
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 22 September 2021
Sec. Cardiovascular Therapeutics
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.685072
 Wence Shi1
Wence Shi1 Lin Ni2
Lin Ni2 Jingang Yang1
Jingang Yang1 Xiaoxue Fan2
Xiaoxue Fan2 Mei Yu3
Mei Yu3 Hongmei Yang4
Hongmei Yang4 Mengyue Yu1*
Mengyue Yu1* Yuejin Yang1* and China Acute Myocardial Infarction (CAMI) Registry study group
Yuejin Yang1* and China Acute Myocardial Infarction (CAMI) Registry study groupBackground: The latest guidelines recommend the use of proton pump inhibitors (PPIs) to minimize gastrointestinal bleeding (GIB) in patients receiving dual antiplatelet therapy (DAPT), even though this co-administration may increase the risk of ischemia due to drug interactions. We have noticed that there are few studies conducted on patients with a lower risk of GIB. Therefore, we investigated the clinical effect of co-administration of PPI on DAPT patients with low GIB risk.
Methods and Results: From January 2013 to September 2014, a total of 17,274 consecutive patients on DAPT from 108 hospitals with low risk for GIB in the China Acute Myocardial Infarction (CAMI) registry were analyzed. The primary endpoints were GIB and major adverse cardiovascular and cerebrovascular events (MACCE). Multivariate logistic regression analysis and Cox proportional hazard models were used to assess the effect of PPIs use. Of the analyzed patients, 66.6% (n = 11,487) were treated with PPIs. PPI use did not show an extra gastrointestinal protective effect in patients with low risk for GIB who were hospitalized and on follow-up after 2 years. Moreover, it was associated with an increased risk of stroke during the 2-year follow-up [hazard ratio (HR) 2.072, 95% confidence interval (CI) 1.388–3.091, p = 0.0003] and an increased risk of MI after 6 months (HR 1.580, 95% CI 1.102–2.265, p = 0.0119). We found the same results after propensity score matching.
Conclusion: PPI use is prevalent in DAPT patients with low GIB risk. PPIs did not show an extra gastrointestinal protective effect, while an increased risk of stroke was observed during the 2-year follow-up.
Clinical Trial Registration: www.clinicaltrials.gov, identifier NCT01874691.
Dual antiplatelet therapy (DAPT), a combination of aspirin and an inhibitor of platelet P2Y12 receptor, is the most clarified medicine in cardiovascular disease, which is widely recommended in the latest guidelines (1, 2). However, it could cause an increased risk of gastrointestinal bleeding (GIB) (3) and other adverse clinical outcomes (4). Although randomized controlled trials have demonstrated that proton pump inhibitors (PPIs) reduce the rate of recurrent GIB (5), especially in high-risk patients [advanced age (>75); concurrent use of anticoagulants, steroids, or non-steroidals; and Helicobacter pylori infection] (6), a potential drug interaction has limited its common use in acute myocardial infarction (AMI) patients (1, 7). We noticed that the impact of PPIs on clinical outcomes when co-administered with DAPT was inconsistent in different studies (8), and the over-prescription of PPIs was increasingly becoming a public health concern (9, 10). Therefore, we investigated the impact of PPIs–DAPT co-medication in patients with low GIB risk and hope to provide more evidence for clinical decisions.
All patients analyzed in our research were from the China Acute Myocardial Infarction (CAMI) registry, which is a prospective, nationwide, multicenter observational study of patients with AMI. The registry includes three levels of hospitals (provincial-, prefectural-, and county-level hospitals, representing typical Chinese governmental and administrative models) covering all provinces and municipalities across mainland China. The CAMI registry was registered with ClinicalTrials.gov (NCT01874691), and this project was approved by the institutional review board central committee at Fuwai Hospital, NCCD of China. All patient data were protected at all times. Detailed descriptions about data management and quality control can be found in the methodological article about the CAMI registry published previously (11).
