
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 09 June 2021
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.671569
This article is part of the Research TopicHighlights in General Cardiovascular Medicine: 2021View all 12 articles
 Abdelrahman I. Abushouk1*
Abdelrahman I. Abushouk1* Ismaeel Yunusa2,3
Ismaeel Yunusa2,3 Ahmed O. Elmehrath4
Ahmed O. Elmehrath4 Abdelmagid M. Elmatboly5
Abdelmagid M. Elmatboly5 Shady Hany Fayek4
Shady Hany Fayek4 Omar M. Abdelfattah6
Omar M. Abdelfattah6 Anas Saad1
Anas Saad1 Toshiaki Isogai1
Toshiaki Isogai1 Shashank Shekhar1
Shashank Shekhar1 Ankur Kalra1
Ankur Kalra1 Grant W. Reed1
Grant W. Reed1 Rishi Puri1
Rishi Puri1 Samir Kapadia1
Samir Kapadia1Objective: Systematic reviews are increasingly used as sources of evidence in clinical cardiology guidelines. In the present study, we aimed to assess the quality of published systematic reviews in high impact cardiology journals.
Methods: We searched PubMed for systematic reviews published between 2010 and 2019 in five general cardiology journals with the highest impact factor (according to Clarivate Analytics 2019). We extracted data on eligibility criteria, methodological characteristics, bias assessments, and sources of funding. Further, we assessed the quality of retrieved reviews using the AMSTAR tool.
Results: A total of 352 systematic reviews were assessed. The AMSTAR quality score was low or critically low in 71% (95% CI: 65.7–75.4) of the assessed reviews. Sixty-four reviews (18.2%, 95% CI: 14.5–22.6) registered/published their protocol. Only 221 reviews (62.8%, 95% CI: 57.6–67.7) reported adherence to the EQUATOR checklists, 208 reviews (58.4%, 95% CI: 53.9–64.1) assessed the risk of bias in the included studies, and 177 reviews (52.3%, 95% CI: 45.1–55.5) assessed the risk of publication bias in their primary outcome analysis. The primary outcome was statistically significant in 274 (79.6%, 95% CI: 75.1–83.6) and had statistical heterogeneity in 167 (48.5%, 95% CI: 43.3–53.8) reviews. The use and sources of external funding was not disclosed in 87 reviews (24.7%, 95% CI: 20.5–29.5). Data analysis showed that the existence of publication bias was significantly associated with statistical heterogeneity of the primary outcome and that complex design, larger sample size, and higher AMSTAR quality score were associated with higher citation metrics.
Conclusion: Our analysis uncovered widespread gaps in conducting and reporting systematic reviews in cardiology. These findings highlight the importance of rigorous editorial and peer review policies in systematic review publishing, as well as education of the investigators and clinicians on the synthesis and interpretation of evidence.
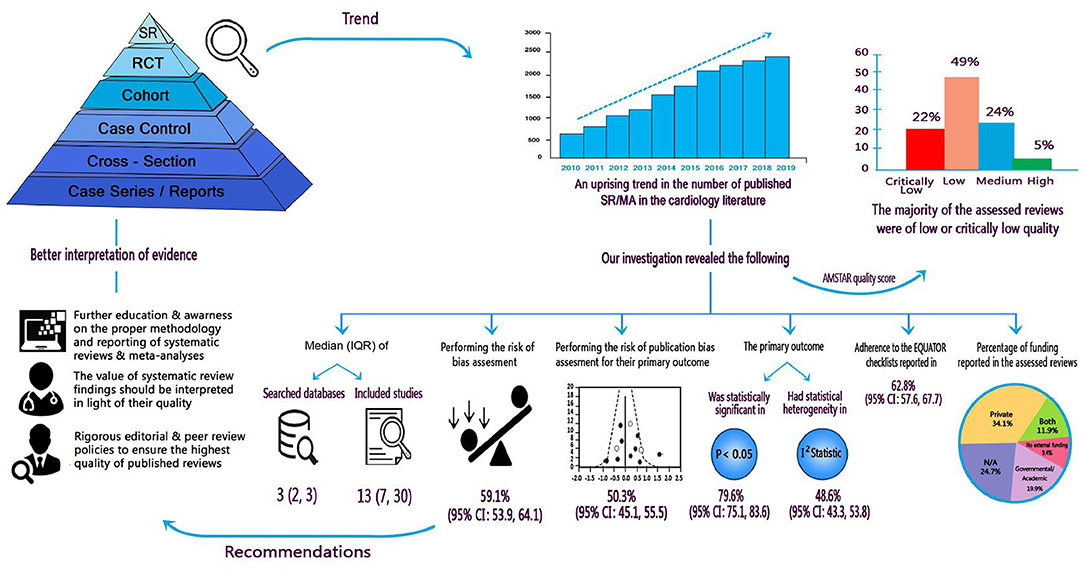
Graphical Abstract. Summary of findings and implications for researchers, clinicians, and stakeholders.
