
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 04 August 2021
Sec. Heart Failure and Transplantation
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.684004
This article is part of the Research Topic Improving Early Detection and Risk Prediction in Heart Failure View all 14 articles
 Xiangkun Xie1,2
Xiangkun Xie1,2 Mingwei Yang1,2,3
Mingwei Yang1,2,3 Shan Xie4
Shan Xie4 Xiaoying Wu1,2
Xiaoying Wu1,2 Yuan Jiang1,2
Yuan Jiang1,2 Zhaoyu Liu4
Zhaoyu Liu4 Huiying Zhao4*
Huiying Zhao4* Yangxin Chen1,2*
Yangxin Chen1,2* Yuling Zhang1,2*
Yuling Zhang1,2* Jingfeng Wang1,2*
Jingfeng Wang1,2*Introduction: Left ventricular reverse remodeling (LVRR) is associated with decreased cardiovascular mortality and improved cardiac survival and also crucial for therapeutic options. However, there is a lack of an early prediction model of LVRR in first-diagnosed dilated cardiomyopathy.
Methods: This single-center study included 104 patients with idiopathic DCM. We defined LVRR as an absolute increase in left ventricular ejection fraction (LVEF) from >10% to a final value >35% and a decrease in left ventricular end-diastolic diameter (LVDd) >10%. Analysis features included demographic characteristics, comorbidities, physical sign, biochemistry data, echocardiography, electrocardiogram, Holter monitoring, and medication. Logistic regression, random forests, and extreme gradient boosting (XGBoost) were, respectively, implemented in a 10-fold cross-validated model to discriminate LVRR and non-LVRR, with receiver operating characteristic (ROC) curves and calibration plot for performance evaluation.
Results: LVRR occurred in 47 (45.2%) patients after optimal medical treatment. Cystatin C, right ventricular end-diastolic dimension, high-density lipoprotein cholesterol (HDL-C), left atrial dimension, left ventricular posterior wall dimension, systolic blood pressure, severe mitral regurgitation, eGFR, and NYHA classification were included in XGBoost, which reached higher AU-ROC compared with logistic regression (AU-ROC, 0.8205 vs. 0.5909, p = 0.0119). Ablation analysis revealed that cystatin C, right ventricular end-diastolic dimension, and HDL-C made the largest contributions to the model.
Conclusion: Tree-based models like XGBoost were able to early differentiate LVRR and non-LVRR in patients with first-diagnosed DCM before drug therapy, facilitating disease management and invasive therapy selection. A multicenter prospective study is necessary for further validation.
Clinical Trial Registration: http://www.chictr.org.cn/usercenter.aspx (ChiCTR2000034128).
Dilated cardiomyopathy (DCM) is the third leading cause of heart failure with decreased ejection fraction and the most important cause of heart transplantation (1, 2). Its 1-year mortality rate is as high as 25–30%, and its 5-year survival rate is <50% (3). Significant improvements in left ventricular end-diastolic diameter (LVDd) and left ventricular ejection fraction (LVEF) are referred to as left ventricular reverse remodeling (LVRR) (4). Despite the use of angiotensin-converting enzyme inhibitors (ACEIs), β-blocker, and mineralocorticoid receptor antagonists, LVRR happened only in approximately 37–52% of DCM patients (5–10). Therapy-induced LVRR has become an important prognostic tool in the management of patients with DCM (5, 11). If a patient is not responsive to medication, not only an early implantable cardioverter defibrillator may be necessary but also the timing of device therapy and insertion in the transplant list are important considerations since these aspects differ from those who are responsive to medication. Despite an increasing understanding of the progression of DCM, prognostic stratification of patients with early phases of DCM remains a challenge (12). It can be seen that early prediction of LVRR will help us to achieve precise management of patients with DCM.
Several early studies have reported the association between some clinical indexes and LVRR in DCM. Kawai et al. (13) first demonstrated that higher systolic blood pressure and lower pulmonary arterial wedge pressure at diagnosis were predictors of LVRR with medical therapy. Afterward, cardiac magnetic resonance was used for the prediction of LVRR. Several studies reported that late gadolinium enhancement at baseline provides a better prediction of LVRR (10, 14–17). However, there is no definite agreement in previous studies in regard to late gadolinium enhancement as an early predictor of LVRR (18). Genotype is also proven to associate with LVRR in DCM. It is reported that an inverse and independent association exists between structural cytoskeleton Z-disk gene rare variants and LVRR (19). Verdonschot et al. (7) also demonstrated that the model including mutation status performs better than the model with only clinical parameters (AUC = 0.760 vs. 0.742, p = 0.008). However, the difficult and expensive measurement limits their clinical application. Ruiz-Zamora et al. (20) found a simple logistic model including five variables with an AUC of 0.83. However, this model included several variables obtained at the end of follow-up, so we cannot make an early prediction for LVRR, which usually happens within 1 to 2 years in patients with DCM. Therefore, if we can identify LVRR in DCM when first diagnosed with a combination of several usual clinical parameters, it could help to make important clinical decisions concerning the need and timing of some therapies in patients with DCM.
Machine learning performs more objectively in selecting predictor variables and handles possible non-linear effects of variables better than traditional statistical methods. A tree-based ensemble algorithm can aggregate multiple weak learners to attain a stronger ensemble model by bagging and boosting two different ensemble ways, among which random forests and extreme gradient boosting (XGBoost) are, respectively, their representative methods. Random forests can use the bootstrap sampling method for avoiding instability of the model, while XGBoost algorithm was developed mainly for penalizing the structure of a decision tree to avoid overfitting (21). It has been found that this XGBoost technique outperforms other machine learning and deep learning methods in many competitions such as Kaggle and KDDCup (22). It has been successfully applied in numerous bioinformatics studies (23, 24) and medical studies (25, 26). Therefore, we conducted a retrospective real-world study and analyzed clinical data by using tree-based learning algorithms to build a predictive model and validate it.