Simply, all elements (especially outcomes events) are collected, validated, and submitted through a secure, password-protected, web-based electronic data capture system (http://www.CAMIRegistry.org) by the local investigators at each participating site. Trained clinical investigators were employed to ensure the accuracy and reliability of data. Element definitions are accessible to investigators automatically at the point of data entry. The front page of the electronic case report form (eCRF) must be filled out and submitted online within 24 h from patient admission who meet the inclusion criteria.
Overall, 26,660 AMI patients from 108 hospitals were enrolled from January 1, 2013 to August 31, 2014. A total of 22,405 patients on DAPT were available, after excluding those with incorrect age (n = 370), no DAPT (n = 1,596), and missing baseline data (n = 2,289). We further excluded 449 patients treated with H2 receptor antagonists for gastrointestinal prophylaxis. A total of 4,709 patients were identified as a high-risk group for GIB [advanced age (>75); concurrent use of anticoagulants, steroids, or non-steroidals; and H. pylori infection] according to the guideline (1, 6). The patients with low risk for GIB were identified, excluding the high-risk population, and the data of 17,247 DAPT patients were finally analyzed (Figure 1).
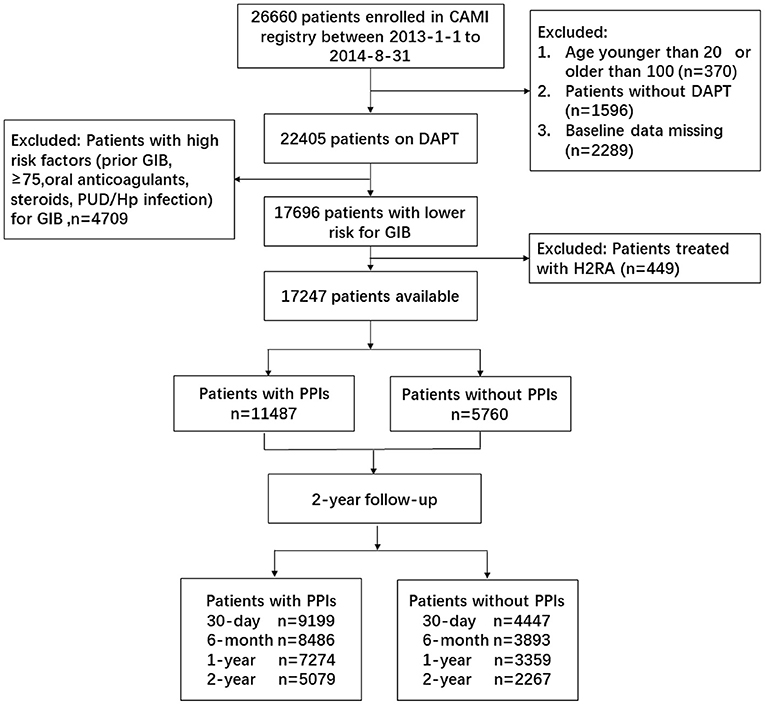
Figure 1. Patient flowchart for the study cohort. CAMI, China Acute Myocardial Infarction; DAPT, dual antiplatelet therapy; GIB, gastrointestinal bleeding; PUD, peptic ulcer disease; PPIs, proton pump inhibitors.
The primary endpoints were GIB and major adverse cardiovascular and cerebrovascular events (MACCE). GIB was defined as clinically evident GIB (gross hematemesis, heme positive coffee-ground emesis, and heme positive melena). MACCE was a composite endpoint of all-cause death, MI, and stroke. Secondary endpoints were all-cause death, MI, and stroke. All the information is collected using the standardized set of variables and standard definitions that were validated by trained investigators. All variables were coded with CDISC, ICD-10, MedDra, and WHO-DD to make them standardized.
Post-discharge study follow-up was conducted via centralized telephone interviews by trained personnel at 30 days, 6 months, 1 year, and 2 years. The clinical events were validated by source documents. PPI use was identified at the study baseline and each study follow-up. Patients were excluded if they had quit their PPI use during the follow-up.
