
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 12 July 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.685904
This article is part of the Research Topic Classical and Novel Biomarkers for Cardiovascular Disease View all 16 articles
Objective: The diagnostic performance of soluble suppression of tumorigenicity (sST2) in heart failure (HF) had been investigated in multiple studies, but the results were inconsistent. This meta-analysis evaluated the diagnostic value of sST2 in HF.
Methods: Pubmed, Web of Science, Embase, and Cochrane Library databases were searched until March 2021. Cohort studies or case-control studies relevant to the diagnostic value of sST2 in HF were screened, and true positive (TP), false positive (FP), false negative (FN), and true negative (TN) data were extracted for calculating sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC). The quality of the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS), the threshold effect was determined by calculating Spearman correlation coefficients and summary receiver operating characteristic (SROC) curve patterns, the heterogeneity was evaluated using the I2 statistic and the Galbraith radial plot, and sensitivity analysis was also performed. Deeks' test was used to assess publication bias.
Results: A total of 11 studies from 10 articles were included in this meta-analysis. The Spearman correlation coefficient was 0.114, p = 0.739, and the SROC curve did not show a “shoulder-arm” shape, which suggests that there was no threshold effect, but study heterogeneity existed because of non-threshold effects. The combined sensitivity was 0.72 [95% confidence interval (CI): 0.65–0.78], specificity was 0.65 (95% CI: 0.45–0.81), PLR was 1.75 (95% CI: 1.33–2.31), NLR was 0.48 (95% CI: 0.37–0.63), DOR was 3.63 (95% CI: 2.29–5.74), and AUC was 0.75. The Deeks' test suggested no significant publication bias in the included studies (P = 0.94).
Conclusion: sST has some diagnostic value in HF, but this should be further evaluated in additional studies with rigorous design and high homogeneity.
Heart failure (HF) is a clinical syndrome of cardiac blood flow impairment caused by ventricular systolic or diastolic insufficiency. It is a global health concern with high morbidity and mortality and has seriously endangered human health (1). Currently, HF is diagnosed based on clinical symptoms, medical history, echocardiography, B-type natriuretic peptide (BNP), and N-terminal (NT)-proBNP (2). However, because of the atypical symptoms and signs of HF, the ancillary tests such as echocardiography and invasive hemodynamics are often limited by factors such as medical condition, and BNP or NT-proBNP levels are easily affected by age, sex, body size, and renal function, which makes the diagnosis and management of HF still a clinical challenge (3). Simple, sensitive, and specific techniques are required to assist in the diagnosis of HF, and HF-related biological markers are the current focus of HF diagnosis (4). Soluble suppression of tumorigenicity 2 (sST2), a marker associated with cardiomyocyte traction, is a potential pathophysiological mediator of myocardial hypertrophy and myocardial fibrosis and an important biomarker of HF (5). Several trials have now confirmed that sST2 levels are significantly elevated in patients with HF and that the elevated levels of sST2 correlate significantly with the degree of HF (6, 7). In recent years, more studies have been reported on the diagnosis of HF using sST2, but the results of these studies vary significantly. In this study, we intend to systematically evaluate the diagnostic value of sST2 in HF using meta-analysis.