This study was a single-center real-world study. The clinical data of patients were collected from consecutively admitted patients with their first diagnosis of DCM at the Sun Yat-sen Memorial Hospital of Sun Yat-sen University between January 2014 and December 2017, and each of the patients had several follow-up records. DCM was diagnosed in keeping with the Chinese guidelines for the diagnosis and treatment of DCM (27) as follows: (1) LVDd >5.0 cm (female) or LVDd >5.5 cm (male); (2) LVEF <45% and left ventricular shortening fraction <25%; and (3) exclusion of valvular heart disease, congenital heart disease, ischemic heart disease, tachycardiomyopathy, and secondary DCM caused by systemic diseases. Patients with any of the following conditions were excluded: (1) alcoholic cardiomyopathy, peripartum cardiomyopathy, and other acquired DCM; (2) a history of HF treatment including ACEIs/angiotensin receptor blockers (ARBs)/angiotensin receptor-neprilysin inhibitors (ARNIs), adrenergic beta-receptor blockers, and mineralocorticoid receptor antagonists; (3) coronary heart disease (having narrowed coronary arteries 50% or more according to coronary angiography or coronary CTA), pulmonary heart disease, organic heart valvular disease, congenital heart disease, hypertensive heart disease, or pericardial disease; (4) not receiving medical therapy recommended by the Chinese Guidelines for the Diagnosis and Treatment of Heart Failure 2018 (28); (5) systemic diseases that may affect the structure and function of the heart, such as hyperthyroidism, hypothyroidism, amyloidosis, pheochromocytoma, systemic lupus erythematosus, or Behcet's disease; (6) cancer, severe infection, or severe renal dysfunction (estimated glomerular filtration rate (eGFR) <15 ml min−1·1.73 m−2); and (7) receiving cardiac resynchronization therapy or left ventricular assist device during follow-up. This study was approved by the institutional review board of Sun Yat-sen Memory Hospital and had therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. No informed consent was required because the data in our study were anonymized. All patients received standard medical therapy according to current guidelines (27, 28).
All data of baselines and return visits were obtained from electronic health records including demographic characteristics, physical sign, comorbidities, laboratory indicators, electrocardiogram, 24-h dynamic electrocardiogram, echocardiographic data, and medication. The blood samples were collected after fasting for 12 h overnight. LVEF was measured using the apical biplane method and transthoracic echocardiography was performed as recommended by the American Society of Echocardiography (29) by a senior echocardiographer at admission and during the follow-up period. The New York Heart Association (NYHA) class was evaluated in this study within the first 8 h of admission.
According to the European Association of Cardiovascular Imaging and the American Society of Echocardiography (30), the relative wall thickness was calculated as the ratio of two times the posterior wall thickness to LVDd. Left ventricular mass (LVM) was calculated according to the formula in (1). The normalization of LVM for body surface area was regarded as the left ventricular mass index. Body surface area was estimated by the formula in (2) (31). The eGFR was calculated using the modification of diet in renal disease equation (32). The doses of ACEIs/ARBs/ARNIs and β-blockers were evaluated by the ratio of the practical dose and target dose of certain drugs within 6 months (28).
The patients underwent a return visit as required. The end of visits was December 2018, the date of death or heart transplantation. Transthoracic echocardiography was performed in all visits. LVRR was defined as an absolute increase in LVEF from >10% to a final value >35% accompanied by a decrease in LVDd ≥10% (10) as assessed at any one visit and lasted until the last visit (median time 24 months, IQR 15–31). Non-LVRR was defined as an absolute increase in LVEF <10% or final value <35% or a decrease in LVDd <10% as assessed at all visits, except those in <9 months. Patients who did not meet the definition of LVRR and have a last visit <9 months were excluded (Figure 1).
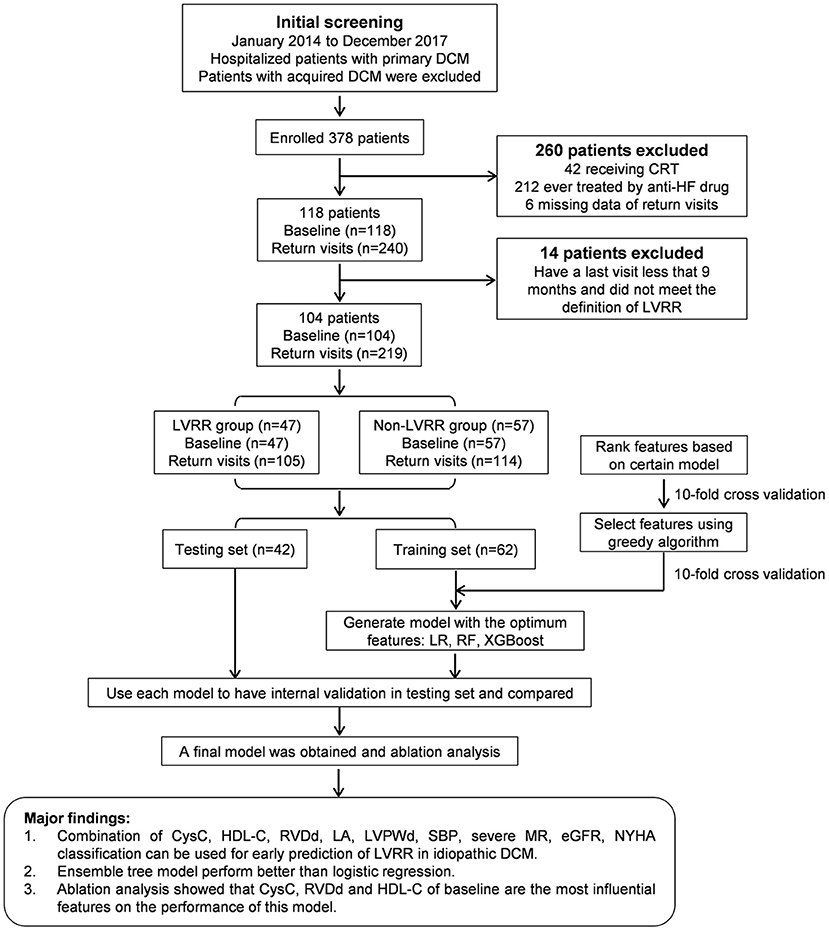
Figure 1. Overall flowchart and main results of this study. CRT, cardiac resynchronization therapy; CysC, cystatin C; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; LA, left atrial dimension; LVDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVPWd, left ventricular posterior wall dimension; LVRR, left ventricular reverse remodeling; LR, logistic regression; MR, mitral regurgitation; NYHA, New York Heart Association; RF, random forest; RVDd, right ventricular end-diastolic dimension; SBP, systolic blood pressure; XGBoost, extreme gradient boosting.
Normally distributed variables are presented as the means ± standard deviations, while non-normally distributed variables are presented as medians with interquartile ranges. NT-proBNP, cTNT, D-dimer, and hsCRP were logarithmically transformed to approximate a normal distribution. The Levene test was used to explore the homogeneity of variance, and a p-value of <0.1 was considered to indicate heterogeneity of variance. Differences between groups were tested by the independent t-test or Mann–Whitney U-test for continuous variables and the chi-square test for categorical variables. De long test was used to detect if the difference between AUCs was statistically significant. Statistical significance was defined as a two-sided p-value of <0.05.
