
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 22 June 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.683281
This article is part of the Research Topic Highlights in General Cardiovascular Medicine: 2021 View all 12 articles
Purpose: The purpose of the study is to evaluate the effect of empagliflozin in patients with heart failure (HF).
Method: We performed a systematic search of PubMed, EMBASE, and the Cochrane Library database through January 20, 2021. Randomized controlled trials (RCTs) were included that compared empagliflozin and placebo in patients with HF. Dichotomous variables were expressed as risk ratios (RRs) with 95% confidence intervals (CIs). Continuous variables were calculated and expressed as mean differences (MD) and standard deviation (SD). Meta-analysis was conducted using a random-effects model on outcomes with high heterogeneity.
Results: Seven studies were included in our meta-analysis (n = 5,150). Significant differences were observed in a composite of cardiovascular death or hospitalization for worsening heart failure [RR: 0.77 (95% CI 0.68–0.87); I2 = 18%; P < 0.0001), hospitalization for worsening heart failure [RR: 0.71 (95% CI 0.61–0.82); I2 = 0%; P < 0.00001], changes in Kansas City Cardiomyopathy Questionnaire (KCCQ) score [MD: 1.70 (95% CI 1.67–1.73); I2 = 0%; P < 0.00001], and changes in body weight [MD: −1.43 (95% CI −2.15 to −0.72); I2 = 84%; P < 0.0001) from baseline. However, empagliflozin did not show a better change in the 6-min walk test (6MWT) [MD: 34.06 (95% CI −29.75–97.88); I2 = 97%; P = 0.30] or NT-proBNP [MD: −98.36 (95% CI, −225.83–29.11); I2 = 68%; P = 0.13] from baseline.
Conclusion: The findings suggest that empagliflozin was effective in reducing a composite of cardiovascular death or hospitalization for worsening heart failure. Further well-designed RCTs are needed to evaluate the long-term effect of empagliflozin in patients with HF.
PROSPERO: CRD42021231712.
Heart failure (HF) leads to a high economic burden, and the prevalence of HF is also increasing gradually (1, 2). It has been estimated that over 26 million people around the world are suffering from heart failure (3). According to the guidelines (4) for HF patients, it is recommended to apply angiotensin-converting enzyme inhibitors, beta-blockers, mineralocorticoids, or aldosterone receptor antagonists. Currently, new drugs such as sodium–glucose cotransporter 2 inhibitors (SGLT2i) and vericiguat also show positive effects across the spectrum of HF (5).
SGLT2i has been used to treat type 2 diabetes mellitus (6, 7). In recent years, SGLT2i has shown a better effect than placebo on the outcomes of all-cause mortality and heart failure hospitalization in patients with type 2 diabetes mellitus in clinical trials, regardless of the presence or absence of heart failure (8, 9). In patients with diabetes mellitus, SGLT2i reduced the risk of all-cause mortality and heart failure hospitalizations by 23% (10). Empagliflozin, one of the frequently used SGLT2i, has been proven to be effective in reducing HF hospitalizations, cardiovascular deaths, and biomarkers in patients with HF (11). However, the results in related articles showed heterogeneity in some outcomes, for example, N-terminal pro-brain natriuretic peptide (NT-proBNP) (12, 13) and the 6-min walk test (6MWT) (14, 15). The effect of empagliflozin in patients with HF has not been evaluated specifically. Previous studies mostly concentrated on SGLT2i rather than empagliflozin. According to previous large-sample trials, empagliflozin also showed different results of a composite cardiovascular endpoint (cardiovascular deaths, non-fatal myocardial infarction, or non-fatal stroke) when compared with dapagliflozin, which means that different drugs may present different results, although all of them belong to SGLT2i (9, 16). Thus, we aim to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the effect of empagliflozin on patients with HF.
Our systematic review and meta-analysis were conducted based on preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Ethical approval was not required. The study protocol was registered in the PROSPERO international prospective register of systematic review (CRD42021231712).
