
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 24 June 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.690790
This article is part of the Research TopicWhat do we know about COVID-19 implications for cardiovascular disease?View all 109 articles
Alterations in cardiac biomarkers have been reported in patients with coronavirus disease 2019 (COVID-19) in relation to disease severity and mortality. We conducted a systematic review and meta-analysis with meta-regression of studies reporting B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) plasma concentrations in COVID-19. We searched PubMed, Web of Science, and Scopus, between January 2020 and 2021, for studies reporting BNP/NT-proBNP concentrations, measures of COVID-19 severity, and survival status (PROSPERO registration number: CRD42021239190). Forty-four studies in 18,856 COVID-19 patients were included in the meta-analysis and meta-regression. In pooled results, BNP/NT-proBNP concentrations were significantly higher in patients with high severity or non-survivor status when compared to patients with low severity or survivor status during follow up (SMD = 1.07, 95% CI: 0.89–1.24, and p < 0.001). We observed extreme between-study heterogeneity (I2 = 93.9%, p < 0.001). In sensitivity analysis, the magnitude and the direction of the effect size were not substantially modified after sequentially removing individual studies and re-assessing the pooled estimates, (effect size range, 0.99 – 1.10). No publication bias was observed with the Begg's (p = 0.26) and Egger's (p = 0.40) t-tests. In meta-regression analysis, the SMD was significantly and positively associated with D-dimer (t = 2.22, p = 0.03), myoglobin (t = 2.40, p = 0.04), LDH (t = 2.38, p = 0.02), and procalcitonin (t = 2.56, p = 0.01) concentrations. Therefore, higher BNP/NT-proBNP plasma concentrations were significantly associated with severe disease and mortality in COVID-19 patients.
A significant number of clinical and demographic factors have been studied in patients with coronavirus disease 19 (COVID-19) in regard to their association with specific clinical presentations and measures of clinical severity (1, 2). The evidence of an excessive activation of inflammatory and immunomodulating pathways in patients with the more severe forms of the disease, typically characterized by the development of respiratory failure with or without multi-organ dysfunction, have prompted the search for specific biomarkers of inflammation and immuno-activation in order to develop better predictive models to assist with management (3). The increasing evidence of significant alterations of different organs and/or systems in patients with COVID-19 has also led to the investigation of the predictive capacity of additional, organ-specific, biomarkers. For example, the presence of myocardial injury, associated with several cardiac manifestations, including myocarditis, acute coronary syndrome, and arrhythmias, has been well-documented in COVID-19 patients with or without pre-existing cardiovascular history (4). Notably, cardiac abnormalities in this group are independently associated with an increased risk of mortality (5). While the exact mechanisms involved in the onset and progression of COVID-19 related myocardial injury remain to be elucidated, several circulating markers of myocardial damage, particularly creatine kinase (CK), and troponin, are being increasingly studied in terms of their predictive capacity (6). Another cardiac complication, heart failure, has been observed in about a quarter of patients with COVID-19 and has been associated with an increased risk of adverse outcomes (7, 8). The active peptide B-type natriuretic peptide (BNP) and the inactive peptide N-terminal proBNP (NT-proBNP) are both derived from the human BNP precursor proBNP in the ventricular myocytes. The increased secretion of BNP and NT-proBNP from the heart, in response to high ventricular filling pressures, is routinely used as a diagnostic and prognostic marker in heart failure and, by some, as a marker of the size or severity of ischaemic insults (9–11). However, its biological and clinical role in patients with COVID-19 is not well-established. We addressed this issue by conducting a systematic review and meta-analysis with meta-regression of studies reporting plasma BNP or NT-proBNP concentrations in COVID-19 patients with different disease severity, based on clinical guidelines or need for hospitalization, mechanical ventilation, or transfer to the intensive care unit (ICU), and clinical outcomes, particularly survival status during follow up.
We conducted a systematic literature search, using the terms “brain natriuretic peptide” or “BNP” or “NT-proBNP” or “N-terminal pro-brain natriuretic peptide” and “coronavirus disease 19” or “COVID-19,” in PubMed, Web of Science and Scopus, from January 2020 to January 2021, to identify peer-reviewed studies reporting BNP/NT-proBNP concentrations in COVID-19 patients according to disease severity and/or mortality. We accessed the references of the retrieved articles to identify additional studies. Eligibility criteria were (a) reporting continuous data on plasma BNP/NT-proBNP concentrations in COVID-19 patients, (b) investigating COVID-19 patients with different disease severity or survival status during follow up, (c) adult patients, (d) English language, (e) >10 patients, and (f) full-text available. Two investigators independently screened individual abstracts. If relevant, they independently reviewed the full articles (PROSPERO registration number: CRD42021239190). We used the Newcastle-Ottawa scale to assess study quality, with a score ≥6 indicating high quality (12).
We calculated standardized mean differences (SMD) and 95% confidence intervals (CIs) in BNP/NT-proBNP concentrations between COVID-19 patients with low vs. high severity or survivor vs. non-survivor status. A p < 0.05 was considered statistically significant. When studies reported medians and interquartile ranges (IQR) the corresponding means and standard deviations were estimated (13). We assessed between-study heterogeneity in SMD values using the Q-statistic (significance level at p < 0.10). Inconsistency across studies was evaluated using the I2 statistic, where I2 <25% indicated no heterogeneity, between 25 and 50% moderate heterogeneity, between 50 and 75% large heterogeneity, and >75% extreme heterogeneity (14, 15). Random-effect models were used to calculate the pooled SMD and 95% CIs if significant heterogeneity was present. In sensitivity analyses, the influence of individual studies on the overall effect size was assessed using the leave-one-out method (16). The presence of publication bias was assessed using the Begg's and the Egger's test, at the p < 0.05 level of significance (17, 18), and the Duval and Tweedie “trim and fill” procedure (19). To identify factors contributing to the between-study variance, we investigated the effects of several biologically and/or clinically plausible factors on the SMD by univariate meta-regression analysis. These factors included age, gender, clinical endpoint, study design (retrospective or prospective), geographical area where the study was conducted, aspartate aminotransferase (AST), alanine aminotransferase (ALT), D-dimer, serum creatinine, myoglobin, troponin, CK, albumin, ferritin, lactate dehydrogenase (LDH), procalcitonin, C-reactive protein (CRP), white blood cell count (WBC), diabetes, hypertension and cardiovascular disease. Statistical analyses were performed using Stata 14 (STATA Corp., College Station, TX, USA). The study was fully compliant with the PRISMA statement (20).
