
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 17 June 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.669110
 Grace Liang1†
Grace Liang1† Xianxi Huang2,3†
Xianxi Huang2,3† James Hirsch4
James Hirsch4 Sanjeev Mehmi4
Sanjeev Mehmi4 Holly Fonda4
Holly Fonda4 Khin Chan4
Khin Chan4 Ngan F. Huang5
Ngan F. Huang5 Oliver Aalami6
Oliver Aalami6 Victor F. Froelicher1,4
Victor F. Froelicher1,4 David P. Lee1
David P. Lee1 Jonathan Myers1,3,4
Jonathan Myers1,3,4 Andrew S. Lee3,7‡
Andrew S. Lee3,7‡ Patricia K. Nguyen1,3,4*‡
Patricia K. Nguyen1,3,4*‡Background: Although engaging in physical exercise has been shown to reduce the incidence of cardiovascular events, the molecular mechanisms by which exercise mediates these benefits remain unclear. Based on epidemiological evidence, reductions in traditional risk factors only accounts for 50% of the protective effects of exercise, leaving the remaining mechanisms unexplained. The objective of this study was to determine whether engaging in a regular exercise program in a real world clinical setting mediates cardiovascular protection via modulation of non-traditional risk factors, such as those involved in coagulation, inflammation and metabolic regulation.
Methods and Results: We performed a prospective, cohort study in 52 sedentary patients with cardiovascular disease or cardiovascular risk factors at two tertiary medical centers between January 1, 2016 and December 31, 2019. Prior to and at the completion of an 8-week exercise program, we collected information on traditional cardiovascular risk factors, exercise capacity, and physical activity and performed plasma analysis to measure levels of fibrinolytic, inflammatory and metabolic biomarkers to assess changes in non-traditional cardiovascular risk factors. The median weight change, improvement in physical fitness, and change in physical activity for the entire cohort were: −4.6 pounds (IQR: +2 pounds, −11.8 pounds), 0.37 METs (IQR: −0.076 METs, 1.06 METs), and 252.7 kcals/week (IQR: −119, 921.2 kcals/week). In addition to improvement in blood pressure and cholesterol, patients who lost at least 5 pounds, expended at least 1,000 additional kcals/week, and/or achieved ≥0.5 MET increase in fitness had a significant reduction in plasminogen activator inhibitor-1 [9.07 ng/mL (95% CI: 2.78–15.35 ng/mL); P = 0.026], platelet derived growth factor beta [376.077 pg/mL (95% CI: 44.69–707.46 pg/mL); P = 0.026); and angiopoietin-1 [(1104.11 pg/mL (95% CI: 2.92–2205.30 pg/mL); P = 0.049)].
Conclusion: Modest improvements in physical fitness, physical activity, and/or weight loss through a short-term exercise program was associated with decreased plasma levels of plasminogen activator inhibitor, platelet derived growth factor beta, and angiopoietin, which have been associated with impaired fibrinolysis and inflammation.
This is a prospective study that compares the effects of exercise on non-traditional and traditional cardiovascular risk factors. In 52 patients, we found that weight loss of ≥5 pounds, an improvement in physical fitness of ≥0.5 METs, and an increase in physical activity of ≥1,000 kcals/week, achieved through a short-term exercise program resulted in decreases in plasma levels of plasminogen activator inhibitor-1, platelet derived growth factor beta-1, and angiopoetin-1, which have been associated fibrinolysis and inflammation. These findings suggest that modest improvements in weight loss, physical fitness, and physical activity levels may reduce cardiovascular risk through modulation of factors involved in coagulation and inflammation.
It is well-known that exercise reduces morbidity and mortality associated with cardiovascular disease. Exercise and physical activity are, thus, Class I indications for the prevention of atherosclerotic cardiovascular disease (ASCVD) (1–3). Performing physical activity assessment and dietary counseling during clinical visits is considered an important strategy to encourage patients to exercise and lead a healthy lifestyle. Although there is a defined minimum amount of recommended activity (e.g., 150 min of moderate intensity physical activity or 75 min of vigorous activity) to reduce the risk of ASCVD (1–3), patients who engage in lower levels of physical activity than the minimum recommended amount have also been found to have significantly lower risk of coronary heart disease (4), suggesting that some physical activity is better than none.
The mechanisms by which exercise mediates cardio-protection remain poorly understood. While physical fitness and activity can reduce cardiovascular risk by as much as 30–50%, changes in individual traditional risk factors are small, with reported decreases of 5% for blood lipids (5), 3–5 mmHg for blood pressure (6), and 1% for hemoglobin A1C (7). Although previous studies have demonstrated exercise reduces novel risk factors in addition to traditional risk factors, these studies have been limited to the measurement of only a handful of biomarkers (8–12) and a reliance on self-reported measures of physical activity, fitness or weight loss (8–10, 13).
To address this knowledge gap, we measured levels of novel and traditional biomarkers associated with cardiovascular disease risk in a cohort of 52 patients recruited from a real world clinical setting. Our objective was to determine whether exercise-induced improvement in weight loss, physical fitness, and activity alone or in combination, correlated with changes in traditional and non-traditional biomarkers of cardiovascular risk.
We conducted a prospective, cohort study that consecutively enrolled patients who were advised by their cardiologist to engage in healthy lifestyle behaviors for primary or secondary prevention of cardiovascular disease. The objective of the study was to correlate improvement in weight loss, physical fitness, and physical activity achieved through a short-term exercise program with traditional and non-traditional biomarkers. We hypothesized that exercise modulates these non-traditional markers of risk independent of its effects on traditional risk factors. The study was approved by the Institutional Human Research Ethics Committee and confirmed to the Declaration of Helsinki. All participants provided written, informed consent before inclusion into the study. Anonymized data supporting the findings in this article are available from the corresponding author upon reasonable request.