For English databases Pubmed, Web of Science, Embase, and Cochrane Library, Heart failure, ST2, and diagnostic test were searched as the key words by the combination of medical subject headings (MeSH) and entry term. The literature search start date was not restricted, and the search end date was March 2021. The search language was only English. The following search strategy was used for pubmed and modified to suit other databases (the detailed retrieval strategy of other databases in Supplementary Documents):
#1 heart failure[MeSH Terms]
#2 ((((((((((((((Cardiac Failure[Title/Abstract]) OR (Heart Decompensation[Title/Abstract])) OR (Decompensation, Heart[Title/Abstract])) OR (Heart Failure, Right-Sided[Title/Abstract])) OR (Heart Failure, Right Sided[Title/Abstract])) OR (Right-Sided Heart Failure[Title/Abstract])) OR (Right Sided Heart Failure[Title/Abstract])) OR (Myocardial Failure[Title/Abstract])) OR (Congestive Heart Failure[Title/Abstract])) OR (Heart Failure, Congestive[Title/Abstract])) OR (Heart Failure, Left-Sided[Title/Abstract])) OR (Heart Failure, Left Sided[Title/Abstract])) OR (Left-Sided Heart Failure[Title/Abstract])) OR (Left Sided Heart Failure[Title/Abstract]))) OR (HF)
#3 #1 OR #2
#4 ((((((Soluble suppression of tumorigenicity 2[Title/Abstract]) OR (Soluble suppression of tumorigenicity-2[Title/Abstract])) OR (suppression of tumorigenicity 2[Title/Abstract])) OR (suppression of tumorigenicity-2[Title/Abstract])) OR (sST2[Title/Abstract])) OR (ST2[Title/Abstract])) OR (soluble ST2[Title/Abstract])
#5 “sensitiv*”[Title/Abstract] OR “sensitivity and specificity”[MeSH Terms] OR (“predictive”[Title/Abstract] AND “value*”[Title/Abstract]) OR (“predictive value of tests”[MeSH Terms] OR (“predictive”[All Fields] AND “value”[All Fields] AND “tests”[All Fields]) OR “predictive value of tests”[All Fields]) OR “accuracy*”[Title/Abstract]
#6 #3 AND #4 AND #5.
Literature inclusion criteria: (1) cohort studies or case-control studies investigating sST2 for the diagnosis of HF; (2) valid data available in the literature for the calculation of true positives (TPs), false positives (FPs), false negatives (FNs), and true negatives (TNs) to obtain information for a four-grid table; and (3) high quality studies using quality evaluation (see below). Exclusion criteria: (1) reviews, conference papers, and letters; (2) literature that cannot provide valid data for a four-grid table; (3) literature with duplicate data; (4) literature reporting results from animals or cellular models; (5) literature with too small a sample size (n <100); (6) literature of low quality using quality evaluation. This systematic evaluation was performed by two authors who independently judged whether the retrieved literature could be included in the study, and the third author made an independent judgment whether to include it in case of disagreement.
The quality assessment of diagnostic accuracy studies (QUADAS) tool provided by the Cochrane Collaboration system was used to evaluate the quality of the literature. The QUADAS tool evaluates the four biases in terms of case selection, trials to be evaluated, gold standard, and flow, and it evaluates the quality of the literature by assessing 11 landmark questions and three types of clinical applicability questions. The 11 landmark issues were evaluated as “Yes” for clear fit, “Unclear” for unclear, and “No” for not meeting the conditions; the four biases were evaluated as “High risk” for clear bias, “Unclear” for unclear bias, and “Low risk” for no clear bias; and the three types of clinical applicability were evaluated as “High concern” for good matches, “Unclear” for unclear matches, and “Low concern” for poor matches. For each included study, two authors evaluated the quality independently, and the third author made an independent judgment in case of disagreement.
The extracted information included the basic information of the study and the four-grid table information. The basic information included authors, year of publication, country, sample size, mean age, sST2 detection method, sST2 cut-off, HF diagnostic criteria, HF type, control population, and study type. TP, FP, TN, and FN data were extracted from the included studies, and data that could not be extracted directly could be obtained by data transformation or by contacting the authors.
Statistical analysis of the data was performed using Stata 15 and Meta-Disc (version 14.0) software. First, threshold effects were determined using Spearman correlation coefficient and the pattern of the summary receiver operating characteristic cure (SROC) curve. Then, the combined effect indicators—sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the curve (AUC) of SROC—were calculated. Heterogeneity was tested with the chi-square test using the I2 of Q statistic, and I2 <50% or P > 0.05 indicated no significant heterogeneity among studies, and the effect indicators were combined using the fixed effect model (FEM); I2 > 50% or P < 0.05 indicated a significant heterogeneity among studies, so the effect indicators were combined using the randomized effect model (REM), and heterogeneity analysis and sensitivity analysis were conducted. The Deeks' test was used to assess publication bias. P < 0.05 was considered a statistically significant difference.
Five hundred and twenty-seven articles were obtained by searching with the proposed input, and a total of 407 articles were retrieved after removing duplicates. By reading the titles and abstracts, 389 articles were initially excluded (55 were not clincal trial; 77 were not heart failure related; 257 were not diagnose related) according to the inclusion and exclusion criteria. A total of 18 articles was investigated, and eight of them were excluded by reading full-text. For the eight excluded articles, one was duplicate publication, one was testing for sST2, four were prognosis related and two with no access to the four-grid table information. Finally, 10 articles with 11 studies were included in the meta-analysis (8–17) (Figure 1).
