- 1Department of Cardiovascular Medicine, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Department of Cardiology, Shanghai Tenth People's Hospital, School of Medicine, Tongji University, Shanghai, China
- 3Department of Laboratory, Taiyuan Hospital Health Center for Woman and Children, Taiyuan, China
Objective: The probability of late recurrent atrial fibrillation (AF) after radiofrequency ablation (RFA) has not yet been fully clarified. This study aims to study the association of left atrial appendage (LAA) morphology with AF recurrence after RFA.
Methods: We retrospectively enrolled 84 patients (24 patients had persistent AF, 60 patients had paroxysmal AF) who underwent RFA in Shanghai East Hospital from June 2014 to May 2018. The mean follow-up of these patients was 618.6 days. According to preoperative transesophageal echocardiography (TEE), the morphology feature of LAA was classified and evaluated by two classification methods. The first method was divided into chicken-wing, windsock, cactus, and cauliflower, and the second method was divided into one lobe, two lobes, and multiple lobes. The correlation between morphological feature of LAA and the recurrence rate of AF after RFA was analyzed.
Results: During follow-up, 12 patients (50%) and 10 patients (16.7%) had AF recurrence in persistent and paroxysmal AF, respectively. The LAA morphology was associated with the recurrence of AF after RFA with the chicken-wing highest recurrence risk (68.2%). The structure type of LAA was also related to the AF recurrence rate (p < 0.01). Compared with one lobe and multiple lobes, two lobes (recurrence, 47.6%) were more likely associated with the recurrence of AF (p < 0.02). Logistic regression analysis showed that the chicken-wing group had a higher risk of recurrence after RFA (OR = 8.13, p = 0.004), and the windsock group had a lower risk of recurrence (OR = 0.17, p = 0.002).
Conclusion: The morphological feature of LAA is related to the recurrence risk of AF after RFA. LAA morphology assessment can predict the risk of AF recurrence.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. The incidence rate of AF is about 1–2% in the whole population (1) and increases with age; for those over 80 years of age, it is up to 9.1–11% (2). According to a retrospective study, the prevalence of AF in Asia was 0.1–4% and 2.8–14% in community and hospital surveys, respectively (3). When AF occurs, the left atrium autonomic pumping function is lost and the atrioventricular synergistic activity is impaired, which influences cardiac function and induced thrombosis. Until now, there have been many treatments for AF besides drug. Circumferential radiofrequency ablation (RFA) of the pulmonary vein is a safe and effective operation for AF (4). Usually, the success rate of RFA in treating paroxysmal AF is 80–90% after surgery, and persistent AF would be 60–80% (5–7). Indeed, the late recurrence rate of AF after a single RFA and repeated RFA are 11–29% and 7–24%, respectively (5–7). The leading cause of recurrence AF is incomplete ablation (8) and resumption of pulmonary vein-left atrial conduction (9).
Left atrial appendage (LAA) is a finger-like projection from the main body of the left atrium. The LAA protrudes from the primordial left atrium, which is formed mainly by the adsorption of the primordial pulmonary veins and their branches. The junction is well-defined by a narrowing at the orifice of the appendage. The orifice of LAA has few cardiomyocytes, while the body of LAA is rich (10). Therefore, the orifice becomes a potential conduction zone for reentry arrhythmia. There are considerable variations in its size, shape, and relationship with adjacent cardiac structure. Veinot et al. define lobes as protrusions from the main body with the tail portion representing a lobe, whereas bends in the tail do not constitute more lobes (10). Studies have shown that the LAA has one-lobe, two-lobe, and multiple-lobe structures Among Asians and Americans (11, 12). They found that two lobes were most common (54%), followed by three lobes (23%), one lobe (20%), and four lobes (3%) (13–16). Di Biase divides the LAA into four types: chicken-wing (48%), windsock (19%), cactus (30%), and cauliflower (3%) (17). The LAA also has a contractile function (18). The pathological state of AF causes the left atrial pressure to increase. The left atrium and the LAA can buffer the left atrial pressure by increasing the inner diameter and contraction force to ensure sufficient blood filling in the left ventricle. With AF progression, the LAA will be enlarged, which will reduce the blood flow and induce thrombosis in LAA.
Many studies have confirmed that the LAA has a stronger contraction and extension function than the left atrium (18, 19). It provides a theoretical basis for the LAA to be a source of AF and possibly affect prognosis. A limited study showed that the LAA volume was an independent factor for AF recurrence following RFA (20). This study focused on the relationship between LAA morphology and AF recurrence after RFA to predict the success rate of RFA.
Methods
Research Population
We retrospectively enrolled 84 patients (58 male/26 female) with AF in the Department of Cardiology, Shanghai East Hospital, from April 2014 to April 2018. Eighty-four patients were selected in this study, and 66 patients were lost to follow-up or could not tolerate medication.
Twenty-four patients with persistent AF and 60 patients with paroxysmal AF were performed by single RFA. Paroxysmal AF is defined as AF that terminates spontaneously or with intervention within 7 days of onset. Persistent AF is defined as AF that fails to self-terminate within 7 days. Moreover, all patients were examined by TEE before RFA to rule out LAA thrombus. Other exclusion criteria are age <20 years, pregnancy, history of cardiac surgery, valvular heart disease, congenital heart disease, thyroid dysfunction, severe liver and kidney dysfunction, electrolyte imbalance, and secondary AF. The study was approved by the hospital's institutional ethics committee, and written informed consent was provided by all patients.