Between January 1, 2016 and December 31, 2019, we recruited 66 patients who were advised by their cardiologist to engage in healthy lifestyle modifications for primary and secondary prevention of cardiovascular disease at Stanford Hospital and the Veterans Affairs Palo Alto Health Care System. Patients were excluded if they were already adhering to the recommended weekly activity levels as defined by the American Heart Association, could not exercise, had a history of non-compliance, a history of active cancer or inflammatory disorder, or were taking immunosuppressive agents. Patients that had any changes in medications during the study period that could affect blood pressure, lipids, inflammation, and coagulation were excluded from the study. Forty-five patients met the inclusion and exclusion criteria and completed a supervised exercise program (e.g., formal in-person cardiac rehabilitation program, or remotely monitored program with active weekly feedback) and 21 patients participated in an unsupervised program (e.g., exercised on their own with advice from study staff. Assessments were performed at baseline and after subjects completed 8 weeks of their program. Of the 66 patients initially recruited, 52 completed both baseline and terminal assessments and were included in the analysis (Figure 1).
Patient demographics, medical history (e.g., hypertension, diabetes, dyslipidemia, family history of premature coronary artery disease, tobacco use), and medication use were collected through chart review. Fasting lipid profile, fasting glucose, glycosylated hemoglobin, lipoprotein A, apolipoprotein B, and high sensitivity C-reactive protein levels were also measured.
Exercise capacity was determined from cardiopulmonary exercise testing using standard protocols. Briefly, subjects were tested using an individualized ramp treadmill protocol with initial ramp rate determined by a questionnaire to estimate exercise capacity (14, 15). The treadmill test was symptom-limited; in the absence of angina, subjects were encouraged to reach maximal effort (defined as exhaustion, a score ≥18 on the Borg scale, or both); target heart rates were not used as testing endpoints. Treadmill tests were performed with breath by breath gas exchange measurements using a CosMed Quark system (Rome, Italy) to determine peak VO2, which was then converted to measured metabolic equivalents (METs). Physical fitness was also assessed by six-minute walk tests, which were performed in accordance with guidelines set forth by the American Thoracic Society (16). To assess physical activity levels, subjects completed the Veterans Physical Activity Questionnaire (17), a well-validated tool to assess weekly physical activity measured in MET-hours that was then converted to weekly kilocalories using an algorithm proposed by the American College of Sports Medicine (ACSM) (18).
The MacNew Heart Disease Health-Related Quality of Life Questionnaire was administered on all patients before and after engaging in the 8-week healthy lifestyle program. The MacNew is designed to assess a patient's feelings about how cardiovascular disease affects their daily function and produces a global score as well sub-scores to evaluate physical limitation, emotional function and social function (19).
Blood samples for biomarker analysis were processed within 1–4 h of collection as follows: samples were collected in BD Vacutainer® CPT™ Mononuclear Cell Preparation Tubes with sodium citrate. Samples were then spun down in a Beckman Coulter Allegra [X-12R] centrifuge at 1800RCF for 20 min with the acceleration set at 9 and deceleration set to 0. Plasma was collected from the CPT tubes after separation from the PBMC layer and aliquoted into cryo-vials for storage at −80°C.
Based on previous studies, we defined subjects who lost ≥5 pounds (20), whose METs increased by 0.5 or greater (21), or had physical activity increase by 1,000 kcals or greater per week (17) as having successfully completed the 8-week program. Otherwise, patients were deemed unsuccessful. The most successful patients were those that were at the top tertile in weight loss, MET increase or physical activity. The least successful were those who were at the top tertile of weight gain, MET decrease or reduction in physical activity. In patients who were deemed the most successful (n = 15) and the least successful (n = 6), plasma was used to measure protein levels for 184 biomarkers including those associated with coagulation, inflammation and metabolism via the OLINK Proseek Multiplex Assay for Cardiovascular II and Cardiovascular III Panels (Olink Proteomics AB, Uppsala, Sweden). Protein names and abbreviations are listed in Supplementary Table 1. Protein levels for the 184 biomarkers were quantified by real-time PCR using a proximity extension assay utilizing oligonucleotide-labeled antibody probe pairs. Quality control and data validation were performed using OLINK's standard quality control pipeline. All Olink samples were analyzed in a blinded fashion.
Findings from this comprehensive screen were confirmed by performing the ELISA assay using the following kits based on manufacturer's protocol: Human PAI-1 Standard ABTS ELISA Development Kit (PeproTech, Rocky Hill, New Jersey), Human PDGF-Beta-1 Standard ABTS ELISA Development Kit (Peprotech, Rocky Hill, New Jersey), and Human Angiopoietin-1 ELISA Kit (ThermoScientific, Frederick, Maryland). Analysis of selected proteins by ELISA was performed on the entire cohort. All ELISA samples were performed in triplicate and analyzed in a blinded fashion.
Data are presented as median with interquartile range for continuous variables or numbers (n) and percentage (%) for categorical variables. Results from the six-minute walk test and cardiopulmonary exercise tests were converted to METs (22). In subjects unable to complete a valid treadmill test, METs from the six-minute walk test were used in the analysis (n = 5). As shown in previous studies (23), a significant correlation was found between METs obtained from the six-minute walk test and cardiopulmonary exercise test for patients who completed both assessments (Spearman's r = 0.32, p-value = 0.039, Supplementary Figure 1).
Unpaired and paired Students' t-tests, Mann Whitney U tests, and Chi-Square tests were used as appropriate. Results from Olink assays were reported in NPX (normalized protein expression), which is expressed in log2 scale and subsequently converted to fold change for comparison against ELISA results (https://www.olink.com/content/uploads/2019/02/Technical-Summary-Olink.pdf). A multivariate logistic regression analysis was performed using demographics, clinical parameters, and improvement in biomarkers as independent predictors of a successful or unsuccessful outcome. All p-values were adjusted for multiple comparisons using the Bonferroni correction method.
A propensity score model was used to balance the effect of covariates between the successful and unsuccessful groups. Patients who were defined as successful and unsuccessful were placed in the “treatment” or “control” group, respectively. An estimation of treatment effects was calculated using propensity-score matching as the estimator, matching as the outcome model, and logistic regression as the treatment model. The following outcomes were entered into the model: (1) change in plasminogen activator inhibitor-1 (PAI-1) between the first and second timepoint; (2) change in platelet derived growth factor beta-1 (PDGF-beta-1) between the first and second timepoint; and (3) change in angiopoetin-1 (ANG-1) between the first and second timepoint. Only values measured by ELISA were entered into the model. Covariates entered into the model included age (continuous), sex, race (categorical), smoking status (never, former, or current), and cardiovascular risk factors (e.g., systolic blood pressure, diastolic blood pressure, lipid profile, lipoproteins, hemoglobin A1C, and C-reactive protein). A p-value < 0.05 was considered significant with adjustment for multiple comparisons if appropriate.
