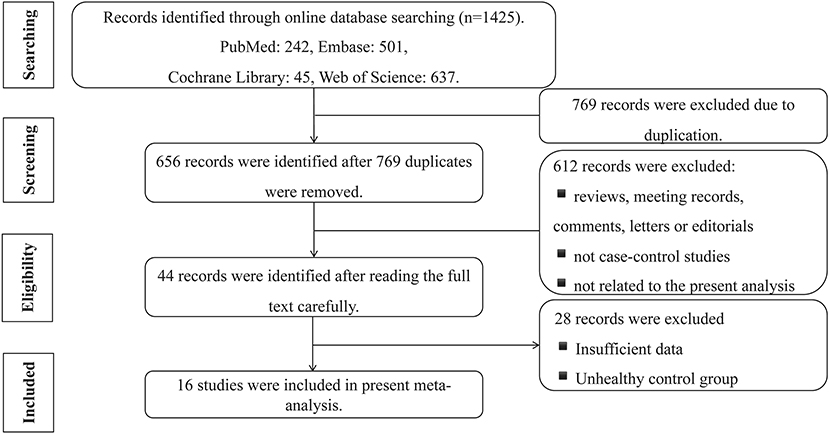
A total of 1,425 studies were searched, comprising 242 from PubMed, 501 from Embase, 45 from Cochrane Library, and 637 from Web of Science. A total of 769 studies were excluded because they duplicated other studies. After reading of the title and abstract, according to the exclusion criteria, 612 studies were removed because the studies were (a) reviews, meeting records, comments, letters, or editorials; (b) not case–control studies; and (c) not related to the present meta-analysis (focused on other diseases, the prognosis of sST2, etc.) Among the remaining 44 studies, two independent researchers read the full studies and excluded 28 records due to insufficient data or the inclusion of control groups with unhealthy individuals. Finally, 16 studies were included in the present meta-analysis (Figure 2).
Characteristics of the Included Literature
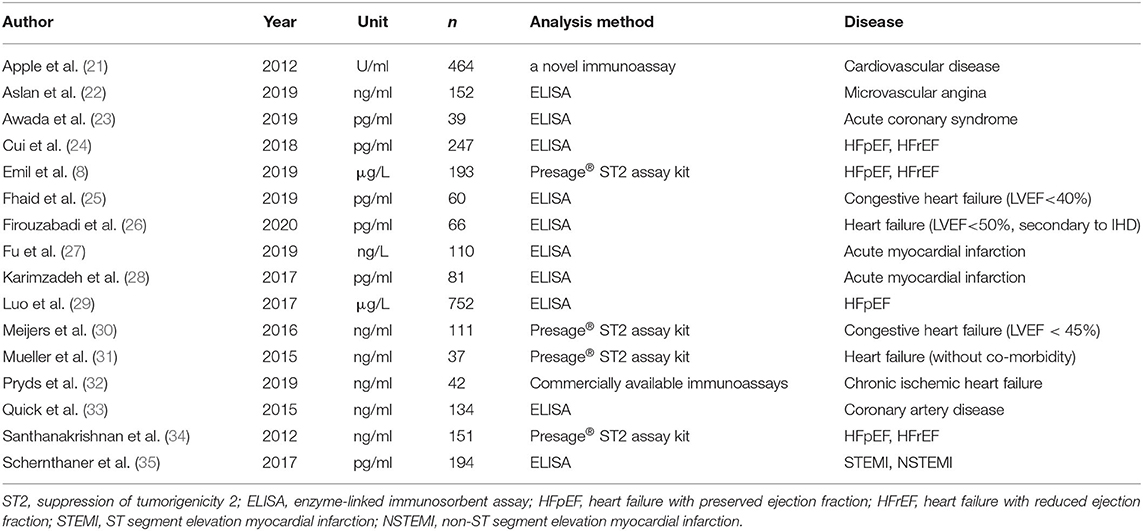
All details of the 16 studies are presented in Table 1. Among the 16 studies, seven focused on IHD (21–23, 27, 28, 33, 35), among which four focused on MI (23, 27, 28, 35), and the others focused on HF. Four studies (30, 31, 34, 36) used a Presage® ST2 assay kit to measure serum sST2 levels, while the remaining studies used ELISA or other immunoassays to complete this task. Most of the studies used weight/volume as the unit of measurement for serum sST2 levels, while Apple et al. used U/ml as the measurement unit (21). Therefore, in the meta-analysis of group 1, standard mean difference (SMD) was used to evaluate the results, thus eliminating the effect of different measurement units used in the various studies. WMD was used to evaluate the effect on the original measurement units for studies in groups 2 and 3. The studies were separated into three groups, as described above, and two subgroups in group 3 based on the method used to determine serum sST2 levels. The NOS scores of all 16 studies were ≥7, indicating that all included studies were of high quality (Table 2).