Transesophageal Ultrasonography
LAA morphology is defined by transesophageal ultrasonography based on the research (13, 17). The Philips IE33 color ultrasound diagnostic apparatus with multi-planar three-dimensional transesophageal probe X7-2t was used to detect the LAA morphology. The patients had fasted for 4–6 h before the examination, and then the oropharynx was anesthetized locally with 2% lidocaine. After that, the patient took a left lateral position. The probe was sent to the back of the left atrium, the cut surface angle was adjusted from 0 to 180°, and the probe was turned counterclockwise or clockwise to show the maximum LAA structure and thrombus (~135°). The pulse-wave sampling volume was then set in the LAA neck to obtain its flow, and the flow stream spectrum, and the image was stored.
Definition of LAA Morphology
Here we displayed those LAA morphology by transesophageal echocardiography (Figure 1).The “chicken-wing” is described as having a dominant lobe that presents with an obvious bend in its proximal or middle part, folding back on itself at some distance from the orifice, and it may have secondary lobes. The “cactus” has a dominant central lobe and secondary lobes arise from it superiorly and inferiorly. The “cauliflower” has a short overall length, more complex internal characteristics, a variable number of lobes with lack of a dominant lobe, and a more irregular shape of the orifice. The “windsock” has a dominant lobe as the primary structure, and there are variations in the location and number of secondary or even tertiary lobes (21).
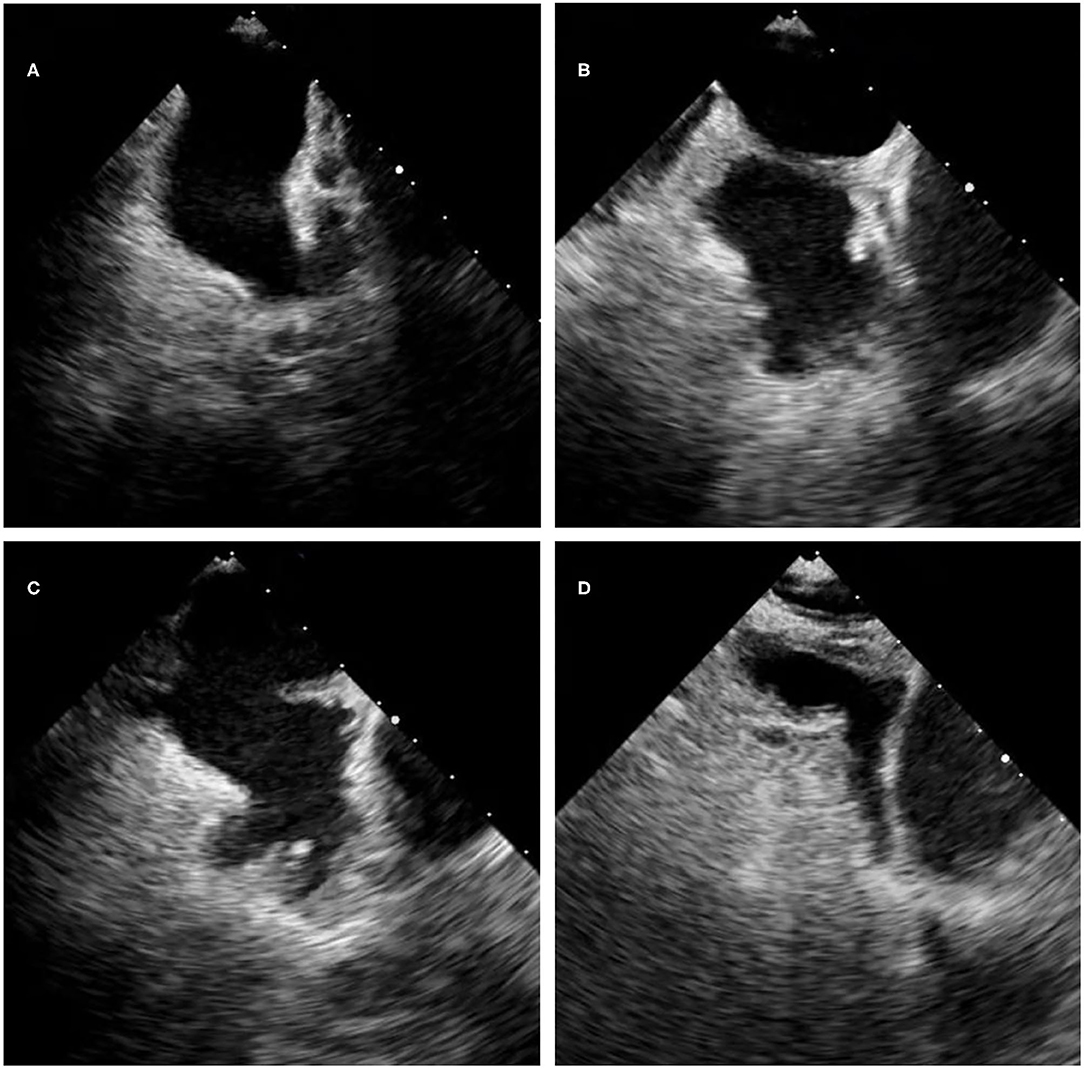
Figure 1. The LAA morphology by 2D transesophageal echocardiography. (A) The chicken-wing morphology. (B) The cauliflower morphology. (C) The cactus morphology. (D) The windsock morphology.
