
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 13 May 2021
Sec. Cardiovascular Epidemiology and Prevention
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.665831
This article is part of the Research Topic Renal Function and Related Biomarkers in Cardiovascular Risk Assessment and Prevention View all 8 articles
Background: Research suggest that albuminuria is not only an independent risk factor for the development of heart failure but may also act as a biomarker for predicting adverse outcomes. To date, no study has synthesized evidence on its role as a prognostic indicator. Thus, the current study aimed to quantitatively assess the prognostic utility of albuminuria as well as dipstick proteinuria in predicting mortality in heart failure patients.
Methods: PubMed, Embase, ScienceDirect, CENTRAL, and Google Scholar databases were searched up to October 10, 2020. All studies reporting multivariable-adjusted hazard ratios (HR) for albuminuria or dipstick proteinuria for mortality and/or hospitalization in heart failure patients were included.
Results: Eleven studies were included. Seven assessed albuminuria and five assessed dipstick proteinuria. Our analysis revealed a statistically significant increased risk of all-cause mortality with microalbuminuria (HR: 1.54; 95% CI, 1.23–1.93; I2 = 79%; p = 0.0002) and macroalbuminuria (HR: 1.76; 95% CI, 1.21–2.56; I2 = 88%; p = 0.003) in heart failure patients. The risk of all-cause mortality and hospitalization was also significantly increased with macroalbuminuria. Microalbuminuria was associated with significantly increased cardiovascular mortality and combined cardiovascular mortality and hospitalization. Positive dipstick test for proteinuria was significantly associated with mortality in heart failure (HR: 1.54; 95% CI, 1.28–1.84; I2 = 67%; p < 0.00001).
Conclusion: Both microalbuminuria and macroalbuminuria are predictors of mortality in patients with heart failure. Dipstick proteinuria may be used as a rapid screening test to predict mortality in these patients.
Heart failure has been identified as a major cardiovascular disease leading to a global public health problem. According to recent estimates, around 64.3 million individuals are affected by heart failure worldwide, with a ratio of 8.5 patients per 1,000 individuals (1). Such high numbers account for 9.9 million years lost due to disability from the disease (1). Heart failure has been associated with significant morbidity, mortality as well as high healthcare expenditure. Despite immense research and developments in the management of the disease, hospitalization for heart failure, incidence of readmissions, and death rates remain high (2). To prevent mortality and hospitalizations due to heart failure, it is necessary to extensively characterize the predictors of such adverse events, amalgamating it with the impact of other comorbidities present in such patients.
Traditional risk factors like diabetes, hypertension, smoking, and hyperlipidemia are well-recognized for predicting mortality with cardiovascular diseases (3, 4). Studies have also reported that albuminuria not only is an independent risk factor for the development of cardiovascular disease but also acts as a biomarker for predicting mortality in such patients (5–7). Specifically, in heart failure patients, the presence of albumin in the urine can represent several pathophysiological conditions like systemic inflammation as well as endothelial and microvascular dysfunction, which may have a role in the prognosis of the disease (8). Furthermore, it may represent kidney injury due to heart failure itself or other comorbidities like diabetes and chronic kidney disease (9). Studies indicate that albuminuria can be a potential target to improve clinical outcomes in heart failure patients (10).
In the past two decades, a few studies have specifically focused on the role of albuminuria in predicting adverse outcomes in heart failure patients (11–13). Owing to the diverse population of the included studies along with adjustment of different confounding factors, there is a need for a pooled analysis to determine the exact value of albuminuria as a biomarker for predicting mortality and hospitalization in heart failure patients. Furthermore, dipstick proteinuria has been suggested as an alternative to the more cumbersome testing of 24-h urinary albumin-to-creatinine ratio for detecting albuminuria (7). Thus, in the current study, we aimed to quantitatively assess the prognostic utility of albuminuria as well as dipstick proteinuria in predicting mortality and hospitalization in heart failure patients.
