
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 20 July 2021
Sec. Cardiac Rhythmology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.690297
 Xin Xie1†
Xin Xie1† Gang Yang2†
Gang Yang2† Xiaorong Li1
Xiaorong Li1 Jinbo Yu1
Jinbo Yu1 Fengxiang Zhang2
Fengxiang Zhang2 Weizhu Ju2
Weizhu Ju2 Hongwu Chen2
Hongwu Chen2 Mingfang Li2
Mingfang Li2 Kai Gu2
Kai Gu2 Dian Cheng1
Dian Cheng1 Xuecheng Wang1
Xuecheng Wang1 Yizhang Wu1
Yizhang Wu1 Jian Zhou1
Jian Zhou1 Xiaoqian Zhou1
Xiaoqian Zhou1 Baowei Zhang1
Baowei Zhang1 Pipin Kojodjojo3
Pipin Kojodjojo3 Kejiang Cao2
Kejiang Cao2 Bing Yang1*
Bing Yang1* Minglong Chen2
Minglong Chen2Background: Pulmonary vein isolation (PVI) is an effective strategy in the treatment of paroxysmal atrial fibrillation (PAF). Yet, there are limited data on additional ablation beyond PVI. In this study, we sought to assess the prevalence, predictors, and outcomes of additional ablation in PAF patients.
Methods: A total of 537 consecutive patients with PAF were retrospectively evaluated for the index procedure. PVI was successfully conducted in all patients, after which electrophysiological study and drug provocation were performed, and additional ablations were delivered for concomitant arrhythmias, non-PV triggers, and low voltage zone (LVZ). The prevalence, predictors, and outcomes of additional ablation were analyzed.
Results: Among 537 consecutive patients, 372 addition ablations were performed in 241 (44.88%) patients, including 252 (67.74%) concomitant arrhythmias in 198 (36.87%) patients, 56 (15.05%) non-PV triggers in 52 (9.68%) patients and 64 (17.20%) LVZ modification in 47 (8.75%) patients. Lower LVEF (OR = 0.937, p = 0.015), AF episode before procedure (OR = 2.990, p = 0.001), AF episode during procedure (OR = 1.998, p = 0.002) and AF episode induced after PVI (OR = 15.958, p < 0.001) were independent predictors of additional ablation. Single-procedure free from atrial arrhythmias at 58.36 ± 7.12 months post-ablation was 70.48%.
Conclusions: Additional ablations were common in patients with PAF for index procedure. Lower LVEF and AF episodes before, during the procedure, and induced after PVI predicts additional ablation.
Since Haissaguerre et al. identified pulmonary veins (PVs) foci as major triggers of atrial fibrillation (AF) (1), pulmonary vein isolation (PVI) has been widely accepted as the basis of AF ablation procedures (2). Nowadays, PVI in paroxysmal atrial fibrillation (PAF) patients has reached a success rate of 46–56% (3–5) after long follow-up.
However, the PV foci are not always the only target, and PVI alone might not guarantee a long-term AF-free outcome in PAF patients. Considering AF is a progressive and complex type of arrhythmia that involves atrial substrate remodeling (2), various strategies have been tried beyond PVI, including linear ablation, complex fractionated atrial electrogram (CFAE) guided ablation, ganglionated plexus modification and focal impulse and rotor modulation (FIRM) targeting (6–9). Meanwhile, non-PV triggers and concomitant supraventricular arrhythmias also account for a large portion of ablation beyond PVI (10–12). In our center, addition ablations were mainly performed due to concomitant arrhythmias, non-PV triggers, and limited substrate modifications in PAF patients who underwent index procedure. Herein, we reported our single-center experience on the prevalence, characteristics, predictors, and outcomes of additional ablation in such population.
We retrospectively evaluated consecutive patients with AF in the First Affiliated Hospital of Nanjing Medical University between Jan 1, 2014, and Dec 31, 2015. The AF ablation volume in our center is more than 600 per year. The procedures were performed by physician with an experience of more than 100 cases per year. PAF was defined as AF that terminated spontaneously or with intervention within 7 days of onset (2). The inclusion criteria included: (1) patients aged 18 to 80 years; (2) patients diagnosed with PAF; (3) no left atrium (LA) thrombosis was detected before the procedure. The exclusion criteria were the following: (1) patients with non-paroxysmal AF; (2) patients who underwent AF ablation before; (3) abandoned procedure due to occurrence of complications. The process of patient enrollment is shown in Figure 1. The study was approved by the Institutional Review Board of Nanjing Medical University. All data were collected from hospital medical record system and stored by a specially-assigned person. All patient identifier were removed before statistical analysis.
