- 1Department of Cardiovascular Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangdong, China
- 2Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease, Department of Pediatric Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangdong, China
- 3Clinical Physiology Laboratory, Guangzhou Women and Children’s Medical Center, Institute of Pediatrics, Guangzhou Medical University, Guangdong, China
Objectives: Pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries (PA/VSD/MAPCAs) is complex and diverse that has led to a variety of treatment strategies. Experience has been largely obtained in the advanced countries. The clinical diversity is greater in China. We evaluated our surgical approaches and outcomes of these patients.
Methods: We reviewed 127 patients undergoing varied surgeries in our center in 2010–2019.
Results: Thirty patients underwent single-stage complete repair by unifocalizing MAPCAs and VSD closure (aged 3.9–131.4 months, median 22) with 3 (10%) early deaths. Ninety-seven underwent the first-stage rehabilitation strategy including systemic-to-pulmonary shunt in 29 (aged 0.5–144 month, median 8), and palliative RV-PA conduit in 68 (aged 2.2–209.6 months, median 14) with 5 (5.2%) early deaths. Eight-one patients (63.8%) eventually achieved complete repair with a median right/left ventricular (RV/LV) pressure ratio of 0.7 (ranged 0.4–1.0). Fourteen patients (11.0%) accepted palliation as final destination. Survival for the entire cohort was 89.5, 85.2, and 76.1% at 1, 5, and 10 years, respectively. Survival for those undergoing complete repair was 88.2 and 76.6% at 1 and 5 year, respectively. RV/LV pressure ratio ≥0.8 was risk factor for mortality (HR10.3, p = 0.003).
Conclusions: Our cohort, the largest from China, had distinctive clinical features with substantially wider age range and higher RV/LV pressure ratio. Using the combined approaches tailored to individual patients, complete repair was achieved in 64% of patients. The early and intermediate outcomes are acceptable compared to many of the previous reports.
Introduction
Surgical strategies for pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries (PA/VSD/MAPCAs) remain controversial. While some reported remarkable outcomes using the early complete unifocalization strategy, others recommended the rehabilitation strategy to promote development of native pulmonary arteries (1, 2). Currently both are used in many centers with favorable results (3–5).
To date, most reports are from the developed countries showing that early surgical intervention is ideal in infants and young children (1, 2, 6–8). However, in China and other developing countries, there is substantial heterogeneity in the age of patients at the first visit and consequent questionable potential for hypoplastic pulmonary arteries to develop and morphologic and structural variabilities in MAPCAs. Selection of surgical strategies for such patients continues to pose challenges. Therefore, we evaluated our strategy combining both rehabilitation and unifocalization and early and intermediate outcomes of the largest single-centered cohort of these patients in China.
Patients and Methods
Following the approval by the Institutional Ethics Board, we retrospectively studied 127 consecutive patients from January 2010 to August 2019. All patients were diagnosed preoperatively using echocardiography and computed tomography. Patients who were initially judged suitable for complete repair underwent further cardiac catheterization and angiography to determine the anatomical features of the native pulmonary arteries, MAPCAs, and bronchopulmonary segment perfusion.
Surgical Strategies
Initial Surgical Strategies
Based on the individualized characteristics of each patient, one of the following initial surgical strategies was chosen:
1. Single-stage complete repair: This involved right ventricular outflow tract (RVOT) reconstruction, complete unifocalization of MAPCAs, and VSD closure via median sternotomy or thoracotomy. RVOT reconstruction was performed with non-valve pericardial conduit, or valved polytetrafluoroethylene conduit, or bovine jugular valved conduit with the outer wall covered with a polyester film to prevent dilation (Balance Medical Technology Co Ltd., Beijing, China) since they became available in 2017. The decision making for complete repair requirements were: (1) preoperative hemoglobin saturation (SaO2) ≥85% at rest; (2) predicted total neopulmonary artery index (TNPAI) ≥150 mm2/m2 after unifocalization (9); (3) More than 3/4 of bronchopulmonary segments perfused by central pulmonary arteries after unifocalization since 2016 (≥2/3 before 2016); (4) pulmonary arterial flow study showing perfusion flow ≥3 L/min/m2 and mean pulmonary arterial pressure (mPAP) ≤25 mmHg since 2016 (1).
2. Rehabilitation surgery: For those with hypoplastic native pulmonary artery, we preferred to choose rehabilitation strategy. According to the diameter of intrapericardial native pulmonary artery, one of the followings was chosen:
Systemic-to-pulmonary (S-P) shunt: A central shunt was used for patients with poor native pulmonary artery development within the pericardium and a diameter ≤2.5 mm. Based on patient’s age and weight, a 4–5-mm polytetrafluoroethylene artificial blood vessel (Gore-Tex, W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) was selected to connect the ascending aorta with the main pulmonary artery or the left and right pulmonary arteries. Since 2017, we used the Melbourne shunt in patients with severely hypoplastic pulmonary artery. A modified Blalock-Taussig shunt was performed in patients from other hospitals.