A total of 11 studies were included. Santhanakrishnan et al. (9) divided HF patients into HF with preserved ejection fraction (HFPEF) and HF with reduced ejection fraction (HFREF) and studied them separately, and thus this literature was considered as two studies. The basic information of the included studies is shown in Table 1. The total sample size of 8,361 patients was included, involving cases from Australia, New Zealand, Singapore, the United Kingdom, Germany, and China. Ten studies investigated middle-aged and elderly populations, and one study focused on children. There were five cohort studies and six case-control studies. sST2 was detected using enzyme-linked immunosorbent assays (ELISAs), and sST2 kits were available from five manufacturers, including MBL, PresageTM, and R&D. Regarding the type of HF, four studies included patients with HFPEF, one included patients with HFREF, and the other six studies did not distinguish between reduced and preserved ejection fractions. The control population included people with dyspnea unrelated to HF, children with hypertension unrelated to HF, healthy populations, and community populations. TP, FP, FN, and TN data were extracted from each study for the meta-analysis (Table 2), and the quality evaluation of the included studies is shown in Figure 2.
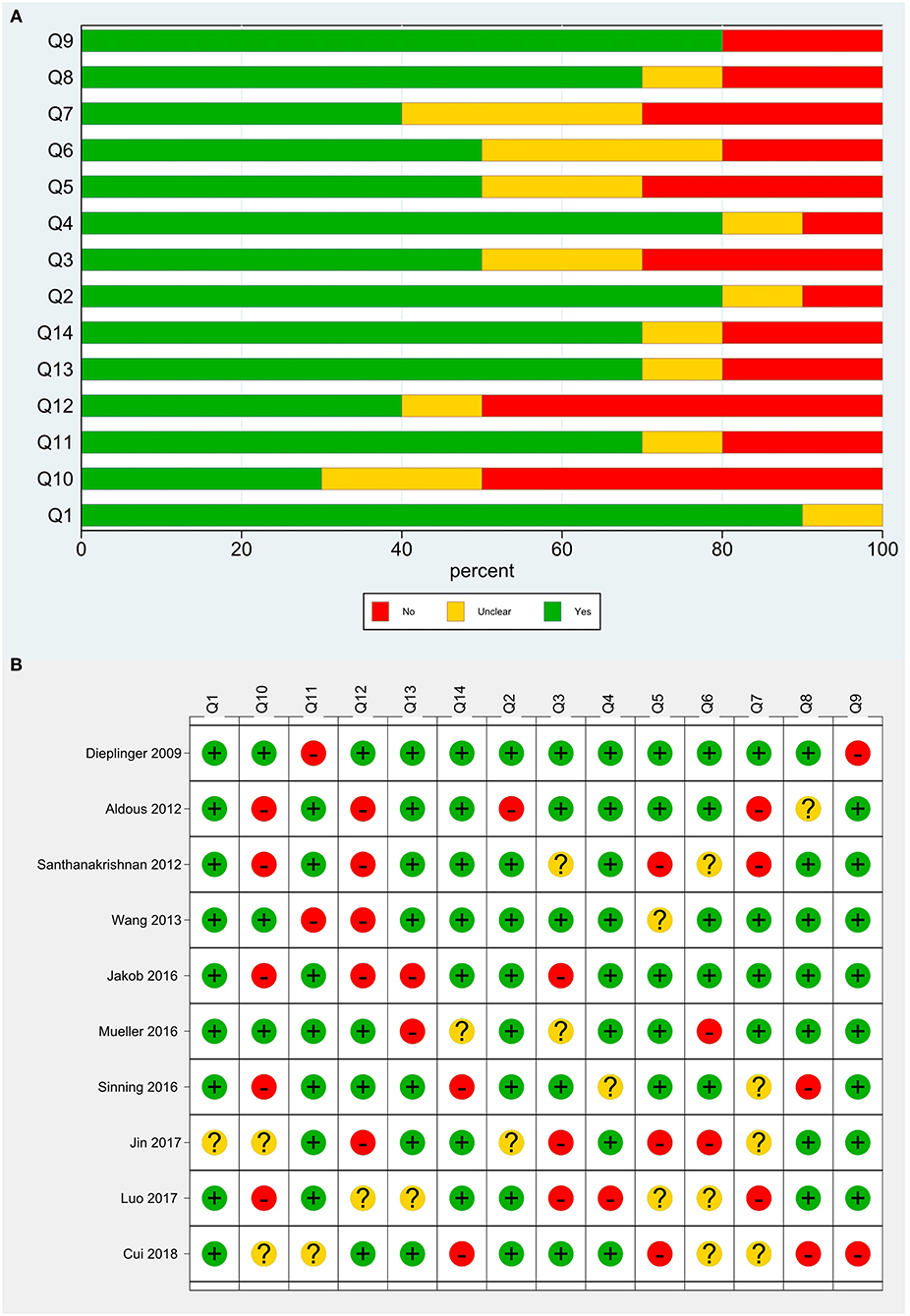
Figure 2. Quality evaluation of the included studies. (A) Review authors' judgments presented as percentages for the included studies; (B) Review authors' judgements for each included study.