A total of 102 features were included for analysis and are described in Supplementary Table 1. Moreover, 65 variables had no missing data, 23 variables had <10% missing data, and the remaining 14 variables had >10% missing data. None of the variables had >50% missing data. All variables were standardized when selecting features and building models to mitigate the effect of the differences in dimensions between variables. The specific method is described in (3), where Xk0 and Xk are the kth values of a certain variable before and after standardization, while Xmin and Xmax are the minimum and maximum values of a certain variable, respectively. K-nearest neighbors were used for the imputation of continuous and discrete variables, which took the average of K samples nearest to the missed point as its value.
We chose three standard supervised machine learning methods for our data: XGBoost (21), random forest (33), and logistic regression with l1 penalty (34). The cases and controls involved in this study were randomly divided into training and testing sets with the ratio, train:test = 6:4. These models were trained on the training set with 10-fold cross-validation and were validated on the testing set (Figure 1). A grid search scheme was performed on the training set through the 10-fold cross-validation to search for the optimal combination of parameters of the model, where the training set was randomly split into 10 subsets. For each combination of parameters, nine subsets were trained for a model and the remaining one was used for validation of the model. The process was repeated for 10 times so that each subset was tested once and the average of their results was collected to measure the performance of the parameter combinations. As a result, we selected the parameter combination that reached the highest AUC to train a model based on the whole training set, and then the model was tested on the independent test set. The discrimination of models was evaluated using the receiver operating characteristic (ROC) curve. The calibration was performed using the isotonic regression (35) and evaluated by a calibration plot.
The distribution of each feature is shown in Supplementary Figure 1. Feature selection was also performed to optimize the feature combination in constructing a prediction model. In this study, we used a greedy feature selection algorithm based on the important features recommended by a specific model.
In general, a specific model was first pretrained to obtain the important features with 10-fold cross-validation on the training set, from which we select the feature greedily according to AUC. The important features included the features with an importance greater than zero. In the greedy searching process, the selection algorithm began with an empty set of features and iteratively searched the best feature from the remaining feature set and added the best feature to the empty set for a higher AUC. This procedure was repeated until the remaining feature set was empty or AUC no longer increased, leading to a best feature subset for building a final prediction model.
The research data of our study were assessed with the machine learning tools of the scikit-learn project. The tool environment we applied was Python 3.7.6 with scikit-learn 0.22 running on Anaconda 3 (4.8.5-Linux-x86_64) for data processing, modeling, and evaluation. SPSS version 22.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA) was used to perform the descriptive statistics.
A total of 378 inpatient clinical data points from 104 patients were collected. Among the 104 patients analyzed, LVRR was observed in 47 individuals (45.2%) (Figure 1). The characteristics and the distribution of the patients are shown in Table 1 and Supplementary Figure 1. Patients who developed LVRR were more likely to have a higher systolic blood pressure, higher platelet count, lower serum D-dimer level, higher high-density lipoprotein cholesterol (HDL-C) level, smaller left atrial dimension, and smaller right ventricular end-diastolic dimension and were less likely to suffer from severe mitral regurgitation (MR). The use or doses of ACEIs/ARBs/ARNIs and β-blockers were not significantly different between the two groups.
All patients completed return visits. The details of the time distributions of visits are shown in Supplementary Figure 2. LVEF and LVDd were similar between the two groups at baselines, but in the LVRR group, LVEF, LVDd, left atrial dimension, and severity of MR were improved significantly and tended to be stable after 1 year (Figures 2A,B,D,G). Right ventricular end-diastolic dimension, left ventricular posterior wall dimension, and interventricular septal dimension showed no obvious change during return visits both in LVRR and non-LVRR groups (Figures 2C,E,F). NYHA functional class in the LVRR group was better than that in non-LVRR groups at each time point (Figure 2H).
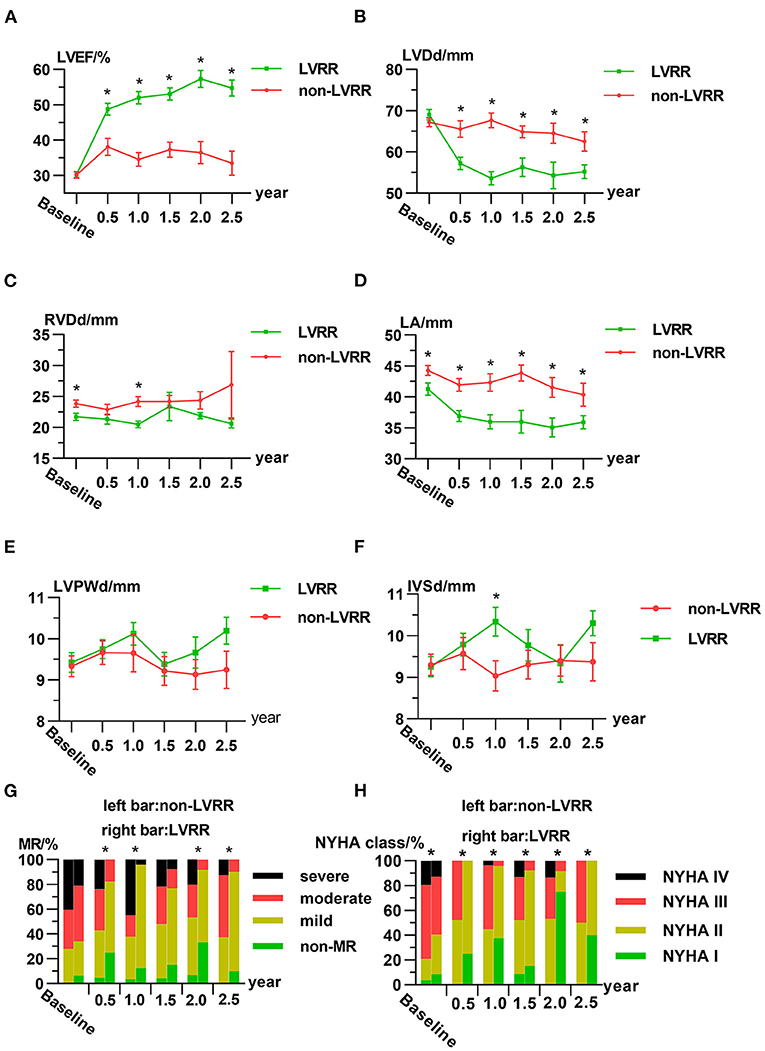
Figure 2. Characteristics of the LVRR group and non-LVRR group at the first visit and return visits. Line chart for the averages of (A) LVEF, (B) LVDd, (C) RVDd, (D) LA, (E) LVPWd, and (F) IVSd. (G,H) Ratio of the severity of MR and NYHA functional class over time. The data are presented as the mean ± standard error (A–F). In (A–F), *p ≤ 0.05 by non-paired Student's t-test between two groups. In (G), *p ≤ 0.05 comparing the percentage of patients who are above moderate or severe in both groups by chi-square test. In (H), *p ≤ 0.05 by Mann–Whitney U-test. IVSd, interventricular septal dimension; LA, left atrial dimension; LVDd, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVPWd, left ventricular posterior wall dimension; LVRR, left ventricular reverse remodeling; MR, mitral regurgitation; NS, no statistically significant difference; NYHA, New York Heart Association; RVDd, right ventricular end-diastolic dimension.