Continuous variables are expressed as mean ± standard deviation or median (25th and 75th percentiles), and categorical variables are presented as percentages. Differences in baseline characteristics and outcomes in patients with and without PPIs were assessed using the chi-square test, Fisher's exact test for categorical variables and analysis of variance test, or the Wilcoxon rank test for continuous variables. Multivariate logistic regression analyses were conducted to evaluate the adjusted effect of PPI use on clinical outcomes. The 2-year follow-up endpoints were modeled using the Cox proportional hazard regression. Clinical characteristics that were imbalanced at a nominal 5% significance level between the two groups, treated or not treated with PPIs, were identified and included in the final adjusted model; other important factors that can affect the clinical endpoints were also included in the final model, although their differences were not significant between the two groups in the univariate analysis (such as the history of diabetes and congestive heart failure). These included age, clinical presentation, and medical therapy (detailed variables included are presented below the relevant tables). Odds ratio (OR) and hazard ratio (HR) were presented with the 95% confidence intervals (CIs). All statistical analyses were performed using SAS version 9.4, and a two-tailed p < 0.05 was considered statistically significant.
We also performed propensity score matching (PSM) to select two comparable patients with balanced observed variables. A propensity score was estimated for each patient using a logistic regression model. Patients were matched on estimated propensity scores, with replacement, using the nearest neighbor approach. The detailed information about the propensity score model can be found in the Supplementary Material.
Among 17,247 DAPT patients with low risk for GIB, 66.6% (n = 11,487) were treated with PPIs. Patients on PPIs tended to be older (58.37 vs. 57.84, p = 0.0042), female (21.8 vs. 20.4%, p = 0.0351), and with a higher Killip class (IV 3.4 vs. 2.7%, p < 0.0001) and hematocrit (Hct; 41.44 vs. 39.76, p = 0.0029) at admission with a history of hypertension (49.6 vs. 46.7%, p = 0.0003), MI (8.9 vs. 7.1%, p = 0.0273), stroke (8.3 vs. 6.8%, p = 0.0007), and malignancy (1.0 vs. 0.7%, p = 0.0331). On hospitalization, they were often treated with a GPIIb/IIIa receptor inhibitor (37.0 vs. 26.4%, p < 0.0001) and heparin (94.2 vs. 89.7%, p < 0.0001). Detailed information on demographic and clinical characteristics is presented in Table 1.
We did not find a protective effect of PPIs against GIB. Another primary efficacy endpoint (composite of all-cause death, MI, and stroke) was similar between patients with PPIs and without PPI use (5.0 vs. 4.7%, adjusted OR 1.026, 95% CI 0.877–1.203, p = 0.7189) (Table 2). Results were consistent across all-cause death and MI as presented in Table 2. Furthermore, PPI use was associated with an increased risk for stroke compared with patients without PPI use (0.6 vs. 0.3%, adjusted OR 2.125, 95% CI 1.216–3.682, p = 0.0062) (Table 2).
Patients were followed up throughout a period of 2 years, and event rates at 30 days, 6 months, 1 year, and 2 years are presented in Figure 2. The mean follow-up for patients finally analyzed in our study was 447.7 days with 57.41% lost-to-follow-up. As for MACCE and all-cause death, we found no difference between patients with and without PPIs during follow-up (Table 3). At 6 months, the risk for all-cause death increased significantly in patients treated with PPIs, and the increased risk was seen consistently across all follow-ups in the PPI group. Moreover, PPI co-administration was associated with stroke events for all follow-up points (Table 3).