Meta-disc analysis showed that the Spearman correlation coefficient between the log of sensitivity and the log of (1-specificity) was 0.114, P = 0.739, and the SROC curve showed no “shoulder-arm” pattern, which suggests that there was no threshold effect in this study.
The combined sensitivity of sST2 for the diagnosis of HF was 0.72 (95% confidence interval (CI): 0.65–0.78) (Figure 3), the combined specificity was 0.65 (95% CI: 0.45–0.81) (Figure 3), the combined PLR was 1.75 (95% CI: 1.33–2.31) (Figure 4A), the combined NLR was 0.48 (95% CI: 0.37–0.63) (Figure 4B), and the combined DOR was 3.63 (95% CI: 2.29–5.74) (Figure 4C). The AUC of the SROC curve was 0.75 (Figure 4D).
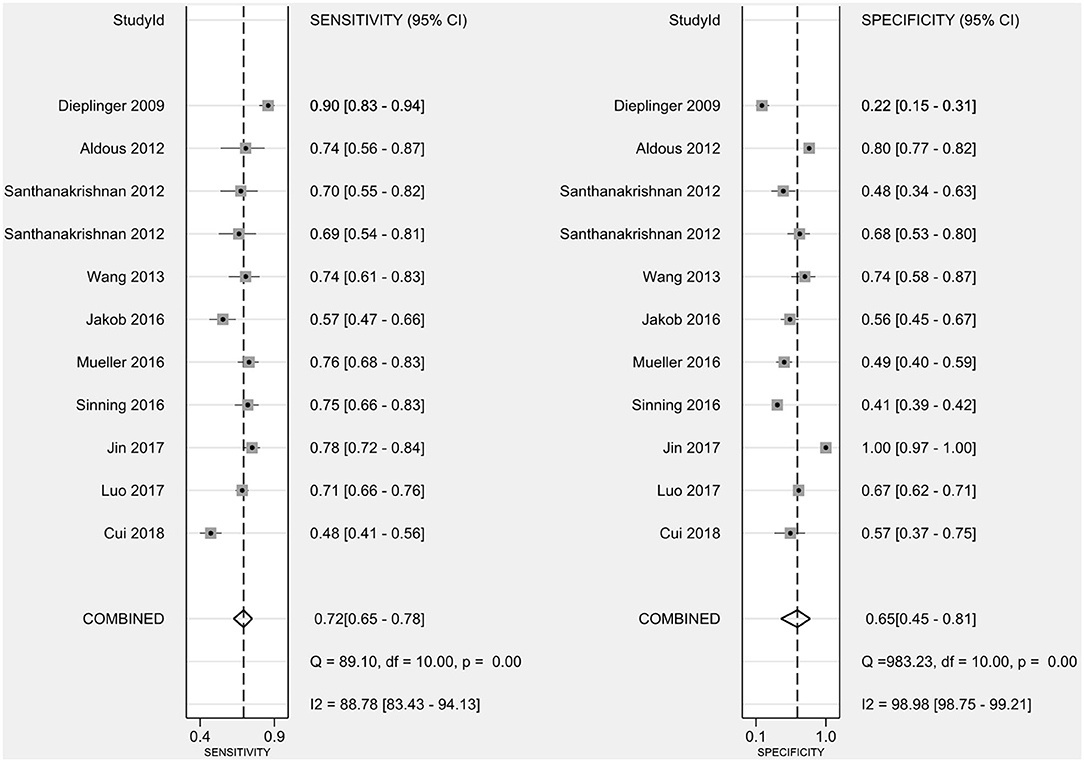
Figure 3. The combined sensitivity and specificity of sST2 for the diagnosis of HF. sST2, soluble suppression of tumorigenicity; HF, heart failure.