The review is conducted as per the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (14). We included studies with the following inclusion criteria: (1) conducted on patients with heart failure, (2) comparing outcomes of patients with and without albuminuria or dipstick proteinuria, (3) reporting mortality data, and (4) data reported as adjusted hazard ratios (HR) with 95% confidence intervals (CI). No restriction was placed on the study design, sample size, or date of publication. The following were the exclusion criteria for the review: (1) studies on a mixed population of cardiovascular diseases, (2) studies not reporting relevant outcomes, (3) studies reporting unadjusted HR, (4) review articles, case reports, and unpublished studies, and (5) non-English language studies.
An electronic search was conducted by two reviewers, independent of each other, for the following databases: PubMed, Embase, ScienceDirect, CENTRAL, and Google Scholar. The time limit was from the inception of the databases to October 10, 2020. The terms used for the literature search included the following: “heart failure,” “cardiac failure,” “albuminuria,” “proteinuria,” “albumin,” “urinary,” “death,” and “mortality.” The search terms were used in different combinations to find relevant articles. After the deduplication of articles, the search records were analyzed separately by their titles and abstracts by the two reviewers. Articles matching the inclusion criteria were identified, and the full texts of these were extracted. Individual studies were then assessed for final inclusion in the study. Any disagreements were resolved by discussion. After completion of the search and identification of included studies, the bibliography of included articles was manually searched for any other potential article.
The following data were extracted from the included studies: names of first authors, publication year, study type and location, use of albuminuria or dipstick proteinuria, definition of albuminuria, sample size, demographic details of the sample, outcomes reported, adjusted variables for outcomes, and follow-up time. The primary outcome of interest was mortality (both all-cause and cardiovascular mortality). The secondary outcome of interest was the risk of hospitalization for heart failure.
The risk of a bias was assessed using the Newcastle–Ottawa Scale (15). Two reviewers independently assessed each study. Studies with more than seven points were judged to be of high quality. Any disagreements were resolved by discussion.
“Review Manager” [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014] was used for the meta-analysis. We extracted data on adjusted HR for mortality and hospitalization for heart failure as reported by the included studies and combined them using inverse variance-weighted averages of logarithmic HRs. Meta-analysis was conducted only if at least two studies reported the same outcome. Sub-group analysis was conducted for microalbuminuria and macroalbuminuria. Studies reporting dipstick proteinuria were pooled separately. In studies reporting data for different sub-groups for evaluating the prognostic significance of albuminuria or dipstick proteinuria, we combined data of the sub-groups into one singular group using the meta-analysis software itself. The pooled data of all sub-groups from a given study was then used for the meta-analysis. We assessed heterogeneity using the I2 statistic. I2-values of 25–50% represented low, values of 50–75% medium, and values more than 75% represented substantial heterogeneity. Due to the different study designs, the difference in factors adjusted, and the varied study populations of the included studies, we chose to use the random-effects model for the meta-analysis. As <10 studies were included in the meta-analysis, funnel plots were not used to assess publication bias. We also conducted a sensitivity analysis for the outcome of all-cause mortality to assess if any study had an undue influence on the results. Each study in the analysis was sequentially excluded to recalculate the effect size in the meta-analysis software itself.
The PRISMA flow chart of the review is presented in Figure 1. A total of 12 studies were included in the review. Seven studies (11–13, 16–19) evaluated albuminuria using 24-h urinary protein excretion in a central laboratory, while five studies (20–24) assessed proteinuria using the dipstick test. We separated these studies for this analysis. The details of seven studies evaluating albuminuria are presented in Table 1. Four studies were secondary analysis of randomized controlled trials (RCTs) from the baseline population, while two were retrospective studies and one was a prospective study. The sample size in these studies ranged from 712 to 4,668 patients. The mean ejection fraction was ≤ 50% in three studies. Similar definitions of microalbuminuria and macroalbuminuria were used in the included studies except for that of Jackson et al. (12). The follow-up duration and variables adjusted for the multivariate analysis differed across studies. None of the included studies were of low quality.
Table 2 presents the details of studies using the dipstick examination method for assessing proteinuria. Of these five studies, three were secondary analyses of RCTs, while two were retrospective studies. The sample size ranged from 1,056 to 24,331 patients in the included studies. One study presented data on in-hospital mortality, while others documented long-term mortality. None of the included studies were of low quality.