Our systematic review and meta-analysis were conducted according to the PRISMA guidelines (17). We performed a systematic search from the following electronic databases: PubMed, EMBASE, and Cochrane Library, from their inception to January 20, 2021. The search terms were as follows: [1-chloro-4-(glucopyranos-1-yl)-2-(4-(tetrahydrofuran-3-yloxy)benzyl] benzene OR BI 10773 OR BI10773 OR BI-10773 OR Jardiance) AND (Cardiac Failure OR Heart Decompensation OR Decompensation, Heart OR Heart Failure, Right-Sided OR Heart Failure, Right Sided OR Right-Sided Heart Failure OR Right Sided Heart Failure OR Myocardial Failure OR Congestive Heart Failure OR Heart Failure, Congestive OR Heart Failure, Left-Sided OR Heart Failure, Left Sided OR Left-Sided Heart Failure OR Left Sided Heart Failure). No restrictions on language, publication date, or publication status were set in our review. We did not include conference abstracts. In addition, we reviewed the reference lists of eligible studies, previous review articles, and registered clinical trials.
All searched articles were imported into EndNote software, and the title and abstract were screened by two reviewers (DP and PC). We entirely included clinical trials that met the following criteria for final analysis: (1) RCTs, (2) the target population was patients with HF, and (3) studies that included the comparison between empagliflozin and placebo.
The exclusion criteria were (1) observation, cohort, case control, case series, qualitative studies, uncontrolled trials, and laboratory studies and (2) duplicate studies with the same population (only the study with the largest participants was included in the meta-analysis if multiple studies included overlapping groups of patients).
If disagreements on study selection were identified, another author (MG) was consulted to solve them.
Two investigators (DP and PC) extracted the included trial data by using a predesigned form independently. The retrieved study characteristics were as follows: (1) first author's name and year of study; (2) study site; (3) intervention, dose of intervention, and comparison; (4) total sample size, and sample size of intervention and control group; (5) primary outcome; and (6) other outcomes.
The primary outcome was determined as a composite of cardiovascular deaths and hospitalization for worsening heart failure. Other outcomes included hospitalization for worsening heart failure, changes in the NT-proBNP, 6WMT, or KCCQ score, and changes in body weight before and after the intervention. The endpoint definitions were those used in the individual trials.
Assessment of the risk of bias for all of the included studies was performed independently by two review authors (DP and PC) through the Cochrane Risk of bias assessment tool. The assessment was performed across the following domains: selection bias (random sequence generation and allocation concealment), performance bias (blinding of patients and investigators), detection bias (blinding of outcome assessors), attrition bias (flawed outcome data), and reporting bias (selective reporting). According to the influence on material biases, the risk of bias was then adjudicated, with low, high, or unclear levels. To evaluate the certainty of the evidence for each outcome, we applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Framework. All disagreements were resolved by the other two reviewers (MG and DS).
We pooled the study results in the meta-analysis using Review Manager software (version 5.4.1; Nordic Cochrane Center, Cochrane Collaboration).
For dichotomous variables, risk ratios (RRs) with 95% confidence intervals (CIs) were calculated. Continuous variables were calculated and expressed in terms of mean differences (MD) and standard deviation (SD). For the articles that expressed continuous outcomes by median and interquartile range (IQR), the mean value and SD value were by the method of Luo et al. and Wan et al. (18, 19). For the articles that reported only the results before and after the intervention, the corresponding change from baseline was calculated according to the Cochrane Handbook for Systematic Reviews of Interventions. In the articles that reported mean and SD before intervention (Mbaseline, SDbaseline), mean and SD after intervention (Mfinal, SDfinal), and mean and SD of the change before and after intervention (Mchange, SDchange), the correlation coefficient (Corr) was calculated as + – /2 * SDbaseline * SDfinal. For the articles that did not report the values of Mchange and SDchange, Mchange and SDchange were calculated by the following equations: Mchange = Mfinal – Mbaseline, SDchange = [ + – (2 * Corr * SDfinal * SDbaseline)]1/2. We assessed heterogeneity by using the X2 test and I2 statistic. Significant heterogeneity was defined as I2 > 50% with a P < 0.10. We used the random-effects model to estimate the pooled effect size from the included data if significant heterogeneity was observed across the trials, and the fixed-effects model was used otherwise. By conducting sensitivity and subgroup analysis, we probed sources of heterogeneity. Sensitivity analyses were conducted by one-by-one elimination. Publication bias was assessed using funnel plots if the included trial number was reasonable (10 or more). The quality of evidence for the main outcomes was assessed by the GRADE (20).