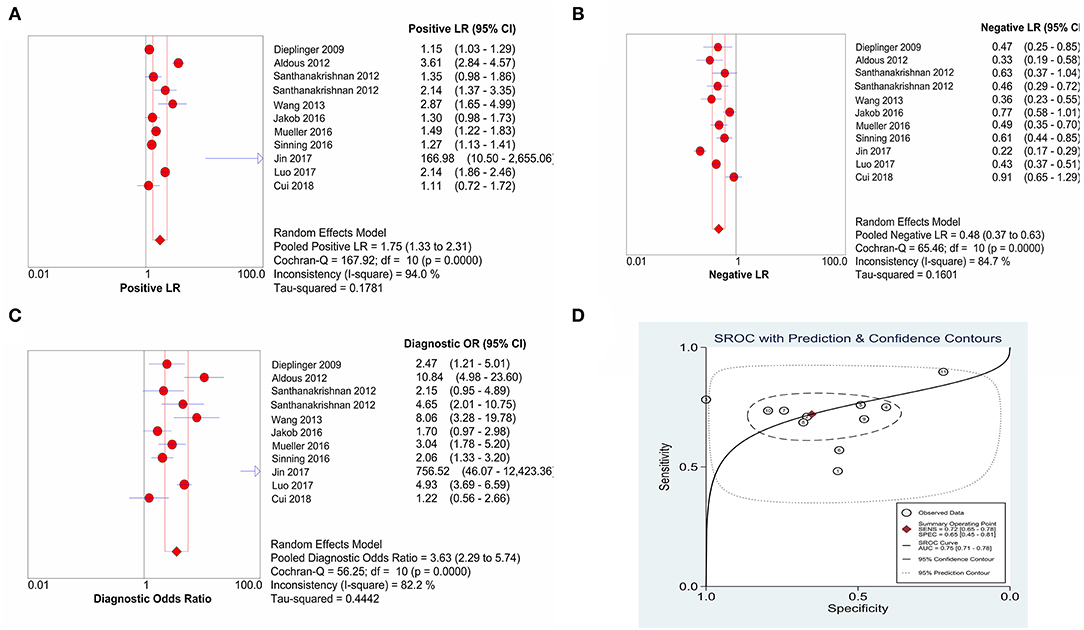
Figure 4. The forest plots of (A) PLR, (B) NLR, (C) DOR, and (D) AUC of SROC. PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; AUC, area under curve; SROC, summary receiver operating characteristic.
Heterogeneity tests showed that I2 = 88.78% (P < 0.0001) for sensitivity, I2 = 98.98% (P < 0.0001) for specificity, I2 = 94.0% (P < 0.0001) for PLR, I2 = 84.7% (P < 0.0001) for NLR, and I2 = 82.2% (P < 0.0001) for DOR, which suggests the presence of heterogeneity unrelated to threshold effects in this study, so the effect sizes were combined using a randomized effect model and the source of heterogeneity was analyzed. The Galbraith radial plot (Figure 5) showed that four studies conducted by Dieplinger et al., Santhanakrishnan et al., Jakob et al., and Cui et al. were the sources of the heterogeneity.
Sensitivity analysis of the data from this study showed that the studies conducted by Santhanakrishnan et al. and Cui et al. had the most impact on the calculation of the results of this study (Figure 6A), while the other original studies had no impact on the calculation of the study results. Taken together, the results of this study were relatively stable. Sensitivity analysis of the impact of individual studies showed that the exclusion of the study conducted by Cui et al. had the most effect on the calculation of results in this meta-analysis (Figure 6B).
The Deeks' test was performed using Stata software to assess publication bias (Figure 7); it showed a P = 0.94, which suggests that there was no significant publication bias in the included studies.