Of the seven studies reporting 24-h urine albuminuria measurements, one study analyzed the relationship of albuminuria and mortality by treating albuminuria as a continuous variable, unlike other studies that grouped the patients into microalbuminuria or macroalbuminuria. Hence, this study was excluded from the meta-analysis. On descriptive analysis, the study of Katz et al. (16) reported a statistically significant increase in mortality per doubling of urinary albumin (HR: 1.15; 95% CI, 1.04–1.28) in a multivariable-adjusted model. On the pooling of data of all-cause mortality from five studies, our analysis revealed a statistically significant increased risk of mortality with microalbuminuria (HR: 1.54; 95% CI, 1.23–1.93; I2 = 79%; p = 0.0002) and macroalbuminuria (HR: 1.76; 95% CI, 1.21–2.56; I2 = 88%; p = 0.003) (Figure 2). On sensitivity analysis, there was no change in the significance of the results on sequential exclusion of each study. Two studies reported HR for all-cause mortality and hospitalization. Pooled analysis revealed a significantly increased risk of this composite outcome for macroalbuminuria (HR: 1.82; 95% CI, 1.14–2.90; I2 = 95%; p = 0.01) but not microalbuminuria (HR: 1.40; 95% CI, 0.96–2.05; I2 = 95%; p = 0.08) (Figure 3). Our analysis also revealed a significantly increased risk of cardiovascular mortality (HR: 1.52; 95% CI, 1.10–2.10; I2 = 0%; p = 0.01) and combined cardiovascular mortality and hospitalization for heart failure (HR: 2.49; 95% CI, 1.04–5.96; I2 = 77%; p = 0.04) in patients with microalbuminuria (Figure 4). Analysis of data from two studies, however, did not reveal statistically significant results for the singular outcome of hospitalization for heart failure in patients with microalbuminuria (HR: 2.42; 95% CI, 0.86–6.84; I2 = 78%; p = 0.09) (Figure 4).
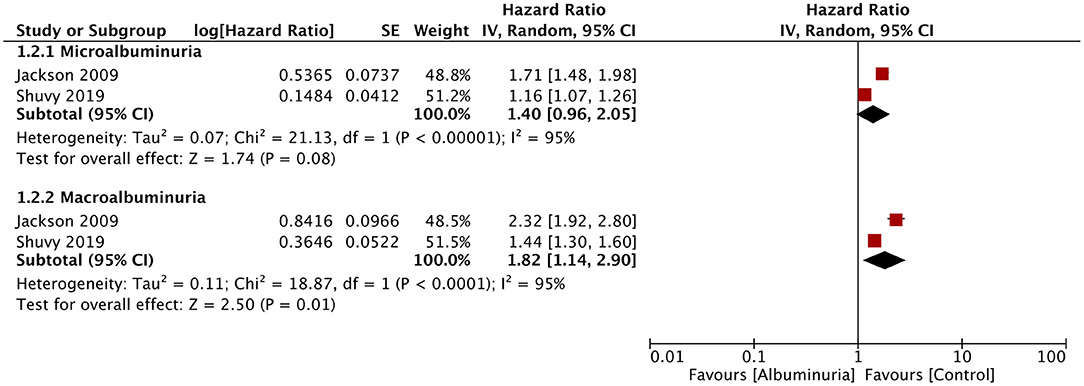
Figure 3. Forest plot for all-cause mortality and hospitalization for heart failure with albuminuria in heart failure patients.