Right ventricle to pulmonary artery (RV-PA) conduit connection: Palliative RV-PA conduit connection was performed in patients with a native pulmonary artery diameter ≥3 mm. The conduits included non-valved autologous pericardial conduit and polytetrafluoroethylene (Gore-Tex) artificial blood vessel which were used in small infants (<10 kg weight) or older children with small native pulmonary arteries, and bovine jugular (Beijing Bairen Medical Technology Co Ltd., Beijing, China) and polytetrafluoroethylene valved conduit which was used in older children. The diameter of conduit was estimated according to this formula: Diameter (mm) = 0.325 × Weight (kg) + 4.629 (10).
Second-Stage and Multi-Stage Surgical Strategies
For patients who had received a S-P shunt, if pulmonary arterial development did not meet the criteria for complete repair as described above, conversion to RV-PA conduit connection was performed at 6 months after the shunt operation in order to further promote the pulmonary artery development.
For patients who had received a S-P shunt or RV-PA conduit, if pulmonary arterial development was satisfactory, complete repair was considered. Starting in 2016, we performed the intraoperative flow study as a functional measure of pulmonary vascular performance and candidacy for complete repair (1), with the additional borderline condition, i.e., mPAP at 25–30 mmHg, the VSD was closed with fenestration. But if mPAP > 30 mmHg, the VSD was left open. At the end of CPB, the RV/LV pressure ratio was measured. If the RV/LV pressure ratio >0.8, a fenestration was carried out. In patients who had undergone fenestration following the flow study, the RV/LV pressure ratio was found <0.8 with a left-to-right shunt at VSD by transesophageal echocardiography, closure of fenestration was performed. The VSD fenestration area was about 0.8–1.0 cm2/m2. Subsequent VSD closure was completed at the third-stage surgery or by transcatheter occlusion.
MAPCAs Management Strategy
For patients with preoperative angiography suggesting that MAPCAs had sufficient communication with native pulmonary arteries, MAPCAs were embolized by transcatheter occlusion or surgical ligation. For patients with MAPCAs as the only source of blood supply to the bronchopulmonary segment, MAPCAs were recruited to the native pulmonary arteries or the newly constructed pulmonary artery. In extreme cases, MAPCAs originated from the descending aorta at the T7–8 level were also connected using artificial blood vessels. Stenosis of MAPCAs was augmented with an autologous pericardial patch, or the proximal segment of the MAPCA up to the stenosis was excised when the distal segment has enough length that it can be recruited to other MAPCAs or the pulmonary artery.
Follow-Up and Reintervention
Echocardiography and cardiac catheterization were performed every 3–6 months after each stage of surgery. Pulmonary arterial development, right and left ventricular function, right ventricular dilation, pulmonary regurgitation, and hemodynamic parameters were qualitatively described. The final clinical status including symptom, exercise tolerance, weigh gain, and death, was collected from the last available follow-up, as described by Amark et al. (11).
For patients who underwent palliative surgery, pulmonary arterial development, and MAPCA changes were assessed, and complete repair was performed if indicated as described above. Balloon dilation was performed for patients with stenosis of the anastomosis, left and right pulmonary arteries following palliative, or complete repair. For those with VSD fenestration, if SaO2 > 95%, and left to right shunt at VSD from echocardiography, and RV/LV pressure ratio <0.75 and Qp:Qs > 1.5, the VSD fenestration was occluded using transcatheter device.
Statistical Analysis
Data were expressed as mean ± SD, median (range), or frequency (%) when appropriate. Between-group differences were compared using a t-test or chi-squared test when appropriate. The Kaplan-Meier method was used to estimate survival time, and the log-rank test was used to compare differences in survival rates. Univariate Cox regression analysis was performed, and variables with a P-value <0.1 were included. A forward stepwise regression method was used based on maximum likelihood estimation. A P-value <0.05 was considered significant. SPSS 22.0 (IBM, Armonk, NY) was used for statistical analyses.
Results
Patient Characteristics
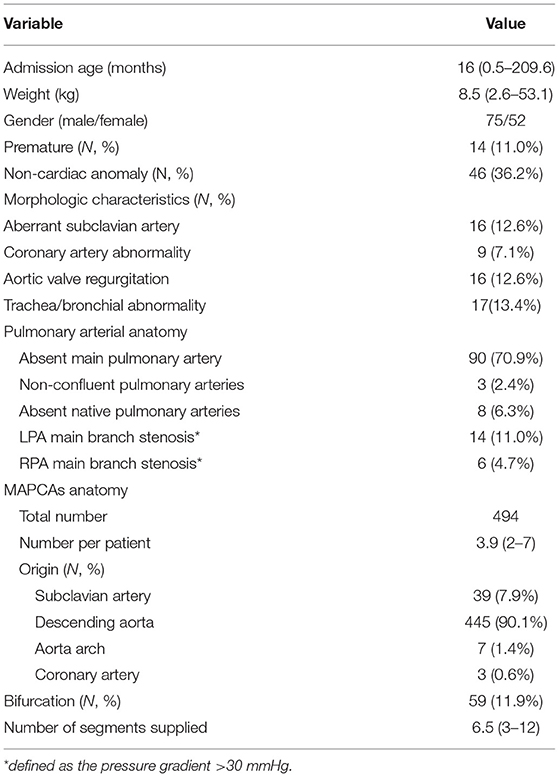
In the 127 patients, the preoperative SaO2 was 77% (range: 45–94%). The median number of MAPCAs per patient was 3.9 (range: 2–7). There were 116 patients with native pulmonary artery confluence, three with intrapericardial pulmonary artery non-confluence, and eight with complete absence of native pulmonary arteries (Table 1).