We initially identified 1,815 studies. A total of 1,758 studies were excluded after the first screening because they were duplicates or irrelevant. Following full-text revision of the remaining 57 articles, 13 were further excluded because they did not meet the inclusion criteria. Thus, 44 studies in 18,856 COVID-19 patients, 14,569 (53% males, mean age 48 years) with low severity or survivor status and 4,287 (59% males, mean age 61 years) with high severity or non-survivor status, were included in the final analysis (Figure 1 and Table 1) (7, 21–63). Thirty-two studies were conducted in Asia (7, 22, 24, 26, 27, 29, 31–33, 35, 38–46, 48, 50, 51, 53–55, 57–63), eight in Europe (25, 28, 30, 34, 47, 49, 52, 56), three in America (23, 36, 37), and one in Africa (21). Thirty-two studies were retrospective (7, 21–23, 27, 29, 31–39, 41–45, 48, 50, 51, 53, 55, 57–63), eight prospective (24, 30, 46, 47, 49, 52, 54, 56), whereas the remaining four did not report the study design (25, 26, 28, 40). Clinical endpoints included disease severity based on current clinical guidelines in 15 studies (21, 24, 33, 39–41, 43, 48, 54, 55, 57–59, 62, 63), hospitalization in one (37), ICU transfer in three (32, 44, 49), or need for mechanical ventilation in one (23), and survival status in 24 studies (7, 22, 25–31, 34–36, 38, 42, 45–47, 50–53, 56, 60, 61). Sixteen studies reported plasma BNP concentrations (23, 24, 26, 33, 35–37, 45, 48, 50, 51, 54, 56–58, 62), whereas the remaining 28 reported plasma NT-proBNP concentrations (7, 21, 22, 25, 27–32, 34, 38–44, 46, 47, 49, 52, 53, 55, 59–61, 63). Only one study reported cumulative 7-day mean plasma NT-proBNP concentrations (34), whereas another reported BNP concentrations on initial presentation to the emergency department (37). The remaining 42 studies reported BNP or NT-proBNP concentrations within the first 24–48 h from admission.
The overall SMD in BNP/NT-proBNP concentrations between COVID-19 patients with low vs. high severity or survivor vs. non-survivor status is shown in Figure 2. In two studies, patients with high severity or non-survivor status had significantly lower BNP/NT-proBNP concentrations when compared to those with low severity or survivor status (mean difference range, −0.45 to −0.67) (31, 59). By contrast, in the remaining studies BNP/NT-proBNP concentrations were lower in patients with low severity or survivor status (mean difference range, 0.02 – 5.09), with a non-significant difference in five studies (35, 38, 44, 50, 63). Pooled results confirmed that BNP/NT-proBNP concentrations were significantly higher in patients with severe disease or non-survivor status (SMD = 1.07, 95% CI: 0.89 – 1.24, and p < 0.001; Figure 2). There was extreme between-study heterogeneity (I2 = 93.9%, p < 0.001). BNP/NT-proBNP concentrations remained significantly higher (SMD = 1.06, 95% CI: 0.86 – 1.26, and p < 0.001; I2 = 93.0%, p < 0.001) in patients with high severity or non-survivor status after excluding two relatively large studies, accounting for nearly 56% of the overall sample size (26, 37).
In sensitivity analysis, the magnitude and the direction of the effect size were not substantially modified after sequentially removing each study and re-assessing the pooled estimates (effect size range, 0.99 – 1.10; Figure 3). No publication bias was observed with the Begg's (p = 0.26) and Egger's (p = 0.40) t-tests. However, using the trim-and-fill method, we identified three potential missing studies to be added to the left side of the funnel plot to ensure symmetry (Figure 4). The adjusted SMD, albeit attenuated, remained significant (SMD = 0.90, 95% CI: 0.70 – 1.09, and p < 0.001).
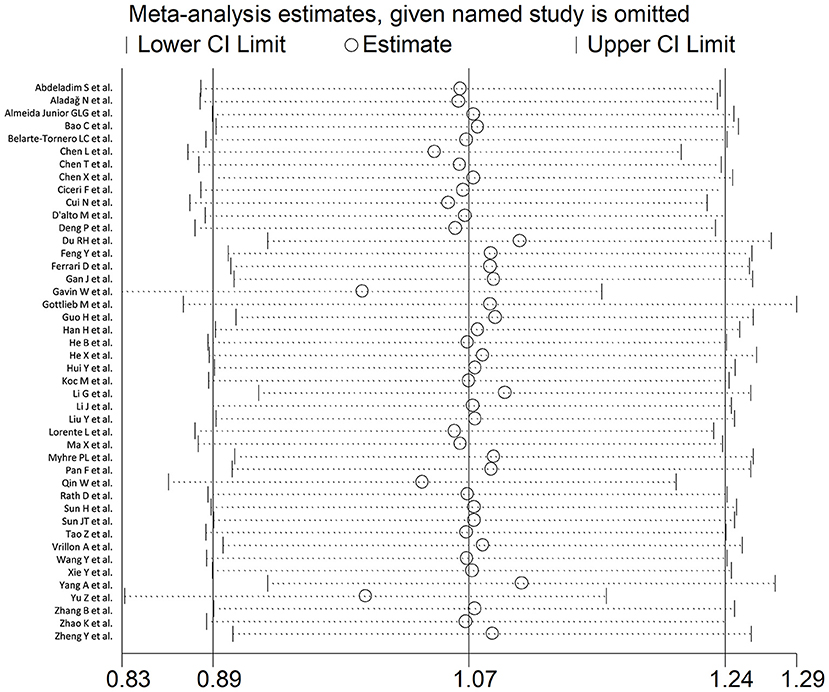
Figure 3. Sensitivity analysis of the association between BNP and COVID-19 disease, assessed by investigating the influence of individual studies on the overall standardized mean difference (SMD). The SMD and the 95% confidence intervals (CIs) are indicated by the middle and the lateral vertical axes, respectively. The pooled SMD and the 95% CIs are indicated by the hollow circles and the two ends of each broken line, respectively.