We conducted the last search on January 20, 2021, and the results of our literature search are shown in Figure 1. A total of 454 publications were identified. After the removal of duplications and screening, 16 studies were selected for eligibility by full-text screening, and seven trials were included in our systematic review and meta-analysis (11–15, 21, 22).
Table 1 reports the characteristics of the included studies. Of the selected seven trials, 5,150 participants were included in total, 2,682 of them were in the empagliflozin group, and 2,468 of them were in the placebo group. Among them, six studies (11–15, 22) were two-arm RCTs. The last was a three-arm RCT (21) comparing two different doses (10 and 25 mg) of empagliflozin with placebo, and it was also the only substudy of a previous trial (9). In the selected trials, Packer's RCT (11) was the study with the largest sample size (n = 3,730). The age ranged from 59 to 70 years old, and the proportion of males was higher than that of females in all of the trials, ranging from 63% to 87%. Except for the three-arm RCT (21) and Mordi et al. (13), the dose of empagliflozin in the remaining trials was 10 mg once daily. The median follow-up time ranged from 6 weeks to 3.1 years.
The outcomes included a composite of cardiovascular death or hospitalization for worsening heart failure [three RCTs (11, 12, 21)], hospitalization for worsening heart failure [three RCTs (11, 12, 21)], change in KCCQ score [three RCTs (11, 12, 14)], change in NT-proBNP from baseline [five RCTs (11–14, 22)], change in 6MWT from baseline [three RCTs (14, 15, 22)], and change in body weight from baseline [four RCTs (11–13, 15)].
All seven included studies reported the generation of random sequences, and all of them also provided generation methods. However, two studies (12, 14) did not report the method of allocation concealment, so their risk level remained unclear. One study (22) did not report the blinding of both treatment providers and participants, while the other studies adopted a double-blind design. Two studies (11, 12) reported blinding of the outcome assessment. All of the studies were evaluated as having a low risk of attrition bias with no loss to follow-up or low and balanced loss to follow-up. There was no selective bias in any of the seven studies. We also detected no other risk in the seven included studies.
Three studies (11, 12, 21) reported the result of a composite of cardiovascular death or hospitalization for worsening heart failure. Empagliflozin significantly reduced the risk of a composite of cardiovascular death or hospitalization for worsening heart failure compared with the placebo group [RR: 0.77 (95% CI 0.68–0.87]; I2 = 18%; P < 0.0001; Figure 2A]. We also conducted a meta-analysis by a random-effects model, and the result was consistent [RR: 0.74 (95% CI 0.56–0.96); I2 = 18%; P = 0.003; Figure 2B].
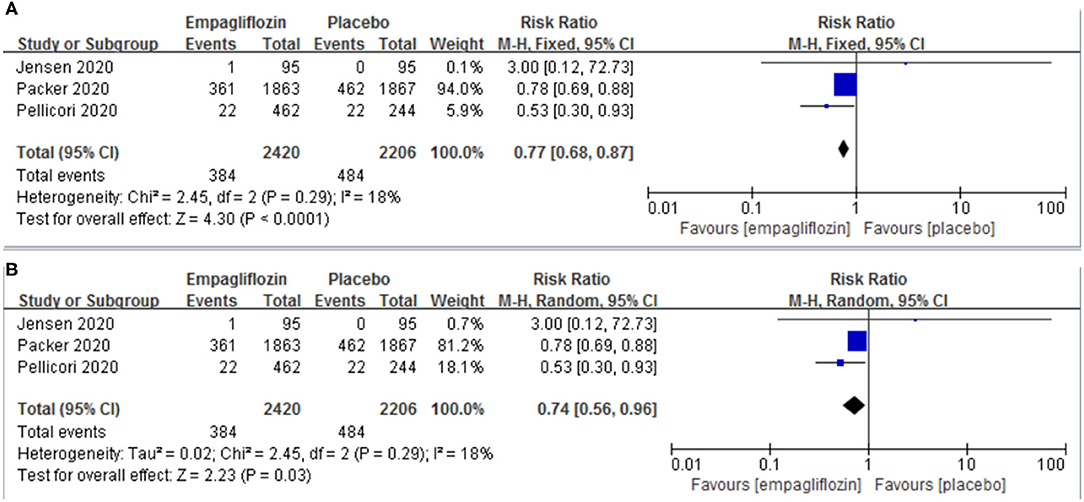
Figure 2. Effect of empagliflozin on a composite of cardiovascular deaths or hospitalizations for worsening heart failure [(A) by fixed-effects model; (B) by random-effects model].