Surgical Algorithm and Early Outcomes
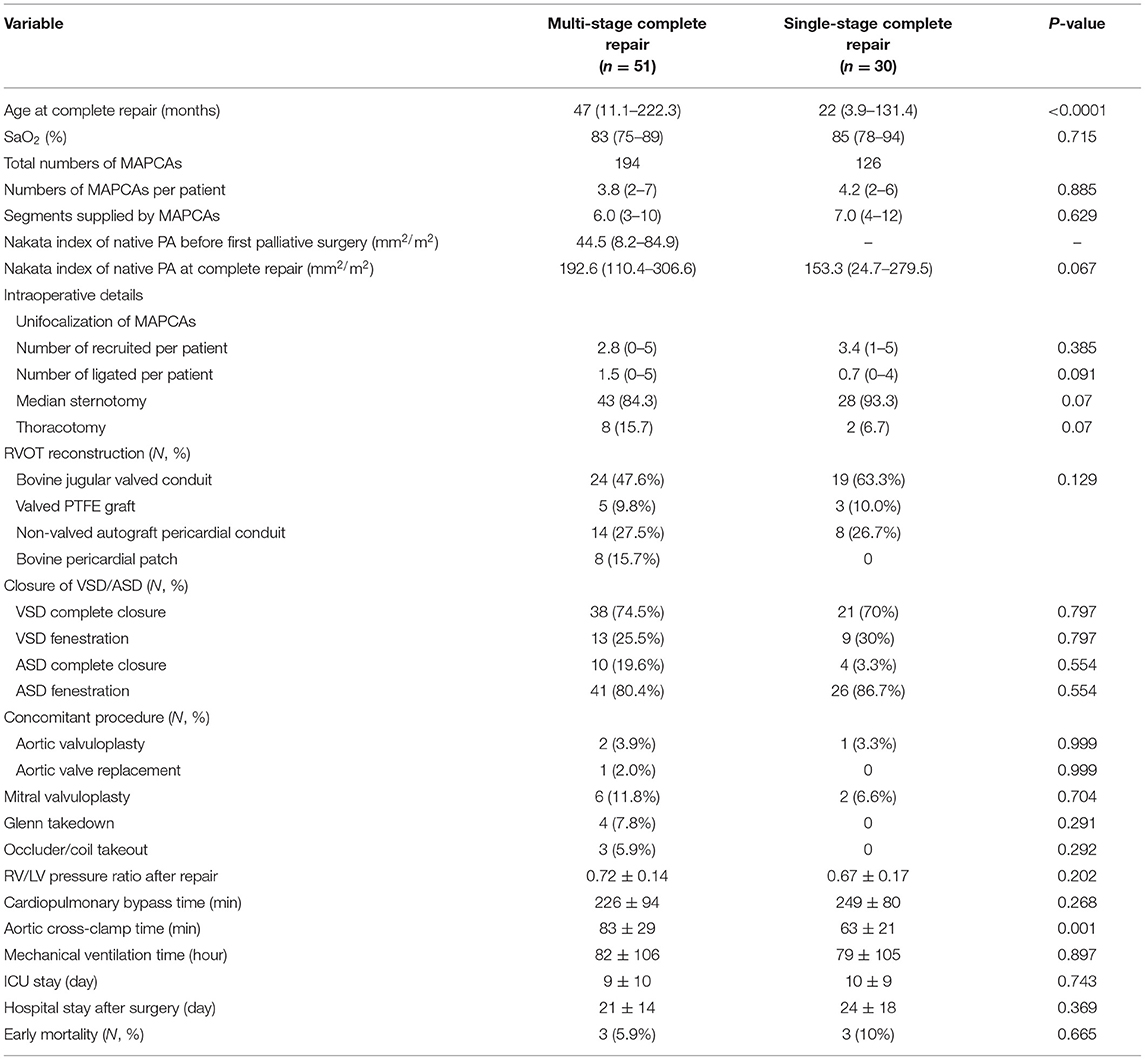
Thirty patients underwent single-stage complete repair at a median age of 22 months (range: 3.9–131.4 months), which was significantly younger than that of multi-stage group (P < 0.0001). Of these, eight patients had absent native pulmonary arteries. The median Nakata index of 22 patients with hypoplastic native pulmonary artery was 153.3 mm2/m2 (range: 24.7–279.5 mm2/m2). A median of 2.9 (range: 1–5) MAPCAs per patient in this group were recruited, and 0.7 (range: 0–4) MAPCAs were ligated intraoperative because of dual supply. Materials for right ventricular outflow tract reconstruction included bovine jugular valved conduit in 19, non-valved autologous pericardial conduit in eight, and polytetrafluoroethylene (Gore-Tex) valved conduit in three patients. Twenty-one patients underwent VSD complete closure, and nine with VSD patch fenestration. Early postoperative death occurred in three patients (10.0%) (the RV/LV pressure ratio >0.9). There was no statistical difference in the early mortality between single-stage and multi-stage complete repair group (10 vs. 5.9%, P = 0.665). The anatomical characteristics and operative details of patients underwent complete repair are summarized in Table 2.
