- 1Department of Cardiology, Yueyang Hospital Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China
Background and Objectives: Acquired coronary fistulas (ACFs) are rare coronary artery abnormalities in patients with chronic total occlusion (CTO). It has been found after revascularization, and it may cause fluster during the CTO percutaneous coronary intervention (CTO PCI). How to distinguish between ACFs and coronary perforation (CP) is very important for CTO operators. Chronic total occlusion reopening may reveal the microchannel of the adventitial vascular layers. Some of ACFs have been seen after revascularization. This study aimed to investigate the characteristics of ACFs after successful CTO PCI.
Methods: The clinical and procedural characteristics, medical history, and findings in electrocardiography (ECG), echocardiography, and coronary angiography were collected from 2,169 consecutive patients undergoing CTO PCI between January 2018 and December 2019 and analyzed retrospectively.
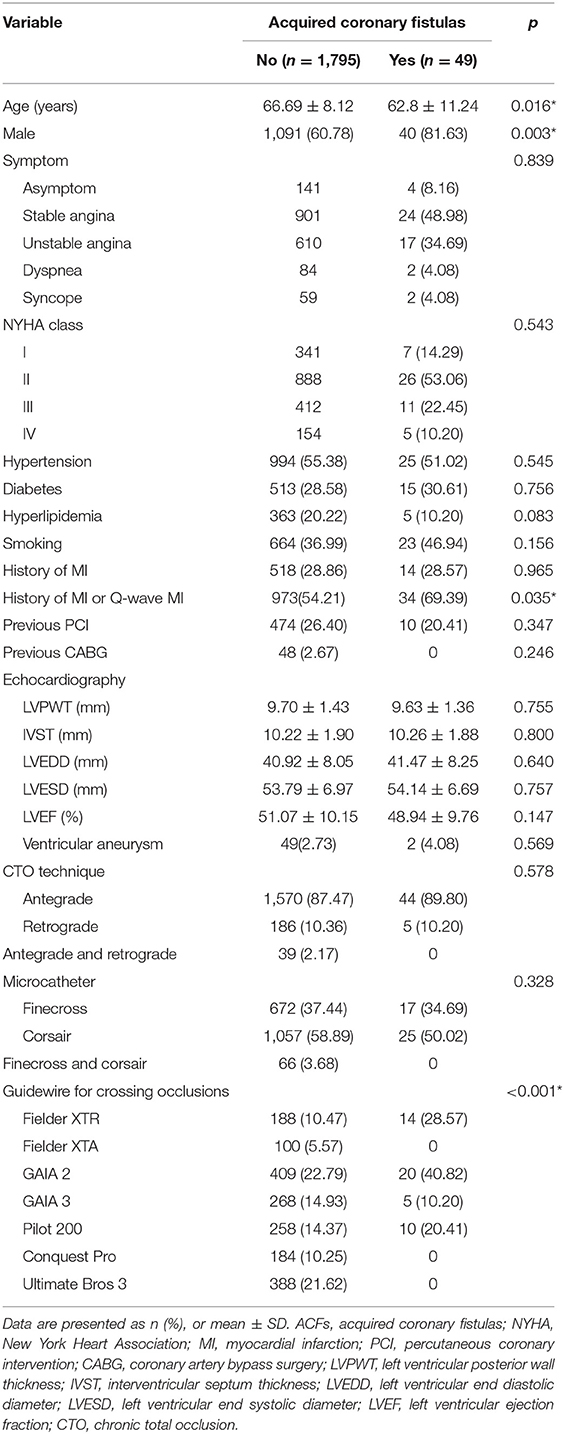
Results: About 1,844 (85.02%) underwent successful CTO PCI with complete revascularization. Acquired coronary fistulas were found in 49 patients (2.66%): the majority of patients with ACFs were men (81.63 vs. 60.78%; p = 0.016) and younger (62.8 vs. 66.69 years; p = 0.003), and had a history of myocardial infarction (MI) or Q-wave (69.39 vs. 54.21%; p = 0.035); 38 (77.55%) patients had multiple fistulas (≥3), and ACFs affected multiple branches of the CTO vessel (≥3) in 29 (59.18%) patients. None had pericardial effusion, tamponade, and hemodynamic abnormality before or after PCI.