Three studies (11, 12, 21) reported the result of hospitalization for worsening heart failure, and we observed a significant difference between the two groups, which favored the empagliflozin group [RR: 0.71 (95% CI 0.61–0.82); I2 = 0%; P < 0.00001; Figure 3].
The outcome of the 6MWT was reported in three studies (14, 15, 22), and we did not observe a significant difference between the two groups with regard to the distance change in the 6MWT before and after intervention [mean difference (MD): 34.06 (95% CI, −29.75–97.88); I2 = 97%; P = 0.30; Figure 4].
The change from baseline of NT-proBNP was reported in five studies (11–14, 22), and no significant difference was observed between the two groups [MD: −98.36 (95% CI −225.83–29.11); I2 = 68%; P = 0.13; Figure 5].
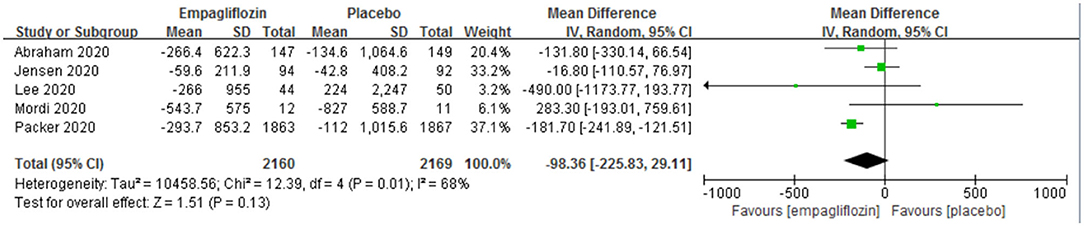
Figure 5. Effect of empagliflozin on the change in N-terminal pro-brain natriuretic peptide (NT-proBNP) from baseline.
Three studies (11, 12, 14) reported the change in KCCQ score. A significant difference was observed between the empagliflozin group and the placebo group, which favored the empagliflozin group [MD: 1.70 (95% CI 1.67–1.73); I2 = 0%; P < 0.00001; Figure 6].
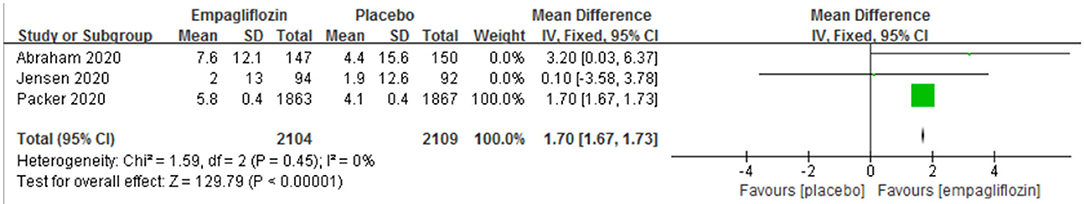
Figure 6. Effect of empagliflozin on the change in Kansas City Cardiomyopathy Questionnaire (KCCQ) score from baseline.
Four studies (11–13, 15) reported the change in body weight before and after the intervention, and a significant difference was detected between the two groups [MD: −1.43 (95% CI, −2.15 to −0.72); I2 = 84%; P < 0.0001; Figure 7].
Five RCTs (11, 12, 14, 21, 22) recruited patients with heart failure and reduced ejection fraction [defined as a left ventricular ejection fraction of <40% (31)]. In HFrEF patients, empagliflozin was associated with significantly better NT-proBNP reduction [MD: −121.56 (95% CI −242.17 to −0.95); I2 = 68%; P = 0.05; Figure 8]. In addition, empagliflozin showed a superior reduction in body weight in HFrEF patients [MD: −0.81 (95% CI −0.82 to −0.80); I2 = 40%; P < 0.00001; Figure 9].