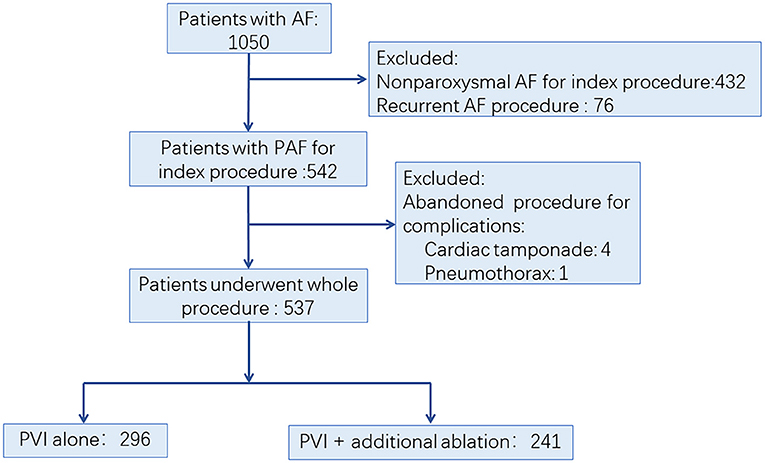
Figure 1. Trial profile. AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation; PVI, pulmonary vein isolation.
All patients were prescribed oral anticoagulants for at least 3 weeks. Antiarrhythmic agents except amiodarone were discontinued for at least 5 half-lives. Informed consent was obtained, and LA thrombus was excluded using transesophageal echocardiography before the procedure. Procedures were performed under local anesthesia and intravenous fentanyl. Access was obtained via bilateral femoral veins. A decapolar catheter was advanced into the coronary sinus and a quadripolar catheter was placed at the His bundle region. Double transseptal access was performed using 8.5F sheaths (SL1, St. Jude Medical, MN, USA). Intravenous heparin was given to maintain an activated clotting time of 300 ± 50 s.
LA was reconstructed using electroanatomic mapping systems (CARTO, Biosense Webster, CA, USA or EnSite-NavX, St Jude Medical, MN, USA). PV orifices were identified by selective venography and catheter drop movement. Antral circumferential ablation was carried out around pairs of ipsilateral PVs using an open-irrigation ablation catheter (Thermocool for CARTO; CoolFlex for ExSite NavX). A power limit of 35 W, a tip temperature limit of 43°C, and an infusion rate of 17 mL/min was adopted. Moreover, power of 30 W was selected when ablating on the posterior wall. PVI was defined as the abolition or dissociation of PV potentials with the circular mapping catheter. AF persisting after PVI was terminated by direct current cardioversion (DCCV).
Perioperative AF episodes were defined as any AF episode lasting >30 s during the perioperative period. The subtype of perioperative AF episode was categorized as: (1) AF episode before the procedure was defined as AF that presented before venous puncture. (2) AF episode during the procedure was defined as AF episode that presented from the venous puncture to PVI. (3) AF episode needing DCCV was defined as AF sustained after PVI and needing DCCV to conversion. (4) AF episode induced after PVI was defined as AF induced by electrophysiological study and/or drug provocation and sustained more than 5 min after PVI.
Concomitant arrhythmia was defined as any pre-procedural documented, spontaneous or induced sustained atrial tachyarrhythmia, supraventricular tachycardia (SVT) or symptomatic premature atrial contraction (PAC) except for AF during the procedure. The non-PV trigger was defined as recurrent PAC that originated outside the PVs and initiates AF (13). All concomitant arrhythmias and non-PV triggers were mapped and ablated (11, 14, 15). Substrate mapping and modification targeting the low voltage zone (LVZ) have been described in our previous study (16). In brief, high-density mapping was performed after PVI during sinus rhythm or high right atrium pacing. Areas with low-voltage (<0.4 mV) and abnormal local intracardiac electrograms (multiphasic electrogram with ≥3 positive or negative distinct peaks and electrogram duration ≥50 ms) were targeted for further ablation.