The individual features were tested in their ability to classify the LVRR and the non-LVRR. As indicated by Figure 3A, there are more than 20 features (30.12%) with an AUC that only reached slightly more than 0.5, and only five features with an AUC larger than 0.65. The maximum AUC of all features is <0.7. Thus, it is necessary to identify the combined effects of the features in discriminating the LVRR and the non-LVRR.
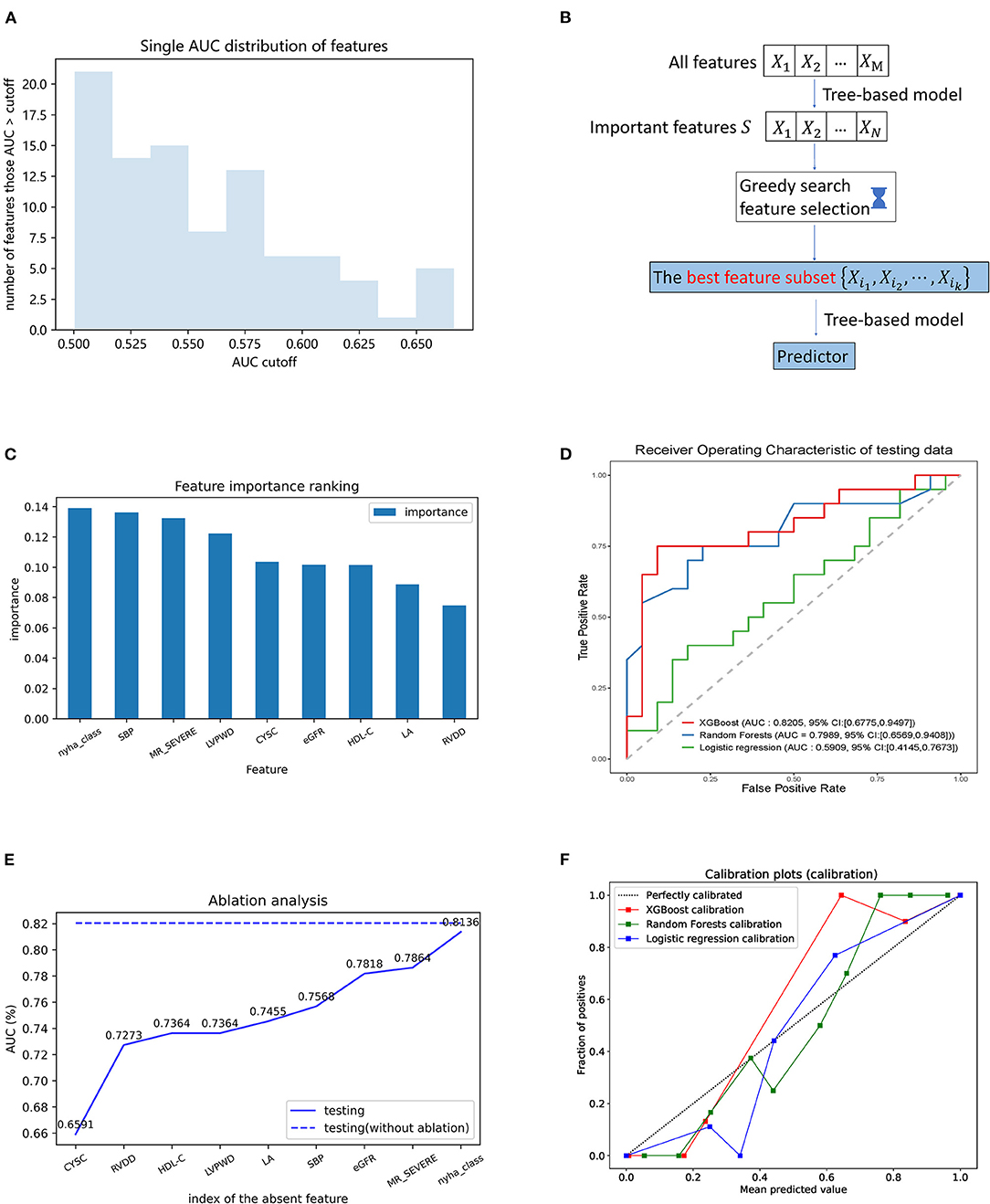
Figure 3. Building model procedure and contributions of the selected features in the prediction. (A) AUC distribution obtained by individual features in the prediction of LVRR and non-LVRR; (B) flowchart of greedy feature selection by XGBoost; (C) greedy feature selection provides the nine best features and the comparison of their importance; (D) receiver operating characteristic curve of three models in testing set. Green, blue, and red curves were generated by the logistic regression, the random forest, and the XGBoost algorithms, respectively; (E) ablation analysis is performed to evaluate the contributions of each feature in the prediction; (F) calibration plot of three models. Blue, green, and red curves were generated by the logistic regression, the random forest, and the XGBoost algorithms, respectively. CysC, cystatin C; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LA, left atrial; LVPWd, left ventricular posterior wall dimension; MR, mitral regurgitation; NYHA, New York Heart Association; RVDd, right ventricular end-diastolic dimension; SBP, systolic blood pressure.
The feature selection procedure is shown in Figure 3B. The tree-based model was first pretrained on the training set to obtain the important features (we describe the result of XGBoost here). Finally, 33 features were selected as important. From these features, we used greedy search to obtain the feature subset which can reach an accurate classification result. The greedy searching provided nine features. Figure 3C shows their importance rank. These features were used to train an XGBoost model with 10-fold cross-validation, which consequently achieved AUC 0.8463 and 0.8205 on the CV (cross-validation) set and test set, respectively (Figure 3D and Supplementary Figure 3). The similarity of the AUC on training and testing set also accounts for the robustness of the model.