Systematic reviews are conducted to synthesize evidence, identify literature gaps and suggest potential areas for research, in a concerted effort to shape clinical practice guidelines and improve patient care outcomes (1, 2). Given their contribution to informing evidence-based practice, the quality of systematic reviews should not be an acceptable area of compromise as poor quality reviews might contribute to the use of low-efficacy or harmful interventions (3, 4). Several guidelines on the conduct and reporting of systematic reviews have been introduced (5–8). However, adherence to these guidelines has not been optimal. In fact, multiple analyses have shown that the quality of systematic reviews has been declining across different medical specialties (9–19).
Among these specialties, Cardiology has witnessed an exponential growth in the number of published systematic reviews and meta-analyses over the past decade, with >2,400 meta-analyses published in 2019, roughly quadruple the number from 2012 (as per our Medline search). In addition, the clinical practice guidelines in cardiology are increasingly reliant on systematic reviews because they are perceived as the highest level of evidence in the evidence-based pyramid (20–22). Despite increasing publication and utilization, concerns have been raised about the poor quality and low methodological standards of such reviews (11, 23). To our knowledge, only one analysis by Rao et al. surveyed 82 cardiology systematic reviews to determine their overall characteristics without in-depth quality assessment or critical appraisal (23).
Herein, we performed a comprehensive quality assessment of published systematic reviews in high impact, general cardiology/cardiovascular medicine journals. Based on our findings, we provide recommendations for researchers, clinicians, journal editors and peer reviewers, as well as policy makers on the conduct and interpretation of systematic reviews in cardiovascular medicine.
We searched PubMed for systematic reviews (with or without meta-analyses) published between 2010 and 2019 in five general cardiology/cardiovascular medicine journals with the highest impact factors according to the 2019 Clarivate Analytics Journal Impact Factor (JIF) list (Circulation [23.6], European Heart Journal [22.7], Journal of the American College of Cardiology [20.6], Circulation Research [14.5], and JAMA Cardiology [12.8]). Although the scope of “Circulation Research” relates mainly to basic cardiovascular research, it publishes systematic reviews of clinical studies on emerging biological and molecular interventions in different cardiovascular diseases. Therefore, it was deemed relevant for this analysis. The detailed search strategies, used in the present analysis, are illustrated in Supplementary Table 1.
A systematic review was defined as per the MeSH database as “A review of primary literature in health and health policy that attempts to identify, appraise, and synthesize all the empirical evidence that meets specified eligibility criteria to answer a given research question,” while a meta-analysis was defined as “Works consisting of studies using a quantitative method of combining the results of independent studies.” Screening for eligible studies was performed by two independent authors (AO and AIA) in two subsequent steps: title and abstract screening followed by full-text screening.
The following data were extracted from eligible systematic reviews: Type (direct, individual-patient data [IPD], network meta-analysis [NMA], others), country of origin (classified into single and multinational collaborations), number and type of included studies (randomized trial, observational and diagnostic test accuracy studies), number of searched databases and filters used during the search, whether the reviewers searched protocol registration sites, conference abstracts, and reference lists of retrieved reports, and used risk of bias assessment tools. We further extracted data on the primary outcome, its statistical significance, and the presence of statistical heterogeneity. Further, we checked the sources of funding and classified them if present into governmental/academic and private sources.