Baseline subject characteristics are described in Table 1. Median age was 67.0 years (IQR 58.3, 70.8 years), 92% were male, 63% were white, and the median BMI was 33.5 kg/m2 (IQR 28.3, 38.6 kg/m2). Ninety-two percent of participants were obese, 42% were diabetic, 69% had hypertension, 65% had hypercholesteremia, and 73% had a history of coronary artery disease. A majority of subjects were on statins (75%), aspirin (69%), and beta blockers (56%) at baseline. There were no significant differences in patient characteristics between those who successfully completed their exercise programs and those who did not.
Of 66 enrolled patients, 52 completed baseline and terminal testing. The median weight change, improvement in physical fitness, and change in physical activity for the entire cohort were −4.6 pounds (IQR: +2 pounds, −11.8 pounds), 0.37 METs (IQR: −0.076 METs, 1.06 METs), and 252.7 kcals/week (IQR: −119, 921.2 kcals/week), respectively. Patients in the supervised exercise program showed significant improvement in weight (−2.96 vs. 0.46 pounds, p = 0.003), physical fitness (0.59 vs. 0.04 METs, p = 0.002), and physical activity (510.0 vs. −13.1 kcals/week, p = 0.01) compared to the unsupervised group (Figure 2A). Sixty-seven percent of patients (35/52) successfully achieved one of the pre-defined cut-offs in weight, physical fitness or physical activity that have been previously defined as thresholds to improve cardiovascular health after engaging in short-term exercise training, and only three patients met all three criteria (Figure 2B, Table 2) (17, 20, 21). Not surprisingly, of patients who were successful, the majority (77%, 27/35) were enrolled in the supervised program. Of the total supervised patients who completed the study (n = 36), remotely supervised patients were more successful than subjects who completed a program in-person [92% (22/24) vs. 42% (5/12), p = 0.001].
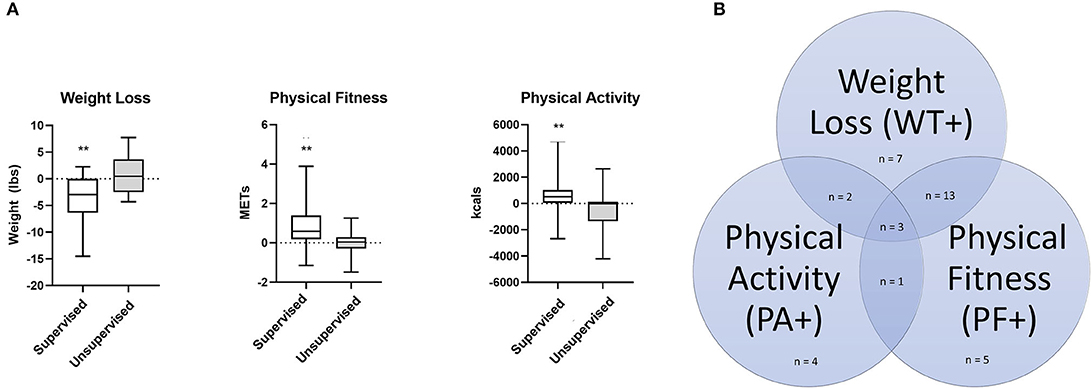
Figure 2. (A) Bar graphs showing in weight loss, physical fitness, and physical activity in patients engaged in supervised (n = 36) and unsupervised (n = 16) programs. **P-value ≤ 0.01. For supervised group: weight loss, p = 0.0029; METs change, p = 0.0025; kcal change, p = 0.0111; Mann-Whitney U Test. (B) Venn diagram showing the number of patients who met the pre-defined criteria for success: Weight loss ≥5 pounds, increase in physical activity of greater or equal to 1,000 kcals per week, and/or improvement in metabolic equivalents of greater or equal to 0.5 METs. Patients who met at least one criteria were deemed successful.
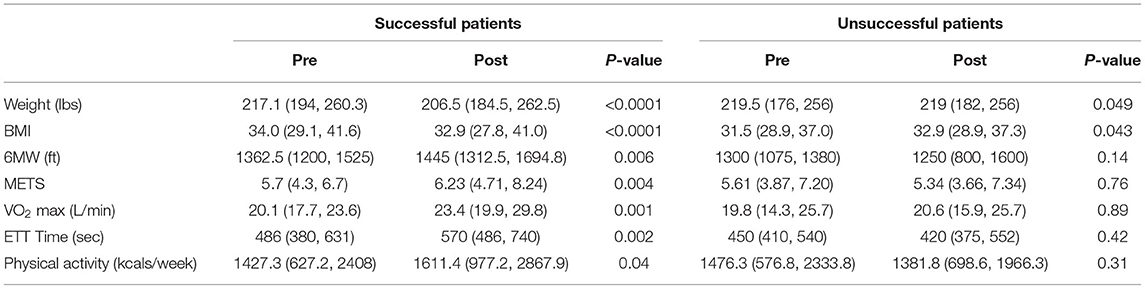
Table 2. Changes in weight loss, physical fitness, and physical activity pre- and post-intervention.