Ninety-seven patients underwent the first-stage palliative surgery to rehabilitate native pulmonary arteries. Of these, 29 patients underwent S-P shunt with a median Nakata index of 33.5 mm2/m2 (range: 11.2–60.6 mm2/m2); 13 of them received a modified Blalock-Taussig shunt and 16 received a central shunt (including five Melbourne shunts) at a median age of 8 months (range: 0.5–144 months). The remaining 68 patients underwent palliative RV-PA conduit connection at a median age of 14 months (range: 2.2–209.6 months) and the median Nakata index was 45.2 mm2/m2 (range: 8.2–184.9 mm2/m2). Conduit materials included non-valved autologous pericardial conduits in 60 patients, Gore-Tex artificial blood vessels in six, and bovine jugular valved conduit in two. Five patients (5.2%) died within 30 days after the first-stage palliative surgery, including three with S-P shunts (10.3%) ((1) center shunt and (2) the Melbourne shunt) and two with RV-PA conduit connection (2.9%). The group with RV-PA connection trended lower early mortality than that of S-P shunt (p = 0.063).
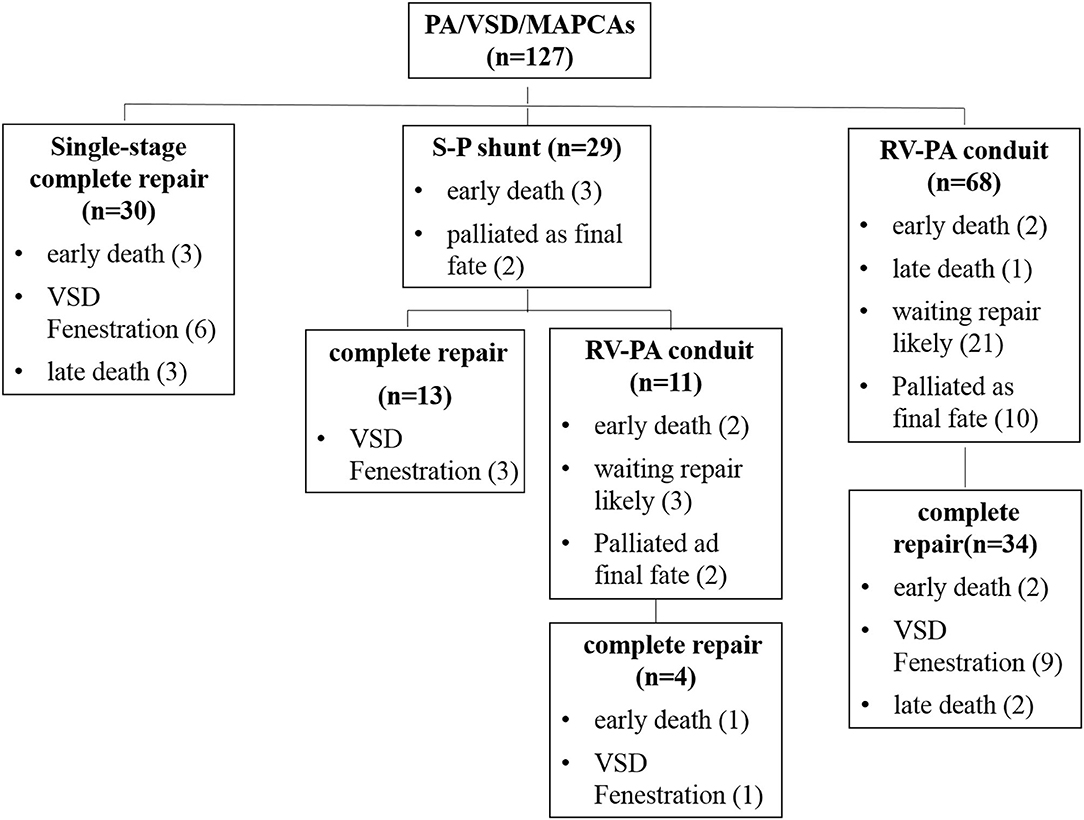
At a median of 24 months (range: 6–116 months) after the first-stage S-P shunt, 13 patients achieved complete repair (three had VSD fenestration) and two were awaiting next-stage surgery. Owing to unsatisfactory native pulmonary artery development, 11 patients were converted to RV-PA conduit connection with two simultaneous unifocalization of MAPCAs. Among these, four underwent complete repair 1–3 years after the conversion and five awaiting the complete closure of VSD; two died early after surgery of cardiac dysfunction as a result of left anterior descending coronary artery injury and poor pulmonary arterial development, respectively. In total, 58% (17/29) achieved complete repair after the first-stage palliative surgery.
In patients undergoing the palliative RV-PA conduit connection, 34 (50%) underwent complete repair after 18 months (range: 6–100 months) at a median age of 47 months (range: 22–222 months) with 3 (8.8%) early deaths. In the 34 patients, the native pulmonary artery Nakata index increased from the median 55.6 mm2/m2 (range: 18.2–84.9 mm2/m2) at the first palliative surgery to the median 126.6 mm2/m2 (range:102.2–176.3 mm2/m2) at complete repair. There was one late death at 5 months postoperatively due to purulent meningitis. In the remaining unrepaired patients, 21 (30.9%) had the subsequent complete repair anticipation, and 10 (14.7%) were felt to never be suitable for close VSD due to poor distal pulmonary artery development (Figure 1).