Conclusion: Acquired coronary fistulas after successful CTO PCI are mainly present in young and male patients with a history of MI, and they often involve multiple fistulas and distal CTO vessels.
Introduction
Coronary fistulas were first described as congenital and abnormal vascular connections between coronary arteries and cardiac chambers or with other vessels, but other factors may also cause coronary fistulas (1), including trauma, surgery, severe coronary atherosclerosis, and myocardial infarction (MI). Myocardial infarction is a common cause of acquired coronary fistulas (ACFs) (2, 3), and ACFs secondary to MI are usually harmless, common in patients with chronic total occlusion (CTO), and hard to identify before complete revascularization because of insufficient collateral filling. In recent years, the increasing percutaneous coronary intervention (PCI) of CTO is done successfully with new equipment and techniques, and more ACFs can be visualized after CTO percutaneous coronary intervention (CTO PCI). It is important to distinguish ACF from coronary perforation (CP), a rare but potentially serious complication in PCI, which can lead to pericardial effusion and tamponade, often necessitating medical treatment and even emergency pericardiocentesis or cardiac surgery (4). To date, the imaging characteristics, medical history, and predictors of ACFs have never been evaluated in a real-word cohort of patients who underwent successful CTO revascularization. This study was undertaken to investigate the prevalence and characteristics of ACFs after successful revascularization of CTO.
Methods
Patients
A total of 2,169 consecutive patients who underwent CTO PCI at ZhongShan Hospital of Fudan University between January 2018 and December 2019 were retrospectively reviewed. Patients were excluded if the PCI was undertaken in the case of acute coronary syndrome. The patients with CP were also excluded because some of them failed to complete revascularization. All original angiograms were reviewed by two physicians to confirm ACFs and to collect other clinical information. The study was approved by the Institutional Review Board (KYSKSSB2020-135).
Procedures
All patients received antiplatelet treatment before the procedures, and unfractionated heparin was administered intravenously at 100 IU/kg followed by further addition of heparin as necessary to achieve a target activated clotting time of 250–350 s. Microcatheters include Finecross (Terumo, Japan) and Corsair (Asahi, Japan); CTO guidewires include Fielder XTR and Fielder XTA (Asahi, Japan), Gaia 2 and 3 (Asahi, Japan), Pilot 150 and 200 (Abbott, America), Conquest Pro (Asahi, Japan), Ultimate Bros 3 (Asahi, Japan), which had been used widely during the procedures.
Study Definitions
Chronic total occlusion was defined as a complete occlusion with thrombolysis in MI (TIMI) flow grade 0 antegrade for ≥3 months (5). Procedural success was defined as <50% residual stenosis with antegrade TIMI flow grade 3 at the end of procedures (6). Acquired Coronary Fistulas was defined as an abnormal connection between coronary arteries and cardiac chambers or with other vessels, secondary to exogenous or endogenous injury. History of MI was identified by medical records, including stent thrombosis-segment elevation MI (STEMI) or non-STEMI (NSTEMI) based on initial electrocardiography (ECG) and clinical and laboratory findings. Adverse events included the death of any cause, stent thrombosis (ST)/Q-wave MI, emergent cardiac surgery, and cardiac tamponade. The coronary collaterals in CTO were classified using the Rentrop grade as follows (7): 0 = none; 1 = filling of side branches of the artery to be dilated via collateral channels without visualization of the epicardial segment; 2 = partial epicardial filling of the occluded artery; and 3 = complete epicardial filling of the occluded artery.
Data Collection
Demographic, procedural, and medical data were obtained by reviewing the catheterization laboratory database and medical records of patients.
Statistical Analysis
Continuous variables are presented as mean ± SD and were compared using Student's or the Welch's t-test, and one-way ANOVA followed by Dunnett's multiple comparisons tests when more than two groups were compared. Categorical variables are presented as numbers and percentages and were compared using the chi-square test or Fisher's exact test. All analyses were performed using SPSS version 26, and a value of p < 0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics
Among the 2,169 consecutive patients who underwent CTO PCI, procedures were successfully conducted in 1,844 (85.01%) patients. Among them, ACFs were found in 49 (2.66%) patients: 25 (51.02%) patients had hypertension, 15 (30.61%) had diabetics, and five (10.20%) had hyperlipidemia; 23 (46.94%) patients were long-term smokers; 10 (20.41%) had a history of prior PCI, and none had previous coronary artery bypass surgery (CABG). As shown in Table 1, patients with ACFs were younger (62.8 vs. 66.69 years; p = 0.003), men (81.63 vs. 60.78%; p = 0.016), and had higher rates of MI history or Q-wave MI (69.39 vs. 54.21%; p = 0.035). Fielder XTR and GAIA 2 were commonly used to cross the lesion in those patients.