As shown in Table 2, compared to baseline, successful patients had significant improvements in weight (217.1 vs. 206.5 pounds, p < 0.0001) and physical fitness as measured by the six-minute walk test (1,362.5 vs. 1,445 feet, p = 0.006), METs (5.7 vs. 6.23 METs, p = 0.004), VO2 max (20.1 vs. 23.4 liters/min, p = 0.001), treadmill time (486 vs. 570 s, p = 0.002), and weekly physical activity (1,427.3 vs. 1,611.4 kcals/week, p = 0.04). In contrast, unsuccessful patients had no significant improvement in weight loss, physical fitness, or physical activity. The median change in weight loss [−7.6 pounds (IQR: −8.2, 21.2 pounds) vs. +7.3 pounds (IQR: +8.0 pounds, −0.55 pounds), p < 0.0001] and physical fitness [0.95 METs [IQR: 0.44 METs, 2.3 METs) vs. 0.04 METs (IQR: 0.19 METs, 0.86 METs), p = 0.003] was significantly different between the successful and unsuccessful groups. Although the median change in physical activity was not significantly different between the two groups [273.5 kcal/week (−101.3, 1090.2 kcal/week) vs. 112.7 kcal/week (−204.8, 721.3 kcal/week)], 27.8% (10/35) of patients in the successful group increased their physical activity level by at least 1,000 kcals/week.
Successful patients had a significant improvement in diastolic blood pressure (80 vs. 75 mmHg, p = 0.0025) and a non-significant decrease in systolic blood pressure (134 vs. 126 mmHg, p = 0.17). Total cholesterol (146 vs. 131 mg/dL, p = 0.03) and LDL (85 vs. 67.5 mg/dl, p = 0.02) were also significantly lower. Although anthropometric measures including waist and hip circumference (44.8 vs. 41.5 inches, p = 0.005; and 42.0 vs. 41.0, inches p = 0.008) as well as fasting glucose (105.5 vs. 97.5 md/dl, p = 0.03) were significantly improved, hemoglobin A1C (6.0 vs. 5.7%, p = 0.38) was not significantly different. Successful patients had a non-significant decrease in C-reactive protein post-intervention (1.9 vs. 1.05 mg/dL, p = 0.69). Unsuccessful patients, however, exhibited a non-significant increase in LDL and C-reactive protein (Table 3).
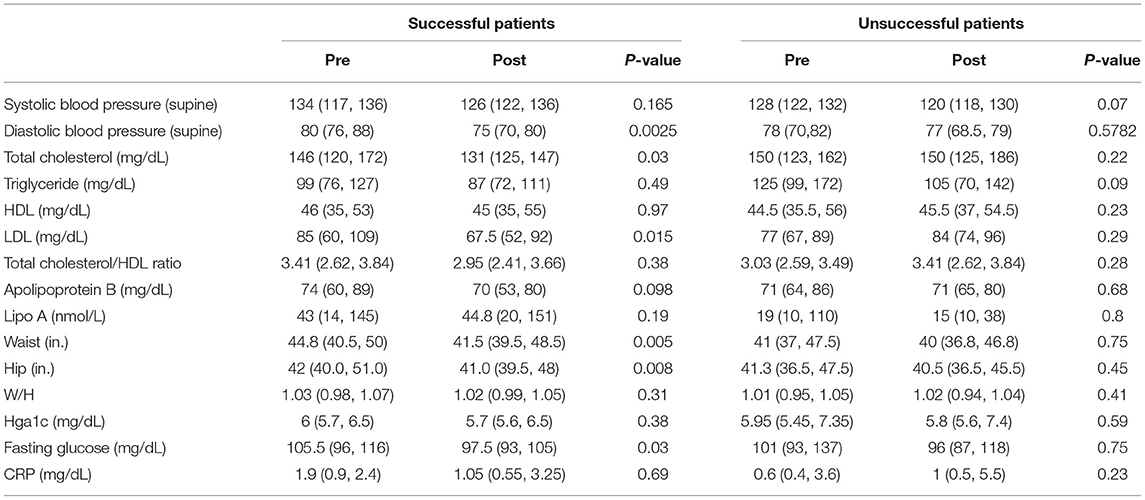
Table 3. Changes in blood pressure, lipids, metabolic parameters, and high-sensitivity C-reactive protein.
Although there was a trend toward improvement in emotional, physical, social, and global MacNew scores, there was no significant changes in quality of life scores in both the successful and unsuccessful groups (Table 4).
Of the 184 biomarkers of cardiovascular risk that we measured, the most significant difference between the 15 most successful patients and the 6 least successful patients was found in PAI-1 (fold change compared to baseline of −2.02 in successful group, p = 0.004), PDGF-beta-1 (fold change compared to baseline of 2.03 in the unsuccessful group, p = 0.027), and ANG-1 (fold change compared to baseline of 2.07 in the unsuccessful group, p = 0.019) (Table 5). To confirm these findings, ELISA was performed in the entire cohort; successful patients had a significant decrease in PAI-1 (16.64 vs. 9.38 ng/mL, p = 0.0002), PDGF-beta-1 (76.71 vs. 60.32 pg/mL, p = 0.0043), and ANG-1 (788.89 vs. 475.28 pg/mL, p = 0.0001), whereas unsuccessful patients either showed a non-significant decrease (PAI-1, 14.47 vs. 10.33 ng/mL, p = 0.098) or had an increase (PDGF-beta-1, 67.77 vs. 70.82 pg/mL, p = 0.42; ANG-1, 534.9 vs. 693.55 pg/mL, p = 0.776) (Figure 3, Table 6). Importantly, there was significant correlation between biomarkers measured by the Olink and ELISA Assays (Supplementary Figure 2). On multivariate logistic regression analysis, changes in PAI-1, PDGF-beta-1, and ANG-1 were not independent from improvement in blood pressure, lipid profile, and glucose control, suggesting that improvement in these biomarkers may be a secondary effect.