Ablation analysis was performed with 10-fold cross-validation to estimate the contributions of each feature in the prediction. As shown in Figure 3E, the absence of each of them could cause a decline of the AUC. Moreover, we observed that cystatin C is the most important feature above all. The ablation of cystatin C can reduce the AUC from 0.8205 to 0.6591.
By comparison, we tested other machine learning methods including logistic regression with l1 penalty and random forests with the same process shown in Figure 3B. As shown in Figure 3D, our method using XGBoost and random forests achieved better AUCs than the linear model on the test set, with AUCs of 0.8205 (95% CI 0.6775–0.9497, p = 0.0119 vs. LR) and 0.7989 (95% CI 0.6589–0.9408, p = 0.0258 vs. LR), respectively. From the confusion matrix of each model shown in Figure 4, we found that the XGBoost can correctly classify 13 of 22 LVRR patients and 16 of 20 non-LVRR patients on the test set, while the random forests can correctly classify 18 of 22 LVRR patients and 13 of 20 non-LVRR patients. The above fact indicated that XGBoost and random forests showed different advantages in classifying the non-LVRR patients and LVRR patients. Moreover, these two tree-based models are both superior to the logistic regression model in classifying LVRR and non-LVRR. Table 2 also reveals the truth by comparing the recall and the sensitivity measurements in classifying LVRR and non-LVRR. Furthermore, we did calibration analysis of the above three models in order to get more statistic evidence for model performance comparison. As shown in Figure 3F, these models had similar calibration.
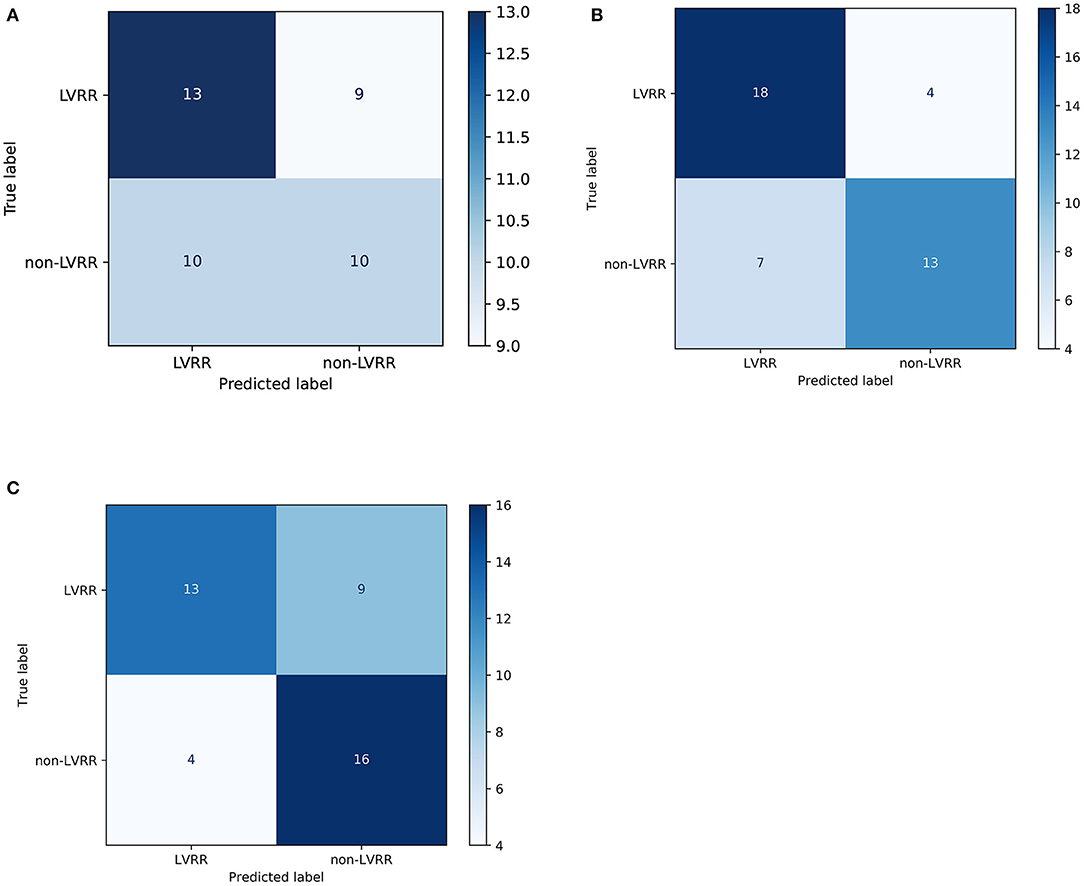
Figure 4. Confusion matrices of the predictive models. The confusion matrix of the logistic regression model (A), random forest (B), and XGBoost (C) in the testing set (~40% of the cohort). Predicted label: the sum of each column represents the predicted sample number of the classes. True label: the sum of each row represents the true sample number of the classes.
In this study, our key findings are as follows: (1) the XGBoost and random forest classifiers combining routine clinical indexes collected before treatment show higher accuracy than logistic regression in predicting LVRR in patients with DCM. (2) Baseline cystatin C, right ventricular end-diastolic dimension, and HDL-C are the most important features in this model, but not LVEF and LVDd. These machine classifiers might be useful to identify the patients who may not respond to the medication and in whom early clinical monitoring and early implementation of preventive strategies may be helpful.
To the best of our knowledge, this is the first study using ensemble tree models of machine learning to predict LVRR. Compared with traditional regression, these models avoid presupposing a linear relation between different variables and the assumptions that are required for correctness of statistical models. In our study, optimized classifiers such as XGBoost and random forest performed with similar better accuracy in predicting LVRR. These ensemble tree models might be useful for improvement in risk factor management in DCM. Unlike the assessment for business risk or the prediction for mortality risk, we pay more attention to better discrimination in the early identification of non-LVRR in DCM, which may be followed more intensively. For the XGBoost model that performed more accurately in differentiating non-LVRR, it was chosen as the final model for subsequent analysis. Moreover, we also found that a single clinical index cannot predict LVRR well, which indicated that LVRR is a consequence of coaction of several factors. At last, we built the XGBoost model including four echocardiogram indexes, three routine laboratory indexes, systolic blood pressure, and NYHA functional class. LVRR is more likely to occur in patients with NYHA functional class I–II, compared with those with NYHA functional class III–IV [61.3% (19/31) vs. 38.4% (28/73), p = 0.032]. Patients with NYHA functional class I–II may be in the early stages of the disease. It has been reported that a shorter duration of disease is associated with a higher likelihood of recovery of LVEF (4). This result is also consistent with some prior reports (20, 36).