Electrophysiological study and drug provocation after PVI were performed in all patients. A 30-min observation period was used to assess spontaneous recovery of PV connection, during which, isoproterenol was intravenously given 4–20 ug/min to achieve a 20% increase of baseline heart rate. Programmed stimulation was given with 3 basal baseline cycle lengths (500 ms, 400 ms, and 330 ms) and up to 3 extra stimulations at either the high right atrium or coronary sinus ostium. A 40 mg of adenosine triphosphate (ATP) was used as an intravenous bolus to evaluate dormant conduction or non-PV trigger. PV reconnection, concomitant arrhythmias, and non-PV trigger were mapped and ablated. Electrophysiological study and drug provocation were re-performed until these could no longer be elicited (Figure 2). If only AF was induced, no additional ablations were delivered. And, if AF persisted, DCCV was performed to restore sinus rhythm.
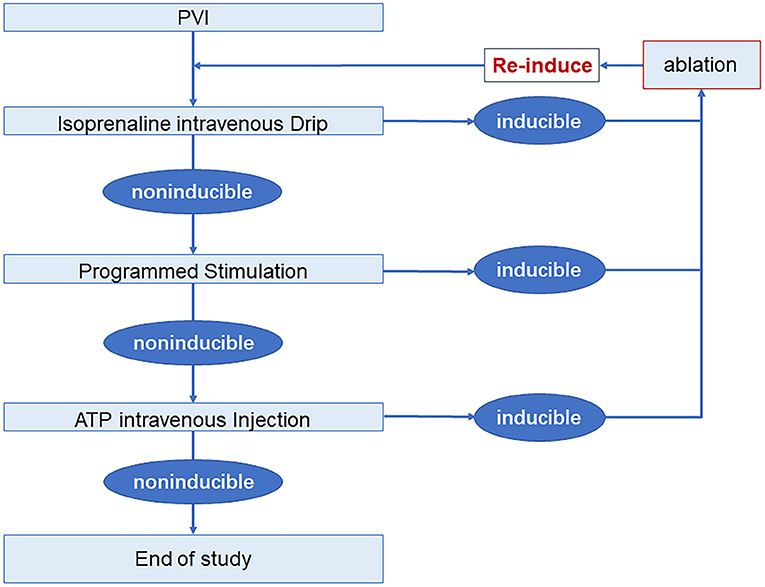
Figure 2. Electrophysiology study and drug provocation protocol. AF, atrial fibrillation; ATP, adenosine triphosphate; PVI, pulmonary vein isolation.
Oral anticoagulation therapy and antiarrhythmic drugs were prescribed for all patients for at least 2–3 months after the procedure. During the first year, all patients were followed-up through clinic visits and 24-h Holter recordings at 1, 3 (the blanking period), 6, and 12 months. In subsequent years, telephone interviews, clinic visits, and 24-h Holter recordings were undertaken every 6 months. A pulse measurement and ECG recording were recommended whenever patients were symptomatic. Successful ablation was defined as no atrial tachyarrhythmias lasting more than 30 s after the blanking period, without antiarrhythmic drugs (2).
The continuous variables are expressed as the mean ± SD. Categorical variables are expressed as number and percentage. The continuous variables were analyzed with an unpaired t-test or Wilcoxon analysis. Categorical variables were compared with the Chi-square test or Fisher's exact test. The event-free rates were calculated using Kaplan–Meier analysis, while log-rank statistics were used for group comparisons. The outcome is unknown for patients who did not reach the event during follow-up due to loss to follow-up or dying. In such cases, the time of follow up was recorded and interpreted as censored data. Univariate and multivariable logistic regression analyses and Cox regression were performed to assess independent predictors associated with additional ablation. The results are expressed as p values. Factors with p < 0.15 in univariate analyses were enrolled in multivariate analyses. A p value of <0.05 was considered as statistically significant. All statistical analyses were performed using SPSS software version 20.0.