HF is a common outcome of multiple cardiovascular diseases. Cardiac overload and myocardial cell injury can lead to reduced cardiac function, which results in compensatory changes such as ventricular hypertrophy and chamber enlargement, as well as corresponding changes in cardiomyocytes, extracellular matrix, and collagen fiber networks followed by ventricular remodeling, leading to further deterioration of cardiac function (18). Multiple factors are involved in the progression of HF, such as myocardial necrosis, apoptosis, autophagy, fibrosis, oxidative stress, inflammatory response, and neurohumoral regulatory disorders, as well as changing levels in a series of biomarkers (19). The American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) guidelines released in 2017 stated that BNP and NT-proBNP provide clear diagnostic value in patients with chronic HF (20). BNP or NT-proBNP has been used clinically as a routine test for HF, but it is susceptible to various factors such as age, sex, and disease condition. Among these biomarkers, the myocardial fibrosis marker sST2 is not affected by factors like age, sex, renal function, and weight (21). Meanwhile, the 2017 ACC/AHA/HFSA guidelines recommend measuring sST2 for risk stratification of patients with chronic HF (20). This suggests that sST2 has some value in the diagnosis and prognosis of HF.
ST2 is a member of the interleukin-1 (IL-1) receptor superfamily, which is encoded by the ST2 gene in cardiomyocytes during myocardial stretch and under mechanical stress. The ST2 gene is located on human chromosome 2q12 and can encode two isoforms of sST2 and the transmembrane receptor form of ST2 (ST2L). Interleukin-33 (IL-33) is a functional ligand for ST2, and the ST2/IL-33 signaling pathway exerts cardioprotective effects by activating ST2L receptors to reduce myocardial fibrosis, inhibit cardiomyocyte hypertrophy, and improve cardiac function, which does not require the sST2 receptor (22). During HF, the increased cardiac load exposes the myocardium to excessive stretch stimulation, and the overproduced sST2 can compete with ST2L for binding IL-33, abrogating the cardioprotective effect of the ST2/IL-33 signaling pathway; this leads to apoptosis, hypertrophy and fibrosis of cardiomyocytes, and further deterioration of cardiac function, aggravating the HF process (23). This suggests that sST2 plays an important role in the development of HF. Clinical studies on the diagnostic value of sST2 in HF have gradually increased in recent years (24). In this study, the clinical diagnostic value of sST2 in HF was evaluated using meta-analysis.
Meta-analysis showed that the combined sensitivity was 0.72, specificity was 0.65, DOR was 3.63, and AUC was 0.75, which indicates that sST2 has a good diagnostic value for HF. The meta-analysis conducted by Huang et al. (24) included 10 original studies, all of which were conducted before 2014 and published in either Chinese or English, and their combined sensitivity was 0.84, specificity was 0.74, DOR was 8.49, and AUC was 0.81. Both the sensitivity and specificity in this study were about 10% lower than those in the Huang et al. (24), which may be related to the inclusion of recent literature and more stringent quality screening performed in this study, but both meta-analyses had a high degree of heterogeneity. Diagnostic studies are generally more heterogeneous than other types of clinical studies because of a possible bias in case selection, trials to be evaluated, gold standards, and flow. This study showed that four studies, those conducted by Dieplinger et al., Santhanakrishnan et al., Jakob et al., and Cui et al. may be the source of heterogeneity in this meta-analysis, and the study conducted by Cui et al. had the most impact on the results of the meta-analysis. The analysis revealed that Dieplinger et al. used a sST2 kit from MBL, Santhanakrishnan et al. conducted a case-control study, Jakob et al. focused on children, and Cui et al. performed a case-control study on patients with HFPEF using a sST2 kit from Shanghai Qiyi Biological Co.; thus, the above-mentioned differences may have contributed to the large heterogeneity observed in this study. In addition, the heterogeneity of this study may have also been caused by the disease typing (different degrees of HF in different studies), the composition of the disease spectrum (the patient group may be combined with other diseases, and the control group includes patients with various cardiovascular diseases without HF), the diagnostic thresholds (the thresholds were not uniform among studies), and differences in the sST2 detection methods. Moreover, there was also heterogeneity because of mixed bias caused by the HF type, control population, sST2 kit, HF diagnostic criteria, study type, and other biases.