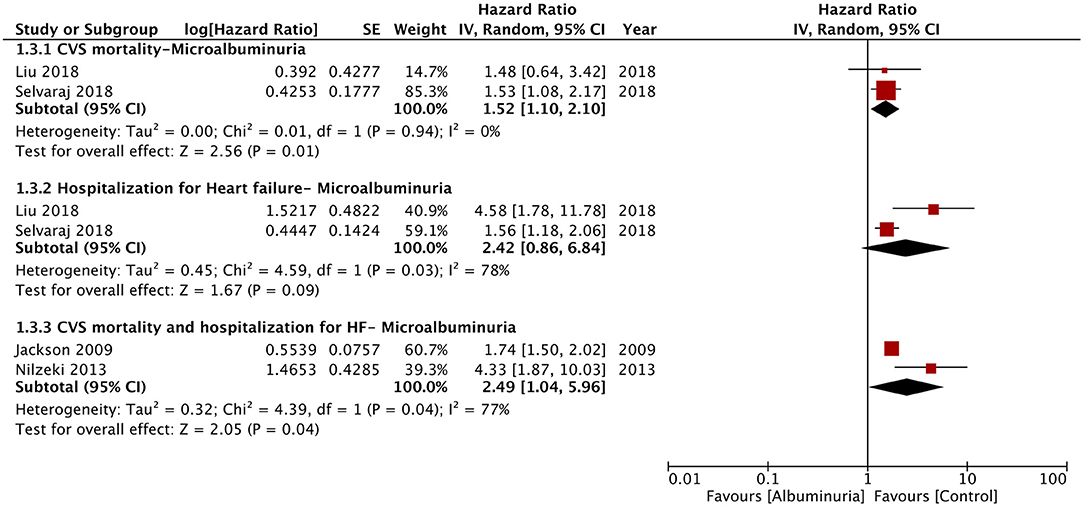
Figure 4. Forest plot for cardiovascular mortality and hospitalization for heart failure associated with microalbuminuria in heart failure patients.
In the second part of the meta-analysis, we pooled the data of the five studies using dipstick proteinuria as a prognostic indicator for mortality in heart failure patients. The pooled analysis indicated a statistically significant increased risk of mortality in heart failure patients with a positive dipstick test for proteinuria (HR: 1.54; 95% CI, 1.28–1.84; I2 = 67%; p < 0.00001) (Figure 5). On sensitivity analysis, there was no change in the significance of the results on sequential exclusion of each study. For this analysis, Miura et al. (22) reported separate data for patients with and without low estimated glomerular filtration rate (eGFR) (defined as eGFR of < 60 ml/min/1.73 m2) but with positive dipstick test. Their results were statistically significant for both groups with HR of 2.71 (95% CI, 1.72–4.27) for patients with low eGFR and HR of 2.44 (95% CI, 1.47–4.05) for patients with normal eGFR. Data for both groups were combined for the meta-analysis. On the other hand, Smith et al. (20) reported separate data based on preservation of systolic function (preserved or reduced) and the amount of proteinuria traced on the dipstick test (+1, +2, and +3). Data for these sub-groups was combined for the analysis. For the study of Brisco et al. (24), data of high blood urea nitrogen/creatinine and low blood urea nitrogen/creatinine groups was combined for the meta-analysis. Owing to the difference in sub-groups of the individual studies, sub-group analysis for dipstick proteinuria could not be conducted in our review.
Our study, which is the first systematic review and meta-analysis in the literature assessing the impact of albuminuria and dipstick proteinuria on the prognosis of heart failure, presents the following important findings: (1) presence of microalbuminuria in heart failure patients increases the risk of all-cause mortality by around 54%, while macroalbuminuria increases the risk by 76%, (2) limited studies indicate that the risk for the combined outcome of all-cause mortality and hospitalization for heart failure is increased by 82% in the presence of macroalbuminuria, (3) microalbuminuria increases the risk of cardiovascular mortality by around 52% and combined cardiovascular mortality and hospitalization by 149%, and (4) positive dipstick proteinuria in heart failure patients is associated with 54% increased risk of all-cause mortality.
Albuminuria or proteinuria is a recognized biomarker for impaired glomerular filtration and kidney disease. Studies in literature have reported a significant prevalence of albuminuria in heart failure patients ranging from 41% to as high as 54% (12, 19). Such high prevalence has also been reported in studies analyzing patients already on medications such as angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists which are known to reduce the prevalence of albuminuria (18). The high prevalence of albuminuria in heart failure can be attributed to the concomitant presence of other illnesses like diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease (5). However, studies have shown that the presence of albuminuria is not only restricted to the sub-population with comorbidities but is also prevalent in patients without them. In the real-world data of Shuvy et al. (19), 22% of patients without comorbidities had the presence of albuminuria. In such cases, the pathological role of heart failure itself has been implicated. The hemodynamic state and raised central venous pressure in heart failure patients can cause vascular congestion in the kidneys and reduced renal blood flow. Glomerular hypertension along with tubulointerstitial hypoxia can reduce the number of nephrons, leading to hyperfiltration and albuminuria (5). The decline in renal function is then further complimented by systemic inflammation, oxidative stress, and endothelial dysfunction due to heart failure and other comorbidities like diabetes which aggravate the filtration of albumin through the glomerular basement membrane and accelerate cardiac damage (5, 13, 25). The association between heart failure and albuminuria seems plausible as increased severity of heart failure has been shown to increase the risk of albuminuria (19).