Among 1,050 consecutive patients who underwent AF ablation between Jan 1, 2014, and Dec 31, 2015, a total of 513 patients were excluded due to non-paroxysmal AF ablation (n = 432), non-index procedure (n = 76), and abandoned procedure (n = 5), respectively. Finally, 537 PAF patients were enrolled in this study (Figure 1). Among them, 296 (55.12%) patients underwent PVI alone (Group I), while 241 (44.88%) patients had additional ablation beyond PVI (Group II). Detailed baseline characteristics of these patients are shown in Table 1. Compared with Group I, there was significantly higher CHA2DS2-VASc score, larger LA diameter, and lower left ventricular ejection fraction (LVEF) in patients in Group II, respectively (Table 1).
AF episodes before the procedure, AF episodes during the procedure, AF episodes needing DCCV and AF episode induced after PVI were documented in 54 (10.06%), 110 (20.48%), 47 (8.75%) patients, and 26 (4.84%) patients, respectively (Table 2). Compared with Group I, the patients in Group II showed a higher prevalence of AF episodes before the procedure (15.35 vs. 5.74%, p < 0.001), AF episodes during the procedure (25.31 vs. 16.55%, p = 0.001), AF episodes needing DCCV (14.11 vs. 4.39%, p < 0.001), and AF episode induced after PVI (9.96 vs. 0.68%, p < 0.001), respectively.
PVI was achieved in all patients. Totally 372 additional ablations were performed in 241 (44.88%) patients. Among them, 145 (27%) patients presented 252 (67.74%) concomitant arrhythmias alone, 24 (4.47%) patients presented non-PV trigger alone, and 18 patients (3.35%) only underwent substrate modifications. Combinations of different categories of additional ablation was performed in 54 (10.6%) patients (Figure 3).
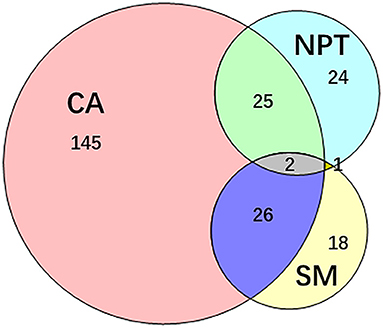
Figure 3. Additional ablation after PVI. 241 patients underwent additional ablation after PVI. The additional ablation mainly contains the ablation of concomitant arrhythmias, non-PV trigger elimination, substrate modification and their combinations. CA, concomitant arrhythmia; NPT, non-PV trigger; PVI, pulmonary vein isolation; SM, substrate modification.
Concomitant arrhythmias, which accounted for the majority of additional ablations, were found in 198 (82.16%) patients. In terms of concomitant arrhythmia type, the most common was atrial flutter (AFL, 55.16%), followed by atrial tachycardia (AT, 24.60%), SVT (13.89%), and non-PV PACs (6.35%), respectively. Concomitant arrhythmias were mostly diagnosed according to previously documented ECG (37.30%) or perioperative episode (30.16%). During PVI, spontaneously AF converted to AFL, and AT was presented in nine and one patient, respectively. Concomitant arrhythmia was induced by electrophysiological study and drug provocation in 51 (20.24%) patients, wherein programmed stimulation, isoproterenol, and ATP accounted for 35 (13.89%), 10 (3.97%), and 6 (2.38%) patients, respectively. Subjective cavotricuspid isthmus linear ablation was performed in 16 patients with the suspicion of the diagnosis of AFL. Previously successfully ablated AFL and SVT were documented in four and one patient, respectively (Table 3).
Fifty-six non-PV triggers were documented in 52 patients (9.68%). Among them, four patients presented more than one origin of non-PV triggers. Non-PV triggers were spontaneous onset that was found in 33 (58.93%) patients, and those induced by electrophysiological study and drug provocation in 19 (33.93%) patients. Arbitrary superior vena cava (SVC) isolation was conducted in 4 (7.14%) patients, according to the operator's opinion. Non-PV triggers mostly originated from SVC and the detailed distribution were listed in Supplemental Table 1.