Although this meta-analysis included a relatively comprehensive literature search, there were still some limitations. First, the heterogeneity of the included studies was high, and the potential sources include HF type, control population, sST2 kit, HF diagnostic criteria, and study type, with possible heterogeneity between subgroups and from other sources. Second, most of the included studies were case-control studies, which could cause selection bias in the selection of study subjects and increase diagnostic sensitivity. Third, the diagnostic cut-off values of sST2 were not uniform, and the diagnostic cut-off values of sST2 varied among the 11 included studies, which may have been related to factors such as kits, test conditions, and sample-handling methods.
In general, sST2 has some diagnostic value for HF, but factors such as HF type, control population, sST2 kits, HF diagnostic criteria, and study type in the original studies may have affected its diagnostic value. Therefore, we still need to design prospective cohort studies with high quality, large sample sizes, uniform study populations, uniform control populations, and uniform test methods to further explore and validate the reliability of the results of this analysis; we also need to establish an accurate cut-off value for sST2 to provide clinical guidance for the diagnosis of HF.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
This study was designed by ZF and JuY. CY and JiY contributed data to the paper. Statistical analysis and interpretation of data were performed by JiY, JZ, WZ, and JW. All authors were involved in drafting and revision of the manuscript for important intellectual content and approved the final version to be published.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81800258, 81770360, and 81670333), Hubei Province Health and Family Planning Scientific Research Project (WJ2019Z004), Hubei Provincial Natural Science Foundation of China (2018CFA044), Hubei Province's Outstanding Medical Academic Leader program, and the Medical and Health Research Project of Yichang city, China (Grant No. A20-2-004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Mark Abramovitz, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.685904/full#supplementary-material
Supplementary Document 1. Literature searches and search strategy.
Supplementary Table 1. Included and excluded reference.
1. Agbor VN, Ntusi NAB, Noubiap JJ. An overview of heart failure in low- and middle-income countries. Cardiovasc Diagn Ther. (2020) 10:244–51. doi: 10.21037/cdt.2019.08.03
2. Shiraishi Y, Kawana M, Nakata J, Sato N, Fukuda K, Kohsaka S. Time-sensitive approach in the management of acute heart failure. ESC Heart Fail. (2021) 8:204–21. doi: 10.1002/ehf2.13139
3. Sabanayagam A, Cavus O, Williams J, Bradley E. Management of heart failure in adult congenital heart disease. Heart Fail Clin. (2018) 14:569–77. doi: 10.1016/j.hfc.2018.06.005
4. Sarhene M, Wang Y, Wei J, Huang Y, Li M, Li L, et al. Biomarkers in heart failure: the past, current and future. Heart Fail Rev. (2019) 24:867–903. doi: 10.1007/s10741-019-09807-z
5. Lotierzo M, Dupuy AM, Kalmanovich E, Roubille F, Cristol JP. sST2 as a value-added biomarker in heart failure. Clin Chim Acta. (2020) 501:120–30. doi: 10.1016/j.cca.2019.10.029
6. Sobieszek G, Powrózek T, Jaroszyński A, Skwarek-Dziekanowska A, Rahnama-Hezavah M, Małecka-Massalska T. Soluble ST2 proteins in male cachectic patients with chronic heart failure. Nutr Metab Cardiovasc Dis. (2021) 31:886–93. doi: 10.1016/j.numecd.2020.11.014
7. Crnko S, Printezi MI, Jansen TPJ, Leiteris L, van der Meer MG, Schutte H, et al. Prognostic biomarker soluble ST2 exhibits diurnal variation in chronic heart failure patients. ESC Heart Fail. (2020) 7:1224–33. doi: 10.1002/ehf2.12673
8. Dieplinger B, Gegenhuber A, Haltmayer M, Mueller T. Evaluation of novel biomarkers for the diagnosis of acute destabilised heart failure in patients with shortness of breath. Heart. (2009) 95:1508–13. doi: 10.1136/hrt.2009.170696