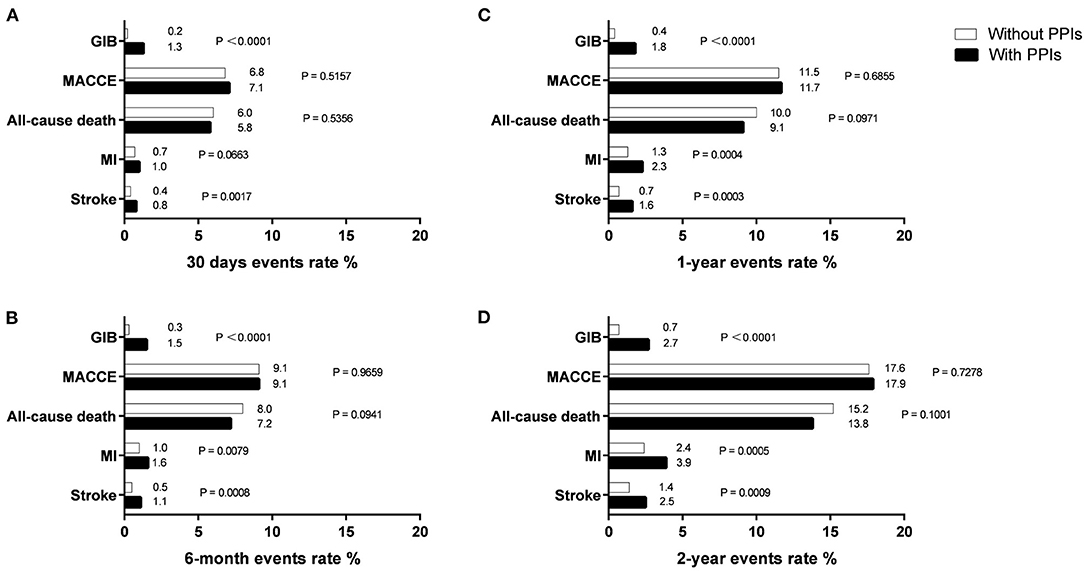
Figure 2. Endpoints events rate at 30 days (A), 6 months (B), 1 year (C), and 2 years (D) in patients with and without PPIs.
After PSM, 5,014 patients with PPIs had an estimated propensity score that matched to 5,014 patients without PPI use. PPIs did not show an extra gastrointestinal protective effect, while an increased risk for stroke was seen during the 2-year follow-up (Supplementary Material).
The main findings of our research were as follows: (1) among the DAPT population, 66% of patients with low GIB risk were treated with PPIs. (2) We did not find an extra protective effect of PPIs on the gastrointestinal tract among DAPT patients with low GIB risk. (3) PPI use was associated with an increased risk of stroke in hospital and during the 2-year follow-up and an increased risk of MI after 6 months. PSM did not change the final results.
In our study, PPI use was common in patients with low risk for GIB. We noticed that PPI over-prescription was becoming a new concern in the field of AMI patient management (9, 10). And our former research also emphasized this in the Chinese AMI population (12). Although our study focused on patients with low GIB risk, the PPI use rate (66%) was still higher than that in the ADAPT-DES study (13) (31.4%), PRODIGY trial (14) (37.4%), and TRANSLATE-ACS study (15) (18.2%). This unexpected finding indicated a lower threshold for prescribing PPIs in China, while clinical practice recommendations made by guidelines were better followed in the US (16). This should attract the attention of physicians and administrative personnel to limit over-prescription, which can help reduce the burden on personal costs and the healthcare system.
Randomized controlled trials have demonstrated that PPIs reduce the rate of recurrent GIB in high-risk patients receiving aspirin (5), while few researchers have investigated the effect of PPI use on low-risk patients. Our results indicated that low-risk patients might not benefit from this gastrointestinal prophylaxis. Moreover, the latest clinical guidelines recommended that PPIs co-administration is applicable for minimizing bleeding while on DAPT. However, pharmacokinetic studies showed a potential drug interaction between PPIs and P2Y12 receptor inhibitors, which could decrease the effect of DAPT. Both clopidogrel and PPIs require bio-transformation into active metabolite via cytochrome P-450 (CYP) enzymes in the liver (17, 18), and physicians raised concerns that competitive inhibition would attenuate its antiplatelet effect, which would increase the risk of ischemic events. However, there were no consistent results in clinical research regarding the effect of co-administration (8, 14, 15). We hypothesized that the benefit of co-administration would be less than the adverse outcomes in patients with low GIB risk.