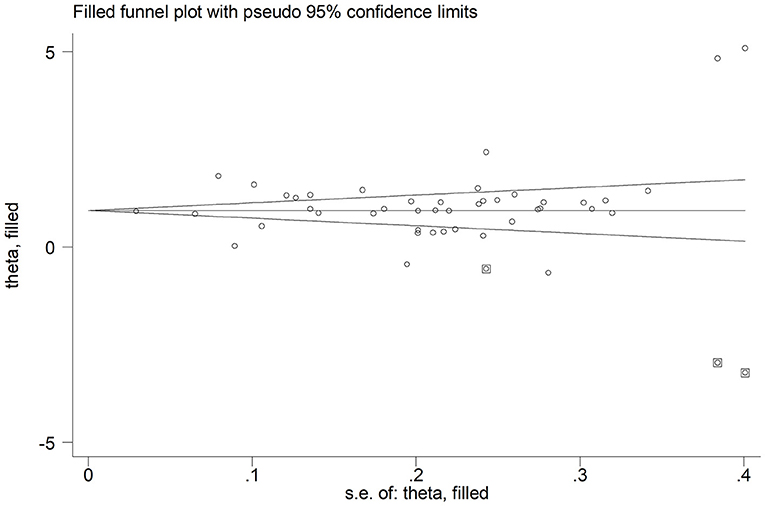
Figure 4. Funnel plot of studies investigating disease severity or survival status after trimming and filling. Enclosed and free circles indicate dummy and genuine studies, respectively.
Sub-group analysis of the 42 studies reporting BNP/NT-proBNP concentrations on admission showed that the SMD remained significantly higher in patients with high severity or non-survivor status (SMD = 1.10, 95% CI: 0.88 – 1.31, and p < 0.001) with an extreme between-study variance (I2 = 94.1%, p < 0.001). Additionally, the pooled SMD value in studies assessing disease severity (SMD = 0.87, 95% CI: 0.68 – 1.07, and p < 0.001; I2 = 79.5, p < 0.001) was non-significantly lower than those investigating survivor status (SMD = 1.37, 95% CI: 1.08 – 1.66, p < 0.001; I2 = 92.3, p < 0.001; t = 1.63, p = 0.11; Figure 5). Similarly, non-significantly higher SMD values were observed in retrospective (SMD = 1.06, 95% CI: 0.86 – 1.27, p < 0.001; I2 = 94.5, p < 0.001) vs. prospective studies (SMD = 0.92, 95% CI: 0.67 – 1.18, p < 0.001; I2 = 59.4, p = 0.016; t = −0.41, p = 0.69; Figure 6). The pooled SMD value in European studies (SMD = 0.96, 95% CI: 0.67 – 1.26, p < 0.002; I2 = 75.5%, p < 0.001) was non-significantly lower than that observed in Asian (SMD = 1.01, 95% CI: 0.77 – 1.24, p < 0.001; I2 = 94.5%, p < 0.001) and American studies (SMD = 2.24, 95% CI: 0.83 – 3.64, p < 0.001; I2 = 98.2%, p < 0.001; t = 1.36, p = 0.18; Figure 7). Finally, the pooled SMD value in studies reporting plasma NT-proBNP concentrations (SMD = 0.98, 95% CI: 0.74 – 1.23, p < 0.001; I2 = 93.2%, p < 0.001) was non-significantly lower than that observed in studies reporting plasma BNP concentrations (SMD = 1.22, 95% CI: 0.93 – 1.52, p < 0.001; I2 = 95.0%, p < 0.001; t = −0.85, p = 0.40; Figure 8). A relatively lower heterogeneity was observed in prospective (I2 = 59.4%) and European studies (I2 = 75.5%), and in those investigating disease severity (I2 = 79.5%).
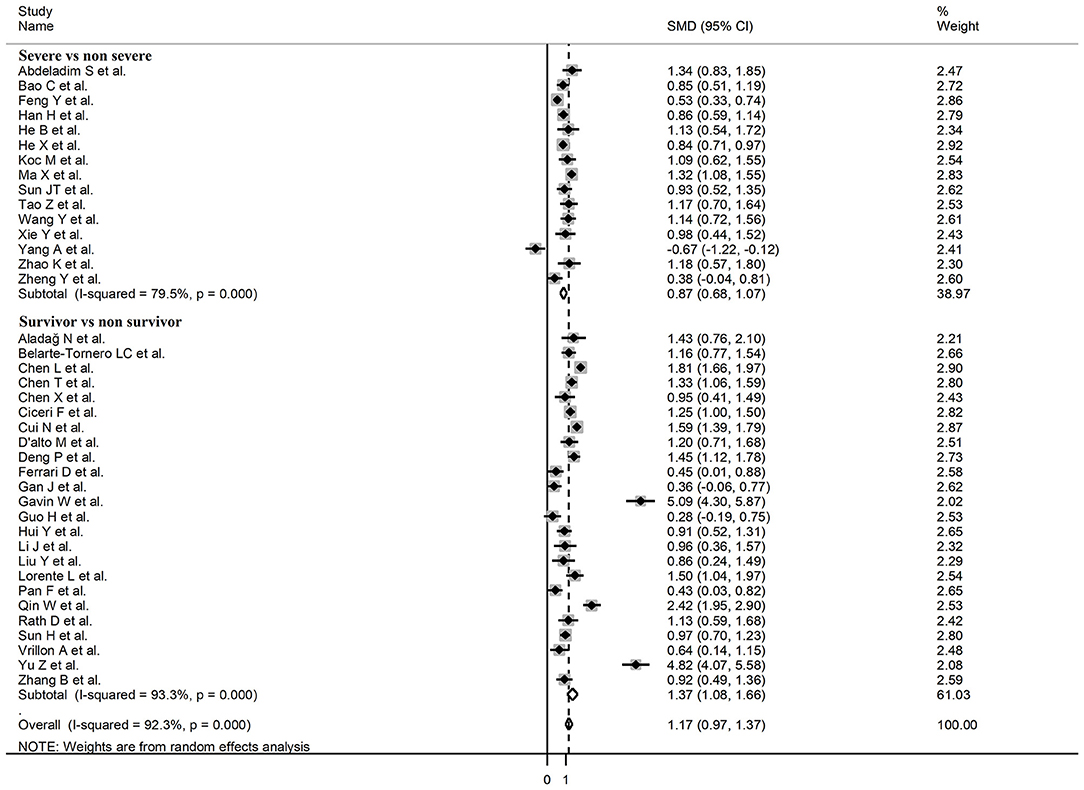
Figure 5. Forest plot of studies reporting BNP concentrations in patients with COVID-19 according to disease severity or survival status.