Meta-Analysis
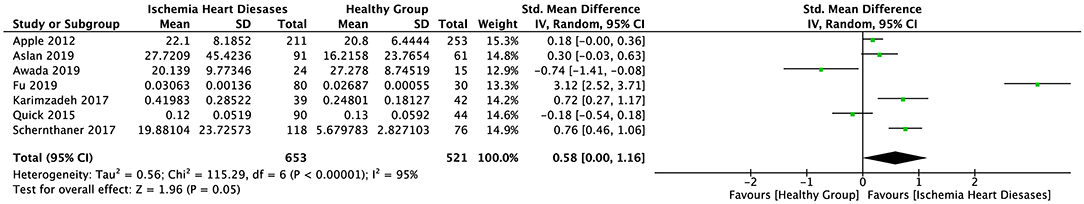
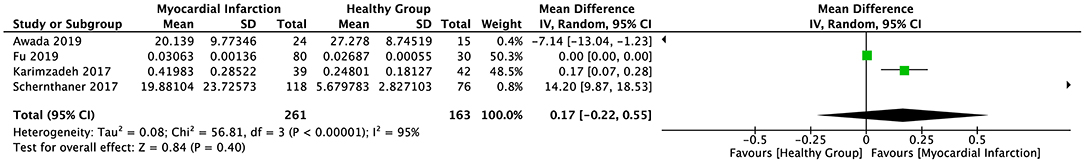
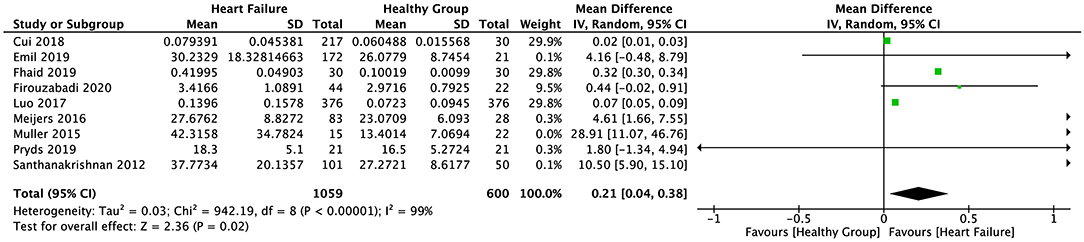
The random-effect model was applied to all three groups due to the presence of significant heterogeneity. No statistical difference was found in the serum sST2 levels between healthy individuals and IHD patients in group 1 (SMD = 0.58, 95% CI = 0.00–1.16, p = 0.05; I2 = 95%, p < 0.00001) (Figure 3) or MI patients in group 2 (WMD = 0.17, 95% CI = −0.22–0.55, p = 0.40; I2 = 95%, p < 0.00001) (Figure 4). However, a statistical difference was determined between the serum sST2 levels of HF patients and healthy individuals in group 3 (WMD = 0.21, 95% CI = 0.04–0.38, p = 0.02; I2 = 99%, p < 0.00001) (Figure 5). Interestingly, no statistical difference was found in subgroup 3.1 (WMD = 0.16, 95% CI = −0.01–0.33, p = 0.06; I2 = 100%, p < 0.00001), whereas a statistical difference was determined in subgroup 3.2 (WMD = 6.17, 95% CI = 2.07–10.28, p = 0.003; I2 = 76%, p = 0.002) (Figure 6).
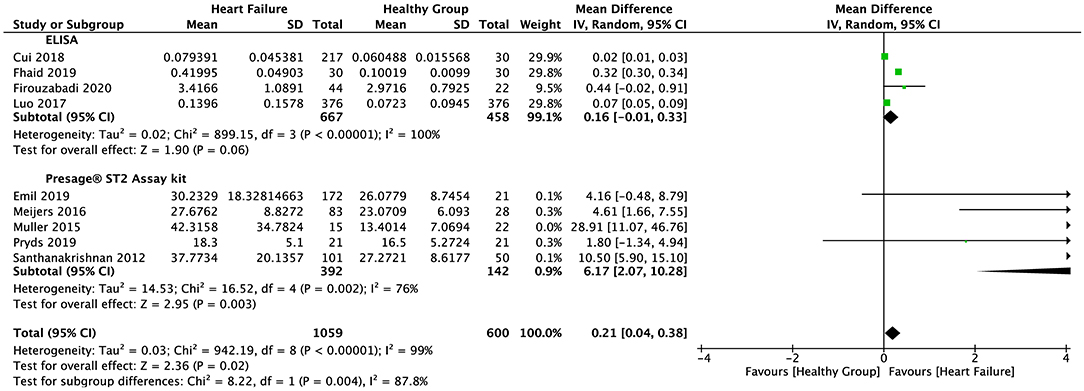
Figure 6. Forest plot of sST2 levels between HF and healthy subgroups with different assay methods (ELISA and Presage® ST2 assay kit).