Cardiac Catheter RFA
An endocardial map of the left atrium was created with an electroanatomic mapping system (CARTO, Biosense Webster, Irvine, CA, USA) and superimposed on the preexisting CMR image of the chamber. With routine hemodynamic and electrocardiographic monitoring, an ablation catheter (CARTO, Thermocool, Biosense Webster) was advanced to the left atrium. Circumferential lesions were applied surrounding the pulmonary veins. Additional ostial lesions were targeted to retain pulmonary vein potentials using a circular multipolar electrode-mapping catheter (Lasso, Biosense Webster). Entrance block into the pulmonary veins was confirmed in all patients as the primary procedural endpoint (22). In cases of persistent AF, the ablation procedure usually included additional ablation lesions, like CFAE ablation (23).
Follow-Up and Assessment of AF Recurrence
All patients were followed up every 3 months at the outpatient clinic for 6–48 months after ablation. Twelve-lead ECGs or 24-h Holter monitoring was performed at 3, 6, and 12 months in clinic. In addition, 12-lead ECGs or 24-h Holter monitoring were also performed if patients reported palpitations. All patients were required to take oral anticoagulant medication (warfarin or new oral anticoagulants) for 2–3 months after RFA except contraindications. In cases of persistent AF, patients would receive oral amiodarone or propafenone for 3 months after surgery. Patients with paroxysmal AF did not need to use oral antiarrhythmic medications regularly. The blanking period is defined as a period post-ablation during which any recurrence of atrial arrhythmias is not considered a failure of the RFA. Here we set 3 months as blanking period according to the 2012 Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation (24). According to the following criteria, recurrence of AF was defined: a documented AF episode of at least 30 s after the traditional blanking period.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed in frequency and percentage. The normal distributed continuous variable data were analyzed by t-test, and the skewness distributed data were compared by the Mann–Whitney U-test. All categorical variables were compared using the χ2-test or Fisher test (if the theoretical frequency is too small in the χ2-test, using the Fisher test). Multivariate logistic regression analysis was performed for variables with a p-value of < 0.05 in the univariate analysis. OR values and 95% confidence intervals (95% CI) were calculated to determine independent predictors associated with AF recurrence. Statistical analyses were performed using SPSS software, version 23 (SPSS Inc., Chicago, IL, USA). p < 0.05 indicated statistical significance.
Results
Subject Characteristics
A total of 84 patients were enrolled in the study. All patients received successful RFA. During the mean follow-up of 618.5 days [6 months−4 years], 22 (35%) patients experienced AF recurrence.
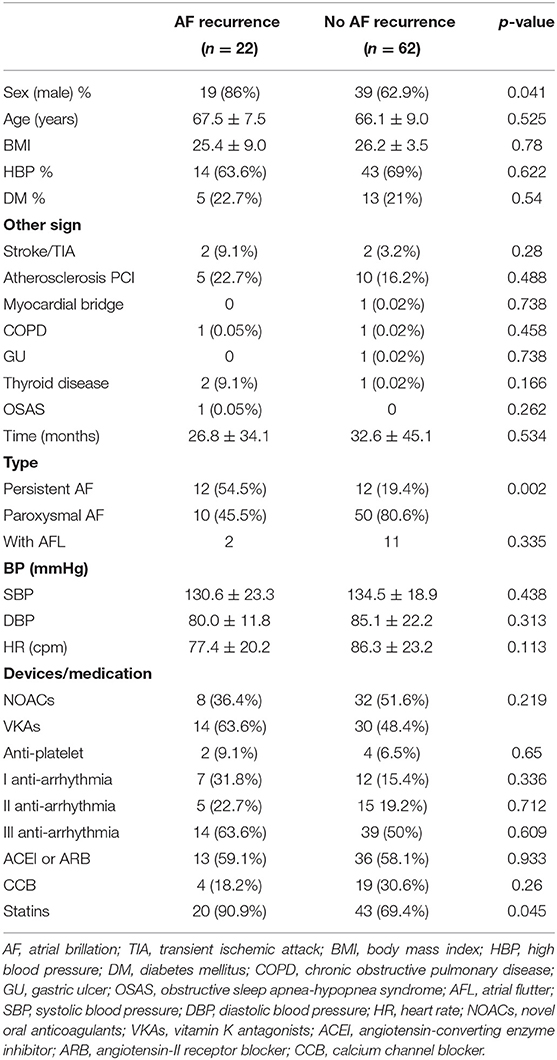
As Table 1 shows, there was a significant gender difference in postoperative AF recurrence. Males had a higher recurrence rate vs. females [19/58 (32.8%) vs. 3/26 (11.5%), p = 0.041]. Patients with persistent AF were more likely to relapse vs. paroxysmal AF patients [12/24 (50.0%) vs. 10/60 (16.7%), p = 0.002]. No significant difference was observed in age, BMI, preoperative blood pressure (systolic and diastolic blood pressure), and heart rate between the recurrent AF patients with the non-recurrent group. Although there were no differences between the two groups in the use of oral anticoagulants, ACEI or ARB, CCB, antiarrhythmics, and antiplatelet agents, the recurrent AF patients had a higher percentage using statin (recurrent AF vs. non-recurrent AF: 90.9 vs. 69.4%; p = 0.045). No differences were found between two groups in patients with hypertension, diabetes, stroke/TIA, coronary heart disease (postoperative PCI), myocardial bridge, and chronic obstructive pulmonary disease (COPD). We also did not observe any correlation of thyroid disease and obstructive sleep apnea syndrome with postoperative recurrence.