Follow-Up, Reintervention, and Intermediate Outcomes
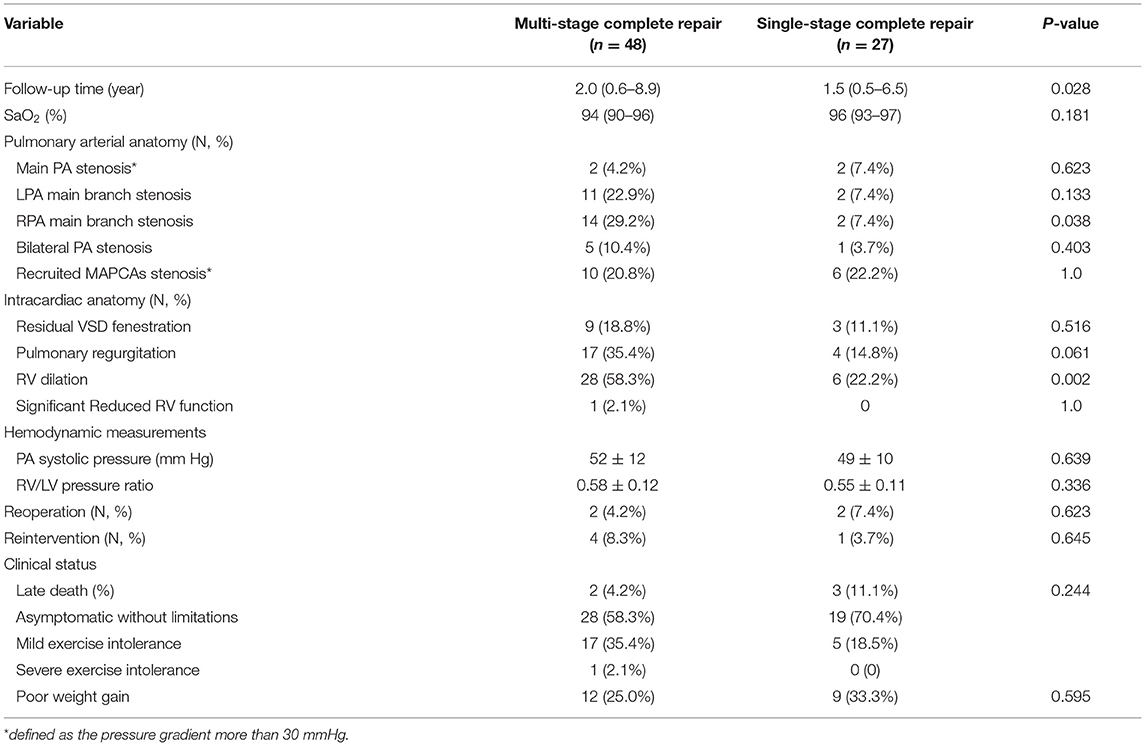
At the latest follow-up, eighty-one patients (63.8%) underwent complete repair with 6 (7.4%) early deaths (3 of 30 single-stage and 3 of 51 multi-stage complete repair) (Figure 1). The surviving 75 patients included 16 with VSD fenestration. The RV/LV pressure ratio was 0.67 ± 0.17 for single-stage complete repair and 0.72 ± 0.14 for multi-stage complete repair (P = 0.2). There were five late deaths; three of them were after single-stage complete repair, one developed RV-PA conduit stenosis 5 years after complete repair and died after conduit replacement due to intracranial infection; two had sudden death (RV/LV pressure ratio ≥0.9). The remaining two deaths were due to right ventricular failure after multi-stage complete repair.
Reintervention was performed in 16 patients, including cardiac catheterization in 12 patients and surgery in four. Balloon dilation was performed in five patients with right and/or left pulmonary artery stenosis in 6–10 months after palliative RV-PA conduit connection, and four with stenosis of unifocalized MAPCAs in 1–3 years after complete repair. Transcatheter VSD occlusion was performed in three patients in 1 month−1 year after complete repair. Three patients with RVOT obstruction underwent surgical replacement; one with severe reflux of the right pulmonary artery conduit underwent subsequent valved conduit replacement. There was no death after reintervention.
The overall 1-, 5-, and 10-year survival rates after initial operation were 89.5% (95% CI: 84.0–95.0%), 85.2% (95% CI: 78.1–92.3%), and 76.1% (95% CI: 64.1–88.1%), respectively (Figure 2A). The 1- and 5-year survival rates after complete repair were 88.2% (95% CI: 81.0–95.5%), and 76.6% (95% CI: 0.4–96.8%), respectively (Figure 2B). Univariate regression analysis showed postoperative RV/LV pressure ≥0.8 (HR: 10.35, 95% CI: 2.19–48.93, P = 0.003), single-stage complete repair (HR: 3.49, 95% CI: 1.06–11.49, P = 0.039) and the era of complete repair surgery before 2016 (HR: 4.48, 95% CI: 1.25–16.03. P = 0.021) were risk factors for mortality after repair. Of note, the three early postoperative deaths following single-stage complete repair occurred before 2016. Multivariate regression analysis showed postoperative RV/LV pressure ≥0.8 (HR: 10.35, 95% CI: 2.19–48.93. P = 0.003) was only risk factor for death. The early mortality rate after 2016 was significantly lower than that of the 2010–2015 period (35.0 vs. 6.7%, P < 0.0001). Despite the high risk of re-stenosis of pulmonary arteries and branches, most patients showed good clinical status with no or mild exercise limitation (Table 3). The clinical status between single- and multi-stage complete repair groups was not significantly different (P = 0.24). Due to the poor pulmonary artery vasculature, 14 (11.0%) (10 in RV-PA conduit connection, 4 in S-P shunt) cases were felt to never be suitable for VSD closure and the palliation was likely to be their final destination.