ROC Curves
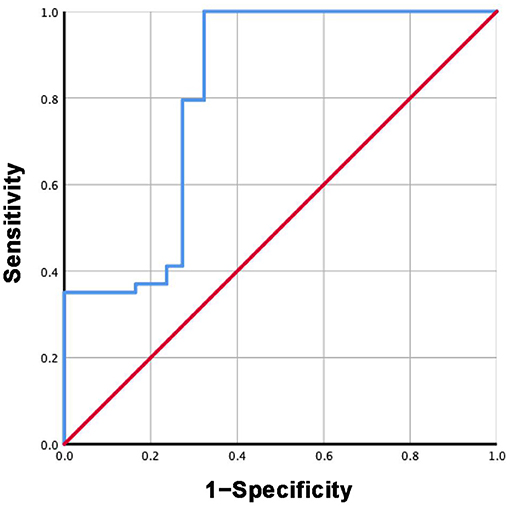
Based on the mean sST2 concentrations reported in each study in group 3 and subgroup 3.2, the specificity and sensitivity of ST2 were calculated. The ROC curves are shown in Figures 7, 8. For group 3, the area under the curve (AUC) is 0.816, p = 0.000, 95% CI = 0.792–0.840. For subgroup 3.2, the AUC is 0.963, p = 0.000, 95% CI = 0.947–0.979, with a cutoff value of 27.4742 ng/ml when the sensitivity was 0.946 and the specificity was 1.000.
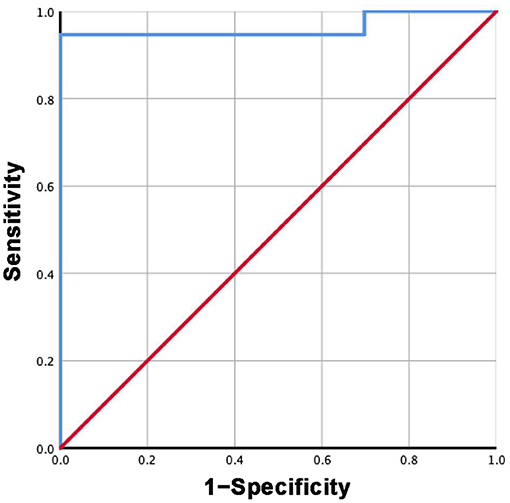
Figure 8. ROC curve of sST2 levels between HF and healthy groups measured by Presage® ST2 assay kit.
Discussion
Biomarkers have made a significant contribution to the diagnosis and prognosis of disease. Although strongly associated with inflammatory and autoimmune diseases, ST2 has also been found to play a role in the diagnosis and prognosis of CVD (11, 36). As one of the isoforms of ST2, sST2 has recently become a promising prognostic indicator for patients diagnosed with HF and a useful tool for risk stratification (37). Due to its prognostic value, sST2 was recommended by the American College of Cardiology Foundation (ACC)/American Heart Association (AHA) as an important biomarker for monitoring HF patients in 2013 (38).
To clearly distinguish sST2 levels in various CVDs, the present study specifically analyzed sST2 levels in patients with IHD, MI, and HF. Among the included studies, seven focused on IHD, which is a disease in which cardiomyocytes do not receive sufficient blood and oxygen supply. Four of these seven studies focused on MI, in which irreversible necrosis of cardiomyocytes occurs as a result of severe ischemia. To investigate sST2 levels in MI patients, we performed a separate meta-analysis of these four studies (group 2).
The meta-analysis findings indicated that serum sST2 levels did not differ significantly in patients with IHD or MI compared with healthy individuals but were remarkably increased in patients with HF. Herein, the biological character of the ST2 molecule; its involvement in heart disease pathophysiology; the diagnostic, prognostic, and treatment values of ST2; and the limitations of the study will be discussed in order to support the application of this biomarker in a clinical setting.
Molecular Biological Characteristic of ST2
ST2 is a member of the Toll-like/IL-1 receptor (TIR) superfamily, the members of which are defined by a common intracellular domain, the TIR domain (32). The functional ligand of ST2 is IL-33. sST2 can also directly bind to IL-33 and act as a decoy receptor, inhibiting membrane-bound ST2 and subsequent signal transduction (22). sST2 is mainly produced by myocardial fibroblasts and stressed or injured cardiomyocytes and is also expressed in mast cells, epithelial cells, smooth muscle cells, and endothelial cells from the cardiac and microvascular systems (39, 40).
The Framingham Heart Study found that age and sex are important determinants of normal sST2 levels (41). Specifically, sex has a potentially crucial influence on sST2, in that female sST2 levels are lower than those of males at corresponding ages (42), which may be influenced by sex hormones (43). In addition, sST2 secretion follows a diurnal rhythm, with lower values in the morning, a peak in the afternoon, and lowest levels at night (43). Understanding the diurnal rhythm and sex differences affecting sST2 expression can help avoid false negatives in prognostic prediction.