The association between albuminuria and increased cardiovascular mortality is established in diseases like diabetes and hypertension. Toyama et al. (26), in a systematic review and meta-analysis of 31 studies, have reported an increased risk of cardiovascular mortality with microalbuminuria [risk ratio (RR), 1.76; 95% CI, 1.38–2.25] and macroalbuminuria (RR, 2.96; 95% CI, 2.44–3.60) as compared to normoalbuminuria in diabetics. Guidelines for the management of hypertension recommend screening as well as targeted management of albuminuria in addition to controlling blood pressure for better clinical outcomes (27). Despite evidence suggesting the deleterious effects of albuminuria independent of kidney function in patients with other cardiovascular diseases (28, 29), there has been limited research on the prognostic significance of this biomarker specifically in heart failure patients. In this context, our review presents important pooled evidence suggesting a significant role of albuminuria in the worsening prognosis of heart failure. The increased risk of mortality was seen with both microalbuminuria and macroalbuminuria, indicating that even mild albuminuria has a prognostic significance in these patients. Evidence was obtained from a pooled analysis of multivariable-adjusted hazard ratios with different studies taking into account multiple confounding factors to arrive at the statistical figure. This lends credibility to the meta-analysis conducted in our review. Furthermore, the finding was stable on sensitivity analysis, with no study exerting an undue influence on the results. It can also be noted from the forest plot of all-cause mortality that the effect size was larger in earlier as compared to more recent studies. While the exact reason for this is difficult to decipher owing to the limited number of studies in our review, the improvement in the management of heart failure as well as associated comorbidities over the period of time may have contributed to this difference.
Our findings have important clinical implications as targeting albuminuria in heart failure patients can be an important therapeutic approach. Studies on diabetic patients have indicated that targeting albuminuria may improve survival (30, 31). However, data on such targeted therapy for albuminuria in heart failure patients is limited. In one of the included studies, Selvaraj et al. (18) demonstrated that the use of spironolactone in heart failure patients reduced albuminuria and improved the outcomes. Thus, further studies are warranted to elucidate the role of targeting albuminuria in heart failure patients to improve outcomes.
For decades, 24-h urine collection has been the gold standard to measure albuminuria. In recent times, dipstick urine analysis has gained popularity owing to its ease of use and rapid results (32). However, dipstick proteinuria is only sensitive to albumin and is a semi-quantitative measure rather than determining the exact values of proteinuria. Furthermore, it solely depends on the urinary protein concentration rather than the actual protein excretion rate (33). Despite its limitations, it has been a popular marker for assessing prognosis in large screening programs owing to its substantially low cost as compared to 24-h urine analysis (34). In a study on the general population, Konta et al. (35) have demonstrated the presence of microalbuminuria in 12, 65, and 92% of patients with dipstick proteinuria results of (–), (±), and (≥1+), respectively. As a supplementary analysis of our main results, we also assessed the prognostic value of this simple test in heart failure patients. Our results demonstrated a statistically significant 54% increased risk of all-cause mortality in heart failure patients with positive dipstick proteinuria. While 24-h urine albuminuria measurements shall remain the gold standard, dipstick proteinuria can be used as a rapid screening tool to predict adverse outcomes in such patients.
The results of our study should be interpreted with the following limitations. Firstly, our review could not include a large number of studies due to the paucity of such investigations in literature. The number of studies included in the meta-analysis of outcomes other than all-cause mortality was very low. Secondly, due to non-availability of data, we could not classify outcomes based on types of heart failure, i.e., with and without reduced ejection fraction. Thirdly, study patients were classified as positive for albuminuria or proteinuria based on single tests. Fluctuations during the course of the disease were not taken into account, and this may have caused a misrepresentation of some patients. The studies also differed in the timing of the albuminuria or dipstick proteinuria test relative to the identification of heart failure. At this point, it is not clear if any treatment strategy, which may have been initiated after the diagnosis, had an influence on the study results. Lastly, the number of confounding factors adjusted and the duration of follow-up varied across studies, and this may have influenced our results.