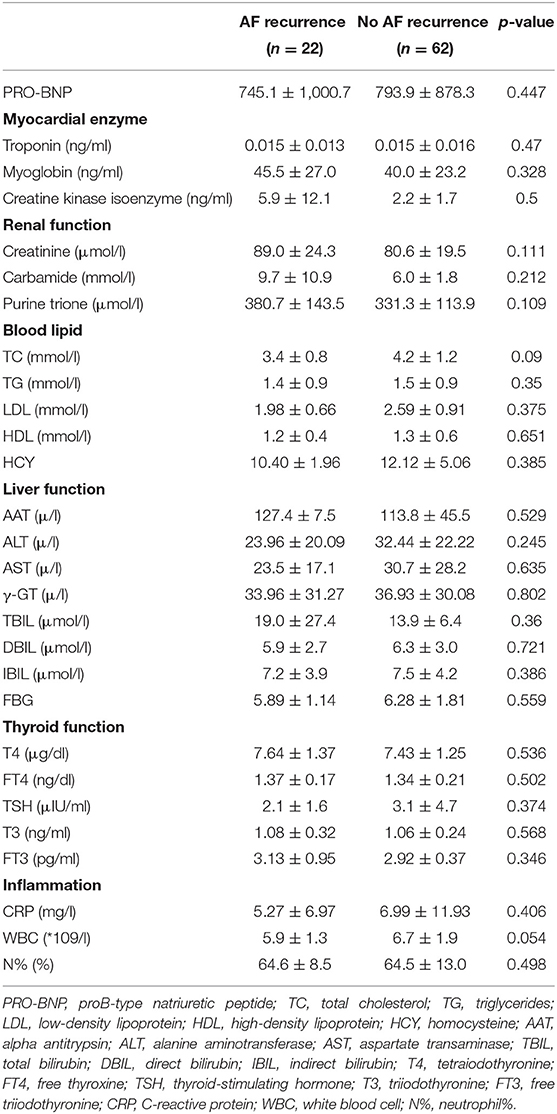
Routine preoperative lab tests for patients were ordered, and the results are shown in Table 2. There was no difference between the recurrence and no recurrence groups.
The Association of LAA Morphology With AF Recurrence
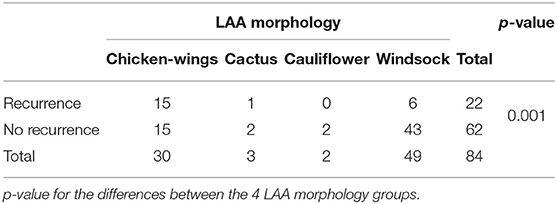
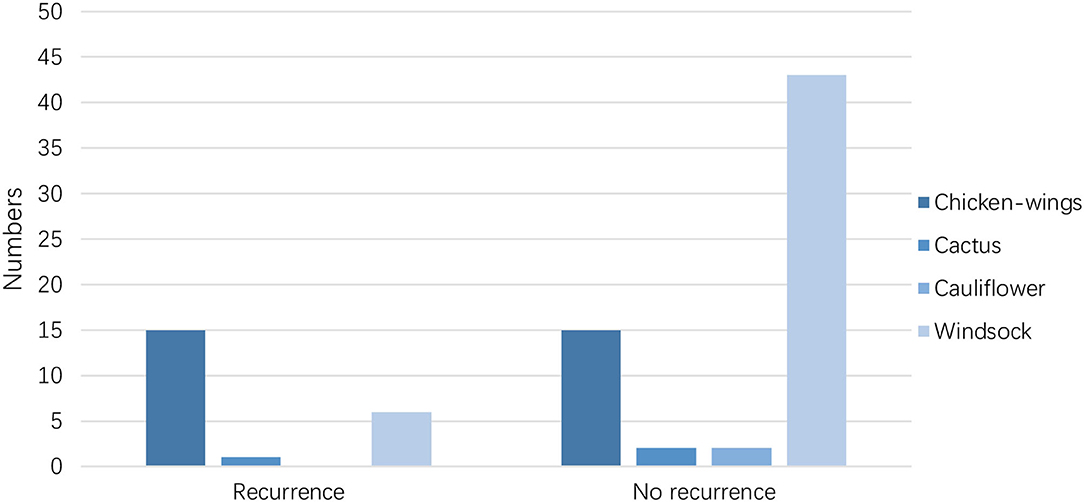
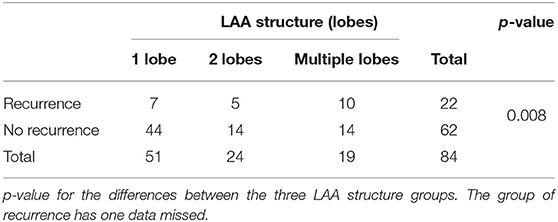
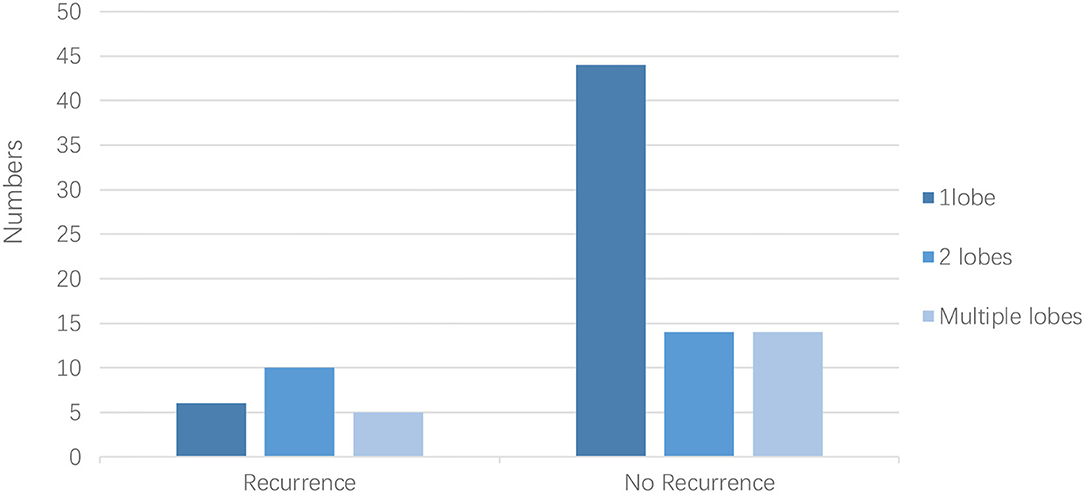
The LAA morphology is shown in Table 3 and Figure 2. The Fisher test results showed a significant difference in AF recurrence among four types of LAA (p = 0.001). Among the four types, the risk of AF recurrent in chicken-wing LAA was higher than in the windsock (p < 0.013) after the adjusted Bonferroni p-value. There was an insignificant difference among the others (p > 0.05). The LAA structure (lobes) was associated with recurrence (p = 0.008) (Table 4 and Figure 3), and the multiple lobes had more risk of recurrence of AF than the one lobe (p < 0.016).