Clinical Symptoms
The most common complaint was angina (41, 83.67%), followed by asymptom (4, 8.16%), dyspnea (2, 4.08%), and syncope (2, 4.08%). By New York Heart Association (NYHA) classification, seven patients were classified as class I, 26 as class II, 11 as class III, and five as class IV. There was no statistical difference with respect to the entire patients with CTO.
Electrocardiography and Echocardiography
As shown in Figure 1, among 49 patients with ACFs, ECG displayed Q-waves in 31 (63.26%) patients, normal in 10 (20.41%) patients, and ST-T changes (depressed ST segment; low and flat T wave) in eight (16.33%) patients; 47 (95.92%) patients had sinus rhythm, and two (4.08%) had atrial fibrillation (AF).
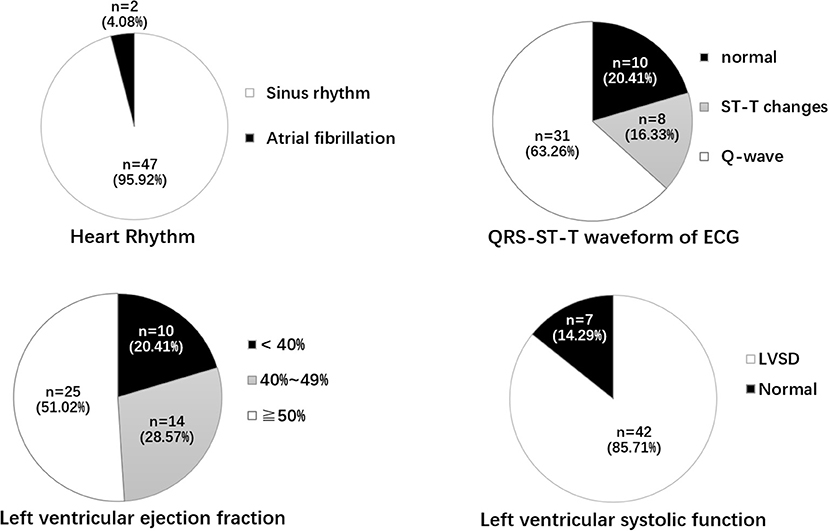
Figure 1. Heart rhythm, QRT-ST-T waveform of ECG, LVEF, and left ventricular systolic function. ECG, electrocardiogram; ST-T change includes depressed ST segment, Low and flat T wave; LVSD, Left ventricular systolic dysfunction; LVEF, left ventricular ejection fraction.
Echocardiographic examination was performed in all the patients. The mean left ventricular ejection fraction (LVEF) was 48.94 ± 9.76%; the LVEF was <40% in 10 (20.41%) patients, 40–49% in 14 (28.57%), and ≥50% in 25 (51.02%). Forty-two (85.71%) patients had left ventricular systolic dysfunction (LVSD), and two (4.08%) had ventricular aneurysms. The mean left ventricular posterior wall thickness (LVPWT), interventricular septum thickness (IVST), left ventricular end-diastolic diameter (LVEDD), and left ventricular end-systolic diameter (LVESD) are shown in Table 1. Pericardial effusion and tamponade were not found in patients with ACFs.