A total of 64 LVZ modifications were performed in 47 patients. Twenty-one (32.81%) LVZ modifications were applied at LA anterior wall, followed by 9 (14.06%) at LA posterior wall, 9 (14.06%) at LA roof, 6(9.38%) at the right atrium, 6 (9.38%) at septal, 6 (9.38%) at right PV antrum, 3 (4.69%) at left atrial appendage, 3 (4.69%) at mitral valve isthmus, 1 (1.56%) at Marshall ligament, respectively. Sole LVZ was located in 33 (70.21%) patients, while 2 and 3 LVZs were noted in 10 (21.28%) and 4 (8.51%) patients, respectively.
Baseline variables and AF episodes patterns were fitted to univariate logistic regression analysis for assessing the predictors of additional ablation. Multi-variable logistic regression analysis revealed that lower LVEF (OR = 0.937, p = 0.015), AF episode before the procedure (OR = 2.990, p = 0.001), AF episode during the procedure (OR = 1.998, p = 0.002) and AF episode induced after PVI (OR = 15.958, p < 0.001) were independent predictors for additional ablation (Supplemental Table 2).
After a mean follow-up period of 58.36 ± 7.12 months, five patients (one in Group I, the other four in Group II) died: three due to respiratory diseases, 1 due to stroke, and 1 due to unknown reason. Ninety-five patients (56 in Group I, 39 in Group II) were lost to follow-up. One hundred and twenty-nine patients, 66 in Group I and 63 in Group II, recurred. The single-procedure success rate was 70.48%, with no significant difference between groups (72.38% vs. 68.18%; log-rank test, p = 0.27, Figure 4).
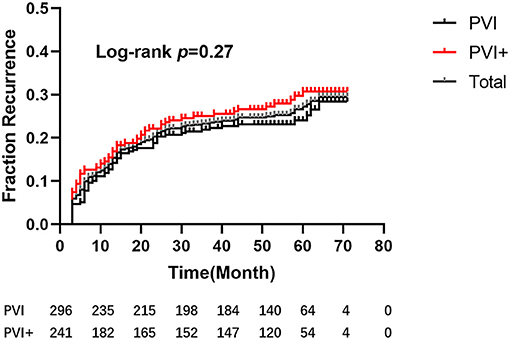
Figure 4. Kaplan–Meier estimation of freedom from atrial tachyarrhythmia after a single procedure. After a mean follow-up period of 58.36 ± 7.12 months, there were no significant differences between the 2 groups (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.58–1.16; P = 0.27).
In multi-variable Cox regression analysis, lower LVEF (OR = 0.947, p = 0.001), additional AT ablation (OR = 1.996, p = 0.002) and spontaneous non-PV trigger (OR = 1.873, p = 0.033) were independent predictors for recurrence (Supplemental Table 3).
In this study, we examined the prevalence, predictors, and outcomes of additional ablations beyond PVI in patients with PAF for the index procedure. Our result revealed that up to 44.88% of patients underwent additional ablation, which was predicted by lower LVEF, AF episode before the procedure, AF episode during the procedure, and AF episode induced after PVI. After a five-year follow-up, 70% of patients were free from AT/AF recurrence.
Since PVI was identified as the basis of AF ablation, the pursuit for better ablation outcome has never stopped (1, 2). In PAF patients, non-PV trigger ablation is commonly performed (17, 18), while some other methods of ablation beyond PVI have also been tried. Routine linear, complex fractionated electrograms, and rotor ablations were reported associated with improved clinical outcomes compared with PVI alone (6, 7, 9, 19). Faustino et al. reported a stepwise ablation strategy that accomplished a 90.7% success rate after 12-month follow-up in PAF patients (20). However, in a more recent study, elimination of triggers as an end point of ablation in patients with PAF showed a lower recurrence compared with stepwise substrate modifications (21). A meta-analysis, which included 145 studies with 23 263 patients, revealed that PVI plus studies were associated with improved outcomes, while the large residual heterogeneity lowered its confidence level (22). These ambiguous results hindered the wide clinical practice of ablation beyond PVI in PAF patients. Moreover, non-contiguous ablation lesions performed may further increase the incidence of iatrogenic arrhythmia (23). Thus, in our center, the lesions were delivered based on objective evidences, including clinical recordings, provocation results, and voltage mapping, which was consistent with previous studies (16). Accordingly, ablations beyond PVI were mainly focused on concomitant arrhythmia, non-PV trigger, and substrate modifications. No complex fractionated atrial electrogram or routine linear ablation was performed.