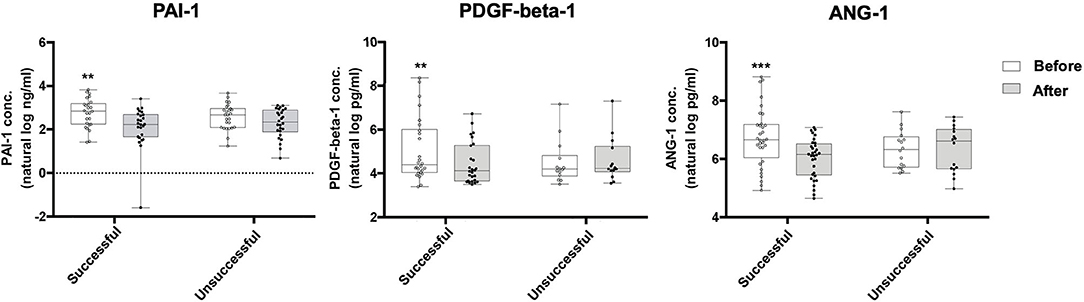
Figure 3. Box and whisker plots comparing ELISA results for the levels of PAI-1, PDGF-beta-1, and ANG-1 in successful and unsuccessful patients before and after engaging in an 8-week exercise program. Successful patients (n = 35) had a significant decrease in PAI-1 (16.64 vs. 9.38 ng/mL, p = 0.0002), PDGF-beta-1 (76.71 vs. 60.32 pg/mL, p = 0.0043), and ANG-1 (788.89 vs. 475.28 pg/mL, p = 0.0001), whereas unsuccessful patients (n = 17) either showed a non-significant decrease (PAI-1, 14.47 vs. 10.33 ng/mL, p = 0.098) or had an increase (PDGF-beta-1, 67.77 vs. 70.82 pg/mL, p = 0.42; ANG-1, 539.4 vs. 693.55 pg/mL, p = 0.776) in these markers. Values on graph shown in natural log. Wilcoxon signed-rank test; **P-value ≤ 0.01; ***P ≤ 0.001. Box defines the first and third quartiles; whiskers show minimum and maximum.
Propensity score matching was used to “correct” the estimation of the effect of the program on the cardiovascular biomarkers by controlling for the existence of confounding factors in the successful and unsuccessful group. The average treatment effects based on propensity score matching are shown in Table 7. Compared to unsuccessful patients, successful patients who completed the 8-week program had a significant average reduction in PAI-1 [ln transformed concentration: 2.03 ng/mL (95% CI: 1.02–2.7 ng/mL); absolute concentration: 9.07 ng/mL (95% CI: 2.78–15.35 ng/mL); p = 0.005], PDGF-beta-1 [ln transformed values: 5.93 pg/mL (95% CI: 3.80–6.56 pg/mL), absolute concentrations (376.077 pg/mL (95% CI: 44.69–707.46 pg/mL); p = 0.026); Ang-1 [ln transformed values 7.01 pg/mL (95% CI: 1.07–7.69 pg/mL), absolute concentrations (1104.11 pg/mL (95% CI: 2.92–2205.30 pg/mL); p = 0.049)].
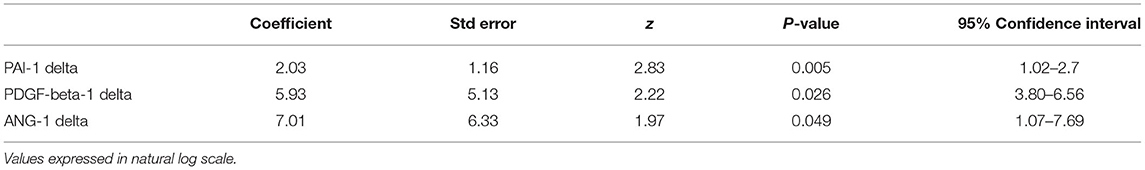
Table 7. Average treatment effect (defined as difference between first time point and second timepoint) based on propensity score matching (n = 52).
In this study, we performed a comprehensive analysis of the effects of a short-term exercise programs on traditional and non-traditional risk factors including 184 biomarkers associated with cardiovascular risk. We found that patients engaged in a supervised, remote-monitoring program compared to those participating in supervised in-person traditional programs or unsupervised programs were the most successful in achieving modest improvements in weight loss (≥5 pounds), physical fitness (≥0.5 METs), and/or physical activity (≥1,000 kcals/week). Successful patients not only improved their blood pressure and cholesterol profile, but also lowered the following non-traditional biomarkers of cardiovascular risk: PAI-1, PDGF-beta-1, and ANG-1. Interestingly, C-reactive protein and other non-traditional risk factors including other factors involved in regulating inflammation, coagulation, and metabolism showed no significant change after exercise.
The safety and effectiveness of remote monitoring programs have been demonstrated previously in several observational and randomized trials (3). In at least 20 studies directly comparing home-based programs with traditional in-person programs, remote-monitoring programs were shown to be non-inferior to traditional-in-person programs in reductions in morbidity and mortality and improvement in weight and physical activity. In our study, a significantly higher number of patients enrolled in the home-based program were successful than those in the traditional programs, which is likely related to program adherence.
In addition to improvement in cardiac risk factors in patients enrolled in short-term exercise programs, we demonstrate that modest gains in weight, physical fitness, and/or activity can reduce PAI, PDGF-beta-1, and ANG-1.
PAI-1 plays an important role in the fibrinolytic cascade. As an inhibitor of plasminogen activators, it regulates the formation of plasmin and the lysis of fibrin clots. Higher levels of blood PAI-1 have been associated with a higher risk of incident coronary heart disease even after adjustment for cardiac risk factors (OR = 1.46; 95% CI: 1.13, 1.88) (24). Mendelian randomization models also suggest a causal effect of an increased PAI-1 level on coronary heart disease risk in patients without prior heart disease (odds ratio = 1.22 per unit increase of log transformed PAI-1; 95% CI: 1.01, 1.47). Importantly, a recent meta-analysis that included 38 studies and 11, 577 patients with and without prior ASCVD confirmed the association of higher levels of PAI-1 [e.g., a mean difference of 6.11 ng/mL (95% CI, 3.27–8.96, P < 0.001) between cases and controls] with the development of major adverse cardiac events including death, myocardial infarction and cerebral vascular events (25). In our study, patients who successfully completed their 8-week exercise program had a >40% decrease in PAI-1 despite modest improvement in their weight, physical activity and physical fitness. While previous studies have also shown a reduction in PAI-1 after 6 months of exercise (26, 27), exercise programs as short as 10 days have shown no benefit (28). Taken together, these findings suggest that the duration of an exercise program seems to be an important factor in mediating reduction in PAI-1 levels and that even modest improvements in weight loss, physical activity and physical fitness through a healthier lifestyle can reduce PAI-1 levels.