Therefore, based on the above characteristics of sST2, relevant inclusion criteria were determined to avoid statistical differences in age and sex between the disease and control groups during the literature screening process. Meanwhile, the exclusion of studies with unhealthy individuals in the control group was strictly followed.
sST2 Involvement in Heart Disease Pathophysiology
Cardiomyocyte Hypertrophy and Cardiac Fibrosis
Studies have shown that the IL-33/ST2 signaling pathway is a biomechanical activation system closely associated with cardiomyocyte hypertrophy and cardiac fibrosis (44). When cardiomyocytes stretch, sST2 is released, neutralizing its ligand IL-33, which is a key component in preventing myocardial fibrosis and hypertrophy (13, 14). Any change in the geometry or load conditions of the heart, such as MI, hypertension, and valvular heart disease, may change the mechanical strain imposed on a single cardiomyocyte, leading to cardiomyocyte hypertrophy, enhanced extracellular protein deposition (ventricular fibrosis), and eventually HF (45–47). As mentioned above, sST2 is positively correlated with cardiomyocyte hypertrophy and cardiac fibrosis. The increased wall thickness in hypertension tends to diminish wall stress and decrease oxygen demand. Cardiomyocyte hypertrophy is the most important of the pathophysiological changes in the heart, eventually contributing to ventricular wall thickening and stiffening (48). Therefore, both cardiomyocyte hypertrophy and cardiac fibrosis contribute to elevated serum sST2 levels in HF patients, which was corroborated by the current findings.
According to the present meta-analysis, no statistical differences in serum sST2 levels were found between the MI and IHD groups compared with the corresponding control groups. Remodeling occurs after MI, but expansion of the infarct zone is only found within 72 h after infarction. Beyond 72 h, remodeling via myocyte hypertrophy and alterations in ventricular architecture begin to affect post-infarction repair (49). The studies investigating MI and IHD included in the meta-analysis reported that blood samples were taken as soon as possible when patients were sent to the emergency room. Thus, increased sST2 levels may not have been observed within the hyperacute or acute phase of MI.
Inflammatory Processes
ST2L is a membrane-bound receptor of IL-33 located on the cardiomyocyte surface (50). Fibroblasts release IL-33, which prevents cardiomyocyte apoptosis and improves cardiac function through ST2 signaling in the myocardium under conditions of damage, high pressure, or biological stress (51). Even if sST2 protects against overload due to inflammatory injury, it could also block the advantageous biological effect of IL-33 because sST2 acts as a competitive inhibitor of ST2L (15). Therefore, high sST2 concentrations enhance apoptosis, cardiomyocyte hypertrophy, and cardiac fibrosis, which eventually cause irreversible damage after MI, leading to HF.
We speculate that higher serum sST2 concentrations after MI may cause a worse prognosis because sST2 blocks the beneficial effects of IL-33, such as reducing fibrosis and hypertrophy, preventing apoptosis, preserving ventricular function, and improving survival (52). Therefore, sST2 may be used to evaluate risk of death after MI.
Clinical Application
Diagnosis
As early as 2013, sST2 was recommended by ACC/AHA as a predictor of hospitalization and death in patients with chronic HF, in addition to BNP (38). The stability of sST2 (48 h at room temperature, 7 days at 4°C, and 2 months at −20°C or −80°C) reflects its suitability for clinical laboratory analysis (42). Some studies have reported an upper reference of 35 ng/ml for sST2 (48), which is higher than the cutoff value found in the current study (27.4742 ng/ml). According to the AUC values obtained in the present meta-analysis, the potential diagnostic value of sST2 in HF is considerable. However, as mentioned above, sex and diurnal rhythm (41–43) should be considered when setting the cutoff value for diagnosis during clinical application.
Moreover, some studies have reported that serum sST2 levels may be higher after MI, especially after 3–5 days (28, 34). However, serum sST2 levels did not differ significantly between patients with MI and healthy individuals in the present meta-analysis. Based on the pathophysiology of sST2 in the CVDs mentioned above, we speculate that sST2 levels might be elevated after the acute phase of MI, which warrants further confirmation. Other CVDs, such as hypertension and valvular heart disease, could also lead to higher sST2 levels; thus, differential diagnoses should be distinguished based on the sST2 level. In addition, elevated sST2 levels have been observed in multiple non-cardiac diseases, such as inflammation, asthma, fibroproliferative diseases, rheumatoid arthritis, autoimmune diseases, sepsis, and trauma (41, 53). Consequently, the diagnosis of HF requires comprehensive assessment. BNP (or NT-pro BNP) is a classic biomarker for the diagnosis of HF. Previous studies have reported that sST2 has a weaker predictive value than NT-proBNP in the diagnosis of HF (54, 55). However, BNP (or NT-pro BNP) has demonstrated weaker differentiating ability in HF and renal failure, while sST2 is hardly influenced by kidney function (56–58). Therefore, sST2 should be used together with BNP (or NT-pro BNP) as an auxiliary diagnostic marker to diagnose HF.
Interestingly, a statistical difference was found between HF patients and healthy individuals when serum sST2 levels were determined using different methods (Presage® ST2 assay kit versus ELISA). We speculate that different operational procedures employed to perform ELISA may cause large heterogeneity and bias, which may decrease the statistical power of meta-analysis. Use of a commercial kit, like the Presage® ST2 assay kit, eliminates differences in operational flow or reagents, enabling repeatable determination of sST2, thus minimizing heterogeneity and bias between laboratories and experiments. Therefore, we recommend that a commercial kit, such as Presage® ST2 assay kit, be employed to evaluate sST2 levels in both research and clinical settings. Furthermore, although we provided a cutoff value of 27.4742 ng/ml, based on the Presage® ST2 assay kit method, this value is only a preliminary value as only five studies were included in subgroup 3.2 and the original data of each individual was not accessible. Thus, a more accurate cutoff value should be investigated in future studies with larger sample sizes.