In our study, additional ablation beyond PVI was frequently performed mainly because of a very high incidence of concomitant arrhythmia, where AFL accounted for the majority. The coexistence of AF and AFL is frequently observed in clinical practice, and their relationship has been well recognized (12). The incidence of AT and atrioventricular node reentrant tachycardia were consistent with previous findings (11, 24), and corresponding successive ablations were associated with improved outcomes. Non-PV trigger ablations in our study accounted for 15.05% of additional ablations involving 52 patients. The reported prevalence and distribution of non-PV triggers varied in different studies, which may be due to different populations, definitions, and provocation protocols (10). Successful detection and elimination of non-PV triggers in PAF patients has been shown associated with better outcomes (17).
In a previous study (25), we used high-density mapping of the LA during sinus rhythm in different AF populations and found that as AF progressed, patients exhibited more low voltage zones, longer LA conduction times, and more complex electrograms. According to Rolf's finding, LVZs can be found in 10% of patients with PAF (26). In our study, considering the internal relationship between atrial fibrosis, LVZ, and AF, substrate modification was accordingly performed in 47 (8.75%) patients, which was comparable to Rolf's work.
Predictors of additional ablations in PAF patients have not been well illustrated in previous studies. Zhao et al. (27) reported a correlation between low-voltage and the presence of non-PV triggers in PAF patients. In the study by Piorkowski et al. age, sex, AF type, and left atrial appendage velocity were independently associated with LVZs (26). As far as we know, our work was the first study that describe all additional ablations, including concomitant arrhythmia, non-PV trigger, and LVZ ablations in one study. In our study, lower LVEF, AF episode before the procedure, AF episode during the procedure, and AF episode induced after PVI resulted as independent predictors for additional ablation. Risk stratification for the needs of additional ablation using these clinical parameters may support perioperative preparation. And, after PVI, special attention should be paid to patients with these risk factors.
Several studies had presented 5–6 years of follow-up data after PVI in PAF patients with a success rate ranging from 46 to 56% (3–5). In our study, a 5-years follow-up revealed a free rate of 70%. With reference to baseline characteristics, patients who underwent additional ablations presented a larger left atrium and lower LVEF, which were previously reported as the risk factors of recurrence (18, 28–30). Moreover, non-PV triggers and worse left atrium substrate may further worsen the outcome (13, 31). Though presenting a relative worse clinic characteristic, patients underwent additional ablation still showed a 68.18% success rate after long follow-up. According to Piorkowski's findings (26), limited substrate modification of LVZs may potentially have a compensatory effect for the impaired outcome in patients with endocardial structural defects. In our study, this effect might be expended with additional ablation in patients with worse baseline conditions. Nonetheless, further prospective, controlled clinical trials containing more variables, such as obstructive sleep apnea and PV anatomy (32), are needed to further clarify these results.
This study has some limitations needed to point out, such as a retrospective design. However, the clinical characteristics, procedure-related data, and follow-up were prospectively collected, and the population was relatively large, helping to minimize bias. Second, although all patients were educated about the follow-up, 17% of patients were lost in 5 years. However, the loss was comparable in both groups. Third, 24-h Holter and telephone interviews, rather than an insert able cardiac monitor, have the potential to underestimate recurrence. Last but not the least, there was no control group of patients undergoing PVI only which would strengthen our findings. Further prospective randomized study would better clarify this hypothesis.