9. Aldous SJ, Richards AM, Troughton R, Than M. ST2 has diagnostic and prognostic utility for all-cause mortality and heart failure in patients presenting to the emergency department with chest pain. J Card Fail. (2012) 18:304–10. doi: 10.1016/j.cardfail.2012.01.008
10. Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. (2012) 14:1338–47. doi: 10.1093/eurjhf/hfs130
11. Wang YC, Yu CC, Chiu FC, Tsai CT, Lai LP, Hwang JJ, et al. Soluble ST2 as a biomarker for detecting stable heart failure with a normal ejection fraction in hypertensive patients. J Card Fail. (2013) 19:163–8. doi: 10.1016/j.cardfail.2013.01.010
12. Hauser JA, Demyanets S, Rusai K, Goritschan C, Weber M, Panesar D, et al. Diagnostic performance and reference values of novel biomarkers of paediatric heart failure. Heart. (2016) 102:1633–9. doi: 10.1136/heartjnl-2016-309460
13. Mueller T, Gegenhuber A, Leitner I, Poelz W, Haltmayer M, Dieplinger B. Diagnostic and prognostic accuracy of galectin-3 and soluble ST2 for acute heart failure. Clin Chim Acta. (2016) 463:158–64. doi: 10.1016/j.cca.2016.10.034
14. Sinning C, Kempf T, Schwarzl M, Lanfermann S, Ojeda F, Schnabel RB, et al. Biomarkers for characterization of heart failure-Distinction of heart failure with preserved and reduced ejection fraction. Int J Cardiol. (2017) 227:272–7. doi: 10.1016/j.ijcard.2016.11.110
15. Jin XL, Huang N, Shang H, Zhou MC, Hong Y, Cai WZ, et al. Diagnosis of chronic heart failure by the soluble suppression of tumorigenicity 2 and N-terminal pro-brain natriuretic peptide. J Clin Lab Anal. (2018) 32:e22295. doi: 10.1002/jcla.22295
16. Luo NS, Zhang HF, Liu PM, Lin YQ, Huang TC, Yang Y, et al. Diagnostic value of combining serum soluble ST2 and interleukin-33 for heart failure patients with preserved left ventricular ejection fraction. Zhonghua Xin Xue Guan Bing Za Zhi. (2017) 45:198–203. doi: 10.3760/cma.j.issn.0253-3758.2017.03.006
17. Cui Y, Qi X, Huang A, Li J, Hou W, Liu K. Differential and predictive value of galectin-3 and soluble suppression of tumorigenicity-2 (sST2) in heart failure with preserved ejection fraction. Med Sci Monit. (2018) 24:5139–46. doi: 10.12659/MSM.908840
18. Hieda M, Sarma S, Hearon CM Jr, Dias KA, Martinez J, Samels M, et al. Increased myocardial stiffness in patients with high-risk left ventricular hypertrophy: the hallmark of stage-b heart failure with preserved ejection fraction. Circulation. (2020) 141:115–23. doi: 10.1161/CIRCULATIONAHA.119.040332
19. Mitic VT, Stojanovic DR, Deljanin Ilic MZ, Stojanovic MM, Petrovic DB, Ignjatovic AM, et al. Cardiac remodeling biomarkers as potential circulating markers of left ventricular hypertrophy in heart failure with preserved ejection fraction. Tohoku J Exp Med. (2020) 250:233–42. doi: 10.1620/tjem.250.233
20. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. (2017) 136:e137–61. doi: 10.1161/CIR.0000000000000509
21. Homsak E, Gruson D. Soluble ST2: a complex and diverse role in several diseases. Clin Chim Acta. (2020) 507:75–87. doi: 10.1016/j.cca.2020.04.011
22. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. (2002) 106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
23. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. (2007) 117:1538–49. doi: 10.1172/JCI30634
Keywords: soluble suppression of tumorigenicity, heart failure, diagnostic value, sensitivity, specificity, meta-analysis
Citation: Yang C, Fan Z, Wu J, Zhang J, Zhang W, Yang J and Yang J (2021) The Diagnostic Value of Soluble ST2 in Heart Failure: A Meta-Analysis. Front. Cardiovasc. Med. 8:685904. doi: 10.3389/fcvm.2021.685904
Received: 07 April 2021; Accepted: 10 June 2021;
Published: 12 July 2021.
Edited by:
Raffaele Maio, University of Catanzaro, ItalyReviewed by:
Zengjun Wang, Nanjing Medical University, ChinaCopyright © 2021 Yang, Fan, Wu, Zhang, Zhang, Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Yang, eWFuZ2ppYW5AY3RndS5lZHUuY24=; Jun Yang, eWFuZ2p1bkBjdGd1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.