In addition, we assessed whether the eligible reviews performed publication bias assessment and the methods used (Egger's test, Begg's test, Funnel plots, correction by trim and fill method, others). For reviews that used any of these tests, we extracted the results of publication bias assessment. For studies that did not perform such assessment, we searched the article for the reason of not performing this analysis.
We used the “Assessing the Methodological Quality of Systematic Reviews (AMSTAR)” checklist online tool to assess the quality of included systematic reviews (https://amstar.ca/Amstar_Checklist.php). The AMSTAR tool was developed by the Universities of Ottawa, Bristol, Bond, and Toronto to assess the methodological quality of systematic reviews. It consists of 16 questions (majority: yes/no answers) that assess the performance of different steps of the systematic review process as determination of inclusion criteria, screening and data extraction, risk of bias and publication bias assessments, as well as analysis methods. It generates a score for each assessed review as either of high, moderate, low, or critically low quality (5).
We used R software (version 3.6.3 for Windows) to conduct the statistical analysis. Data were expressed as count (proportion, 95% confidence interval [CI]) or median (interquartile range) for categorical and numerical data, respectively. We used Chi-Square, ordinal logistic, and Poisson regression tests to evaluate the association between methodological characteristics, publication bias assessment, AMSTAR quality scores, and citations (after adjusting for the journal and publication year). We used the Wilson method without continuity correction to calculate 95% confidence intervals for proportions (24). A p-value < 0.05 was considered statistically significant.
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Among 659 retrieved search results, we excluded 278 after title/abstract screening. Eventually, we identified 352 eligible systematic reviews that were published in the target cardiology journals between 2010 and 2019. In systematic reviews that included a meta-analysis, the most frequent type was direct head-to-head comparison, followed by IPD meta-analyses, while only few studies used NMA. Only eight studies performed a qualitative systematic review without meta-analysis. The eligibility criteria in the assessed meta-analyses focused on RCTs (N = 164; 46.6%), observational studies (N = 104; 17.6%), or both RCTs and observational studies (N = 65; 16.8%); Table 1. The majority of eligible reviews (N = 193) were the result of a multinational collaboration; other reviews were published most frequently from the United States (N = 80), followed by the Netherlands (N = 13), Italy (N = 12), and Canada (N = 12).
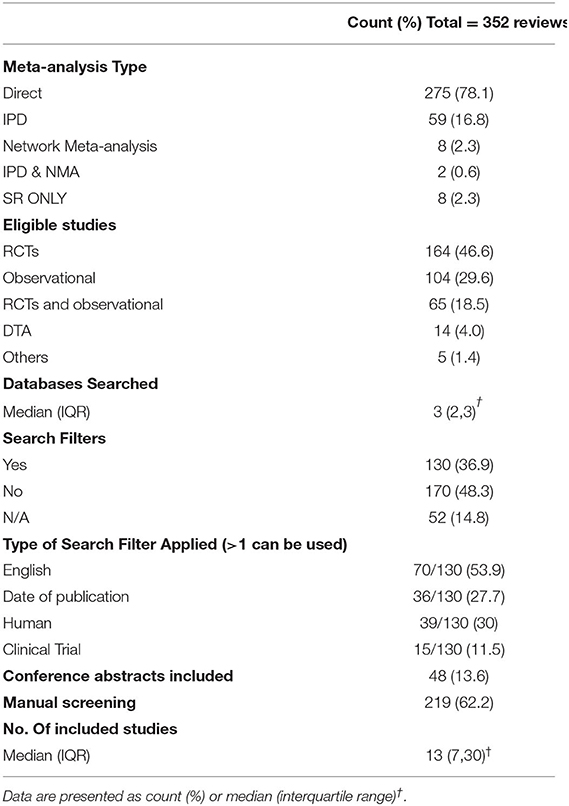
Table 1. Characteristics and literature search methods of the assessed systematic reviews in the current analysis.