We performed sensitivity analysis to evaluate the influence of any individual study on the overall effect. For NT-proBNP, empagliflozin was significantly better than placebo when Jensen et al. (12) was removed [MD: −173.07 (95% CI −230.05 to −116.08); I2 = 35%; P < 0.00001]. For the 6MWT, the placebo group showed slightly better performance than the empagliflozin group when Santos-Gallego et al. (15) was removed [MD: −2.65 (95% CI −5.16 to −0.15); I2 = 0%; P = 0.04].
Table 2 shows the GRADE assessment of the certainty of the effect of empagliflozin in patients with HF. Because the participants in one study were non-diabetic patients (15), whereas another study focused on patients with type 2 diabetes mellitus (13), the certainty level of body weight was downgraded to moderate. The certainty level of the 6MWT was downgraded to moderate for one article focused on non-diabetic patients (15), and another focused on patients with type 2 diabetes mellitus or prediabetes (22). The other outcomes, a composite of cardiovascular death or hospitalization for worsening heart failure, hospitalization for worsening heart failure, and changes in NT-proBNP and the KCCQ score, were evaluated as high certainty, with no evidence for downgrading.
In this systematic review and meta-analysis, we included seven RCTs with 5,150 participants in total, and with regard to the primary outcome of a composite of cardiovascular death or hospitalization for worsening heart failure, the empagliflozin group showed significant superiority to the placebo group. In terms of hospitalization for worsening heart failure, the change in KCCQ score, and the change in body weight, significant differences were observed between the two groups and favored the empagliflozin group. For the results of the changes in 6MWT and NT-proBNP, we did not observe notable differences between the two groups except when studies that caused high heterogeneity were removed, when 6MWT favored the placebo, and NT-proBNP favored empagliflozin.
To our knowledge, this is the first systematic review and meta-analysis that concentrated on the effect of empagliflozini in patients with HF. Instead of assuming the parameter Corr to be 0.5, we calculated Corr, which made the results more convincing. We also included four new high-quality RCTs in our study and pooled two new outcomes (6MWT and body weight). Hence, our results provided more robust and comprehensive evidence for evaluating the effect of empagliflozin on HF. We observed significant improvements in heart failure hospitalizations and cardiovascular deaths, which is in line with a previous study (32) focused on the effect of SGLT2i in heart failure patients. However, regarding the two outcomes of a composite of cardiovascular death or hospitalization for worsening heart failure and hospitalization for worsening heart failure, our study showed greater RR reduction than the previous meta-analysis (32), which may indicate that empagliflozin is superior in patients with reduced ejection fraction. It is also worth noting that the results of our study and a previous trial with dapagliflozin intervention (33) demonstrated no significant difference in the change in NT-proBNP. However, some trials showed that SGLT2i, especially empagliflozin or dapagliflozin, were associated with a reduction in plasma NT-proBNP levels (11, 22, 30), which is consistent with the result of the HFrEF subgroup in our meta-analysis. Therefore, the results on NT-proBNP were controversial. However, with pooled results including new high-quality trials, we believe that our results are more convincing.
SGLT2i localize at the brush border of the early proximal tubule and function by reabsorbing nearly all of the filtered glucose (23, 24). Because the excretion of glucose was promoted, the blood glucose level was decreased. With lower blood glucose, lower heart failure mortality was observed in a cohort trial (25). However, the outcome cannot be simply explained by lower blood glucose, since other antidiabetic drugs with stronger effects did not show the same cardiovascular protective results (26). In addition to the aforementioned mechanism, SGLT2i prevents the reabsorption of glucose in the proximal tubule, further causing secondary osmotic effects and then leading to natriuretic and diuretic effects (23). This mechanism could account for the reduction in body weight, which was found in our study. Moreover, it has been proven that empagliflozin significantly improved left ventricular diastolic function and reduced mortality in mice, probably by reducing spontaneous diastolic sarcoplasmic reticulum calcium release. Leakage was thought to be the mechanism of diastolic dysfunction (27). In addition, empagliflozin also promoted cardiac function in non-diabetic rats, which may be associated with improved cardiac metabolism and cardiac ATP production (28).