The Association of Ablation Strategy With AF Recurrence
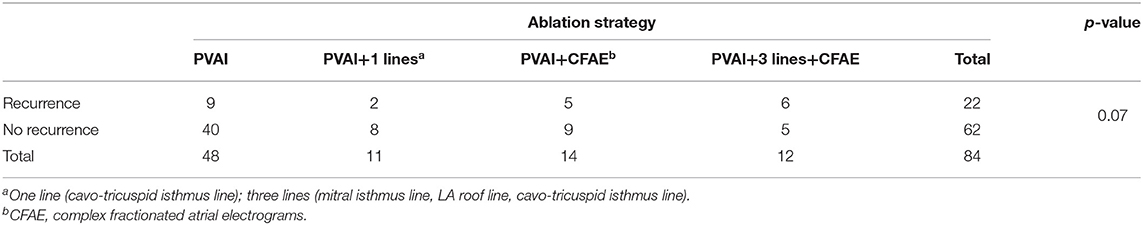
All the patients experienced those ablation strategies: PVAI, PVAI+1Lines, PVAI+CFAE, or PVAI+3 Lines+CFAE; there was no significant difference among the four types of surgery by the χ2-test (p = 0.07, Table 5).
The Association of LAA Function With AF Recurrence
TEE showed an insignificant correlation between the AF recurrent group and non-AF recurrent group in LAA max/min area, max/min orifice diameter, flow emptying velocity, and flow filling velocity (Table 6).
Univariate and Multivariate Logistic Regression of the AF Recurrence After RFA
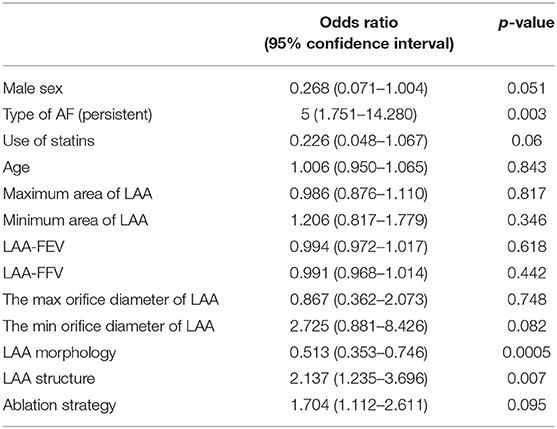
The type of AF (p = 0.003), LAA morphology (p = 0.0005), and LAA structure (p = 0.007) had statistical significance in univariate logistic regression (Table 7). Multivariate logistic regression is shown in Table 8. There was no significant correlation between AF recurrence and sex, type of AF, statin use, ablation strategy, and LAA function. In the classification of LAA, chicken-wing and windsock LAA were significantly associated with postoperative recurrence. The risk of AF recurrence after RFA in the chicken-wing group was higher than in other groups. The chicken-wing LAA was a risk factor for AF recurrence after RFA [OR = 8.13 (1.94–34.02)]. The AF recurrence of the windsock LAA group was lower than that of the other group [OR = 0.17 (0.06–0.51)]. There was an insignificant difference between cactus and cauliflower LAA [OR = 0.34 (0.01–14.38), and OR = 0.53 (0.04–6.51)]. In the LAA structure (lobe) classification, the risk of recurrence in one lobe was lower than in other structures [OR = 0.12 (0.02–0.56)], and there was no statistically significant difference in the recurrence of AF between the multiple lobes and the two lobes.
Kaplan–Meier Survival Curves of AF Recurrence After RFA
Kaplan–Meier survival curves showed that the AF recurrence rate after ablation was significantly different between the four different LAA morphology (Figure 4, p < 0.01) during the 1,460 days follow-up, the LAA morphology of chicken wings were more likely to have a high proportion of AF recurrence. Kaplan–Meier survival curves also showed that the proportion of AF recurrence was significantly different between the three different lobes (Figure 5, p = 0.01); the AF recurrence rate was high in the multiple-lobe group.