In contrast, PDGF-beta-1, which can be secreted from platelets, macrophages, endothelial cells and vascular smooth muscle cells (29, 30), is a primary growth regulatory molecule that modulates vascular smooth muscle migration and proliferation. PDGF-beta-1 has been shown to promote the development of advanced atherosclerotic lesions in vitro and in animal models. Administration of PDGF-beta-1 along with interleukin-1B to human aortic vascular smooth muscle cells in vitro results in a phenotypic transition from a contractile to a synthetic state, which is a hallmark of atherosclerosis (31). In animal models, PDGF-beta-1 from vascular smooth muscles leads to the accumulation of leukocytes in the adventitia and media, promoting advanced plaque formation (32). Although serum levels of PDGF-beta-1 are increased after strenuous exercise (33), little information is available on the effects of low to moderate intensity exercise on levels of PDGF-beta-1. We observed that patients who lost weight, improved their physical fitness and/or increased their physical fitness had a >20% decrease in PDGF-beta-1 levels. The reduction of PDGF-beta-1 levels is, thus, another potential mechanism by which exercise may mediate cardiovascular benefits.
ANG-1, on the other hand, is an important regulator of angiogenesis and inflammation (34–36). Although its role in vascular remodeling and development is better defined, studies have also shown that ANG-1 directly activates endothelial cells, neutrophils, and monocytes/macrophages toward a pro-inflammatory state (37). Moreover, animal studies have shown that ANG-1 increases the number of innate immune cells as well as their retention in atherosclerotic lesions (38). Finally, ANG-1 may increase the size of atherosclerotic plaques by inhibiting cholesterol efflux from macrophages as well as stimulating their production of pro inflammatory cytokines (39). We found that patients who successfully completed the program had a 39.8% decrease in ANG-1. To our knowledge, this is the first study to measure ANG-1 levels after exercise in humans and demonstrate a reduction with regimented physical activity, providing an additional mechanism in support for regular exercise and its impact on vascular health.
Although it is unknown the exact mechanisms by which exercise mediates reductions in these non-traditional biomarkers, studies have shown that exercise-induced shear stress in the arteries of both contracting and non-contracting tissue alters the function of endothelial cells, which are the major producers of PAI-1, PDGF-beta-1, and ANG-1 (40–42). Exercise may improve endothelial function through the upregulation of athero-protective genes with concomitant downregulation of atherogenic genes (41, 42), such as PAI-1, PDGF-beta-1, and ANG-1. In a previous randomized control trial, for example, exercise was shown to lower PAI-1, but the greatest reduction was found in patients who had 4G/5G polymorphism of the PAI-1 gene, which resulted in higher baseline levels of PAI (43). Further studies are needed to understand how exercise affects the levels of these non-traditional biomarkers.
Unlike other studies evaluating changes in biomarkers after regular exercise, we did not find significant differences in other markers of inflammation including C-reactive protein. In a recent meta-analysis of 3,769 patients from 83 randomized and non-randomized trials that evaluated changes in CRP after at least 2 weeks of exercise training, exercise training was associated with a reduction in CRP even in the absence of weight loss (44). The greatest improvement in CRP, however, occurred in patients who achieved a significant reduction in BMI or percent of fat. The authors further cite several examples where exercise training did not result in a significant change in CRP including a study of 162 individuals engaged in exercise where only those achieving weight loss >6.4 pounds had a notable improvement in CRP and a study in 421 women where change in weight >5.7 pounds but not improvement in physical fitness was associated with a substantial decrease in CRP (45, 46). Similarly, we did not see a significant improvement in CRP in our cohort, given that the median weight loss in our study was −4.6 pounds (IQR: +2 pounds, −11.8 pounds). Alternatively, changes CRP may not be accurately detected because it is an extremely non-specific marker of inflammation. Because the presence of inflammation anywhere in the body can increase CRP levels, significant reductions in CRP induced by exercise and weight loss may be counter balanced by other factors that increase systemic CRP including infection, stress (47), and poor sleep (48). Taken together, these results suggest CRP may be less sensitive in detecting modest improvements in cardiovascular health after exercise than PAI-1, PDGF-beta 1, or ANG-1.
One of the limitations of this study was that patients were able to select their own exercise program and were not randomized. Consecutive recruitment, however, mimics a real world clinical setting where patients choose their own behavioral modification program. Importantly, there were no significant differences in baseline demographic and clinical factors in patients who were successful or unsuccessful in achieving the defined improvement in weight, physical fitness, and physical activity.
A second limitation of the study is the sample size. Although we were able to find significant improvements in PAI-1, PDGF-beta-1, and ANG-1, we may not have been powered to detect differences in other measured proteomic markers. In addition, the majority of patients recruited were male because the VA is the primary recruitment site and men tend to have coronary artery disease at a younger age (< 70), an age bracket that is less frail and more likely to engage in structured exercise programs. Hence, these findings may not be generalizable to the women.
Thirdly, we do not have long-term follow-up on the patients. During the median follow-up period of 1.9 years (IQR: 1.5, 2.3 years), however, no patients died of cardiovascular related deaths. Of the three patients who suffered a major adverse cardiovascular event (e.g., one patient who had a heart attack with subsequent revascularization and two patients who had elective percutaneous coronary interventions), all three had no significant decrease or an increase in PAI-1, PDGF-1-beta, and ANG-1. These data, however, are limited and further investigation is needed.
Finally, changes in these non-traditional biomarkers may be not only due to improvement in weight loss, physical fitness, and physical activity, but also due to changes in traditional risk factors. This finding is not surprising given these factors have also been associated with hypertension (49), dyslipidemia (50), and diabetes (51, 52). Despite these study limitations, we believe our study provides important information on how an exercise program administered in a real-world setting can improve non-traditional markers of cardiovascular risk.
Patients achieving modest improvement in weight loss, fitness and/or physical activity after engaging in a short-term exercise program had decreases in PAI-1, PDGF-beta-1, and ANG-1, which are associated with impaired fibrinolysis and inflammation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by VA Palo Alto and Stanford. The patients/participants provided their written informed consent to participate in this study.