During hospitalization and the 2-year follow-up, we found an increased risk of stroke in patients with PPI use; few studies have reported the same finding previously. Stroke is more prevalent in the Chinese population (19, 20). Therefore the effect of adverse drug interaction was amplified in patients with low GIB risk. Moreover, we noticed that the adverse effects of PPIs on MI occurred after 6 months. This indicated that PPI use could help improve DAPT compliance within 6 months and patients could benefit from this gastrointestinal prophylaxis. This result was similar to another research from the Netherlands (21). However, long-term co-administration (especially over 6 months) would pose ischemic risk for patients with a low risk of GIB. Although PPI use was recommended for reducing bleeding while on DAPT in the latest guidelines, a definite duration of co-administration was not specified. It is hard for physicians to decide when to quit PPI use to ensure maximum benefit for patients. Our results provide some insight into this problem, and we derive that <6-month co-administration might be suitable.
There are some limitations in our manuscript: (1) Although CAMI is a large-scale and multicenter registry, our research is a retrospective study. Therefore, the two groups were not comparable to some extent. This would affect the assessment of the effect of PPIs on clinical outcomes. We have used statistical approaches (PSM or multiple regression) to diminish the bias. (2) CAMI could not evaluate the individual effect of PPIs on endpoints; we admitted that drug interaction between different PPIs and P2Y12 could affect the clinical events in patients and further better-designed research is warranted. (3) The CAMI project was launched in 2016, and clopidogrel was prevalent in that period because limited research and guidelines recommended other P2Y12. Further sub-analysis and studies evaluating the effect of PPIs on other P2Y12 inhibitors are warranted. (4) The inclusion of only the Chinese population might limit its applicability to other populations.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The original study on which this paper is based was reviewed and approved by Ethics Committee of Fuwai Hospital. Reference Number: 2012-398. The patients/participants provided their written informed consent to participate in the original study.
WS was responsible for literature search, study design, data management, data interpretation, and writing. XF and LN was responsible for data analysis, data interpretation, and writing. JY, MeiY, and HY was involved in study design, statistical analysis plan, and data interpretation. MenY and YY were involved in data interpretation and writing of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-009), Twelfth Five-Year Planning Project of the Scientific and Technological Department of China (2011BAI11B02), and National Natural Science Foundation of China (No. 81670415).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge the work of the entire CAMI registry research group and Professor Yang's guidance and leadership.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.685072/full#supplementary-material
1. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
2. Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. (2019) 55:4–90. doi: 10.1093/eurheartj/ehy394
3. Agewall S, Cattaneo M, Collet JP, Andreotti F, Lip GY, Verheugt FW, et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J. (2013) 34:1708–13. doi: 10.1093/eurheartj/eht042
4. Nikolsky E, Stone GW, Kirtane AJ, Dangas GD, Lansky AJ, McLaurin B, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. (2009) 54:1293–302. doi: 10.1016/j.jacc.2009.07.019
5. Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. (2002) 346:2033–8. doi: 10.1056/NEJMoa012877
6. Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, Clark CB, et al. ACCF/ACG/AHA (2010) Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. (2010) 122:2619–33. doi: 10.1161/CIR.0b013e318202f701
7. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. (2011) 124:e574–651. doi: 10.1161/CIR.0b013e31823a5596