Our ablation analysis showed that serum cystatin C contributes remarkably for the predictive model, which is a similar finding to those of previous studies on prognosis of dilated cardiomyopathy. It has been reported that cystatin C was the best predictor of LVEF increase in DCM patients (37). Chatterjee et al. (38) revealed that baseline cystatin C showed incremental benefit in the prediction of cardiac resynchronization therapy non-response compared with conventional renal markers. As we all know, cystatin C is not subject to variability in renal filtration and is considered to be a more stable renal marker, which is less sensitive to gender and age. However, cystatin C may not only serve as a marker of intersecting cardio-renal pathways in patients with DCM but also associate with cathepsin B inhibition, collagen accumulation, and myocardial fibrosis, as an inhibitor of cathepsins, which play a role in the degradation of the extracellular matrix (39). It has been reported that an excess of cystatin C leads to extracellular tissue inhibitor of metalloproteinase-1 and osteopontin accumulation in human cardiac fibroblast cells (40). We speculate that cystatin C takes part in alterations in collagen metabolism and the process of cardiac fibrosis in DCM, which was shown as a key determinant of left ventricular remodeling in DCM (14). Hence, the combination of cystatin C and eGFR (calculated by creatinine) leads to obvious improvement in our model for LVRR in DCM.
In the ablation analysis, we can see that there are four important clinical indexes of cardiac structure obtained by echocardiography. Echocardiography represents the first-line examination in patients with DCM. Our results are similar to those of previous studies on prognosis and dilated cardiomyopathy. Barison et al. (41) reported that prognosis in patients with <35% LVEF was not significantly worse than those with LVEF >35% (p = 0.476). La Vecchia et al. (42) reported that right ventricular end-diastolic volume but not LVEF was demonstrated as an independent predictor of transplant-free survival. Recent studies also found that right ventricular function can be used for prediction in the prognosis of DCM (42, 43). Furthermore, baseline right ventricular dysfunction was proven as a stronger predictor than other known prognostic factors, such as NYHA functional class, functional mitral regurgitation (43), and systolic blood pressure (5, 13). Right ventricular dysfunction may reflect an increased pulmonary artery pressure (44), which may represent an advance stage of ventricular remodeling. Although, right ventricular end-diastolic dimension did not adequately reflect right ventricular function, the combination of adverse remodeling characteristics, such as functional mitral regurgitation and enlargement of other chambers, can provide valuable information for prediction.
HDL-C was another important variable that contributes much in a predictive model from ablation analysis. Emmens et al. (45) reported an inverse association between HDL-C and all-cause mortality or MACE in HFrEF, but not in HFpEF. Freitas et al. (46) also obtained a similar result. The mechanism underlying the association between HDL-C and left ventricular reverse remodeling is not yet clear. Emerging evidence shows that subfractions of HDL have antioxidant, anti-inflammatory, and endothelial cell protective capacity (47–49). Sampietro et al. (50) also found a significant association between HDL-C level and idiopathic DCM and a negative correlation between HDL-C level and inflammation markers, which are similar to our results (Supplementary Figure 4). It may be because serum NT-proBNP levels at first admission can indicate only a short congestive state (51), and there are several novel mechanisms between HDL-C level and left ventricular reverse remodeling in patients with DCM; in our study, there are obvious differences in the HDL-C level but not in hsCRP and NT-proBNP between the LVRR and non-LVRR groups. In addition, DCM is a kind of clinical syndrome which has an impact on multiple organ systems and diverse etiologies. We need the timely identification of LVRR, which can be helpful for their precise management. Machine learning applications might be an attractive option to provide a solution to this problem.
A limitation of our study is that it is a single-center and retrospective study, so we should obtain stronger evidence by performing a large sample prospective study and external validation. A further limitation is that we focused on the predictive performance rather than statistical inference. Therefore, we cannot draw a conclusion about risk factors. In addition, compared with the linear models, tree-based models usually own some unexplainable feature mechanism.
XGBoost and random forest algorithms exhibit good performance for predicting LVRR in patients with DCM. The combination of routine laboratory indicators and echocardiography indexes can be used for predicting LVRR in DCM. These machine learning classifiers might be useful for accurate management and risk evaluation of patients with DCM.
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Sun Yat-Sen Memorial Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JW, YZ, YC, and HZ contributed to the conception and design of the study. XX and MY contributed to the collection of data. XX, MY, HZ, and SX contributed to the analysis and interpretation of the data. XX, XW, YJ, ZL, and YZ contributed to the drafting of the article. All authors have revised the manuscript critically for important intellectual content, read, and approved the final manuscript.