Coronary Angiography
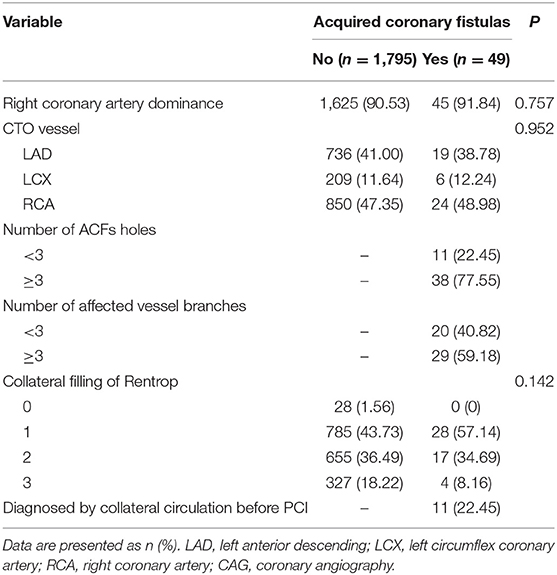
The angiographic characteristics of ACFs are shown in Table 2. Because more right coronary artery (RCA) CTO PCIs were performed, the most frequent origin of the ACFs was RCA (n = 24; 48.98%), followed by left anterior descending (LAD) (n = 19; 38.78%) and left circumflex coronary artery (LCX) (n = 6; 12.24%); 38 (77.55%) patients had multiple drainage sites (≥3), and ACFs involved multiple branches of CTO vessel (≥3) in 29 (59.18%) patients. Images of ACFs are shown in Figure 2 and Supplementary Movies 1–6. In 11 (22.45%) patients, the collateral blood supply was identified on angiography, indirectly indicating ACFs before PCI (Supplementary Movies 7, 8).
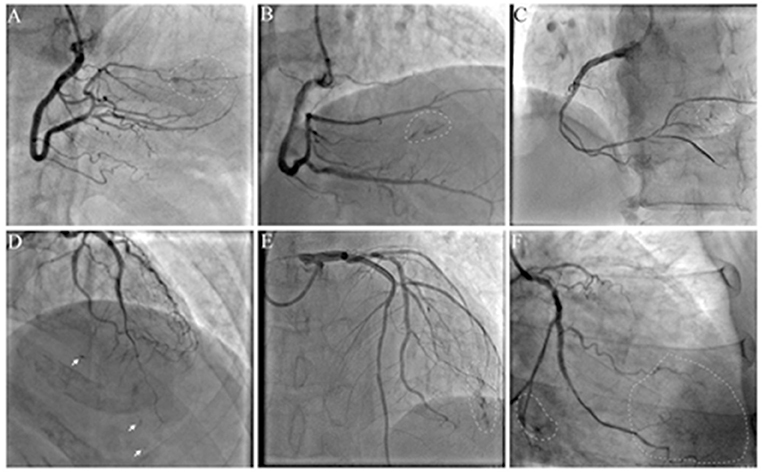
Figure 2. Angiographic characteristics of acquired coronary fistulas (ACFs): multiple, diffuse, and tiny. (A–C) ACFs were found after right coronary artery (RCA) chronic total occlusion-percutaneous coronary intervention (CTO-PCI); (D,E) ACFs were found after left anterior descending (LAD) CTO-PCI; (F) ACFs were found after left circumflex coronary artery (LCX) CTO-PCI. Arrow: spot fistulas; circle: flake fistulas; Supplementary Movies 1–6 show angiographic findings.
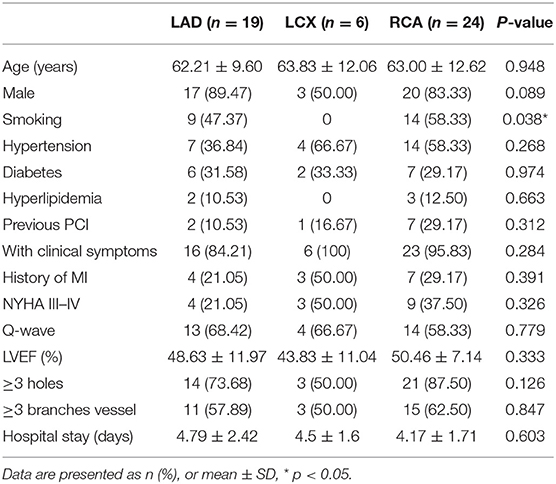
As shown in Table 3, clinical and imaging characteristics and medical history were similar among patients with ACF originating from RCA, LAD, and LCX, except that smoking, was more frequent in patients with ACFs originating from RCA and LAD than in those with ACFs from LCX. The mean length of hospital stay was 4.45 ± 1.99 days, and there was no death, emergent cardiac surgery, or MI during hospitalization.