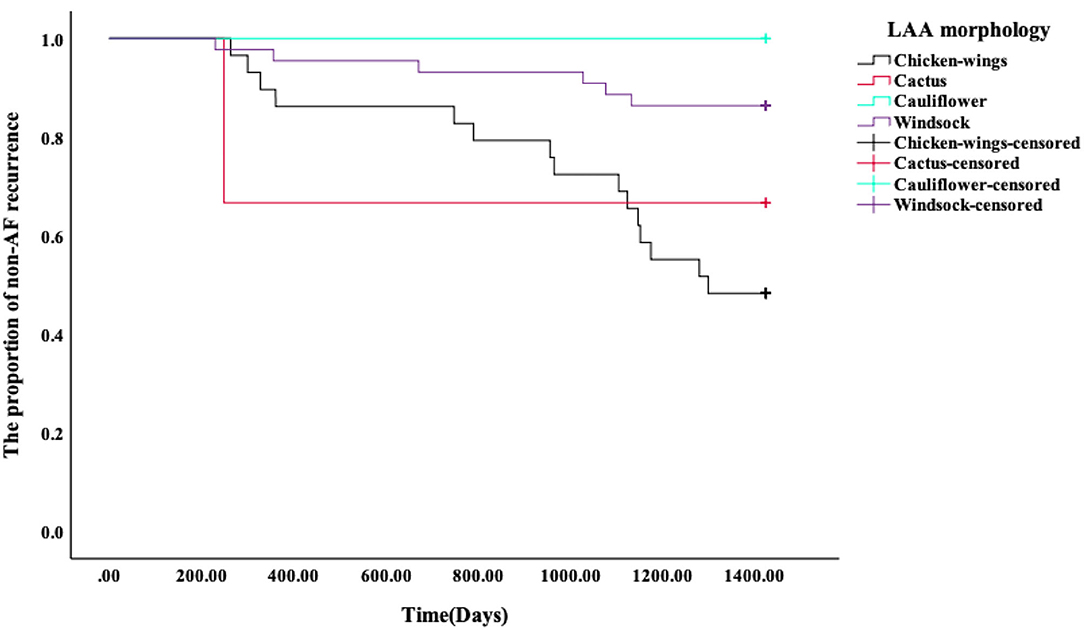
Figure 4. Kaplan–Meier analysis for recurrence after RFA. The Kaplan–Meier survival curves of AF recurrence after ablation and the log-rank test between the four types' LAA morphology during 1,460 days of follow-up.
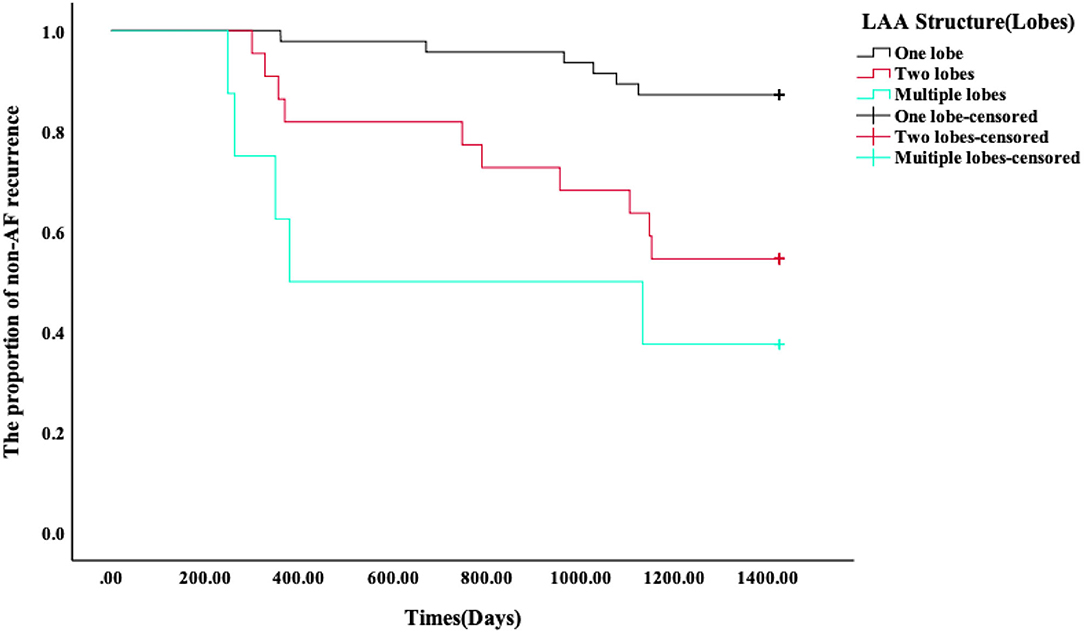
Figure 5. Kaplan–Meier analysis for recurrence after RFA. The Kaplan–Meier survival curves of AF recurrence after ablation and the log-rank test between the three different lobes during 1,460 days of follow-up.
Discussion
Our research found that the morphological structure of LAA is associated with AF recurrence after RFA. The chicken-wing shape is a risk factor for postoperative AF recurrence. The one-lobe type is less likely to cause AF recurrence compared with other types. We speculate that the main reason may be related to anatomy and electrophysiology. The bend angle of chicken-wing LAA is larger and longer, and some of them have multiple distal lobes, making deeper and more complex electrophysiological activities, leading to easy recurrence after ablation. It is also explained that one-lobe LAA have a low recurrence rate. During the development of the left atrium, the LAA is considered to be an unimportant accessory structure. However, as the research progresses, the understanding of the LAA becomes more and more profound. Di Biase et al. report that LAA isolation could prevent secondary RFA caused by AF recurrence (25). Studies have shown that 27% of patients have an LAA local ectopic pacemaker after pulmonary vein isolation, and in 8.7% of patients, LAA is the only source of recurrent arrhythmias (26). Ablation of the LAA's anterior wall near the LAA's neck and prolonging or electrically isolating the LAA can effectively prevent AF recurrence (18). These studies suggested that LAA is an essential factor for the recurrence of AF and the structure or the morphology of LAA may influence the recurrence of AF. Our study confirmed that LAA morphology can influence AF recurrence. The first reason is that the contraction and extension of LAA are stronger than those of the left atrium, and it plays a buffering role in decreasing left atrial pressure (27, 28). Secondly, the Bachmann beam and Marshall ligament near the LAA are the main conduction pathways of atrial electrical activity. The efferent fibers of the LAA sympathetic and vagus nerves are essential for maintaining the regular electrophysiological activity of the LAA. The left atrium with different structures of LAA may have different electrophysiological activities. Lastly, the LAA is a well-known source of atrial natriuretic peptide (ANP). It is associated with B-type natriuretic peptide levels (BNP), adiponectin, insulin, and free fatty acids. Studies have shown that surgical epicardial LAA closure can lead to downregulation of the adrenergic system and RAAS (29, 30).