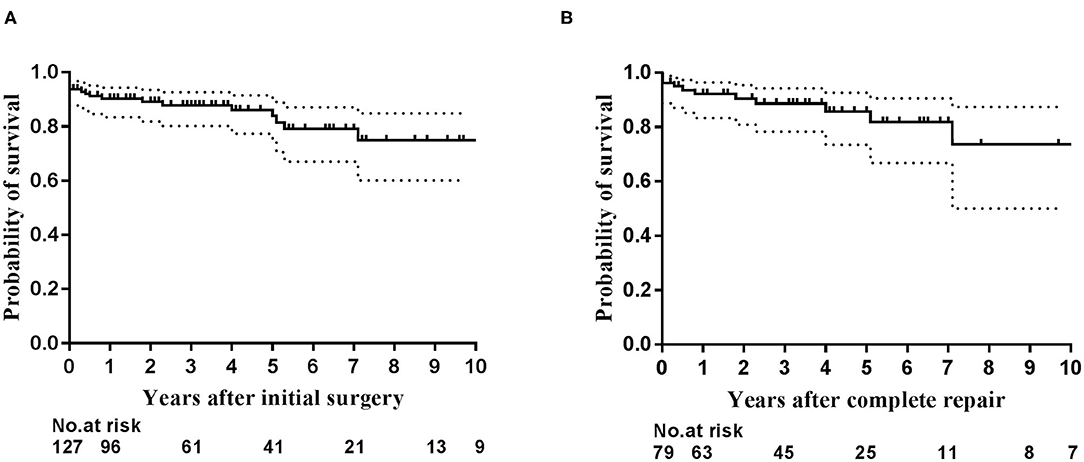
Figure 2. The Kaplan–Meier curves depict estimated survival. (A) Survival for the entire cohort of 127 patients after initial operation; (B) Survival for patients after complete repair.
Discussion
This study reviewed our experience that combined the distinctive patient characteristics in China with literature reports from advanced countries in the management of 127 patients with PA/VSD/MAPCAs in our center over the past decade, representing the developing course of surgical, and interventional management of these patients. This is the largest cohort report from China and the third largest in the world to date. Our data showed that despite of even greater clinical diversity, particularly older age, and higher RV/LV pressure ratio after complete repair, 63.8% of patients ultimately achieved complete repair with satisfactory early and intermediate outcomes.
Regarding the shunt, our strategy was to use a S-P shunt for those with native right and left pulmonary artery diameters of ≤2.5 mm. If, at 6 months after surgery, pulmonary arterial development was unsatisfactory, we converted to RV-PA conduit connection. In this group, 44.8% (13/29) achieved complete repair at the second stage, while 37.9% (11/29) showed unsatisfactory pulmonary arterial development and were converted to RV-PA conduit connection at the second stage. Four of these patients achieved complete repair at the third stage, making the cumulative complete repair rate 58.6% (17/29).
The palliative RV-PA connection includes the use of various conduits to reconstruct RV-PA continuity. We preferred non-valved autologous pericardial conduit in small infants to reconstruct RV-PA connection. Its advantages include easily suture, less bleeding, no extra cost, and longer term of patency. When the autologous pericardium is not available, the Gore-tex artificial blood vessel is considered. Hovever, for older children with hypoplastic pulmonary arteries, we preferred using a valved conduit, such as, bovine jugular valved conduit with the outer wall covered with a polyester film to prevent dilation or polytetrafluoroethylene (Gore-Tex) valved conduit, because high distal resistance may cause significant regurgitation and lead to right ventricular dysfunction. Besides, in order to avoid overflow of the pulmonary circulation, the diameter of conduit is generally restrictive calculated from the established formula (10). Of the 68 patients undergoing palliative RV-PA conduit connection, we found that the risk of unexpected over-circulation and significant regurgitation and right ventricular dysfunction is low. Despite of the older age at the first-stage palliative surgery (age range 2.2–209.6 months (median 14 months) in our patients as compared to the age range 2–6 months in previous reports) (12–14), RV-PA conduit connection still promoted native pulmonary arterial development as indicated by the development in Nakata index, and 47.1% (32/68) patients achieved complete repair.
A valved conduit is adopted at the complete repair in the current era. Nonetheless, if the development of pulmonary vascular bed is good without elevated pulmonary artery pressure and pulmonary vascular resistance, i.e., the RV / LV pressure ratio <0.8 at the end of CPB and stable systemic arterial blood pressure, a non-valved conduit was applicable, particularly when the valved conduit was unavailable as in our experience. This situation is similar to the use of a transannular patch in the complete repair of tetralogy of Fallot. In our cohort, 8 (26.7%) in one-stage and 22 (43.2%) in multistage complete repair used non-valved conduit, and in the intermediate follow-up, only 1 (2.1%) of patients in multistage complete repair group showed significant right ventricular dilation and dysfunction.