Prognosis
Even if no diagnostic value had been found in the present meta-analysis, the prognostic value of sST2 in MI and IHD has been clarified. In patients with AMI, elevated ST2 levels are often associated with poor prognosis (59). Measurement of sST2 early after AMI predicts left ventricle (LV) function and recovery after AMI, which may interlink the RAAS and IL-33/sST2 pathways (60). The prognostic value of sST2 in AMI patients has been demonstrated, but its pathogenesis requires further research.
For HF, sST2 has not only diagnostic but also prognostic value. In HF patients classified as chronic New York Heart Association (NYHA) classes III to IV, sST2 is an independent predictor of subsequent mortality (61). Some studies have demonstrated that sST2 levels have predictive value for mortality in HF patients in both the short and long term (62, 63). A higher sST2 concentration (upper reference limit of 35 ng/ml) provides a substantial predictive risk of cardiovascular events, such as progressive LV failure, hospitalization for HF, and death (64). It is worth noting that measuring sST2 combined with BNP or NT-proBNP is better than merely measuring NT-proBNP, because sST2 has a better predictive value than NT-proBNP in myocardial fibrosis and remodeling, which is a known indicator of HF severity (65, 66). Wu et al. also reported that the low biological variability of sST2 compared to BNP or NT-proBNP enables serial monitoring of sST2, which is preferred over single measurement (65).
The predictive role of sST2 in HF is somewhat limited in some situations. A study investigating cardiac resynchronization therapy (CRT) and markers and response to CRT (MARC) (67) reported that sST2 did not reflect changes in HF and LV ejection fluid (LVEF) before and after CRT. Further research is required to explore this phenomenon.
Treatment
Studies have shown that increased IL-33/ST2 signaling may induce heart protective effects, and MyD88 may represent a common point of ST2L and Toll-like receptor 4 (TLR-4) heart protection signaling. The following strategies can be applied: direct administration or enhanced IL-33 release from cardiac fibroblasts, administration of ST2L agonists or sST2 antagonists, or administration of an adaptor protein numerator (68–70). Unique cardiovascular targets may be discovered if precise cytoplasmic and nuclear effects can be elucidated. Experiments have shown that a short course of IL-33/ST2 can prevent cardiomyocyte apoptosis and improve the results of experimental MI without lung inflammation (53). Therefore, transient IL-33 treatment may improve cardiovascular outcomes and minimize adverse inflammatory reactions.
Limitations
Among the 16 studies included in the current meta-analysis, four measurement methods were used to evaluate serum sST2 levels: the Presage® ST2 assay kit, ELISA, commercially available immunoassays, and a novel immunoassay. Some researchers have reported that different methods may cause up to a 100-fold difference in results. Further, although the Presage® ST2 assay kit has been cleared by the U.S. Food and Drug Administration and has received the Conformitè Europèenne (CE) mark, ELISA remains a research-only method (42). Different methods yield different results, which may cause significant heterogeneity and weaken the statistical power of the analysis. This is one of the limitations of the present meta-analysis. Use of a commercial kit, such as the Presage® ST2 assay kit, would standardize the methodology and enable accurate comparisons between studies. Furthermore, one study used U/L as the measurement unit, which cannot be converted into ng/ml. SMD was used for this analysis, which weakens the value of the results for application in clinical diagnosis. Therefore, we suggest that researchers and doctors use the Presage® ST2 assay kit to evaluate the level of sST2 on both bench and bedside.
Although we provided a cutoff sST2 value at the optimal sensitivity/specificity, the sample and study sizes were small, which is also one of the limitations of the present study. Meanwhile, inaccessibility of the original data from each individual sST2 level may also have led to the same problem. The present analysis is thus a preliminary investigation on the topic; more studies and original data are required to determine a precise cutoff value for sST2.
Finally, only four and seven studies were included in the MI and IHD groups, respectively. The resulting error and bias cannot be ignored during the statistical analysis and discussion of the results. Meanwhile, p = 0.05 was applied in group 1, but we believe a statistical difference exists when p < 0.05. In both groups 1 and 2, the random-effect model was employed because high heterogeneity existed, which reduced the statistical power of the results. These factors may have caused a bias in the meta-analysis results. Therefore, more research assessing the diagnostic value of sST2 in MI and IHD is needed, which will lead to more accurate results than those presented here.