To conclude, our review indicates that both microalbuminuria and macroalbuminuria are predictors of mortality in patients with heart failure. Dipstick proteinuria may be used as a rapid screening test to predict mortality in these patients. Owing to the review limitations, the results of our study should be supplemented with future high-quality research. Studies should also be planned to analyze if albuminuria can be an independent target for therapy in heart failure patients to improve outcomes.
Publicly available datasets were analyzed in this study. This data can be found here: PubMed, Embase, ScienceDirect, CENTRAL, and Google Scholar databases.
WL and QL conceived, designed the study, and were involved in the writing of the manuscript. Q-yW, HY, and JY collected the data and performed the literature search. All the authors have read and approved the final manuscript.
This study was supported by Gansu Province Health Research Project (GSWSKY-2019-92).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J. (2020) 5:15. doi: 10.21037/amj.2020.03.03
2. Roger VL. Epidemiology of heart failure. Circ Res. (2013) 113:646–59. doi: 10.1161/CIRCRESAHA.113.300268
3. Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. (2008) 1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146
4. Buddeke J, Valstar GB, van Dis I, Visseren FLJ, Rutten FH, den Ruijter HM, et al. Mortality after hospital admission for heart failure: improvement over time, equally strong in women as in men. BMC Public Health. (2020) 20:36. doi: 10.1186/s12889-019-7934-3
5. Stehouwer CDA, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. (2006) 17:2106–11. doi: 10.1681/ASN.2005121288
6. Nagata M, Ninomiya T, Kiyohara Y, Murakami Y, Irie F, Sairenchi T, et al. Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: pooled analysis of 39,000 individuals from 7 cohort studies in Japan. Am J Epidemiol. (2013) 178:1–11. doi: 10.1093/aje/kws447
7. Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. (2013) 7:13–24. doi: 10.2147/IJNRD.S40522
8. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. (2013) 62:263–71. doi: 10.1016/j.jacc.2013.02.092
9. Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. (2007) 2:581–90. doi: 10.2215/CJN.03190906
10. Ghali JK. A new direction for albuminuria: an enigmatic multibiomarker. JACC Heart Fail. (2014) 2:597–9. doi: 10.1016/j.jchf.2014.07.006
11. Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure data from the GISSI-Heart failure trial. Circ Hear Fail. (2010) 3:65–72. doi: 10.1161/CIRCHEARTFAILURE.109.881805
12. Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. (2009) 374:543–50. doi: 10.1016/S0140-6736(09)61378-7
13. Niizeki T, Takeishi Y, Sasaki T, Kaneko K, Sugawara S, Watanabe T, et al. Usefulness of albuminuria as a prognostic indicator in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. (2013) 111:1180–6. doi: 10.1016/j.amjcard.2012.12.050
14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed October 30, 2020).
16. Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Hear Fail. (2014) 2:586–96. doi: 10.1016/j.jchf.2014.05.016
17. Liu M, Liang Y, Zhu J, Yang Y, Ma W, Zhang G. Albumin-to-creatinine ratio as a predictor of all-cause mortality and hospitalization of congestive heart failure in Chinese elder hypertensive patients with high cardiovascular risks. Clin Hypertens. (2018) 24:12. doi: 10.1186/s40885-018-0095-3
18. Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, O'Meara E, et al. Prognostic value of albuminuria and influence of spironolactone in heart failure with preserved ejection fraction. Circ Heart Fail. (2018) 11:e005288. doi: 10.1161/CIRCHEARTFAILURE.118.005288
19. Shuvy M, Zwas DR, Lotan C, Keren A, Gotsman I. Albuminuria: associated with heart failure severity and impaired clinical outcomes. Can J Cardiol. (2020) 36:527–34. doi: 10.1016/j.cjca.2019.09.001
20. Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction : the Cardiovascular Research Network PRESERVE study. Circ Cardiovasc Qual Outcomes. (2013) 6:333–42. doi: 10.1161/CIRCOUTCOMES.113.000221
21. Anand IS, Bishu K, Rector TS, Ishani A, Kuskowski MA, Cohn JN. Proteinuria, chronic kidney disease, and the effect of an angiotensin receptor blocker in addition to an angiotensin-converting enzyme inhibitor in patients with moderate to severe heart failure. Circulation. (2009) 120:1577–84. doi: 10.1161/CIRCULATIONAHA.109.853648
22. Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, et al. Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail. (2012) 14:367–76. doi: 10.1093/eurjhf/hfs001
23. Chen Y, Cai L, Du Z, Xu J, Tan N, Ye Z, et al. Dipstick proteinuria is a prognostic indicator of short-term mortality in patients with heart failure. Int J Cardiol. (2017) 230:59–63. doi: 10.1016/j.ijcard.2016.12.096
24. Brisco MA, Zile MR, Ter Maaten JM, Hanberg JS, Wilson FP, Parikh C, et al. The risk of death associated with proteinuria in heart failure is restricted to patients with an elevated blood urea nitrogen to creatinine ratio. Int J Cardiol. (2016) 215:521–6. doi: 10.1016/j.ijcard.2016.04.100
25. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage—The Steno hypothesis. Diabetologia. (1989) 32:219–26. doi: 10.1007/BF00285287
26. Toyama T, Furuichi K, Ninomiya T, Shimizu M, Hara A, Iwata Y, et al. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: meta-analysis. PLoS ONE. (2013) 8:71810. doi: 10.1371/journal.pone.0071810
27. Chua DCY, Bakris GL. Is proteinuria a plausible target of therapy? Curr Hypertens Rep. (2004) 6:177–81. doi: 10.1007/s11906-004-0066-9
28. Åkerblom A, Clare RM, Lokhnygina Y, Wallentin L, Held C, Van De Werf F, et al. Albuminuria and cardiovascular events in patients with acute coronary syndromes: results from the TRACER trial. Am Heart J. (2016) 178:1–8. doi: 10.1016/j.ahj.2016.04.013
29. Mok Y, Ballew SH, Sang Y, Grams ME, Coresh J, Evans M, Barany P, et al. Albuminuria as a predictor of cardiovascular outcomes in patients with acute myocardial infarction. J Am Heart Assoc. (2019) 8:546. doi: 10.1161/JAHA.118.010546
30. Jansson FJ, Forsblom C, Harjutsalo V, Thorn LM, Wadén J, Elonen N, et al. Regression of albuminuria and its association with incident cardiovascular outcomes and mortality in type 1 diabetes: the FinnDiane Study. Diabetologia. (2018) 61:1203–11. doi: 10.1007/s00125-018-4564-8
31. Anyanwagu U, Donnelly R, Idris I. Albuminuria regression and all-cause mortality among insulin-treated patients with type 2 diabetes: analysis of a large UK primary care cohort. Am J Nephrol. (2019) 49:146–55. doi: 10.1159/000496276
32. Bakris GL. Slowing nephropathy progression: focus on proteinuria reduction. Clin J Am Soc Nephrol. (2008) 3:S3–10. doi: 10.2215/CJN.03250807
33. Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton). (2010) 15(Suppl. 2):53–6. doi: 10.1111/j.1440-1797.2010.01314.x
34. Iseki K, Konta T, Asahi K, Yamagata K, Fujimoto S, Tsuruya K, et al. Dipstick proteinuria and all-cause mortality among the general population. Clin Exp Nephrol. (2018) 22:1331–40. doi: 10.1007/s10157-018-1587-x
Keywords: proteinuria, albumin, mortality, cardiac failure, meta-analysis
Citation: Liang W, Liu Q, Wang Q-y, Yu H and Yu J (2021) Albuminuria and Dipstick Proteinuria for Predicting Mortality in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:665831. doi: 10.3389/fcvm.2021.665831
Received: 10 February 2021; Accepted: 29 March 2021;
Published: 13 May 2021.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Julie K. K. Vishram-Nielsen, Rigshospitalet, DenmarkCopyright © 2021 Liang, Liu, Wang, Yu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yu, yujing2304@126.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.