Balk et al. (31) confirmed that patients with non-paroxysmal AF had an average of 60% higher probability of AF recurrence after RFA than patients with paroxysmal AF. This finding is consistent with our research. However, any individual factor or a set of patient's underlying eigenvalues do not consistently and independently predict the recurrence of AF, possibly due to a particular relationship and interaction between the patient's disease characteristics and cardiac measurements.
In this study, statins were associated with AF recurrence after RFA in univariate analysis. However, multivariate regression analysis did not yield any positive association positive results. Peng et al. (32) studied the relationship between statins and the recurrence rate of AF after RFA, indicating that statins have no advantage in reducing AF recurrence after AF ablation. However, in a subgroup analysis, statins were associated with a lower AF recurrence rate after RFA. More randomized controlled trials are needed to confirm it. Statins have anti-inflammatory and antioxidant actions that may act on atrial structural and electrical remodeling (31). Atorvastatin can inhibit ROS production by downregulating NOX2 and alleviating atrial fibrosis, thereby preventing AF (32). For these reasons, we infer that statins may have an effect of preventing AF recurrence after catheter ablation.
In the current study, we explored some risk factors of AF recurrence after RFA. We found that the morphology of the LAA, including morphology (shape) and structure (lobes), is associated with postoperative recurrence, which may be associated with different electrical conductivities and structural specificities of LAA. The TEE can measure the shape and structure of LAA. It will enable us to understand better LAA morphology and the relationship between the LAA morphology and AF recurrence.
Limitations
First, this is a small-sample, retrospective study, because there is a limited number of patients who had TEE. Prospective research with more samples is needed to confirm the results. Secondly, the AF recurrence after RFA is a combination of the related factors, including genetic polymorphism, inflammatory factors, and left atrial fibrosis. The associated basic research investigation will clarify the mechanism, and the basic research and clinical combination need to be further studied.
Conclusions
The morphological structure of LAA is one of the causes of AF recurrence after RFA. The chicken-wing LAA is a risk factor for postoperative AF recurrence. The risk of AF recurrence in windsock LAA is lower than that of others. Compared with the multiple-lobe type, the one-lobe type is less likely to cause AF recurrence. A better understanding of the LAA morphology before RFA of AF contributes to predicting postoperative AF recurrence risk.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XZ and SG contributed to conception and design of the study. XM and YC organized the database. XZ and JZ performed the RFA of AF. YG performed the transesophageal echocardiography. SG and BL wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the general program of the Shanghai Municipal Science and Technology Commission (grant numbers 15411968600 and 18411966000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank XZ at Shanghai East Hospital, Tongji University for technical advice and support, and Wenhui Peng for his precious help in preparing the manuscript, thanks RH for statistical support, thanks BL for the English language review.
References
1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. (2014) 129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
2. Fuster V, Ryden LE, Cannom DS, Crijns JH, Curtis AB, Ellenbogen KA, et al. 2011ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation:a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. (2011) 123:E269–367. doi: 10.1161/CIR.0b013e318214876d
3. Gyh L, Brechin CM. Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. (2012) 142:1489–98. doi: 10.1378/chest.11-2888
4. Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia a new anatomic approach for curing atrial fibrillation. Circulation. (2000) 102:2619–28. doi: 10.1161/01.CIR.102.21.2619
5. Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation lessons from a 5-year followu. Circulation. (2010) 122:2368. doi: 10.1161/CIRCULATIONAHA.110.946806
6. Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ-Arrhythmia Electrophysiol. (2010) 3:237. doi: 10.1161/CIRCEP.109.923771
7. Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, et al. Catheter ablation for atrial fibrillation are results maintained at 5 years of follow-up. J Am Coll Cardiol. (2011) 57:160. doi: 10.1016/j.jacc.2010.05.061
8. Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circul Arrhythmia Electrophysiol. (2010) 3:243. doi: 10.1161/CIRCEP.109.924878
9. Shah AN, Mittal S, Sichrovsky TC, Cotiga D, Arshad A, Maleki K, et al. Long-term outcome following successful pulmonary vein isolation:pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol. (2008) 19:661–7. doi: 10.1111/j.1540-8167.2008.01101.x
10. Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. Cardiovasc Imag. (2014) 7:1251–65. doi: 10.1016/j.jcmg.2014.08.009
11. Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, et al. Anatomy of the Normal Left Atrial Appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. (1997) 96:3112–5. doi: 10.1161/01.CIR.96.9.3112