Autophagy plays a crucial role in relieving cellular stress caused by different metabolites, such as glucose and lipids (29, 34), and clearing accumulated metabolites and dysfunctional organelles, thus, preventing cells from dysfunction or death. In heart failure patients, the autophagy capacity of cardiomyocytes is markedly impaired (35). Autophagic vacuoles, which reflect the extent of autophagy, were found to be associated with a better prognosis of heart failure (36). SGLT2i promotes urinary caloric loss, which mimics nutrient deprivation, in which autophagy is activated, leading to cellular survival, and homeostasis instead of growth (37). In non-diabetic mice, SGLT2i upregulated SIRT1 and AMPK, which promote autophagy, and downregulated the Akt-mTOR pathway, which has the opposite function (35, 37). Through the mechanisms mentioned above, autophagy was promoted, thereby protecting cardiomyocytes from dysfunction and death.
There are some limitations that should be noted. First, the doses and durations of follow-up in the included trials were not uniform. Consequently, we conducted sensitivity analysis to evaluate heterogeneity. The results of the 6MWT and NT-proBNP changed after sensitivity analysis, indicating that the two results should be interpreted with great caution. Second, the forms of reported results varied across trials, and the transformation to a consistent form may have introduced some inaccuracy. Nonetheless, we transformed the results according to relevant articles (18, 19), which minimized the error as much as possible. Third, we obtained trial-level data instead of individual-level data; thus, we could not conduct subgroup analysis based on type 2 diabetes mellitus, different drug therapies, New York Heart Association (NYHA) functional class, estimated glomerular filtration rate (eGFR), and so on.
In conclusion, compared with placebo, empagliflozin significantly reduced a composite of cardiovascular deaths or hospitalizations for worsening heart failure but showed no statistically significant change in NT-proBNP. However, in HFrEF patients, empagliflozin showed a significant reduction in NT-proBNP. More high-quality, large-scale RCTs are needed to comprehensively evaluate the long-term effect of empagliflozin in patients with HF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
DP, LX, and MG designed the study, assessed the risk of bias, analyzed the data, and wrote the first and revised version of the manuscript. DP and PC screened and extracted the data. DP, DS, HJ, and MG modified the final manuscript. All authors read and approved the final manuscript, contributed to the conceptualization of the research questions, interpretation of the results, and article writing.
This work was supported by the National Natural Science Foundation of China (Grant No. 81904025) and the Fundamental Research Funds for the Central Public Welfare Research Institutes (Grant No. ZZ13-YQ-016 and ZZ13-YQ-016-C1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, et al. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. (2002) 4:531–9. doi: 10.1016/S1388-9842(02)00034-X
2. Eriksson H. Heart failure: a growing public health problem. J Intern Med. (1995) 237:135–41. doi: 10.1111/j.1365-2796.1995.tb01153.x
3. Ambrosy P, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. (2014) 63:1123–33. doi: 10.1016/j.jacc.2013.11.053
4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
5. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. (2020). 324:488–504. doi: 10.1001/jama.2020.10262
6. M. Hanefeld T. Forst dapagliflozin, an SGLT2 inhibitor, for diabetes. Lancet. (2010) 375:2196–8. doi: 10.1016/S0140-6736(10)60749-0
7. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. (2012) 35:1473–8. doi: 10.2337/dc11-1693
8. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. (2019) 139:2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130
9. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
10. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. (2019) 393:31–9. doi: 10.1016/S0140-6736(18)32590-X
11. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. doi: 10.1056/NEJMoa2022190
12. Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure reduced ejection fraction: a double-blinded, randomized, placebo-controlled trial. Am Heart J. (2020) 228:47–56. doi: 10.1016/j.ahj.2020.07.011
13. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. J Circulation. (2020) 142:1713–24. doi: 10.1161/CIRCULATIONAHA.120.048739
14. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability symptoms in heart failure patients with reduced preserved ejection fraction, with without type 2 diabetes. Eur Heart J. (2020) 42:700–10. doi: 10.1093/eurheartj/ehaa943
15. Santos-Gallego G, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2021) 77:243–55. doi: 10.1016/j.jacc.2020.11.008
16. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
18. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
19. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
20. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
21. Pellicori P, Ofstad AP, Fitchett D, Zeller C, Wanner C, George J, et al. Early benefits of empagliflozin in patients with or without heart failure: findings from EMPA-REG OUTCOME. ESC Heart Fail. (2020) 7:3401–3407. doi: 10.1002/ehf2.12891
22. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. (2020) 143:516–25. doi: 10.1161/CIRCULATIONAHA.120.052186
23. Shentu Y, Li Y, Xie S, Jiang H, Sun S, Lin R, et al. Empagliflozin, a sodium glucose cotransporter-2 inhibitor, ameliorates peritoneal fibrosis via suppressing TGF-β/Smad signaling. Int Immunopharmacol. (2021) 93:107374. doi: 10.1016/j.intimp.2021.107374
24. Dharmalingam M, Aravind SR, Thacker H, Paramesh S, Mohan B, Chawla M, et al. Efficacy and safety of remogliflozin etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: a 24-week, randomized, double-blind, active-controlled trial. Drugs. (2020) 80:587–600. doi: 10.1007/s40265-020-01285-0
25. Barsheshet A, Garty M, Grossman E, Sandach A, Lewis BS, Gottlieb S, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. (2006) 166:1613–9. doi: 10.1001/archinte.166.15.1613
26. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. (2020) 43:508–11. doi: 10.2337/dci19-0074
27. Moellmann J, Klinkhammer BM, Droste P, Kappel B, Haj-Yehia E, Maxeiner S, et al. Empagliflozin improves left ventricular diastolic function of db/db mice. Biochim Biophys Acta. (2020) 1866:165807. doi: 10.1016/j.bbadis.2020.165807
28. Yurista SR, Sillje HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. (2019) 21:862–73. doi: 10.1002/ejhf.1473
29. Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. (2004) 18:1692–700. doi: 10.1096/fj.04-2263com
30. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
31. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of america, heart failure association of the european society of cardiology, japanese heart failure society and writing committee of the universal definition of heart failure. J Card Fail. (2021). doi: 10.1016/j.cardfail.2021.01.022
32. Chambergo-Michilot D, Tauma-Arrué A, Loli-Guevara S. Effects and safety of SGLT2 inhibitors compared to placebo in patients with heart failure: A systematic review and meta-analysis. Int J Cardiol Heart Vasc. (2021) 32:100690. doi: 10.1016/j.ijcha.2020.100690
33. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. (2019) 140:1463–76. doi: 10.1161/CIRCULATIONAHA.119.042929
34. Nozyński J, Zakliczyński M, Konecka-Mrowka D, Zielinska T, Zakliczynska H, Nikiel B, et al. Advanced glycation end product accumulation in the cardiomyocytes of heart failure patients with and without diabetes. Ann Transplant. (2012) 17:53–61. doi: 10.12659/AOT.883223
35. Packer M. Molecular, cellular, and clinical evidence that sodium-glucose cotransporter 2 inhibitors act as neurohormonal antagonists when used for the treatment of chronic heart failure. J Am Heart Assoc. (2020) 9:e016270. doi: 10.1161/JAHA.120.016270
36. Saito T, Asai K, Sato S, Hayashi M, Adachi A, Sasaki Y, et al. Autophagic vacuoles in cardiomyocytes of dilated cardiomyopathy with initially decompensated heart failure predict improved prognosis. Autophagy. (2016) 12:579–87. doi: 10.1080/15548627.2016.1145326
Keywords: empagliflozin, sodium-glucose cotransporter 2 inhibitors, heart failure, systematic review, cardiovascular
Citation: Pan D, Xu L, Chen P, Jiang H, Shi D and Guo M (2021) Empagliflozin in Patients With Heart Failure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 8:683281. doi: 10.3389/fcvm.2021.683281
Received: 20 March 2021; Accepted: 30 April 2021;
Published: 22 June 2021.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Rahul Gupta, Lehigh Valley Health Network, United StatesCopyright © 2021 Pan, Xu, Chen, Jiang, Shi and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Guo, Z3VvbWluZ21pbmcxOTg3QGFsaXl1bi5jb20=; Dazhuo Shi, c2hpZGF6aHVvQGNhY21zLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.