Conclusion
The results of the present meta-analysis of serum sST2 levels in different CVDs demonstrate that serum sST2 levels in HF patients are remarkably higher than those in healthy individuals. However, serum sST2 levels did not differ significantly between IHD or MI patients and healthy individuals. Therefore, we believe that ST2 can be used as an auxiliary diagnostic biomarker of HF.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZC contributed to the conception of the study. TZ and CX performed the meta-analyses and wrote the manuscript. RZ edited and revised the original manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant Number: 82002001), the Natural Science Foundation of Liaoning Province (Grant Number: 20180530004), and the Open Project of Forensic Pathology Key Laboratory, Ministry of Public Security of China (Grant Number: GABFYBL201804).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Skourtis D, Stavroulaki D, Athanasiou V, Fragouli PG, Iatrou H. Nanostructured polymeric, liposomal and other materials to control the drug delivery for cardiovascular diseases. Pharmaceutics. (2020) 12:1160. doi: 10.3390/pharmaceutics12121160
2. Hösch O, Ngyuen TT, Lauerer P, Schuster A, Kutty S, Staab W, et al. BNP and haematological parameters are markers of severity of Ebstein's anomaly: correlation with CMR and cardiopulmonary exercise testing. Eur Heart J Cardiovasc Imaging. (2015) 16:670–5. doi: 10.1093/ehjci/jeu312
3. Prontera C, Masotti S, Franzini M, Emdin M, Passino C, Zucchelli GC, et al. Comparison between BNP values measured in capillary blood samples with a POCT method and those measured in plasma venous samples with an automated platform. Clin Chem Lab Med. (2015) 53:e125–7. doi: 10.1515/cclm-2014-0873
4. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the gbd 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
5. Cao Z, Zhao M, Xu C, Zhang T, Jia Y, Wang T, et al. Diagnostic roles of postmortem ctn i and ctn t in cardiac death with special regard to myocardial infarction: a systematic literature review and meta-analysis. Int J Mol Sci. (2019) 20:3351. doi: 10.3390/ijms20133351
6. Cao Z, Zhao M, Xu C, Zhang T, Jia Y, Wang T, et al. Evaluation of agonal cardiac function for sudden cardiac death in forensic medicine with postmortem brain natriuretic peptide (BNP) and NT-proBNP: a meta-analysis. J Forensic Sci. (2020) 65:686–91. doi: 10.1111/1556-4029.14232
7. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of america, heart failure association of the european society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure: endorsed by canadian heart failure society, heart failure association of india, the cardiac society of Australia and New Zealand, and the Chinese heart failure association. Eur J Heart Fail. (2021) 23:352–80. doi: 10.1002/ejhf.2115
8. Najjar E, Faxén UL, Hage C, Donal E, Daubert J-C, Linde C, et al. ST2 in heart failure with preserved and reduced ejection fraction. Scand Cardiovasc J. (2019) 53:21–7. doi: 10.1080/14017431.2019.1583363
9. Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. (1989) 258:301–4. doi: 10.1016/0014-5793(89)81679-5
10. Klemenz R, Hoffmann S, Werenskiold AK. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc Natl Acad Sci U S A. (1989) 86:5708–12. doi: 10.1073/pnas.86.15.5708
11. Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. (2001) 164:277–81. doi: 10.1164/ajrccm.164.2.2008120
12. Dieplinger B, Mueller T. Soluble ST2 in heart failure. Clin Chim Acta. (2015) 443:57–70. doi: 10.1016/j.cca.2014.09.021
13. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. (2002) 106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
14. Parikh RH, Seliger SL, Christenson R, Gottdiener JS, Psaty BM, deFilippi CR. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community-dwelling population. J Am Heart Assoc. (2016) 5:e003188. doi: 10.1161/JAHA.115.003188
15. Marino R, Magrini L, Orsini F, Russo V, Cardelli P, Salerno G, et al. Comparison between soluble ST2 and high-sensitivity troponin i in predicting short-term mortality for patients presenting to the emergency department with chest pain. Ann Lab Med. (2017) 37:137–46. doi: 10.3343/alm.2017.37.2.137
16. Dudek M, Kałuzna-Oleksy M, Migaj J, Straburzyńska-Migaj E. Clinical value of soluble ST2 in cardiology. Adv Clin Exp Med. (2020) 29:1205–10. doi: 10.17219/acem/126049
17. Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupón J, Latini R, et al. sST2 Predicts outcome in chronic heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol. (2018) 72:2309–20. doi: 10.1016/j.jacc.2018.08.2165
18. McCarthy CP, Januzzi JL Jr. Soluble ST2 in Heart Failure. Heart Fail Clin. (2018) 14:41–8. doi: 10.1016/j.hfc.2017.08.005
19. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
20. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
21. Apple FS, Steffen LM, Pearce LA, Murakami MM, Luepker RV. Increased cardiac troponin i as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: The minnesota heart survey. Clin Chem. (2012) 58:930–5. doi: 10.1373/clinchem.2011.179176
22. Aslan G, Polat V, Bozcali E, Opan S, Çetin N, Ural D. Evaluation of serum sST2 and sCD40L values in patients with microvascular angina. Microvasc Res. (2019) 122:85–93. doi: 10.1016/j.mvr.2018.11.009
23. Awada A, Unger P. Potential involvement of the IL-33/ST2 axis in the pathogenesis of tatosubo syndrome. Acta Cardiol. (2019) 74:10. doi: 10.1136/annrheumdis-2012-203187
24. Cui Y, Qi X, Huang A, Li J, Hou W, Liu K. Differential and Predictive Value of Galectin-3 and Soluble Suppression of Tumorigenicity-2 (sST2) in Heart Failure with Preserved Ejection Fraction. Med Sci Mon Int Med J Exp Clin Res. (2018) 24:5139–46. doi: 10.12659/MSM.908840
25. Fhaid NW, Mostafa TM, Ibrahim OM, Ashmawy MM. Correlation between sST2, IL-34 and mortality in CHF Egyptian patients. Int J Pharm Sci Rev Res. (2019) 54:37–44.