Sixty-four reviews (18.2%, 95% CI: 14.5–22.6) registered/published their protocol. The median number of searched databases was 3 (IQR: 2, 3). The frequently searched databases were Medline (N = 310; 88.1%), EMBASE (N = 196; 55.7%), Cochrane Central Register of Clinical Trials (N = 168; 47.7%), and Web of Science (N = 49; 13.9%). A full list of the used databases and search engines in the assessed reviews is illustrated in Supplementary Table 2. Among the assessed systematic reviews, 130 (36.9%) used search filters, most frequently limiting search results by language, date of publication, human species, and study type. Manual screening was employed in 219 reviews (62.2%), while 48 (13.6%) reviews added conference abstracts to their eligibility criteria. The median number of included studies was 13 (IQR: 7, 30); Table 1.
Among the retrieved systematic reviews, 208 (58.4%, 95% CI: 53.9–64.1) reported their risk of bias assessment in adequate details using validated tools and 177 (52.3%, 95% CI: 45.1–55.5) examined the risk of publication bias. Among the latter, publication bias was present in 35 reviews. The commonly used methods to assess for publication bias were Funnel plots, Egger's and Begg's tests. Among reviews that did not assess for publication bias, only 21 reviews cited reasons, that were mostly related to the small number of included studies.
The primary outcomes in the assessed reviews were commonly related to mortality and stroke. In 274 (79.6%, 95% CI: 75.1–83.6) reviews, the comparison for the primary outcome was statistically significant and statistical heterogeneity was significant in 167 (48.5%, 95% CI: 43.3–53.8) meta-analyses.
Of the assessed studies, 87 (24.7%, 95% CI: 20.5–29.5) did not report on whether they received funding or not, 33 (9.4%, 95% CI: 6.8–12.9) reported receiving no funding, 70 (19.9%, 95% CI: 16.1–24.4) reviews reported receiving governmental/academic funding, 120 (34.1%, 95% CI: 29.3–39.2) reported private funding from pharmaceutical companies, while 42 (11.9%, 95% CI: 9–15.7) reviews received funding from both sources. The distribution of the number of citations for the assessed reviews was clearly skewed (median 92, IQR: 38, 196); the majority was the publications with smaller number of citations; Table 2.
The number of reviews with critically low, low, moderate, and high quality scores were 78 (22.2%, 95% CI: 18–27), 171 (48.6%, 95% CI: 43.2–53.9), 85 (24.1%, 95% CI: 19.6–28.8), and 18 (5.1%, 95% CI: 3.1–8), respectively. This means that the majority of assessed meta-analyses (71%, 95% CI: 65.7–75.4) had low or critically low quality. Only 221 (62.8%, 95% CI: 57.6–67.7) reviews stated clearly that they followed the EQUATOR reporting guidelines (PRISMA, MOOSE, etc.).
We found a significant association between the existence of publication bias and statistical heterogeneity of the primary outcome (OR, 3.93; 95% CI, 1.43–10.85), but not significance of the primary outcome (OR, 1.79; 95% CI, 0.58–5.51). Interestingly, regression analysis showed that meta-analyses with complex design (e.g., IPD), those that included RCTs, included a larger number of studies, had a significant primary outcome, adhered to EQUATOR reporting checklist, or had a higher AMSTAR quality score were associated with a significantly higher citation count (p < 0.05) after adjusting for journal and publication year. On the other hand, we found no significant association between AMSTAR quality score and publication journal, registering the review protocol (OR, 1.33; 95% CI, 0.69–2.56), multinational collaboration (OR, 1.09; 95% CI, 0.73–1.61) or receiving private funding (OR, 1.02; 95% CI, 0.03–29.43). Interestingly, the publication year positively predicted AMSTAR quality score (OR, 1.03, 95% CI: 1.02–1.04).