Discussion
In the present study, the most common characteristics of ACFs in patients with successful CTO PCI included multiplicity and diffusibility with three or more branch vessels and drainage sites, and a history of MI or Q-wave (69.40 vs. 54.21%), which was 56.3–58.4% in the overall CTO population (8). Smoking is a risk factor for coronary artery disease (CHD) and is more common among patients with ACFs originating from LAD and RCA, which could be ascribed to the smaller sample size or lower incidence in LCX. In the presence of ACFs, the contrast agent quickly diffuses out of the coronary artery without retention during angiography.
In our study, the prevalence of ACFs in the successful CTO PCI patients was 2.66%, which is lower than CP (up to 8.9%) during CTO PCI (9). On occasions, CP and ACFs have a similar angiographic appearance, and Ellis classification has been used to classify CP as type I–III (10). Unlike CP, ACFs are drained into the cardiac chambers, and therefore, pericardial effusion and tamponade do not develop, thereby rendering procedures safe. The incorrect judgment of the operator and lack of bedside echocardiography may lead to unnecessary treatment. For example, coil embolization is feasible and effective for the treatment of CP, but useless and unnecessary for ACFs (Figure 3; Supplementary Movies 9, 10). It is therefore important to distinguish ACFs from CP during CTO PCI. However, contrast echocardiography is helpful to determine whether there is a true pericardial effusion from ACFs (11). A flow chart may be helpful to distinguish between CP and ACFs after the CTO reopening (Figure 4).
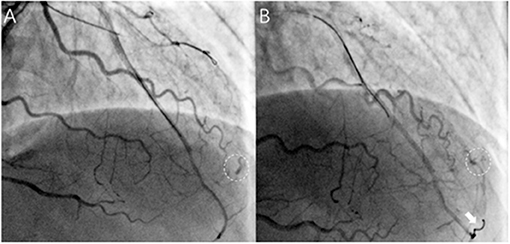
Figure 3. Acquired coronary fistulas uselessly and unnecessarily treated with coil embolization in a patient. (A) After successful recanalization of the LAD, ACFs were visualized and difficult to identify with coronary perforation timely (circle); (B) Rattled and successful delivery of one coil through the microcatheter (Finecross, Terumo, Japan) (arrows), but ACFs still existed (as shown in circle), and pericardial effusion was not found on echocardiography. (Supplementary Movies 9, 10 show angiographic findings).
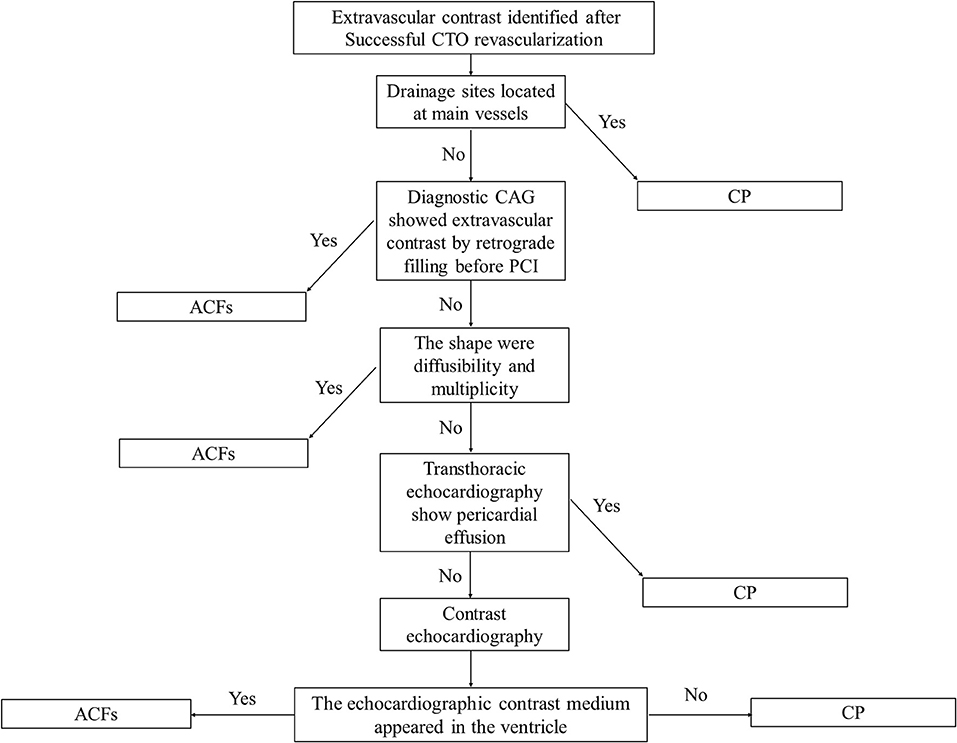
Figure 4. A flow chart used to distinguish between coronary perforation (CP) and ACFs after CTO reopening.