8. Melloni C, Washam JB, Jones WS, Halim SA, Hasselblad V, Mayer SB, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes. (2015) 8:47–55. doi: 10.1161/CIRCOUTCOMES.114.001177
9. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. (2012) 29:681–90. doi: 10.1007/BF03262283
10. Schepisi R, Fusco S, Sganga F, Falcone B, Vetrano DL, Abbatecola A, et al. Inappropriate use of proton pump inhibitors in elderly patients discharged from acute care hospitals. J Nutr Health Aging. (2016) 20:665–70. doi: 10.1007/s12603-015-0642-5
11. Xu H, Li W, Yang J, Wiviott SD, Sabatine MS, Peterson ED, et al. The China Acute Myocardial Infarction (CAMI) Registry: a national long-term registry-research-education integrated platform for exploring acute myocardial infarction in China. Am Heart J. (2016) 175:193–201.e3. doi: 10.1016/j.ahj.2015.04.014
12. Shi W, Ni L, Yang J, Fan X, Yu M, Yang H, et al. Appropriateness of gastrointestinal prophylaxis use during hospitalization in patients with acute myocardial infarction: analysis from the China Acute Myocardial Infarction Registry. Clin Cardiol. (2021) 44:43–50. doi: 10.1002/clc.23449
13. Weisz G, Smilowitz NR, Kirtane AJ, Rinaldi MJ, Parvataneni R, Xu K, et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: the ADAPT-DES study. Circ Cardiovasc Interv. (2015) 8:e001952. doi: 10.1161/CIRCINTERVENTIONS.114.001952
14. Gargiulo G, Costa F, Ariotti S, Biscaglia S, Campo G, Esposito G, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. (2016) 174:95–102. doi: 10.1016/j.ahj.2016.01.015
15. Jackson LR, Peterson ED, McCoy LA, Ju C, Zettler M, Baker BA, Messenger JC, et al. Impact of proton pump inhibitor use on the comparative effectiveness and safety of prasugrel versus clopidogrel: insights from the treatment with adenosine diphosphate receptor inhibitors: longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) study. J Am Heart Assoc. (2016) 5:e003824. doi: 10.1161/JAHA.116.003824
16. Farhat N, Haddad N, Crispo J, Birkett N, McNair D, Momoli F, et al. Trends in concomitant clopidogrel and proton pump inhibitor treatment among ACS in patients, 2000-2016. Eur J Clin Pharmacol. (2019) 75:227–35. doi: 10.1007/s00228-018-2564-8
17. Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. (2009) 301:937–44. doi: 10.1001/jama.2009.261
18. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. (2009) 360:354–62. doi: 10.1056/NEJMoa0809171
19. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study (2010). Lancet. (2013) 381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1
20. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study (2013). Lancet. (2016) 387:251–72. doi: 10.1016/S0140-6736(15)00551-6
21. Hoedemaker N, Damman P, Ottervanger JP, Dambrink JHE, Marcel Gosselink AT, Kedhi E, et al. Trends in cardiovascular and bleeding outcomes in acute coronary syndrome patients treated with or without proton-pump inhibitors during the introduction of novel P2Y12 inhibitors: a five-year experience from a single-center observational registry. Eur Heart J Cardiovasc Pharmacother. (2019) 5:127–38. doi: 10.1093/ehjcvp/pvy030
Keywords: proton pump inhibitors, acute myocardial infarction, gastrointestinal bleeding (GIB), co-medication, lower risk
Citation: Shi W, Ni L, Yang J, Fan X, Yu M, Yang H, Yu M, Yang Y and China Acute Myocardial Infarction (CAMI) Registry study group (2021) The Clinical Impact of Proton Pump Inhibitors When Co-Administered With Dual Antiplatelet Therapy in Patients Having Acute Myocardial Infarction With Low Risk of Gastrointestinal Bleeding: Insights From the China Acute Myocardial Infarction Registry. Front. Cardiovasc. Med. 8:685072. doi: 10.3389/fcvm.2021.685072
Received: 24 March 2021; Accepted: 19 August 2021;
Published: 22 September 2021.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Fix Press, Unitelma Sapienza University, ItalyCopyright © 2021 Shi, Ni, Yang, Fan, Yu, Yang, Yu, Yang and China Acute Myocardial Infarction (CAMI) Registry study group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengyue Yu, eXVtZW5neXVlQGZ1d2FpaG9zcGl0YWwub3Jn; Yuejin Yang, eWFuZ3lqZndAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.