GL: data collection and manuscript preparation. XH, OA, and NH: data analysis and manuscript preparation. JH, SM, HF, KC, DL, and VF: data collection. JM: data collection, data analysis, and manuscript preparation. AL and PN: concept, data collection, data analysis, and manuscript preparation. All authors contributed to the article and approved the submitted version.
This work was supported by the National Institutes of Health (R01 HL 134830-01 to PN).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.669110/full#supplementary-material
Supplementary Figure 1. Two-way scatter plot showing the relationship between ETT METs and METS obtained from cardiopulmonary testing. Spearman correlation coefficient (r2) = 0.315; p-value = 0.0384. Open circles: unsupervised baseline; Closed circles: unsupervised post; Gray plus sign: supervised baseline; Black plus sign: supervised post.
Supplementary Figure 2. Two-way scatter plot showing the relationship between biomarkers measured by the OLINK and ELISA assays. Spearman correlation coefficient (r2) = r2 = 0.7925, p-value < 0.0001 for plasminogen activator inhibitor-1 (PAI-1); Spearman correlation coefficient (r2) = 0.717; p-value < 0.0001 for platelet derived growth factor beta-one (PDGF-beta-1); Spearman correlation coefficient (r2) = 0.569; p-value = 0.0001 for angiopoetin-1 (ANG-1). White Bar: baseline; Gray Bar: post.
Supplementary Table 1. List of cardiovascular biomarkers measured by OLINK.
ANG-1, angiopoetin; ASCVD, atherosclerotic cardiovascular disease; METs, metabolic equivalents; PAI-1, plasminogen activator inhibitor-1; PDGF-beta-1, platelet derived growth factor beta-1.
1. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. (2013) 127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6
3. Thomas RJ, Beatty AL, Beckie TM, Brewer LPC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. (2019) 140:e69–89. doi: 10.1016/j.jacc.2019.03.008
4. Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. (2011) 124:789–95. doi: 10.1161/CIRCULATIONAHA.110.010710
5. Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. (2002) 347:1483–92. doi: 10.1056/NEJMoa020194
6. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. (2002) 137:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006
7. Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. (2007) 147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005
8. Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. (2007) 116:2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939
9. Wannamethee SG, Lowe GDO, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. (2002) 105:1785–90. doi: 10.1161/hc1502.107117
10. Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. (2002) 162:1286–92. doi: 10.1001/archinte.162.11.1286
11. Elhakeem A, Cooper R, Whincup P, Brage S, Kuh D, Hardy R. Physical activity, sedentary time, and cardiovascular disease biomarkers at age 60 to 64 years. J Am Heart Assoc. (2018) 7:e007459. doi: 10.1161/JAHA.117.007459
12. Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. (2015) 4:e002014. doi: 10.1161/JAHA.115.002014
13. Stattin K, Lind L, Elmståhl S, Wolk A, Lemming EW, Melhus H, et al. Physical activity is associated with a large number of cardiovascular-specific proteins: cross-sectional analyses in two independent cohorts. Eur J Prev Cardiol. (2019) 26:1865–73. doi: 10.1177/2047487319868033
14. Myers J, Buchanan N, Walsh D, Kraemer M, McAuley P, Hamilton-Wessler M, et al. Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol. (1991) 17:1334–42. doi: 10.1016/s0735-1097(10)80144-5
15. Myers J, Do D, Herbert W, Ribisl P, Froelicher VF. A nomogram to predict exercise capacity from a specific activity questionnaire and clinical data. Am J Cardiol. (1994) 73:591–6. doi: 10.1016/0002-9149(94)90340-9
16. Brooks D, Solway S. ATS statement on six-minute walk test. Am J Respir Crit Care Med. (2003) 167:1287. doi: 10.1164/ajrccm.167.9.950
17. Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. (2004) 117:912–8. doi: 10.1016/j.amjmed.2004.06.047
18. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. (2011) 43:1575–81. doi: 10.1249/MSS.0b013e31821ece12
19. Höfer S, Lim L, Guyatt G, Oldridge N. The MacNew heart disease health-related quality of life instrument: a summary. Health Qual Life Outcomes. (2004) 2:3. doi: 10.1186/1477-7525-2-3
20. Willis EA, Huang WY, Saint-Maurice PF, Leitzmann MF, Salerno EA, Matthews CE, et al. Increased frequency of intentional weight loss associated with reduced mortality: a prospective cohort analysis. BMC Med. (2020) 18:248. doi: 10.1186/s12916-020-01716-5
21. Blair SN. Physical fitness and all-cause mortality. A prospective study of healthy men and women. J Am Med Assoc. (1989) 262:2395–401. doi: 10.1001/jama.262.17.2395
22. Pescatello, Linda S. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health (2014).
23. Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. (2010) 10:31. doi: 10.1186/1471-2466-10-31
24. Song C, Burgess S, Eicher JD, O'Donnell CJ, Johnson AD, Huang J, et al. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. (2017) 6:e004918. doi: 10.1161/JAHA.116.004918
25. Jung RG, Motazedian P, Ramirez FD, Simard T, Di Santo P, Visintini S, et al. Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thromb J. (2018) 16:12. doi: 10.1186/s12959-018-0166-4
26. Killewich LA, MacKo RF, Montgomery PS, Wiley LA, Gardner AW. Exercise training enhances endogenous fibrinolysis in peripheral arterial disease. J Vasc Surg. (2004) 40:741–5. doi: 10.1016/j.jvs.2004.07.030
27. Aznar J, Estellés A, Tormo G, Sapena P, Tormo V, Blanch S, et al. Plasminogen activator inhibitor activity and other fibrinolytic variables in patients with coronary artery disease. Heart. (1988) 59:535–41. doi: 10.1136/hrt.59.5.535
28. Bodary PF, Yasuda N, Watson DD, Brown AS, Davis JM, Pate RR. Effects of short-term exercise training on plasminogen activator inhibitor (PAI-1). Med Sci Sports Exerc. (2003) 35:1853–8. doi: 10.1249/01.MSS.0000093751.82616.F0
29. Huang M, DuHadaway JB, Prendergast GC, Laury-Kleintop LD. RhoB regulates PDGFR-β trafficking and signaling in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. (2007) 27:2597–605. doi: 10.1161/ATVBAHA.107.154211
30. Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, et al. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. (1990) 248:1009–12. doi: 10.1126/science.2343305
31. Chen CN, Li YSJ, Yeh YT, Lee PL, Usami S, Chien S, et al. Synergistic roles of platelet-derived growth factor-BB and interleukin-1β in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci USA. (2006) 103:2665–70. doi: 10.1073/pnas.0510973103
32. He C, Medley SC, Hu T, Hinsdale ME, Lupu F, Virmani R, et al. PDGFRβ signalling regulates local inflammation and synergizes with hypercholesterolaemia to promote atherosclerosis. Nat Commun. (2015) 6:7770. doi: 10.1038/ncomms8770
33. Czarkowska-Paczek B, Bartlomiejczyk I, Przybylski J. The serum levels of growth factors: PDGF, TGF-beta and VEGF are increased after strenuous physical exercise. J Physiol Pharmacol. (2006) 57:189–97.