This study was funded by the National Science Foundation of China Grant Nos. 81870170 (JW), 81970388 (YZ), and 81900387 (ZL); Guangdong Province Natural Science Foundation Grant Nos. 2019A1515011682 (YZ) and 2019A1515011806 (ZL); Guangdong Science and Technology Major Foundation Grant No. 2015B010131010 (JW); Guangdong Laboratory of Regenerative Medicine and Health Foundation Grant No. 2019GZR110406004 (JW, YZ, and YC); and Guangzhou Science and Technology Major Foundation Grant Nos. 201803040010 (JW) and 201704020044 (YZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.684004/full#supplementary-material
1. Taylor DO, Edwards LB, Boucek MM, Trulock EP, Waltz DA, Keck BM, et al. Registry of the International society for heart and lung transplantation: twenty-third official adult heart transplantation report−2006. J Heart Lung Transplant. (2006) 25:869–79. doi: 10.1016/j.healun.2006.05.002
2. Song L. Clinical and pathological findings of 400 heart transplants in fuwai hospital. Chin Circ J. (2015) 30:204–5. doi: 10.3969/j.issn.1000-3614.2015.03.002
3. Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. (1994) 331:1564–75. doi: 10.1056/NEJM199412083312307
4. Wilcox J, Fang J, Margulies K, Mann D. Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. J Am Coll Cardiol. (2020) 76:719–34. doi: 10.1016/j.jacc.2020.05.075
5. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. (2011) 57:1468–76. doi: 10.1016/j.jacc.2010.11.030
6. Choi JO, Kim EY, Lee GY, Lee SC, Park SW, Kim DK, et al. Predictors of left ventricular reverse remodeling and subsequent outcome in nonischemic dilated cardiomyopathy. Circ J. (2013) 77:462–9. doi: 10.1253/circj.CJ-12-0507
7. Verdonschot JAJ, Hazebroek MR, Wang P, Wijk SSV, Merken JJ, Adriaansen YA, et al. Clinical phenotype and genotype associations with improvement in left ventricular function in dilated cardiomyopathy. Circ Heart Fail. (2018) 11:e005220. doi: 10.1161/CIRCHEARTFAILURE.118.005220
8. Ikeda Y, Inomata T, Iida Y, Iwamoto-Ishida M, Nabeta T, Ishii S, et al. Time course of left ventricular reverse remodeling in response to pharmacotherapy: clinical implication for heart failure prognosis in patients with idiopathic dilated cardiomyopathy. Heart Vessels. (2016) 31:545–54. doi: 10.1007/s00380-015-0648-2
9. Hoshikawa E, Matsumura Y, Kubo T, Okawa M, Yamasaki N, Kitaoka H, et al. Effect of left ventricular reverse remodeling on long-term prognosis after therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and β blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. (2011) 107:1065–70. doi: 10.1016/j.amjcard.2010.11.033
10. Kubanek M, Sramko M, Maluskova J, Kautznerova D, Weichet J, Lupinek P, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. (2013) 61:54–63. doi: 10.1016/j.jacc.2012.07.072
11. McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (intervention in myocarditis and acute cardiomyopathy)-2 study. J Am Coll Cardiol. (2011) 58:1112–8. doi: 10.1016/j.jacc.2011.05.033
12. Merlo M, Caiffa T, Gobbo M, Adamo L, Sinagra G. Reverse remodeling in dilated cardiomyopathy: insights and future perspectives. Int J Cardiol Heart Vasc. (2018) 18:52–7. doi: 10.1016/j.ijcha.2018.02.005
13. Kawai K, Takaoka H, Hata K, Yokota Y, Yokoyama M. Prevalence, predictors, and prognosis of reversal of maladaptive remodeling with intensive medical therapy in idiopathic dilated cardiomyopathy. Am J Cardiol. (1999) 84:671–6. doi: 10.1016/S0002-9149(99)00414-2
14. Masci PG, Schuurman R, Andrea B, Ripoli A, Coceani M, Chiappino S, et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. (2013) 6:790–9. doi: 10.1161/CIRCIMAGING.113.000438
15. Ikeda Y, Inomata T, Fujita T, Iida Y, Nabeta T, Ishii S, et al. Cardiac fibrosis detected by magnetic resonance imaging on predicting time course diversity of left ventricular reverse remodeling in patients with idiopathic dilated cardiomyopathy. Heart Vessels. (2016) 31:1817–25. doi: 10.1007/s00380-016-0805-2
16. Ishii S, Inomata T, Fujita T, Iida Y, Ikeda Y, Nabeta T, et al. Clinical significance of endomyocardial biopsy in conjunction with cardiac magnetic resonance imaging to predict left ventricular reverse remodeling in idiopathic dilated cardiomyopathy. Heart Vessels. (2016) 31:1960–8. doi: 10.1007/s00380-016-0815-0
17. Xu Y, Li W, Wan K, Liang Y, Jiang X, Wang J, et al. Myocardial tissue reverse remodeling after guideline-directed medical therapy in idiopathic dilated cardiomyopathy. Circ Heart Fail. (2021) 14:e007944. doi: 10.1161/CIRCHEARTFAILURE.120.007944
18. Tayal U, Prasad SK. Myocardial remodelling and recovery in dilated cardiomyopathy. JRSM Cardiovasc Dis. (2017) 6:204800401773447. doi: 10.1177/2048004017734476
19. Ferro MD, Stolfo D, Altinier A, Gigli M, Perrieri M, Ramani F, et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. (2017) 103:1704–10. doi: 10.1136/heartjnl-2016-311017
20. Ruiz-Zamora I, Rodriguez-Capitan J, Guerrero-Molina A, Morcillo-Hidalgo L, Rodriguez-Bailon I, Gomez-Doblas JJ, et al. Incidence and prognosis implications of long term left ventricular reverse remodeling in patients with dilated cardiomyopathy. Int J Cardiol. (2016) 203:1114–21. doi: 10.1016/j.ijcard.2015.11.099
21. Chen T, Guestrin C. Xgboost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. San Francisco, CA: ACM (2016). p. 785–94. doi: 10.1145/2939672.2939785
22. Dhaliwal S, Nahid AA, Abbas R. Effective intrusion detection system using XGBoost. Information. (2018) 9:149. doi: 10.3390/info9070149
23. Ke Y, Rao J, Zhao H, Lu Y, Xiao N, Yang Y. Accurate prediction of genome-wide RNA secondary structure profile based on extreme gradient boosting. Bioinformatics. (2019) 36:4576–82. doi: 10.1093/bioinformatics/btaa534
24. Lv X, Chen J, Lu Y, Chen Z, Xiao N, Yang Y. Accurately predicting mutation-caused stability changes from protein sequences using extreme gradient boosting. J Chem Inf Model. (2020) 60:2388–95. doi: 10.1021/acs.jcim.0c00064
25. Al'Aref SJ, Maliakal G, Singh G, van Rosendael AR, Ma X, Xu Z, et al. Machine learning of clinical variables and coronary artery calcium scoring for the prediction of obstructive coronary artery disease on coronary computed tomography angiography: analysis from the CONFIRM registry. Eur Heart J. (2020) 41:359–67. doi: 10.1093/eurheartj/ehz565
26. Tse G, Lee S, Zhou J, Liu T, Wong ICK, Mak C, et al. Territory-wide Chinese cohort of long QT syndrome: random survival forest and Cox analyses. Front Cardiovasc Med. (2021) 8:608592. doi: 10.3389/fcvm.2021.608592
27. Chinese Society of Cardiology of Chinese Medical Chinese Myocarditis and Cardiomyopathy Association. Chinese guidelines for the diagnosis and treatment of dilated cardiomyopathy. J Clin Cardiol. (2018) 34:421–34. doi: 10.13201/j.issn.1001-1439.2018.05.001
28. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association Chinese Heart Failure Association of Chinese Medical Doctor Association Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi. (2018) 46:760–89. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004
29. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. (1989) 2:358–67. doi: 10.1016/S0894-7317(89)80014-8
30. Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European association of cardiovascular imaging (EACVI) and the American society of echocardiography (ASE)†. Eur Heart J Cardiovasc Imaging. (2015) 16:577–605. doi: 10.1093/ehjci/jev076
31. Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism. (2006) 55:515–24. doi: 10.1016/j.metabol.2005.11.004
32. Levey AS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. (2000) 11:A0828. Available online at: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/658418
34. Lee S, Lee H, Abbeel P, Ng A. Efficient L~ 1 regularized logistic regression. In: Proceedings, the Twenty-First National Conference on Artificial Intelligence and the Eighteenth Innovative Applications of Artificial Intelligence Conference. Boston, MA: AAAI Press (2006). p. 401–8.