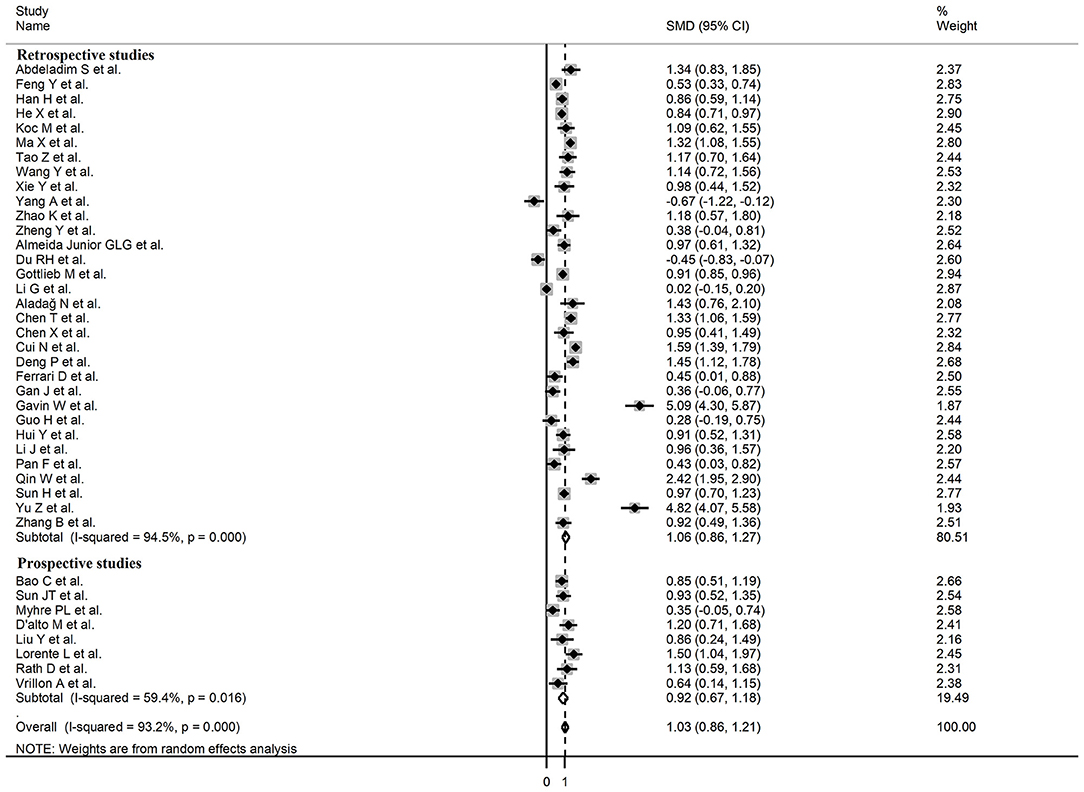
Figure 6. Forest plot of studies reporting BNP concentrations in patients with COVID-19 according to study design (retrospective or prospective).
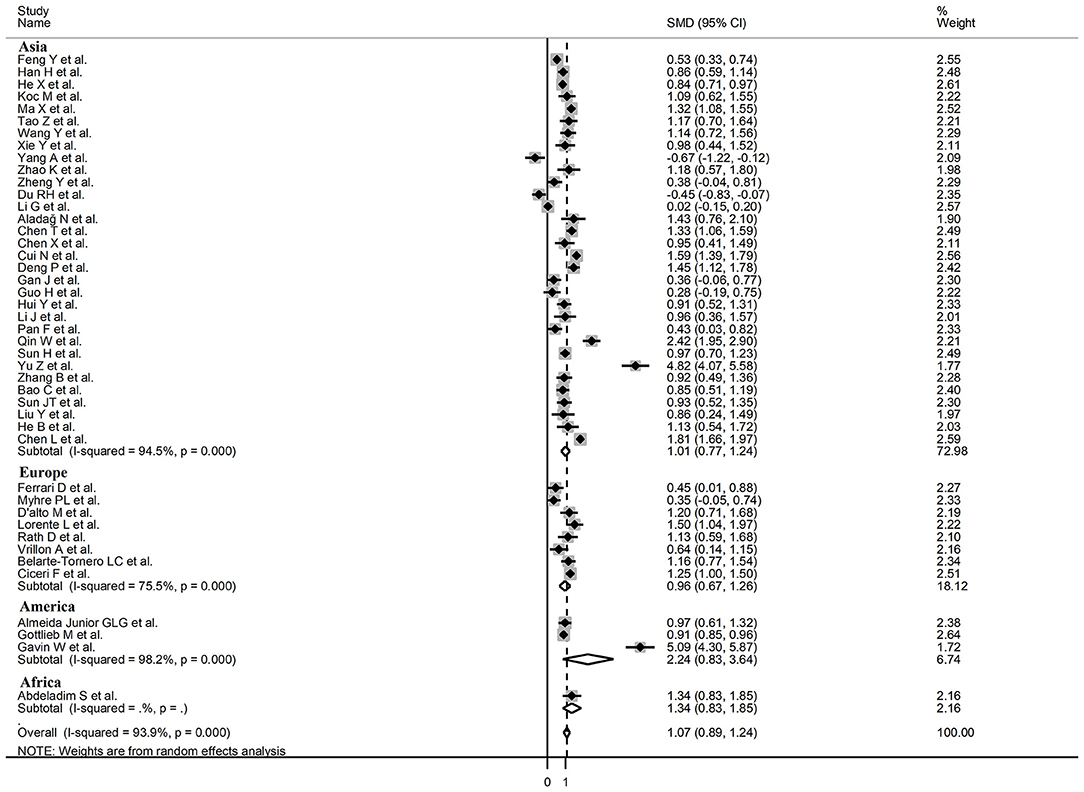
Figure 7. Forest plot of studies examining BNP concentrations in patients with COVID-19 according to the geographic area where the study was conducted.