34. Seok SH, Heo JI, Hwang JH, Na YR, Yun JH, Lee EH, et al. Angiopoietin-1 elicits pro-inflammatory responses in monocytes and differentiating macrophages. Mol Cells. (2013) 35:550–6. doi: 10.1007/s10059-013-0088-8
35. Ahmad S, Cudmore MJ, Wang K, Hewett P, Potluri R, Fujisawa T, et al. Angiopoietin-1 induces migration of monocytes in a tie-2 and integrin-independent manner. Hypertension. (2010) 56:477–83. doi: 10.1161/HYPERTENSIONAHA.110.155556
36. Sturn DH, Feistritzer C, Mosheimer BA, Djanani A, Bijuklic K, Patsch JR, et al. Angiopoietin affects neutrophil migration. Microcirculation. (2005) 12:393–403. doi: 10.1080/10739680590960296
37. Lemieux C, Maliba R, Favier J, Théorêt JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. (2005) 105:1523–30. doi: 10.1182/blood-2004-09-3531
38. Fujisawa T, Wang K, Niu XL, Egginton S, Ahmad S, Hewett P, et al. Angiopoietin-1 promotes atherosclerosis by increasing the proportion of circulating Gr1+ monocytes. Cardiovasc Res. (2017) 113:81–89. doi: 10.1093/cvr/cvw223
39. Ou X, Gao JH, He LH, Yu XH, Wang G, Zou J, et al. Angiopoietin-1 aggravates atherosclerosis by inhibiting cholesterol efflux and promoting inflammatory response. Biochim Biophys Acta. (2020) 1865:158535. doi: 10.1016/j.bbalip.2019.158535
40. Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda). (2011) 26:132–45. doi: 10.1152/physiol.00052.2010
41. Qiao C, Meng F, Jang I, Jo H, Chen YE, Zhang J. Deep transcriptomic profiling reveals the similarity between endothelial cells cultured under static and oscillatory shear stress conditions. Physiol Genomics. (2016) 48:660–6. doi: 10.1152/physiolgenomics.00025.2016
42. Ajami NE, Gupta S, Maurya MR, Nguyen P, Li JYS, Shyy JYJ, et al. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc Natl Acad Sci USA. (2017) 114:10990–5. doi: 10.1073/pnas.1707517114
43. Väisänen SB, Humphries SE, Luong LA, Penttilä I, Bouchard C, Rauramaa R. Regular exercise, plasminogen activator inhibitor-1 (PAI-1) activity and the 4G/5G promoter polymorphism in the PAI-1 gene. Thromb Haemost. (1999) 82:1117–20.
44. Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. (2017) 51:670–6. doi: 10.1136/bjsports-2016-095999
45. Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, et al. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. (2010) 42:708–16. doi: 10.1249/MSS.0b013e3181c03a43
46. Stewart LK, Earnest CP, Blair SN, Church TS. Effects of different doses of physical activity on C-reactive protein among women. Med Sci Sports Exerc. (2010) 42:701–7. doi: 10.1249/MSS.0b013e3181c03a2b
47. Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73131 individuals. JAMA Psychiatry. (2013) 70:176–84. doi: 10.1001/2013.jamapsychiatry.102
48. Leng Y, Ahmadi-Abhari S, Wainwright NWJ, Cappuccio FP, Surtees PG, Luben R, et al. Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open. (2014) 4:e006071. doi: 10.1136/bmjopen-2014-006071
49. Fogari R, Zoppi A, Mugellini A, Maffioli P, Lazzari P, Derosa G. Role of angiotensin II in plasma PAI-1 changes induced by imidapril or candesartan in hypertensive patients with metabolic syndrome. Hypertens Res. (2011) 34:1321–6. doi: 10.1038/hr.2011.137
50. Levine JA, Oleaga C, Eren M, Amaral AP, Shang M, Lux E, et al. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci Rep. (2021) 11:430. doi: 10.1038/s41598-020-79948-x
51. Yarmolinsky J, Bordin Barbieri N, Weinmann T, Ziegelmann PK, Duncan BB, Inês Schmidt M. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. (2016) 6:17714. doi: 10.1038/srep17714
Keywords: exercise, coronary artery disease, biomarkers, thrombosis, inflammation
Citation: Liang G, Huang X, Hirsch J, Mehmi S, Fonda H, Chan K, Huang NF, Aalami O, Froelicher VF, Lee DP, Myers J, Lee AS and Nguyen PK (2021) Modest Gains After an 8-Week Exercise Program Correlate With Reductions in Non-traditional Markers of Cardiovascular Risk. Front. Cardiovasc. Med. 8:669110. doi: 10.3389/fcvm.2021.669110
Received: 18 February 2021; Accepted: 13 April 2021;
Published: 17 June 2021.
Edited by:
Gabrielle Fredman, Albany Medical College, United StatesReviewed by:
Francine Welty, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesCopyright © 2021 Liang, Huang, Hirsch, Mehmi, Fonda, Chan, Huang, Aalami, Froelicher, Lee, Myers, Lee and Nguyen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia K. Nguyen, cGtuZ3V5ZW5Ac3RhbmZvcmQuZWR1
†These authors have contributed equally to this work
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.