35. Zadrozny B, Elkan C. Transforming Classifier Scores into Accurate Multiclass Probability Estimates. Edmonton, AB: KDD (2002). p. 694–9. doi: 10.1145/775047.775151
36. Moliner-Abós C, Mojón Álvarez D, Rivas-Lasarte M, Belarte LC, Pamies Besora J, Solé-González E, et al. A simple score to identify super-responders to sacubitril/valsartan in ambulatory patients with heart failure. Front Physiol. (2021) 12:642117. doi: 10.3389/fphys.2021.642117
37. Bielecka-Dabrowa A, von Haehling S, Aronow WS, Ahmed MI, Rysz J, Banach M. Heart failure biomarkers in patients with dilated cardiomyopathy. Int J Cardiol. (2013) 168:2404–10. doi: 10.1016/j.ijcard.2013.01.157
38. Chatterjee NA, Singh JP, Szymonifka J, Deaño RC, Thai W, Wai B, et al. Incremental value of cystatin C over conventional renal metrics for predicting clinical response and outcomes in cardiac resynchronization therapy: the BIOCRT study. Int J Cardiol. (2016) 205:43–9. doi: 10.1016/j.ijcard.2015.12.002
39. Xie L, Terrand J, Xu B, Tsaprailis G, Boyer J, Chen QM. Cystatin C increases in cardiac injury: a role in extracellular matrix protein modulation. Cardiovasc Res. (2010) 87:628–35. doi: 10.1093/cvr/cvq138
40. Huerta A, López B, Ravassa S, José GS, Querejeta R, Beloqui Ó, et al. Association of cystatin C with heart failure with preserved ejection fraction in elderly hypertensive patients: potential role of altered collagen metabolism. J Hypertens. (2016) 34:130–8. doi: 10.1097/HJH.0000000000000757
41. Barison A, Aimo A, Ortalda A, Todiere G, Grigoratos C, Passino C, et al. Late gadolinium enhancement as a predictor of functional recovery, need for defibrillator implantation and prognosis in non-ischemic dilated cardiomyopathy. Int J Cardiol. (2018) 250:195–200. doi: 10.1016/j.ijcard.2017.10.043
42. La Vecchia L, Varotto L, Zanolla L, Spadaro GL, Fontanelli A. Right ventricular function predicts transplant-free survival in idiopathic dilated cardiomyopathy. J Cardiovasc Med. (2006) 7:706–10. doi: 10.2459/01.JCM.0000243006.90170.ce
43. Merlo M, Gobbo M, Stolfo D, Losurdo P, Ramani F, Barbati G, et al. The prognostic impact of the evolution of RV function in idiopathic DCM. JACC Cardiovasc Imaging. (2016) 9:1034–42. doi: 10.1016/j.jcmg.2016.01.027
44. La Vecchia L, Zanolla L, Varotto L, Bonanno C, Spadaro GL, Ometto R, et al. Reduced right ventricular ejection fraction as a marker for idiopathic dilated cardiomyopathy compared with ischemic left ventricular dysfunction. Am Heart J. (2001) 142:181–9. doi: 10.1067/mhj.2001.116071
45. Emmens JE, Jones DJL, Cao TH, Chan DCS, Romaine SPR, Quinn PA, et al. Proteomic diversity of high-density lipoprotein explains its association with clinical outcome in patients with heart failure. Eur J Heart Fail. (2017) 20:260–7. doi: 10.1002/ejhf.1101
46. Freitas HFG, Barbosa EA, Rosa FHFP, Lima ACP, Mansur AJ. Association of HDL cholesterol and triglycerides with mortality in patients with heart failure. Braz J Med Biol Res. (2009) 42:420–5. doi: 10.1590/S0100-879X2009000500004
47. Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, et al. High-density lipoproteins: a consensus statement from the national lipid association. J Clin Lipidol. (2013) 7:484–525. doi: 10.1016/j.jacl.2013.08.001
48. Kingwell BA, Chapman MJ, Kontush A, Miller NE. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov. (2014) 13:445–64. doi: 10.1038/nrd4279
49. McGarrah RW. Refocusing the AIM on HDL in the metabolic syndrome. Atherosclerosis. (2016) 251:531–3. doi: 10.1016/j.atherosclerosis.2016.06.051
50. Sampietro T, Neglia D, Bionda A, Dal Pino B, Bigazzi F, Puntoni M, et al. Inflammatory markers and serum lipids in idiopathic dilated cardiomyopathy. Am J Cardiol. (2005) 96:1718–20. doi: 10.1016/j.amjcard.2005.07.093
Keywords: idiopathic dilated cardiomyopathy, heart failure, reverse remodeling, predictive model, machine learning
Citation: Xie X, Yang M, Xie S, Wu X, Jiang Y, Liu Z, Zhao H, Chen Y, Zhang Y and Wang J (2021) Early Prediction of Left Ventricular Reverse Remodeling in First-Diagnosed Idiopathic Dilated Cardiomyopathy: A Comparison of Linear Model, Random Forest, and Extreme Gradient Boosting. Front. Cardiovasc. Med. 8:684004. doi: 10.3389/fcvm.2021.684004
Received: 22 March 2021; Accepted: 07 June 2021;
Published: 04 August 2021.
Edited by:
Vinicius Tragante, University Medical Center Utrecht, NetherlandsReviewed by:
Shinichi Okuda, Yamaguchi Prefectural Grand Medical Center, JapanCopyright © 2021 Xie, Yang, Xie, Wu, Jiang, Liu, Zhao, Chen, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingfeng Wang, d2ppbmdmQG1haWwuc3lzdS5lZHUuY24=; Yuling Zhang, emh5dWxAbWFpbC5zeXN1LmVkdS5jbg==; Yangxin Chen, Y2hlbnl4MzlAbWFpbC5zeXN1LmVkdS5jbg==; Huiying Zhao, emhhb2h5OEBtYWlsLnN5c3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.