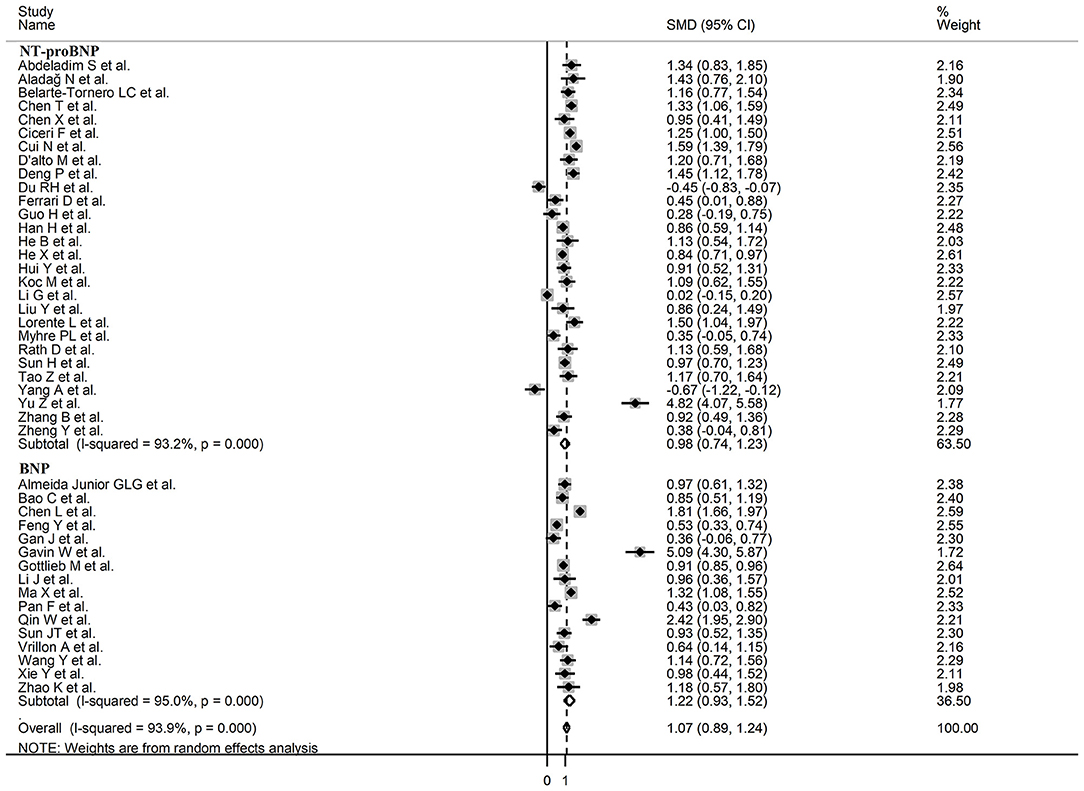
Figure 8. Forest plot of studies examining BNP concentrations in patients with COVID-19 according to whether plasma NT-proBNP or BNP was measured.
The D-dimer (t = 2.22, p = 0.03), myoglobin (t = 2.40, p = 0.04), LDH (t = 2.38, p = 0.02), and procalcitonin (t = 2.56, p = 0.01) concentrations were significantly and positively associated with the pooled SMD. By contrast, no significant correlations were observed between the SMD and age (t = −0.30, p = 0.76), gender (t = 0.26, p = 0.80), AST (t = 0.25, p = 0.81), ALT (t = −0.89, p = 0.38), creatinine (t = 0.93, p = 0.36), troponin (t = 0.18, p = 0.86), CK (t = 0.85, p = 0.41), albumin (t = 0.70, p = 0.49), ferritin (t = −1.29, p = 0.22), CRP (t = 0.96, p = 0.34), WBC (t = 0.08, p = 0.94), diabetes (t = −0.59, p = 0.56), hypertension (t = −0.01, p = 0.99), and cardiovascular disease (t = −0.53, p = 0.60).
In our study, plasma concentrations of BNP and NT-proBNP, generally measured within the first 24–48 h from admission, were significantly higher in COVID-19 patients with severe disease, based on clinical assessment or the need for hospitalization, mechanical ventilation, or ICU transfer, and in those who did not survive when compared to patients with mild disease or who survived during follow up. The observed SMD values for combined natriuretic peptide concentrations or BNP and NT-proBNP separately, 1.07, 1.22, and 0.98, respectively, suggest a biologically and clinically significant effect size (64). Although between-study heterogeneity was extreme, in sensitivity analysis the effect size was not influenced when individual studies were sequentially removed. The Begg's and Egger's t-tests did not show any evidence of publication bias. In meta-regression analysis, significant associations were observed between the SMD value and D-dimer, myoglobin, LDH, and procalcitonin, but not with age, gender, AST, ALT, creatinine, troponin, CK, albumin, ferritin, CRP, WBC, diabetes, hypertension, or cardiovascular disease.