The current analysis of 352 systematic reviews, published in high impact cardiology journals, showed serious gaps in conducting and reporting systematic reviews. These gaps include: (1) protocol registration is often a neglected step of the systematic review process; (2) risk of bias assessment is an integral piece of the systematic review process; however, poor tool selection and reporting of findings influences its credibility; (3) publication bias assessment is often neglected or poorly reported in published systematic reviews; and (4) despite the increasing mandate by publishers, the compliance of published systematic reviews to the EQUATOR network checklists is not yet optimal. Overall, the quality of the assessed systematic reviews was often low or critically low. Although we limited our analysis to the most renowned cardiology journals, we expect published systematic reviews in less reputable journals to have similar gaps and quality scores. However, variations may exist based on the availability of methodological and statistical expertise in the journal editorial and peer review pools.
Several tools have been devised to assess the quality of systematic reviews. The 2009 PRISMA statement was designed to address reporting bias and missing data (6). Most journals now ask authors to be PRISMA-compliant while submitting systematic reviews for publication. However, the adherence is not yet uniform (as per our findings). Moreover, PRISMA does not address the methodological and statistical quality of systematic reviews. Another checklist (AMSTAR) was designed to overcome this caveat (5). In our analysis, almost ¾ of the assessed systematic reviews scored critically low to low quality. Besides cardiology, this phenomenon of declining quality has been observed in other disciplines of clinical medicine (9–19). Berlin et al. linked such occurrence to limited expertise, inaccurate methodology and poor adherence to quality evaluation tools (25). On the positive side, we found that publication year was positively associated with improving review quality score. While the magnitude of this association was small and needs further confirmation, it may reflect increasing expertise of systematic review methods among authors and reviewers.
In addition, the current analysis showed that most published reviews in high-impact cardiology journals do not register or refer to their protocols. Preparing and publishing protocols for systematic reviews can improve the consistency between the team members' decisions, reduce the risk of selective outcome reporting, and eliminate redundant reviews in the literature (26). Ideally, the protocol should be a collaboration between clinicians and methodologists to promote transparency, minimize bias and ensure the reproducibility of the review steps (27). A recommended platform for protocol registration is PROSPERO (Centre for Reviews and Dissemination, York University); however, authors may opt to publish their protocol in a peer-reviewed journal to get expert opinion that might improve the quality of their systematic review (28).
Another aspect, assessed in our analysis, was reporting on publication bias assessment. Publication bias occurs when authors, reviewers, and editors tend to not submit or accept manuscripts for publication according to the direction of the study results. Negative studies are less likely to be published than positive studies and tend to take longer to be published. This can affect the reliability of meta-analyses since those including only positive outcomes are likely to overestimate the true effect of an intervention (29). Thus, assessing publication bias is a fundamental part of the systematic review process. In consistence with prior reports (30–32), our analysis showed that only 52.3% of retrieved systematic reviews in high impact cardiology journals reported on publication bias, further confirming that publication bias reporting is often omitted in systematic review publications.
In a statement from the American Heart Association, Rao and colleagues narratively described the statistical and risk of bias assessment tools in a sample of 82 cardiology meta-analyses published in 2014. The writing group then proceeded to assign methodological standards for conducting systematic reviews that addressed protocol development and dissemination, quality assessment, and choosing appropriate statistical methods (23). Based on our analysis, we stress the importance of some of these recommendations (which are also relevant beyond the field of cardiology):
A) Protocol registration improves the methodological quality and reporting standards of systematic reviews. An ideal systematic review protocol requires collaboration between clinicians and methodologists.
B) Database search: A minimum of two databases should be searched. The selection of search filters should be justified.
C) Additional search: Manual search of the bibliography of relevant studies and searching protocol registries should supplement the electronic database search.
D) Risk of bias assessment is a core element of the systematic review process. Further, adopting the GRADE system to assess the certainty of evidence is highly recommended.
E) Dealing with heterogeneity: This starts with appreciating clinical and methodological heterogeneity between the included studies before commencing analysis. Using a proper statistical model, as well as exploration of the source of heterogeneity using methods as sensitivity and subgroup analysis or meta-regression are essential.
F) Publication bias assessment: The assessment of publication bias is an important step in the systematic review process. If not feasible, the authors should at least mention the reason for not conducting such assessment.
G) EQUATOR checklist compliance: Ideally, each manuscript should contain a PRISMA/MOOSE checklist as an appendix. Journal editors should apply stricter editorial rules that enforce compliance with EQUATOR network checklist.