Acquired coronary fistulas may result from MI, hypertrophic cardiomyopathy, dilated cardiomyopathy, tumor, PCI, CABG, cardiac transplant, cardiac biopsy, pacemaker placement, and others (12–14). Microvasculature rupture may be the most important cause of ACFs (15, 16). Sudden complete occlusion of a coronary artery may block the blood supply to the myocardium, leading to myocardial necrosis. The myocardial rupture will occur within the first 2 weeks in 90% of MI because of extensive myocardial necrosis (17, 18). Complete rupture triggers hemopericardium and sudden death, and incomplete rupture will cause the formation of a ventricular aneurysm because the pericardium closes the ventricular perforation. In the patients with CTO, in contrast, the coronary artery is obturated slowly, allowing the development of collateral circulation; myocardial necrosis does not cause symptoms and patients can survive the attack, but endocardial necrosis may cause damage to the microvasculature. Myocardial scarring and microvasculature rupture may cause ACFs, which become apparent on coronary angiography after successful CTO-PCI. Young and male patients were more likely to survive after MI attack, so our data show the ACFs were more common in them. Other underlying mechanisms for ACFs include newly developed collaterals, neovascularization of mural thrombus formation, and reopening of the Thebesian vessels. Moreover, coronary steal syndrome or hemodynamic impairment was not evident after CTO PCI in our patients with ACFs.
Limitations
Contrast echocardiography is an ultrasound technique, which is safe and non-invasive for the assessment of drainage sites (19). This technique was not employed in the present study, and the specific location of drainage sites of ACFs was still unclear, but no pericardial effusion and tamponade were found at 2 h after PCI on transthoracic echocardiogram. Acquired coronary fistula have been reported to cause myocardial ischemia, endocarditis, and congestive heart failure (20), but long-term investigations are needed to evaluate whether ACFs are associated with poor prognosis in patients with CTO.
Conclusion
Although ACFs are mostly benign, it is very important to recognize that ACFs may cause unnecessary treatments. The ACFs are characterized by diffusibility, multiplicity, and leakage of contrast on angiography, often found in the branches of CTO vessels, and more common in patients with a history of MI.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RF drafted the manuscript. HT collected data and conducted the statistical analysis. WY reviewed all original angiograms. YS provided the figures and their interpretation. MF, ZH, and JG helped revise the manuscript. All the authors have read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (No. 2016YFC1301200), the National Natural Science Foundation of China (No. 81870269), and the Foundation of Shanghai Construction of TCM Medical Service System (No. ZY[2018-2020]-FWTX-8003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the research staff for their kind help.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.690890/full#supplementary-material
References
1. Said SA, Schiphorst RH, Derksen R, Wagenaar LJ. Coronary-cameral fistulas in adults: acquired types (second of two parts). World J Cardiol. (2013) 5:484–94. doi: 10.4330/wjc.v5.i12.484
2. Ryan C, Gertz EW. Fistula from coronary arteries to left ventricle after myocardial infarction. Br Heart J. (1977) 39:1147–9. doi: 10.1136/hrt.39.10.1147
3. Cassese S, Cirillo P, Dellegrottaglie S, Piscione F, Chiariello M. Acquired left coronary artery fistula draining to the cardiac vein system after acute myocardial infarction revealed by CT scan. Clin Imaging. (2011) 35:395–7. doi: 10.1016/j.clinimag.2010.08.012
4. DePersis M, Khan SU, Kaluski E, Lombardi W. Coronary artery perforation complicated by recurrent cardiac tamponade: a case illustration and review. Cardiovasc Revasc Med. (2017) 18:S30–4. doi: 10.1016/j.carrev.2017.03.003