Differently from the inactive NT-proBNP, the BNP exerts significant biological effects through its binding to the guanylyl cyclase-coupled natriuretic receptors A and B. The consequent increase in cyclic guanosine monophosphate causes vasodilatation, diuresis, natriuresis, inhibition of the renin-angiotensin-aldosterone system, inhibition of fibrosis, hypertrophy, cell apoptosis and inflammation, including suppression of superoxide generation by neutrophils, and improvement in myocardial relaxation (10). Notably, there is no evidence of significant associations between BNP and cyclic guanosine monophosphate concentrations in human studies. Furthermore, specific BNP-mediated protective effects, particularly the suppression of neutrophil-mediated generation of superoxide via nicotinamide adenine dinucleotide phosphate oxidase, are impaired in the context of acute heart failure, even in the presence of increased BNP concentrations (65). Whilst such effects are partially restored with pharmacological treatment, the failure of BNP-related suppression of superoxide release might lead to sustained tissue inflammation in heart failure, with or without concomitant COVID-19. There are other differences between the BNP and the NT-proBNP, with the latter being characterized by a higher molecular mass, a longer half-life (>60 vs. 15–20 min), a higher degree of in vivo glycosylation, and a lower degree of intra-individual biological variation (66). The better analytical characteristics of the available immunoassay methods for the measurement of NT-proBNP concentrations, when compared to those for the assessment of the BNP, have prompted some experts to advocate the measurement and monitoring of NT-proBNP concentrations as the best strategy for the management of patients with heart failure (66). These issues notwithstanding, in our meta-analysis the studies reporting BNP vs. NT-proBNP plasma concentrations had similar SMD values and degrees of heterogeneity.
The significant association observed between plasma BNP/NT-proBNP concentrations, disease severity and mortality in patients with COVID-19 is likely to reflect the presence of heart failure and its adverse sequelae in this group. In this context, these routine and relatively inexpensive biomarkers might assist the clinician with the early diagnosis of cardiac dysfunction and the prompt initiation of appropriate pharmacological and non-pharmacological therapies (67). Further research is warranted to determine whether the assessment of BNP/NT-proBNP might also be incorporated into predictive tools that are specifically developed and validated in COVID-19 patients. The reported associations, in meta-regression analysis, between the SMD of BNP/NT-proBNP and D-dimer, myoglobin, LDH, and pro-calcitonin suggests that the effect size is particularly correlated with markers of pro-coagulant activity, skeletal muscle and other tissue damage, and severe sepsis, respectively. Notably, these markers have, in turn, been shown to have significant associations with COVID-19 severity and outcomes (68–70). By contrast, the lack of associations observed with other cardiac biomarkers, e.g., troponin and CK, suggests that the measurement of BNP/NT-proBNP may provide complementary, rather than redundant, information regarding the presence of cardiac abnormalities in patients with COVID-19.
The extreme between-study heterogeneity represents a limitation of our study. However, we did not observe significant publication bias and the overall effect size was not substantially affected in sensitivity analyses. The lack of significant associations between the SMD and several patient and study characteristics, except for D-dimer, myoglobin, LDH, and procalcitonin concentrations, suggests that other unreported factors might have contributed to the observed heterogeneity. Such factors may include the relationship between the SMD values and the presence of new onset vs. acute on chronic heart failure and the information regarding the specific analytical methods used for the determination of BNP and NT-proBNP plasma concentrations (66). In this context, the lack of available information on indexes of left ventricular function prevented the conduct of further meta-regression analyses of the association between such indexes and the SMD values. Furthermore, virtually all selected studies reported isolated, rather than serial, measurements of natriuretic peptide shortly after hospital admission. This issue is particularly important as the routine monitoring of BNP/NT-proBNP concentrations has been shown to be beneficial in heart failure (71). Further studies should investigate the prognostic value of single vs. serial BNP/NT-proBNP measurements also in patients with COVID-19.
In conclusion, higher plasma concentrations of BNP or NT-proBNP are significantly associated with higher disease severity and increased mortality in COVID-19. Additional studies are required to determine whether these cardiac biomarkers can be incorporated into robust predictive tools that further assist with early management and monitoring in this patient group.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
AZ: initial idea. AZ and SS: data collection and analysis. AZ, SS, CC, and AM: data interpretation and writing—review and editing. AM: writing—first draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.690790/full#supplementary-material
1. Huang C, Soleimani J, Herasevich S, Pinevich Y, Pennington KM, Dong Y, et al. Clinical characteristics, treatment, and outcomes of critically ill patients with COVID-19: a scoping review. Mayo Clin Proc. (2021) 96:183–202. doi: 10.1016/j.mayocp.2020.10.022
2. Pijls BG, Jolani S, Atherley A, Derckx RT, Dijkstra JIR, Franssen GHL, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. (2021) 11:e044640. doi: 10.1136/bmjopen-2020-044640
3. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. (2020) 383:2255–73. doi: 10.1056/NEJMra2026131
4. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. (2020) 17:543–58. doi: 10.1038/s41569-020-0413-9
5. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
6. Yang J, Liao X, Yin W, Wang B, Yue J, Bai L, et al. Elevated cardiac biomarkers may be effective prognostic predictors for patients with COVID-19: a multicenter, observational study. Am J Emerg Med. (2021) 39:34–41. doi: 10.1016/j.ajem.2020.10.013
7. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
9. Maisel AS, Duran JM, Wettersten N. Natriuretic peptides in heart failure: atrial and B-type natriuretic peptides. Heart Fail Clin. (2018) 14:13–25. doi: 10.1016/j.hfc.2017.08.002
10. Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. (2009) 191:341–66. doi: 10.1007/978-3-540-68964-5_15
11. Omland T, Persson A, Ng L, O'Brien R, Karlsson T, Herlitz J, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. (2002) 106:2913–8. doi: 10.1161/01.CIR.0000041661.63285.AE
12. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. The Ottawa Hospital Research Institute (2013). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed June 11, 2021).
13. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
14. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
16. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. (1999) 47:15–7.