H) Funding disclosure: All systematic review funding should be disclosed. Journals should use standardized forms to help authors disclose all potential sources of funding.
Although our study, to our knowledge, provides the first comprehensive analysis on the methodological characteristics and gaps of systematic reviews in the cardiology literature, some limitations should be addressed. First, journal selection in the present study was based on the impact factor. While we recognize the limitations of such metric, the selected journals are indeed the most reputable in our field. Second, our analysis focused more on methodological rather than statistical properties of systematic reviews, such as analysis models or effect estimates. Third, the number of reviews with significant publication bias was relatively small to fully analyse the possible contributing factors that can be improved in the systematic review methodology.
Our analysis uncovered serious gaps with published systematic reviews in the cardiology literature, including issues with protocol registration and conducting and reporting bias assessments. These findings highlight the importance of rigorous editorial and peer review policies in systematic review publishing, as well as education of the investigators and clinicians on the synthesis and interpretation of evidence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
AA: idea conception and study design. OA, AS, IY, AElme, AElma, and SF: data collection and initial drafting. AA and IY: data analysis. TI, SS, GR, RP, AK, and SK: final drafting and revision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Mr. Abdelrahman M. Metwally for his help with data illustration.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.671569/full#supplementary-material
1. Chalmers I, Hedges LV, Cooper H. A brief history of research synthesis. Eval Health Prof. (2002) 25:12–37. doi: 10.1177/0163278702025001003
2. Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. (1997) 126:376–80. doi: 10.7326/0003-4819-126-5-199703010-00006
3. Sutton AJ. Evidence concerning the consequences of publication and related biases. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis-Prevention, Assessment and Adjustments. Chichester: John Wiley & Sons (2005). p. 175–92. doi: 10.1002/0470870168.ch10
4. Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al. Dissemination and publication of research findings: an updated review of related biases. Heal Technol Assess. (2010) 14:1–193. doi: 10.3310/hta14080
5. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. (2009) 62:1013–20. doi: 10.1016/j.jclinepi.2008.10.009
6. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
7. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons (2019). doi: 10.1002/9781119536604
8. Morton S, Berg A, Levit L, Eden J. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: National Academies Press (2011).
9. Campbell JM, Kavanagh S, Kurmis R, Munn Z. Systematic reviews in burns care: poor quality and getting worse. J Burn Care Res. (2017) 38:e552–67. doi: 10.1097/BCR.0000000000000409
10. Dixon E, Hameed M, Sutherland F, Cook DJ, Doig C. Evaluating meta-analyses in the general surgical literature: a critical appraisal. Ann Surg. (2005) 241:450. doi: 10.1097/01.sla.0000154258.30305.df
11. Ioannidis JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. (2016) 94:485–514. doi: 10.1111/1468-0009.12210
12. Delaney A, Bagshaw SM, Ferland A, Manns B, Laupland KB, Doig CJ. A systematic evaluation of the quality of meta-analyses in the critical care literature. Crit care. (2005) 9:1–8. doi: 10.1186/cc3803
13. Rudmik LR, Walen SG, Dixon E, Dort J. Evaluation of meta-analyses in the otolaryngological literature. Otolaryngol Neck Surg. (2008) 139:187–94. doi: 10.1016/j.otohns.2008.03.020
14. Dijkman BG, Abouali JAK, Kooistra BW, Conter HJ, Poolman RW, Kulkarni AV, et al. Twenty years of meta-analyses in orthopaedic surgery: has quality kept up with quantity? JBJS. (2010) 92:48–57. doi: 10.2106/JBJS.I.00251
15. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: critical evaluation. Bmj. (2000) 320:537–40. doi: 10.1136/bmj.320.7234.537
16. Jadad AR, McQuay HJ. Meta-analyses to evaluate analgesic interventions: a systematic qualitative review of their methodology. J Clin Epidemiol. (1996) 49:235–43. doi: 10.1016/0895-4356(95)00062-3
17. Schmitter M, Sterzenbach G, Faggion CM, Krastl G. A flood tide of systematic reviews on endodontic posts: methodological assessment using of R-AMSTAR. Clin Oral Investig. (2013) 17:1287–94. doi: 10.1007/s00784-013-0945-z
18. Wasiak J, Tyack Z, Ware R, Goodwin N, Faggion Jr CM. Poor methodological quality and reporting standards of systematic reviews in burn care management. Int Wound J. (2017) 14:754–63. doi: 10.1111/iwj.12692
19. Gianola S, Gasparini M, Agostini M, Castellini G, Corbetta D, Gozzer P, et al. Survey of the reporting characteristics of systematic reviews in rehabilitation. Phys Ther. (2013) 93:1456–66. doi: 10.2522/ptj.20120382
20. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320
21. Han M, Chen Q, Liu L, Li Q, Ren Y, Zhao Y, et al. Stage 1 hypertension by the 2017 American College of Cardiology/American Heart Association hypertension guidelines and risk of cardiovascular disease events: systematic review, meta-analysis, and estimation of population etiologic fraction of prospective cohort studies. J Hypertens. (2020) 38:573–8. doi: 10.1097/HJH.0000000000002321
22. Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2019) 139:e1144–61. doi: 10.1161/CIR.0000000000000626
23. Rao G, Lopez-Jimenez F, Boyd J, D'Amico F, Durant NH, Hlatky MA, et al. Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American Heart Association. Circulation. (2017) 136:e172–94. doi: 10.1161/CIR.0000000000000523
24. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. (1998) 17:857–72. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E
25. Berlin JA, Golub RM. Meta-analysis as evidence: building a better pyramid. Jama. (2014) 312:603–6. doi: 10.1001/jama.2014.8167
26. Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. (2012) 1:1–4. doi: 10.1186/2046-4053-1-7
27. Riaz I. Bin, Khan MS, Riaz H, Goldberg RJ. Disorganized systematic reviews and meta-analyses: time to systematize the conduct and publication of these study overviews? Am J Med. (2016) 129:339–e11. doi: 10.1016/j.amjmed.2015.10.009
28. Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. (2012) 1:1–9. doi: 10.1186/2046-4053-1-2
29. Torgerson CJ. Publication bias: the Achilles' heel of systematic reviews? Br J Educ Stud. (2006) 54:89–102. doi: 10.1111/j.1467-8527.2006.00332.x
30. Onishi A, Furukawa TA. Publication bias is underreported in systematic reviews published in high-impact-factor journals: metaepidemiologic study. J Clin Epidemiol. (2014) 67:1320–6. doi: 10.1016/j.jclinepi.2014.07.002
31. Hedin RJ, Umberham BA, Detweiler BN, Kollmorgen L, Vassar M. Publication bias and nonreporting found in majority of systematic reviews and meta-analyses in anesthesiology journals. Anesth Analg. (2016) 123:1018–25. doi: 10.1213/ANE.0000000000001452
Keywords: cardiology, publication bias, systematic review, quality assessment, critical appraisal
Citation: Abushouk AI, Yunusa I, Elmehrath AO, Elmatboly AM, Fayek SH, Abdelfattah OM, Saad A, Isogai T, Shekhar S, Kalra A, Reed GW, Puri R and Kapadia S (2021) Quality Assessment of Published Systematic Reviews in High Impact Cardiology Journals: Revisiting the Evidence Pyramid. Front. Cardiovasc. Med. 8:671569. doi: 10.3389/fcvm.2021.671569
Received: 24 February 2021; Accepted: 04 May 2021;
Published: 09 June 2021.
Edited by:
Jinwei Tian, The Second Affiliated Hospital of Harbin Medical University, ChinaReviewed by:
Luiz Felipe Pinho Moreira, University of São Paulo, BrazilCopyright © 2021 Abushouk, Yunusa, Elmehrath, Elmatboly, Fayek, Abdelfattah, Saad, Isogai, Shekhar, Kalra, Reed, Puri and Kapadia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdelrahman I. Abushouk, YWJ1c2hvYUBjY2Yub3Jn; YWJkZWxyYWhtYW4uYWJ1c2hvdWtAbWVkLmFzdS5lZHUuZWc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.