5. Stone GW, Kandzari DE, Mehran R, Colombo A, Schwartz RS, Bailey S, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation. (2005) 112:2364–72. doi: 10.1161/circulationaha.104.481283
6. Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation. (2019) 140:420–33. doi: 10.1161/CIRCULATIONAHA.119.039797
7. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. (1985) 5:587–92. doi: 10.1016/s0735-1097(85)80380-6
8. Kadi H, Ceyhan K, Koc F, Celik A, Onalan O. Relation between fragmented QRS and collateral circulation in patients with chronic total occlusion without prior myocardial infarction. Anadolu Kardiyol Derg. (2011) 11:300–4. doi: 10.5152/akd.2011.079
9. Hirai T, Nicholson WJ, Sapontis J, Salisbury AC, Marso SP, Lombardi W, et al. A detailed analysis of perforations during chronic total occlusion angioplasty. JACC Cardiovasc Interv. (2019) 12:1902–12. doi: 10.1016/j.jcin.2019.05.024
10. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation. (1994) 90:2725–30. doi: 10.1161/01.cir.90.6.2725
11. Bagur R, Bernier M, Kandzari DE, Karmpaliotis D, Lembo NJ, Rinfret S, et al. A novel application of contrast echocardiography to exclude active coronary perforation bleeding in patients with pericardial effusion. Catheter Cardiovasc Interv. (2013) 82:221–9. doi: 10.1002/ccd.24564
12. Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G. Coronary arteriovenous fistulae: a review. Int J Angiol. (2014) 23:1–10. doi: 10.1055/s-0033-1349162
13. Ali M, Kassem KM, Osei K, Effat M. Coronary artery fistulae. J Thromb Thrombolysis. (2019) 48:345–51. doi: 10.1007/s11239-019-01897-8
14. Subash S, Thimmarayappa A, Patel GP, Dhananjaya M, Gopal D, Manjunatha N, et al. Rare case of left atrial myxoma vascularity causing acquired coronary cameral fistula: role of transesophageal echocardiography. Heart Views. (2018) 19:12–5. doi: 10.4103/heartviews.Heartviews_79_17
15. Lee CH, Lemos PA, Serruys PW. Acquired coronary artery fistula leading to acute myocardial infarction after endomyocardial biopsy. Heart. (2003) 89:495. doi: 10.1136/heart.89.5.495
16. Schanzenbacher P, Bauersachs J. Acquired right coronary artery fistula draining to the right ventricle: angiographic documentation of first appearance following reperfusion after acute myocardial infarction, with subsequent spontaneous closure. Heart. (2003) 89:e22. doi: 10.1136/heart.89.8.e22
17. Bajaj A, Sethi A, Rathor P, Suppogu N, Sethi A. Acute complications of myocardial infarction in the current era: diagnosis and management. J Invest Med. (2015) 63:844–55. doi: 10.1097/jim.0000000000000232
18. Marchandot B, Crimizade U, El Ghannudi S, Morel O. Giant ventricular pseudoaneurysm following inferior myocardial infarction: insights from multimodal imaging approach. Eur Heart J Case Rep. (2018) 2:yty019. doi: 10.1093/ehjcr/yty019
19. Chai SC, Tan PJ, Tong KL. A review of the safety and clinical utility of contrast echocardiography. Singapore Med J. (2020) 61:181–3. doi: 10.11622/smedj.2019169
Keywords: acquired coronary fistulas, chronic total occlusion, percutaneous coronary intervention, coronary perforation, revascularization
Citation: Fan R, Tan H, Song Y, Yao W, Fan M, Huang Z and Ge J (2021) Prevalence and Characteristics of Acquired Coronary Fistulas After Successful Revascularization of Chronic Total Occlusion. Front. Cardiovasc. Med. 8:690890. doi: 10.3389/fcvm.2021.690890
Received: 04 April 2021; Accepted: 27 September 2021;
Published: 22 December 2021.
Edited by:
Salah D. Qanadli, University of Lausanne, SwitzerlandReviewed by:
Yundi Feng, Peking University, ChinaGeorge Vetrovec, Virginia Commonwealth University Richmond, United States
Copyright © 2021 Fan, Tan, Song, Yao, Fan, Huang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Fan, ZmFubWluQHNoeXVleWFuZ2hvc3BpdGFsLmNvbQ==; Zheyong Huang, emhleW9uZ2h1YW5nQDEyNi5jb20=
 Rong Fan1
Rong Fan1 Min Fan
Min Fan Zheyong Huang
Zheyong Huang