12. Wang Fan, Zhu MY, Wang XY, Zhang W, Su Y, Lu YY, et al. Predictive value of left atrial appendage lobes on left atrial thrombus or spontaneous echo contrast in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. (2018) 18:153. doi: 10.1186/s12872-018-0889-y
13. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339:659. doi: 10.1056/NEJM199809033391003
14. Kim MN, Lee JJ, Kim SA, Kim YH, Choi JI, Park SM, et al. The difference of predictors for recurrence after catheter ablation of non-paroxysmal atrial fibrillation according to follow-up period. Int Heart. (2014) 55:312–8. doi: 10.1536/ihj.13-370
15. Malmborg H, Christersson C, Lonnerholm S, Blomstrom-Lundqvist C. Comparison of effects on coagulation and inflammatory markers using a duty-cycled bipolar and unipolar radiofrequency pulmonary vein ablation catheter vs. a cryoballoon catheter for pulmonary vein isolation. Europace. (2013) 15:798–804. doi: 10.1093/europace/eus411
16. Haeusler KG, Koch L, Herm J, Kopp UA, Heuschmann PU, Endres M, et al. 3 Tesla MRI-detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. Cardiovasc Electrophysiol. (2013) 24:14–21. doi: 10.1111/j.1540-8167.2012.02420.x
17. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. (2012) 60:531–8. doi: 10.1016/j.jacc.2012.04.032
18. Kainuma S, Masai T, Yoshitatsu M, Miyagawa S, Yamauchi T, Takeda K, et al. Advanced leftatrial fibrosis is associated with unsuccessful maze operation for valvular atrial fibrillation. Eur J Cardiothorac Surg. (2011) 40:61–9. doi: 10.1016/j.ejcts.2010.11.008
19. Mathew S, Metzner A, Ouyang F, Kuck KH, Tilz RR. Catheter ablation of paroxysmal atrial fibrillation. Optimal approach and result. Herzschrittmacherther Elektrophysiol. (2013) 24:7–14. doi: 10.1007/s00399-013-0244-z
20. Tian X, Zhang XJ, Yuan YF Li CY, Zhu LX, Gao BL. Morphological and functional parameters of left atrial appendage play a greater role in atrial fibrillation relapse after radiofrequency ablation. Sci Rep. (2020) 20:3. doi: 10.1038/s41598-020-65056-3
21. Khurram IM, Habibi M, Gucuk Ipek E, Chrispin J, Yang E, Fukumoto K, et al. Left atrial LGE and arrhythmia recurrence following pulmonary vein isolation for paroxysmal and persistent AF. JACC Cardiovasc Imag. (2016) 10:15. doi: 10.1016/j.jcmg.2015.10.015
22. Ciuffo L, Tao S, Gucuk Ipek E, Zghaib T, Balouch M, Lima JAC, et al. Intra-atrial dyssynchrony during sinus rhythm predicts recurrence after the first catheter ablation for atrial fibrillation. JACC Cardiovasc Imag. (2019) 122:28. doi: 10.1016/j.jcmg.2017.11.028
23. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. (2012) 33:171–257. doi: 10.1007/s10840-012-9672-7
24. Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, et al. Left atrial appendage: an under-recognized trigger site of atrial fibrillation. Circulation. (2010) 122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903
25. Di Biase L, Santangeli P, Natale A. How to ablate long-standing persistent atrial fibrillation? Curr Opin Cardiol. (2013) 28:26–35. doi: 10.1097/HCO.0b013e32835b59bb
26. Park HC, Lee D, Shim J, Choi JI, Kim YH. The clinical efficacy of left atrial appendage isolation caused by extensive left atrial anterior wall ablation in patients with atrial fibrillation. J Interv Card Electrophysiol. (2016) 46:287–97. doi: 10.1007/s10840-016-0116-7
27. Ballermann BJ, Brenner BM. Atrial natriuretic peptide and the kidney. Am J Kidney Dis. (1987) 10:7–12.
28. Lakkireddy D, Turagam M, Afzal MR, Rajasingh J, Atkins D., Dawn B, et al. Left atrial appendage closure and systemic homeostasis the LAA HOMEOSTASIS study. JACC. (2018) 71:135–44. doi: 10.1016/j.jacc.2017.10.092
29. Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S, et al. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. (2010) 21:1798. doi: 10.1111/j.1540-8167.2010.01798.x
30. Peng H, Yang Y, Zhao Y, Xiao H. The effect of statins on the recurrence rate of atrial fibrillation after catheter ablation: a meta-analysis. Pacing Clin Electrophysiol. (2018) 11:41. doi: 10.1111/pace.13485
31. Naji F, Sabovic M. The current role of statin therapy in the treatment of atrial fibrillation. Cardiovasc Hematol Agents Med Chem. (2013) 11:9–13. doi: 10.2174/1871525711311010004
32. Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, et al. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. (2011) 124:1107–17. doi: 10.1161/CIRCULATIONAHA.111.029223
Keywords: atrial fibrillation, recurrence, radiofrequency ablation, left atrial appendage morphology structure, recurrence risk
Citation: Gong S, Zhou J, Li B, Kang S, Ma X, Cai Y, Guo Y, Hu R and Zhang X (2021) The Association of Left Atrial Appendage Morphology to Atrial Fibrillation Recurrence After Radiofrequency Ablation. Front. Cardiovasc. Med. 8:677885. doi: 10.3389/fcvm.2021.677885
Received: 08 March 2021; Accepted: 09 July 2021;
Published: 12 August 2021.
Edited by:
Antonio Sorgente, EpiCURA, BelgiumReviewed by:
Nassir Marrouche, Tulane University, United StatesOsmar Antonio Centurion, National University of Asunción, Paraguay
Copyright © 2021 Gong, Zhou, Li, Kang, Ma, Cai, Guo, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xumin Zhang, emhhbmd4dW1pbjg4OCYjeDAwMDQwO2hvdG1haWwuY29t
†These authors have contributed equally to this work
 Shiyu Gong
Shiyu Gong Jian Zhou1†
Jian Zhou1† Sheng Kang
Sheng Kang