Additional ablations were common in patients with PAF for index procedure. Lower LVEF and AF episodes before, during the procedure, and induced after PVI may predicts additional ablation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Institutional Review Board of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was funded by Top-level Clinical Discipline Project of Shanghai Pudong District (PWYgf2018-02) and Health Commission of Shanghai Pudong District (PW2019D-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.690297/full#supplementary-material
1. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339:659–66. doi: 10.1056/NEJM199809033391003
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2020) 42:373–498. doi: 10.1093/eurheartj/ehaa612
3. Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. (2010) 122:2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806
4. Sawhney N, Anousheh R, Chen WC, Narayan S, Feld GK. Five-year outcomes after segmental pulmonary vein isolation for paroxysmal atrial fibrillation. Am J Cardiol. (2009) 104:366–72. doi: 10.1016/j.amjcard.2009.03.044
5. Uchiyama T, Miyazaki S, Taniguchi H, Komatsu Y, Kusa S, Nakamura H, et al. Six-year follow-up of catheter ablation in paroxysmal atrial fibrillation. Circ J. (2013) 77:2722–7. doi: 10.1253/circj.CJ-13-0468
6. Hocini M, Jaïs P, Sanders P, Takahashi Y, Rotter M, Rostock T, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. (2005) 112:3688–96. doi: 10.1161/CIRCULATIONAHA.105.541052
7. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. (2004) 43:2044–53. doi: 10.1016/j.jacc.2003.12.054
8. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. (2011) 8:672–8. doi: 10.1016/j.hrthm.2010.12.047
9. Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol. (2014) 63:1761–8. doi: 10.1016/j.jacc.2014.02.543
10. Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. (2017) 14:1087–96. doi: 10.1016/j.hrthm.2017.02.030
11. Sauer WH, Alonso C, Zado E, Cooper JM, Lin D, Dixit S, et al. Atrioventricular nodal reentrant tachycardia in patients referred for atrial fibrillation ablation: response to ablation that incorporates slow-pathway modification. Circulation. (2006) 114:191–5. doi: 10.1161/CIRCULATIONAHA.106.621896
12. Waldo AL. Atrial fibrillation and atrial flutter: Two sides of the same coin! Int J Cardiol. (2017) 240:251–2. doi: 10.1016/j.ijcard.2017.02.146
13. Chang HY, Lo LW, Lin YJ, Chang SL, Hu YF Li CH, et al. Long-term outcome of catheter ablation in patients with atrial fibrillation originating from nonpulmonary vein ectopy. J Cardiovasc Electrophysiol. (2013) 24:250–8. doi: 10.1111/jce.12036
14. Chen SA, Tai CT. Catheter ablation of atrial fibrillation originating from the non-pulmonary vein foci. J Cardiovasc Electrophysiol. (2005) 16:229–32. doi: 10.1046/j.1540-8167.2005.40665.x
15. Tai CT, Chen SA, Chiang CE, Lee SH, Wen ZC, Huang JL, et al. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J Cardiovasc Electrophysiol. (1998) 9:115–21. doi: 10.1111/j.1540-8167.1998.tb00892.x
16. Yang B, Jiang C, Lin Y, Yang G, Chu H, Cai H, et al. STABLE-SR (Electrophysiological Substrate Ablation in the Left Atrium During Sinus Rhythm) for the Treatment of Nonparoxysmal Atrial Fibrillation: A Prospective, Multicenter Randomized Clinical Trial. Circ Arrhythm Electrophysiol. (2017) 10:e005405. doi: 10.1161/CIRCEP.117.005405
17. Hayashi K, An Y, Nagashima M, Hiroshima K, Ohe M, Makihara Y, et al. Importance of nonpulmonary vein foci in catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm. (2015) 12:1918–24. doi: 10.1016/j.hrthm.2015.05.003
18. Tilz RR, Heeger CH, Wick A, Saguner AM, Metzner A, Rillig A, et al. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. (2018) 11:e005250. doi: 10.1161/CIRCEP.117.005250
19. Fassini G, Riva S, Chiodelli R, Trevisi N, Berti M, Carbucicchio C, et al. Left mitral isthmus ablation associated with PV Isolation: long-term results of a prospective randomized study. J Cardiovasc Electrophysiol. (2005) 16:1150–6. doi: 10.1111/j.1540-8167.2005.50192.x