17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
18. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/S0895-4356(01)00377-8
19. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
21. Abdeladim S, Oualim S, Elouarradi A, Bensahi I, Aniq Filali R, El Harras M, et al. Analysis of cardiac injury biomarkers in COVID-19 patients. Arch Clin Infect Dis. (2020) 15:e105515. doi: 10.5812/archcid.105515
22. Aladag N, Atabey RD. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID-19 patients. Acta Cardiol. (2021) 76:132–9. doi: 10.1080/00015385.2020.1810914
23. Almeida Junior GLG, Braga F, Jorge JK, Nobre GF, Kalichsztein M, Faria PMP, et al. Prognostic value of troponin-T and B-type natriuretic peptide in patients hospitalized for COVID-19. Arq Bras Cardiol. (2020) 115:660–6. doi: 10.36660/abc.20200385
24. Bao C, Tao X, Cui W, Yi B, Pan T, Young KH, et al. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. (2020) 9:16. doi: 10.1186/s40164-020-00172-4
25. Belarte-Tornero LC, Valdivielso-More S, Vicente Elcano M, Sole-Gonzalez E, Ruiz-Bustillo S, Calvo-Fernandez A, et al. Prognostic implications of chronic heart failure and utility of NT-proBNP levels in heart failure patients with SARS-CoV-2 infection. J Clin Med. (2021) 10:323. doi: 10.3390/jcm10020323
26. Chen L, Yu J, He W, Chen L, Yuan G, Dong F, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. (2020) 34:2173–83. doi: 10.1038/s41375-020-0911-0
27. Chen X, Yan L, Fei Y, Zhang C. Laboratory abnormalities and risk factors associated with in-hospital death in patients with severe COVID-19. J Clin Lab Anal. (2020) 34:e23467. doi: 10.1002/jcla.23467
28. Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, Galli L, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. (2020) 217:108509. doi: 10.1016/j.clim.2020.108509
29. Cui N, Yan R, Qin C, Zhao J. Clinical characteristics and immune responses of 137 deceased patients with COVID-19: a retrospective study. Front Cell Infect Microbiol. (2020) 10:595333. doi: 10.3389/fcimb.2020.595333
30. D'Alto M, Marra AM, Severino S, Salzano A, Romeo E, De Rosa R, et al. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care. (2020) 24:670. doi: 10.1186/s13054-020-03385-5
31. Deng P, Ke Z, Ying B, Qiao B, Yuan L. The diagnostic and prognostic role of myocardial injury biomarkers in hospitalized patients with COVID-19. Clin Chim Acta. (2020) 510:186–90. doi: 10.1016/j.cca.2020.07.018
32. Du RH, Liu LM, Yin W, Wang W, Guan LL, Yuan ML, et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. (2020) 17:839–46. doi: 10.1513/AnnalsATS.202003-225OC
33. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. (2020) 201:1380–8. doi: 10.1164/rccm.202002-0445OC
34. Ferrari D, Seveso A, Sabetta E, Ceriotti D, Carobene A, Banfi G, et al. Role of time-normalized laboratory findings in predicting COVID-19 outcome. Diagnosis (Berl). (2020) 7:387–94. doi: 10.1515/dx-2020-0095
35. Gan J, Li J, Li S, Yang C. Leucocyte subsets effectively predict the clinical outcome of patients with COVID-19 pneumonia: a retrospective case-control study. Front Public Health. (2020) 8:299. doi: 10.3389/fpubh.2020.00299
36. Gavin W, Campbell E, Zaidi SA, Gavin N, Dbeibo L, Beeler C, et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. (2021) 49:158–65. doi: 10.1016/j.ajic.2020.07.005
37. Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med. (2020) 27:963–73. doi: 10.1111/acem.14104
38. Guo H, Shen Y, Wu N, Sun X. Myocardial injury in severe and critical coronavirus disease 2019 patients. J Card Surg. (2021) 36:82–8. doi: 10.1111/jocs.15164
39. Han H, Xie L, Liu R, Yang J, Liu F, Wu K, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. (2020) 92:819–23. doi: 10.1002/jmv.25809
40. He B, Wang J, Wang Y, Zhao J, Huang J, Tian Y, et al. The metabolic changes and immune profiles in patients with COVID-19. Front Immunol. (2020) 11:2075. doi: 10.3389/fimmu.2020.02075
41. He X, Wang L, Wang H, Xie Y, Yu Y, Sun J, et al. Factors associated with acute cardiac injury and their effects on mortality in patients with COVID-19. Sci Rep. (2020) 10:20452. doi: 10.1038/s41598-020-77172-1
42. Hui Y, Li Y, Tong X, Wang Z, Mao X, Huang L, et al. The risk factors for mortality of diabetic patients with severe COVID-19: a retrospective study of 167 severe COVID-19 cases in Wuhan. PLoS One. (2020) 15:e0243602. doi: 10.1371/journal.pone.0243602
43. Koc M, Sumbul HE, Gulumsek E, Koca H, Bulut Y, Karakoc E, et al. Disease severity affects ventricular repolarization parameters in patients with COVID-19. Arq Bras Cardiol. (2020) 115:907–13. doi: 10.36660/abc.20200482
44. Li G, Zhou CL, Ba YM, Wang YM, Song B, Cheng XB, et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. (2021) 40:2154–61. doi: 10.1016/j.clnu.2020.09.040
45. Li J, Xu G, Yu H, Peng X, Luo Y, Cao C. Clinical characteristics and outcomes of 74 patients with severe or critical COVID-19. Am J Med Sci. (2020) 360:229–35. doi: 10.1016/j.amjms.2020.05.040
46. Liu Y, Xie J, Gao P, Tian R, Qian H, Guo F, et al. Swollen heart in COVID-19 patients who progress to critical illness: a perspective from echo-cardiologists. ESC Heart Fail. (2020) 7:3621–32. doi: 10.1002/ehf2.12873