Regarding the treatment strategies for MAPCAs, early complete unifocalization, even in patients with absence of the native pulmonary arteries, was recommended to obtain low-pressure pulmonary circulation (15, 16). Patients whose MAPCAs had sufficient communication with native pulmonary arteries underwent transcatheter occlusion or surgical ligation. Recruitment was performed wherever possible for MAPCAs that supply individual bronchopulmonary segments during complete repair. Some centers such as Stanford and Birmingham prefer the S-P shunt or RV-PA conduit connection at early complete unifocalization and have reported excellent results (4, 16). However, our earlier experiences before 2010 (before the study period) with this strategy showed a fairly high early mortality. Subsequently we adopted the rehabilitation strategy.
The indications for complete repair remain controversial. In our experience, among the four requirements described above, two are more important, i.e., more than 3/4 of bronchopulmonary segments perfused by central pulmonary arteries after unifocalization, and the result of the flow study. SaO2 and Nakata index, on the other hand, are less so. In total, there were three early deaths and three late deaths after single-stage complete repair, all with the RV/LV pressure ratio ≥0.8. Of note, the three early deaths following single-stage complete repair occurred before 2016. The statistical result showing single-stage complete repair as a risk factor is not applicable to later era.
In the entire cohort, the 1-, 5-, and 10-year survival rates were 89.5, 85.2, and 76.1%, respectively. The 1- and 5-year survival rates after complete repair were 88.2 and 76.6%, respectively. The results are not optimal, nonetheless acceptable when compared to many of the previous reports (1, 2, 8, 16–18). As described above, some progressions were made since 2016, including the implementation of the pulmonary arterial flow study to predict VSD closure and more strict criteria for complete VSD closure in 2016, the availability of valved conduit in 2017, and implementation of follow-up cardiovascular angiography and balloon dilation in 2019. As shown in our data, mortality rate significantly reduced from 35.0% in 2010–2015 to 6.7% since 2016.
Limitations
Our study has several limitations. (1) This is a single-centered study. Our center has the most cases of PA/VSD/MAPCAs in China, thus, our results do not represent the overall practice and outcomes in China as there is large disparity of patient populations and treatment strategies among centers. (2) The 10-year study period represents our learning course whereby the surgical strategies were continuously evolved. (3) The follow-up and catheterization protocol that is still being refined.
Conclusion
Using an approach tailored to the distinctive patient characteristics in China, about two-thirds of PA/VSD/MAPCAs patients achieved complete repair with satisfactory early and intermediate survival rates that, although suboptimal, are acceptable compared to many of the previous results, despite of older age, and higher RV/LV pressure ratio after complete repair. Refinements and improvements in perioperative and follow-up management are warranted to further improve clinical outcomes of this particularly complex group of patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further, inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Guangzhou Women and Children’s Medical Center. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
M-HZ: conceptualization, data curation, investigation, and writing - review & editing. LM: conceptualization, data curation, and writing - review & editing. Y-QC: data curation, formal analysis, and investigation. H-ZW: data curation and statistical analysis. W-LL: writing - original draft. JL: conceptualization and writing - review & editing. X-XC: conceptualization, investigation, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81800280), Key-Area Research and Development Program of Guangdong Province (2019B020227001), and High-Level Clinical Key Subjects Construction Project of Guangzhou City (011102-02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ASD, Atrial septal defect; LV, Left ventricle; PA/VSD/MAPCAs, ulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries; RV, Right ventricle; RV-PA, Right ventricle to pulmonary artery; RVOT, Right ventricular outflow tract; TNPAI, Total neopulmonary artery index.