20. Faustino M, Pizzi C, Agricola T, Xhyheri B, Costa GM, Flacco ME, et al. Stepwise ablation approach versus pulmonary vein isolation in patients with paroxysmal atrial fibrillation: Randomized controlled trial. Heart Rhythm. (2015) 12:1907–15. doi: 10.1016/j.hrthm.2015.06.009
21. Lee KN, Roh SY, Baek YS, Park HS, Ahn J, Kim DH, et al. Long-term clinical comparison of procedural end points after pulmonary vein isolation in paroxysmal atrial fibrillation: elimination of nonpulmonary vein triggers versus noninducibility. Circ Arrhythm Electrophysiol. (2018) 11:e005019. doi: 10.1161/CIRCEP.117.005019
22. Cluckey A, Perino AC, Yunus FN, Leef GC, Askari M, Heidenreich PA, et al. Efficacy of ablation lesion sets in addition to pulmonary vein isolation for paroxysmal atrial fibrillation: findings from the smash - af meta-analysis study cohort. J Am Heart Assoc. (2019) 8:e009976. doi: 10.1161/JAHA.118.009976
23. Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. (2007) 115:2606–12. doi: 10.1161/CIRCULATIONAHA.107.691386
24. Hara H, Yoshinaga M, Matsui Y, Yamamoto S, Ishido T, Yutaka K, et al. Clinical significance of induced left atrial macro-reentrant tachycardia after pulmonary vein isolation. J Interv Card Electrophysiol. (2016) 46:167–76. doi: 10.1007/s10840-015-0055-8
25. Lin Y, Yang B, Garcia FC, Ju W, Zhang F, Chen H, et al. Comparison of left atrial electrophysiologic abnormalities during sinus rhythm in patients with different type of atrial fibrillation. J Interv Card Electrophysiol. (2014) 39:57–67. doi: 10.1007/s10840-013-9838-y
26. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. (2014) 7:825–33. doi: 10.1161/CIRCEP.113.001251
27. Zhao Y, Di Biase L, Trivedi C, Mohanty S, Bai R, Mohanty P, et al. Importance of non-pulmonary vein triggers ablation to achieve long-term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm. (2016) 13:141–9. doi: 10.1016/j.hrthm.2015.08.029
28. Gokoglan Y, Mohanty S, Gunes MF, Trivedi C, Santangeli P, Gianni C, et al. Pulmonary Vein Antrum Isolation in Patients With Paroxysmal Atrial Fibrillation: More Than a Decade of Follow-Up. Circ Arrhythm Electrophysiol. (2016) 9. doi: 10.1161/CIRCEP.115.003660
29. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. (2013) 2:e004549. doi: 10.1161/JAHA.112.004549
30. Cheng WH, Lo LW, Lin YJ, Chang SL, Hu YF, Hung Y, et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm. (2019) 16:1327–33. doi: 10.1016/j.hrthm.2019.03.028
31. Yamaguchi T, Tsuchiya T, Fukui A, Kawano Y, Otsubo T, Takahashi Y, et al. Impact of the extent of low-voltage zone on outcomes after voltage-based catheter ablation for persistent atrial fibrillation. J Cardiol. (2018) 72:427–33. doi: 10.1016/j.jjcc.2018.04.010
32. Bose A, Chevli PA, Berberian G, Januszkiewicz J, Ahmad G, Hashmath Z, et al. Presence of a left common pulmonary vein and pulmonary vein anatomical characteristics as predictors of outcome following cryoballoon ablation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol. (2020). doi: 10.1007/s10840-020-00916-6. [Epub ahead of print].
Keywords: atrial fibrillation, catheter ablation, additional ablation, concomitant arrhythmia, non-pulmonary vein trigger
Citation: Xie X, Yang G, Li X, Yu J, Zhang F, Ju W, Chen H, Li M, Gu K, Cheng D, Wang X, Wu Y, Zhou J, Zhou X, Zhang B, Kojodjojo P, Cao K, Yang B and Chen M (2021) Prevalence and Predictors of Additional Ablation Beyond Pulmonary Vein Isolation in Patients With Paroxysmal Atrial Fibrillation. Front. Cardiovasc. Med. 8:690297. doi: 10.3389/fcvm.2021.690297
Received: 02 April 2021; Accepted: 28 June 2021;
Published: 20 July 2021.
Edited by:
Mehdi Namdar, Geneva University Hospitals (HUG), SwitzerlandReviewed by:
François Regoli, University of Zurich, SwitzerlandCopyright © 2021 Xie, Yang, Li, Yu, Zhang, Ju, Chen, Li, Gu, Cheng, Wang, Wu, Zhou, Zhou, Zhang, Kojodjojo, Cao, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Yang, eWJoZWFydEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.