47. Lorente L, Martin MM, Argueso M, Sole-Violan J, Perez A, Marcos YRJA, et al. Association between red blood cell distribution width and mortality of COVID-19 patients. Anaesth Crit Care Pain Med. (2020) 40:100777. doi: 10.1016/j.accpm.2020.10.013
48. Ma X, Li A, Jiao M, Shi Q, An X, Feng Y, et al. Characteristic of 523 COVID-19 in henan province and a death prediction model. Front Public Health. (2020) 8:475. doi: 10.3389/fpubh.2020.00475
49. Myhre PL, Prebensen C, Strand H, Roysland R, Jonassen CM, Rangberg A, et al. Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation. (2020) 142:2128–37. doi: 10.1161/CIRCULATIONAHA.120.050360
50. Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. (2020) 17:1281–92. doi: 10.7150/ijms.46614
51. Qin WD, Bai W, Liu K, Liu Y, Meng X, Zhang K, et al. Clinical course and risk factors of disease deterioration in critically ill patients with COVID-19. Hum Gene Ther. (2021) 32:310–5. doi: 10.1089/hum.2020.255
52. Rath D, Petersen-Uribe A, Avdiu A, Witzel K, Jaeger P, Zdanyte M, et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. (2020) 109:1491–9. doi: 10.1007/s00392-020-01683-0
53. Sun H, Ning R, Tao Y, Yu C, Deng X, Zhao C, et al. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J Am Geriatr Soc. (2020) 68:E19–E23. doi: 10.1111/jgs.16533
54. Sun JT, Chen Z, Nie P, Ge H, Shen L, Yang F, et al. Lipid profile features and their associations with disease severity and mortality in patients with COVID-19. Front Cardiovasc Med. (2020) 7:584987. doi: 10.3389/fcvm.2020.584987
55. Tao Z, Xu J, Chen W, Yang Z, Xu X, Liu L, et al. Anemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol. (2021) 93:1478–88. doi: 10.21203/rs.3.rs-39184/v1
56. Vrillon A, Hourregue C, Azuar J, Grosset L, Boutelier A, Tan S, et al. COVID-19 in older adults: a series of 76 patients aged 85 years and older with COVID-19. J Am Geriatr Soc. (2020) 68:2735–43. doi: 10.1111/jgs.16894
57. Wang Y, Zhou Y, Yang Z, Xia D, Hu Y, Geng S. Clinical characteristics of patients with severe pneumonia caused by the SARS-CoV-2 in Wuhan, China. Respiration. (2020) 99:649–57. doi: 10.1159/000507940
58. Xie Y, You Q, Wu C, Cao S, Qu G, Yan X, et al. Impact of cardiovascular disease on clinical characteristics and outcomes of coronavirus disease 2019 (COVID-19). Circ J. (2020) 84:1277–83. doi: 10.1253/circj.CJ-20-0348
59. Yang A, Qiu Q, Kong X, Sun Y, Chen T, Zuo Y, et al. Clinical and Epidemiological Characteristics of COVID-19 Patients in Chongqing China. Front Public Health. (2020) 8:244. doi: 10.3389/fpubh.2020.00244
60. Yu Z, Ke Y, Xie J, Yu H, Zhu W, He L, et al. Clinical characteristics on admission predict in-hospital fatal outcome in patients aged >/=75 years with novel coronavirus disease (COVID-19): a retrospective cohort study. BMC Geriatr. (2020) 20:514. doi: 10.1186/s12877-020-01921-0
61. Zhang B, Dong C, Li S, Song X, Wei W, Liu L. Triglyceride to high-density lipoprotein cholesterol ratio is an important determinant of cardiovascular risk and poor prognosis in coronavirus disease-19: a retrospective case series study. Diabetes Metab Syndr Obes. (2020) 13:3925–36. doi: 10.2147/DMSO.S268992
62. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. (2020) 7:ofaa250. doi: 10.1093/ofid/ofaa250
63. Zheng Y, Xu H, Yang M, Zeng Y, Chen H, Liu R, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. (2020) 127:104366. doi: 10.1016/j.jcv.2020.104366
64. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale, NJ: Erlbaum (1988).
65. Liu S, Ngo DT, Chong CR, Amarasekera AT, Procter NE, Licari G, et al. Suppression of neutrophil superoxide generation by BNP is attenuated in acute heart failure: a case for ‘BNP resistance'. Eur J Heart Fail. (2015) 17:475–83. doi: 10.1002/ejhf.242
66. Clerico A, Zaninotto M, Passino C, Plebani M. New issues on measurement of B-type natriuretic peptides. Clin Chem Lab Med. (2017) 56:32–9. doi: 10.1515/cclm-2017-0433
67. Zhang Y, Coats AJS, Zheng Z, Adamo M, Ambrosio G, Anker SD, et al. Management of heart failure patients with COVID-19: a joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2020) 22:941–56. doi: 10.1002/ejhf.1915
68. Paliogiannis P, Mangoni AA, Dettori P, Nasrallah GK, Pintus G, Zinellu A. D-Dimer Concentrations and COVID-19 severity: a systematic review and meta-analysis. Front Public Health. (2020) 8:432. doi: 10.3389/fpubh.2020.00432
69. Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. (2020) 25:30. doi: 10.1186/s40001-020-00432-3
70. Qin JJ, Cheng X, Zhou F, Lei F, Akolkar G, Cai J, et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension. (2020) 76:1104–12. doi: 10.1161/HYPERTENSIONAHA.120.15528
Keywords: B-type natriuretic peptide, COVID-19, disease severity, mortality, biomarkers
Citation: Zinellu A, Sotgia S, Carru C and Mangoni AA (2021) B-Type Natriuretic Peptide Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front. Cardiovasc. Med. 8:690790. doi: 10.3389/fcvm.2021.690790
Received: 04 April 2021; Accepted: 03 June 2021;
Published: 24 June 2021.
Edited by:
Shuyang Zhang, Peking Union Medical College Hospital, ChinaReviewed by:
John David Horowitz, University of Adelaide, AustraliaCopyright © 2021 Zinellu, Sotgia, Carru and Mangoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arduino A. Mangoni, YXJkdWluby5tYW5nb25pQGZsaW5kZXJzLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.