References
1. Bauser-Heaton H, Borquez A, Han B, Ladd M, Asija R, Downey L, et al. Programmatic approach to management of tetralogy of fallot with major aortopulmonary collateral arteries: a 15-year experience with 458 patients. Circ Cardiovasc Interv. (2017) 10:e004952. doi: 10.1161/CIRCINTERVENTIONS.116.004952
2. Soquet J, Liava’a M, Eastaugh L, Konstantinov IE, Brink J, Brizard CP, et al. Achievements and limitations of a strategy of rehabilitation of native pulmonary vessels in pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Ann Thorac Surg. (2017) 103:1519–26. doi: 10.1016/j.athoracsur.2016.08.113
3. Zhang Y, Hua Z, Yang K, Zhang H, Yan J, Wang X, et al. Outcomes of the rehabilitative procedure for patients with pulmonary atresia, ventricular septal defect, and hypoplastic pulmonary arteries beyond the infant period. Eur J Cardiothorac Surg. (2014) 46:297–303; discussion 03. doi: 10.1093/ejcts/ezt622
4. Fouilloux V, Bonello B, Kammache I, Fraisse A, Mace L, Kreitmann B. Management of patients with pulmonary atresia, ventricular septal defect, hypoplastic pulmonary arteries and major aorto-pulmonary collaterals: focus on the strategy of rehabilitation of the native pulmonary arteries. Arch Cardiovasc Dis. (2012) 105:666–75. doi: 10.1016/j.acvd.2012.08.003
5. Fang M, Wang H, Jin Y, Wang Z, Wang Z, Zhang C. Development of pulmonary arteries after a central end-to-side shunt in patients with pulmonary atresia, ventricular septal defect, and diminutive pulmonary arteries. Thorac Cardiovasc Surg. (2014) 62:211–5. doi: 10.1055/s-0033-1354247
6. Ma M, Mainwaring RD, Hanley FL. Comprehensive management of major aortopulmonary collaterals in the repair of tetralogy of fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2018) 21:75–82. doi: 10.1053/j.pcsu.2017.11.002
7. Soquet J, Barron DJ, d’Udekem Y. A review of the management of pulmonary atresia, ventricular septal defect, and major aortopulmonary collateral arteries. Ann Thorac Surg. (2019) 108:601–12. doi: 10.1016/j.athoracsur.2019.01.046
8. Davies B, Mussa S, Davies P, Stickley J, Jones TJ, Barron DJ, et al. Unifocalization of major aortopulmonary collateral arteries in pulmonary atresia with ventricular septal defect is essential to achieve excellent outcomes irrespective of native pulmonary artery morphology. J Thorac Cardiovasc Surg. (2009) 138:1269–75.e1. doi: 10.1016/j.jtcvs.2009.08.011
9. Reddy VM, Petrossian E, McElhinney DB, Moore P, Teitel DF, Hanley FL. One-stage complete unifocalization in infants: when should the ventricular septal defect be closed? J Thorac Cardiovasc Surg. (1997) 113:858–66; discussion 66–8. doi: 10.1016/S0022-5223(97)70258-7
10. Zheng S, Yang K, Li K, Li S. Establishment of right ventricle-pulmonary artery continuity as the first-stage palliation in older infants with pulmonary atresia with ventricular septal defect may be preferable to use of an arterial shunt. Interact Cardiovasc Thorac Surg. (2014) 19:88–94. doi: 10.1093/icvts/ivu052
11. Amark KM, Karamlou T, O’Carroll A, MacDonald C, Freedom RM, Yoo SJ, et al. Independent factors associated with mortality, reintervention, and achievement of complete repair in children with pulmonary atresia with ventricular septal defect. J Am Coll Cardiol. (2006) 47:1448–56. doi: 10.1016/j.jacc.2005.10.068
12. Hibino N, He D, Yuan F, Yu JH, Jonas R. Growth of diminutive central pulmonary arteries after right ventricle to pulmonary artery homograft implantation. Ann Thorac Surg. (2014) 97:2129–33. doi: 10.1016/j.athoracsur.2013.10.046
13. Dragulescu A, Kammache I, Fouilloux V, Amedro P, Metras D, Kreitmann B, et al. Long-term results of pulmonary artery rehabilitation in patients with pulmonary atresia, ventricular septal defect, pulmonary artery hypoplasia, and major aortopulmonary collaterals. J Thorac Cardiovasc Surg. (2011) 142:1374–80. doi: 10.1016/j.jtcvs.2011.05.010
14. Lenoir M, Pontailler M, Gaudin R, Gerelli S, Tamisier D, Bonnet D, et al. Outcomes of palliative right ventricle to pulmonary artery connection for pulmonary atresia with ventricular septal defect. Eur J Cardiothorac Surg. (2017) 52:590–8. doi: 10.1093/ejcts/ezx194
15. Carrillo SA, Mainwaring RD, Patrick WL, Bauser-Heaton HD, Peng L, Reddy VM, et al. Surgical repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals with absent intrapericardial pulmonary arteries. Ann Thorac Surg. (2015) 100:606–14. doi: 10.1016/j.athoracsur.2015.03.110
16. Barron DJ, Kutty RS, Stickley J, Stumper O, Botha P, Khan NE, et al. Unifocalization cannot rely exclusively on native pulmonary arteries: the importance of recruitment of major aortopulmonary collaterals in 249 cases. Eur J Cardiothorac Surg. (2019) 56:679–87. doi: 10.1093/ejcts/ezz070
17. Brawn WJ, Jones T, Davies B, Barron D. How we manage patients with major aorta pulmonary collaterals. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2009) 12:152–7. doi: 10.1053/j.pcsu.2009.01.018
Keywords: pulmonary atresia, ventricular septal defect, major aortopulmonary collateral arteries, unifocalization, rehabilitation
Citation: Zou M-H, Ma L, Cui Y-Q, Wang H-Z, Li W-L, Li J and Chen X-X (2021) Outcomes After Repair of Pulmonary Atresia With Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries: A Tailored Approach in a Developing Setting. Front. Cardiovasc. Med. 8:665038. doi: 10.3389/fcvm.2021.665038
Received: 06 February 2021; Accepted: 16 March 2021;
Published: 14 April 2021.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Xiangming Fan, Capital Medical University, ChinaAdriano Carotti, Bambino Gesù Children Hospital (IRCCS), Italy
Copyright © 2021 Zou, Ma, Cui, Wang, Li, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Xin Chen, emluZ2VyY2hlbkAxNjMuY29t
†These authors have contributed equally to this work
 Ming-Hui Zou
Ming-Hui Zou Li Ma1,2†
Li Ma1,2†