26. Firouzabadi N, Dashti M, Dehshahri A, Bahramali E. Biomarkers of IL-33 and SST2 and lack of association with carvedilol therapy in heart failure. Clin Pharmacol. (2020) 12:53–8. doi: 10.2147/CPAA.S256290
27. Fu ZQ, Gao WQ, Zhang FY, Tan GJ, Han CG, Liu YX, et al. Lymphocyte GRK2 as a significant predictor for heart failure among elderly patients with acute non-ST segment elevation myocardial infarction. J Am Geriatr Soc. (2019) 67:S628. doi: 10.1111/jgs.16120
28. Karimzadeh S, Zamani S, Amirzade-Fard F, Ardekani AA, Doroudchi M. Correlation between IL-33 and sST2 levels in sera of patients with acute myocardial infarction. Biomed Res-India. (2017) 28:7027–34.
29. Luo NS, Zhang HF, Liu PM, Lin YQ, Huang TC, Yang Y, et al. Diagnostic value of combining serum soluble ST2 and interleukin-33 for heart failure patients with preserved left ventricular ejection fraction. Zhonghua Xin Xue Guan Bing Za Zhi. (2017) 45:198–203. doi: 10.3760/cma.j.issn.0253-3758.2017.03.006
30. Meijers WC, van der Velde AR, Muller Kobold AC, Dijck-Brouwer J, Wu AH, Jaffe A, et al. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail. (2017) 19:357–65. doi: 10.1002/ejhf.669
31. Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin Chim Acta. (2015) 445:155–60. doi: 10.1016/j.cca.2015.03.033
32. Pryds K, Rahbek Schmidt M, Bjerre M, Thiel S, Refsgaard J, Bøtker HE, et al. Effect of long-term remote ischemic conditioning on inflammation and cardiac remodeling. Scand Cardiovasc J: SCJ. (2019) 53:183–91. doi: 10.1080/14017431.2019.1622770
33. Quick S, Waessnig NK, Kandler N, Poitz DM, Schoen S, Ibrahim K, et al. Soluble ST2 and myocardial fibrosis in 3T cardiac magnetic resonance. Scand Cardiovasc J: SCJ. (2015) 49:361–6. doi: 10.3109/14017431.2015.1076936
34. Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. (2012) 14:1338–47. doi: 10.1093/eurjhf/hfs130
35. Schernthaner C, Lichtenauer M, Wernly B, Paar V, Pistulli R, Rohm I, et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Invest. (2017) 47:638–48. doi: 10.1111/eci.12785
36. AbouEzzeddine OF, McKie PM, Dunlay SM, Stevens SR, Felker GM, Borlaug BA. ST2 in Heart Failure With Preserved Ejection Fraction. Circulation. (2015) 132.
37. Boisot S, Beede J, Isakson S, Chiu A, Clopton P, Januzzi J, et al. Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Card Fail. (2008) 14:732–8. doi: 10.1016/j.cardfail.2008.06.415
38. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. (2013) 128:e240–327. doi: 10.1161/CIR.0b013e31829e8776
39. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. (2005) 23:479–90. doi: 10.1016/j.immuni.2005.09.015
40. Villacorta H, Maisel AS. Soluble ST2 testing: a promising biomarker in the management of heart failure. Arq Bras Cardiol. (2016) 106:145–52. doi: 10.5935/abc.20150151
41. Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. (2012) 58:1673–81. doi: 10.1373/clinchem.2012.192153
42. Dieplinger B, Januzzi JL, Steinmair M, Gabriel C, Poelz W, Haltmayer M, et al. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma — The Presage™ ST2 assay. Clinica Chimica Acta. (2009) 409:33–40. doi: 10.1016/j.cca.2009.08.010
43. Crnko S, Printezi MI, Jansen TPJ, Leiteris L, van der Meer MG, Schutte H, et al. Prognostic biomarker soluble ST2 exhibits diurnal variation in chronic heart failure patients. ESC Heart Failure. (2020) 7:1224–33. doi: 10.1002/ehf2.12673
44. Yao HC, Li XY, Han QF, Wang LH, Lou T, Zhou YH, et al. Elevated serum soluble ST2 levels may predict the fatal outcomes in patients with chronic heart failure. Int J Cardiol. (2015) 186:303–4. doi: 10.1016/j.ijcard.2015.03.269
45. Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. (2005) 2:209–16. doi: 10.1038/ncpcardio0158
46. Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. (1997) 59:551–71. doi: 10.1146/annurev.physiol.59.1.551
47. McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. (2005) 115:538–46. doi: 10.1172/JCI24144
48. Dattagupta A, Immaneni S. ST2: Current status. Indian Heart J. (2018) 70:S96–101. doi: 10.1016/j.ihj.2018.03.001
49. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. (2000) 101:2981–8. doi: 10.1161/01.CIR.101.25.2981
50. De la Fuente M, MacDonald TT, Hermoso MA. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. (2015) 26:615–23. doi: 10.1016/j.cytogfr.2015.07.017
51. Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. (2009) 2:684–91. doi: 10.1161/CIRCHEARTFAILURE.109.873240
52. Homsak E, Gruson D. Soluble ST2: A complex and diverse role in several diseases. Clinica Chimica Acta. (2020) 507:75–87. doi: 10.1016/j.cca.2020.04.011
53. Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. (2008) 7:827–40. doi: 10.1038/nrd2660
54. Dieplinger B, Gegenhuber A, Haltmayer M, Mueller T. Evaluation of novel biomarkers for the diagnosis of acute destabilised heart failure in patients with shortness of breath. Heart. (2009) 95:1508–13. doi: 10.1136/hrt.2009.170696
55. Henry-Okafor Q, Collins SP, Jenkins CA, Miller KF, Maron DJ, Naftilan AJ, et al. Soluble ST2 as a Diagnostic and Prognostic Marker for Acute Heart Failure Syndromes. Open Biomark J. (2012) 2012:1–8. doi: 10.2174/1875318301205010001
56. Homšak E. ST2-a new prognostic marker on the heart failure scene. Clin Chem Lab Med. (2016) 54:eA140.
57. Jin XL, Huang N, Shang H, Zhou MC, Hong Y, Cai WZ, et al. Diagnosis of chronic heart failure by the soluble suppression of tumorigenicity 2 and N-terminal pro-brain natriuretic peptide. J Clin Lab Anal. (2018) 32:e22295. doi: 10.1002/jcla.22295
58. Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Invest. (2014) 44:303–8. doi: 10.1111/eci.12234
59. Zhang K, Zhang XC Mi YH, Liu J. Predicting value of serum soluble ST2 and interleukin-33 for risk stratification and prognosis in patients with acute myocardial infarction. Chin Med J (Engl). (2013) 126:3628–31. doi: 10.3760/cma.j.issn.0366-6999.20130145
60. Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. (2010) 55:243–50. doi: 10.1016/j.jacc.2009.08.047
61. Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. (2003) 107:721–6. doi: 10.1161/01.CIR.0000047274.66749.FE
62. Januzzi JL. Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. (2007) 50:607–13. doi: 10.1016/j.jacc.2007.05.014
63. O'Meara E, Prescott MF, Claggett B, Rouleau JL, Chiang LM, Solomon SD, et al. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM-HF trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail. (2018) 11:e004446. doi: 10.1161/CIRCHEARTFAILURE.117.004446
64. Januzzi JL, Pascual-Figal D, Daniels LB. ST2 Testing for chronic heart failure therapy monitoring: the international ST2 consensus panel. Am J Cardiol. (2015) 115:70B–5B. doi: 10.1016/j.amjcard.2015.01.044
65. Wu AHB, Wians F, Jaffe A. Biological variation of galectin-3 and soluble ST2 for chronic heart failure: Implication on interpretation of test results. Am Heart J. (2013) 165:995–9. doi: 10.1016/j.ahj.2013.02.029
66. Lopes D, Menezes Falcão L. Mid-regional pro-adrenomedullin and ST2 in heart failure: Contributions to diagnosis and prognosis. Revista Portuguesa de Cardiologia. (2017) 36:465–72. doi: 10.1016/j.repc.2016.11.009
67. Maass AH, Vernooy K, Wijers SC., van't Sant J, Cramer MJ, Meine M, et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the Markers and Response to CRT (MARC) study. EP Europace. (2018) 20:e1–10. doi: 10.1093/europace/euw445
68. Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. (2008) 102:257–64. doi: 10.1161/CIRCRESAHA.107.158220
69. Riad A, Jäger S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immun (Baltimore, Md: 1950). (2008) 180:6954–61. doi: 10.4049/jimmunol.180.10.6954
Keywords: sST2, meta-analysis, cardiovascular diseases, biomarker, heart failure
Citation: Zhang T, Xu C, Zhao R and Cao Z (2021) Diagnostic Value of sST2 in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:697837. doi: 10.3389/fcvm.2021.697837
Received: 20 April 2021; Accepted: 24 June 2021;
Published: 23 July 2021.
Edited by:
Christian Schulte, University Heart and Vascular Center Hamburg (UHZ), GermanyReviewed by:
Alexander H. Maass, University Medical Center Groningen, NetherlandsDonato Santovito, Hospital of the University of Munich, Germany
Copyright © 2021 Zhang, Xu, Zhao and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Zhao, cnpoYW9AY211LmVkdS5jbg==; Zhipeng Cao, enBjYW9AY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tianyi Zhang
Tianyi Zhang Chengyang Xu
Chengyang Xu Rui Zhao1,2*
Rui Zhao1,2*