- 1Department of Endocrinology, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Endocrine and Metabolic Diseases, Shanghai Clinical Center for Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Institute of Endocrinology and Metabolism, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Shanghai Key Laboratory of Children's Digestion and Nutrition, Department of Gastroenterology, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Aims: To evaluate the prospective association of circulating PCSK9 levels with the cardiometabolic risk profiles (high LDL-cholesterol, high triglycerides, low HDL-cholesterol, hypertension, type 2 diabetes, and metabolic syndrome).
Methods: A population-based prospective study was conducted among 7,104 Chinese individuals (age 56.2 ± 7.5 years; 32.0% men). Circulating PCSK9 levels were measured using ELISA.
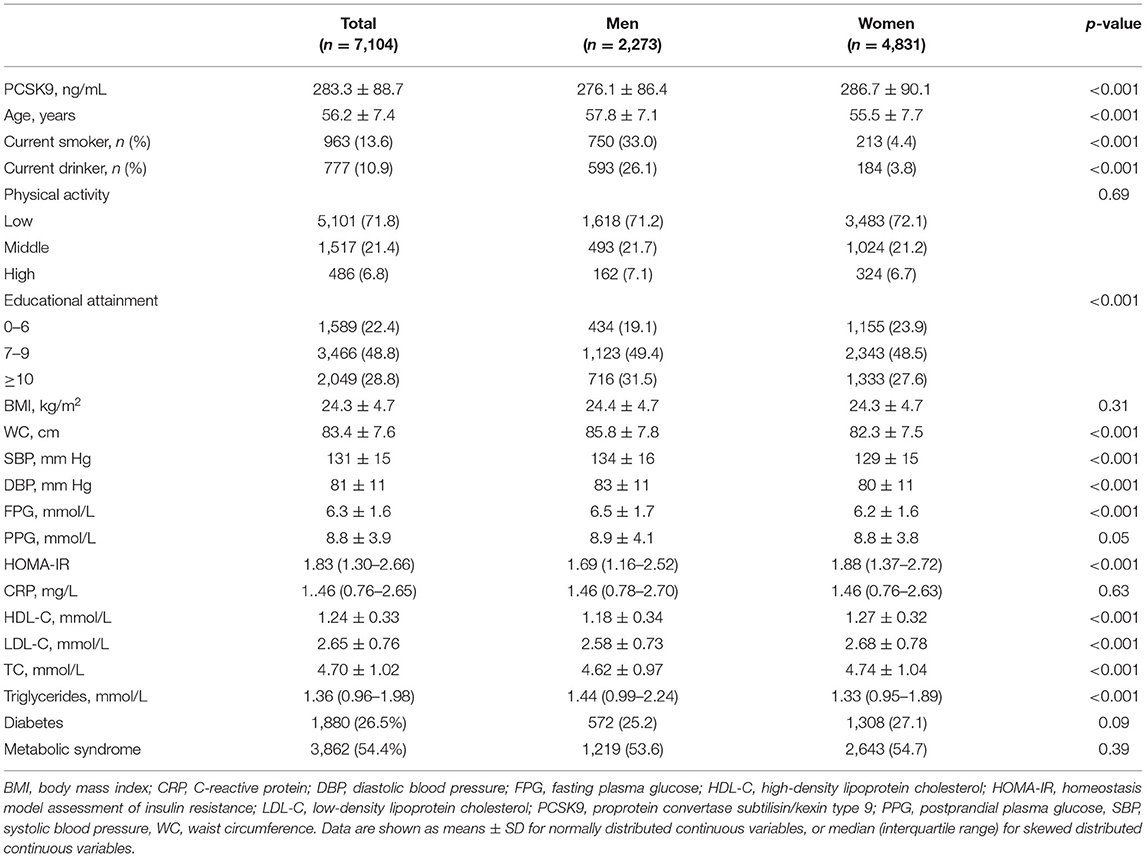
Results: Circulating PCSK9 levels were higher in women than men (286.7 ± 90.1 vs. 276.1 ± 86.4 ng/ml, p < 0.001). And circulating PCSK9 was positively correlated with LDL-cholesterol, total cholesterol, and triglycerides both in men and women (all p < 0.001). The positive correlation between PCSK9 and waist circumference, fasting glucose, insulin resistance, systolic blood pressure, diastolic blood pressure and C-reactive protein (all p < 0.01) was observed in women only. According to Cox regression analysis, circulating PCSK9 was positively associated with incidence of high LDL-cholesterol both in men (HR 1.33, 95% CI 1.09–1.65, p < 0.001) and women (HR 1.36, 95% CI 1.12–1.69, p < 0.001). Moreover, PCSK9 was significantly associated with incident high triglycerides (HR 1.31, 95% CI 1.13–1.72, p < 0.001), hypertension (HR 1.28, 95% CI 1.08–1.53, p = 0.011), type 2 diabetes (HR 1.34, 95% CI 1.09–1.76, p = 0.005), and metabolic syndrome (HR 1.30, 95% CI 1.11–1.65, p = 0.009) per SD change in women only. No statistically significant association was observed between circulating PCSK9 and incidence of low HDL-cholesterol (p > 0.1).
Conclusions: Elevated circulating PCSK9 was significantly associated with cardiometabolic risk factors and independently contributed to the prediction of cardiometabolic risks in women.
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), the ninth member of the proprotein convertase family, is a key lipid metabolic regulator (1, 2). It is reported that PCSK9 is a serine protease produced primarily by the liver and the intestine, but only the liver releases it into circulation (3). This protease regulates cholesterol homeostasis by promoting degradation of low-density lipoprotein (LDL) receptor, the major route of clearance of circulating LDL-cholesterol, through an endosomal/lysosomal pathway (4). Subsequently, reduced LDL receptor levels resulted in impaired clearance of LDL-cholesterol, leading to hypercholesterolemia and metabolic disorders. Genetic studies have shown that gain-of-function mutations of the PCSK9 gene caused hypercholesterolemia (5), whereas loss-of-function mutations in PCSK9 resulted in hypocholesterolemia and reduced risk of cardiovascular disease (6, 7). On account of the involvement of PCSK9 in the degradation of LDL receptor, this crucial lipid metabolic regulator has recently emerged as new and promising pharmacological targets for the management of atherosclerotic cardiovascular disease. Indeed, treatment targeted at PCSK9 inhibition markedly reduced atherogenic lipoproteins and confers additional cardiovascular benefit beyond that achieved by lipid-lowering treatment alone (8–10).
Although the best-described impact of PCSK9 is on the levels of LDL-cholesterol, data from epidemiological studies have shown that PCSK9 is also associated with certain features of cardiometabolic profiles including high-density lipoprotein (HDL) cholesterol, triglycerides, blood pressure, and fasting glucose, as well as insulin resistance (11–13). However, to date, evidence from prospective study about the relationship between circulating PCSK9 and incidence of cardiometabolic risk factors is scarce.
To further evaluate whether PCSK9 contributes to the cluster of cardiometabolic risk factors involved in atherosclerotic cardiovascular diseases, we investigated prospectively the incidence of these metabolic abnormalities in relation to baseline PCSK9 levels in a large-scale Chinese population.
Materials and Methods
Study Population
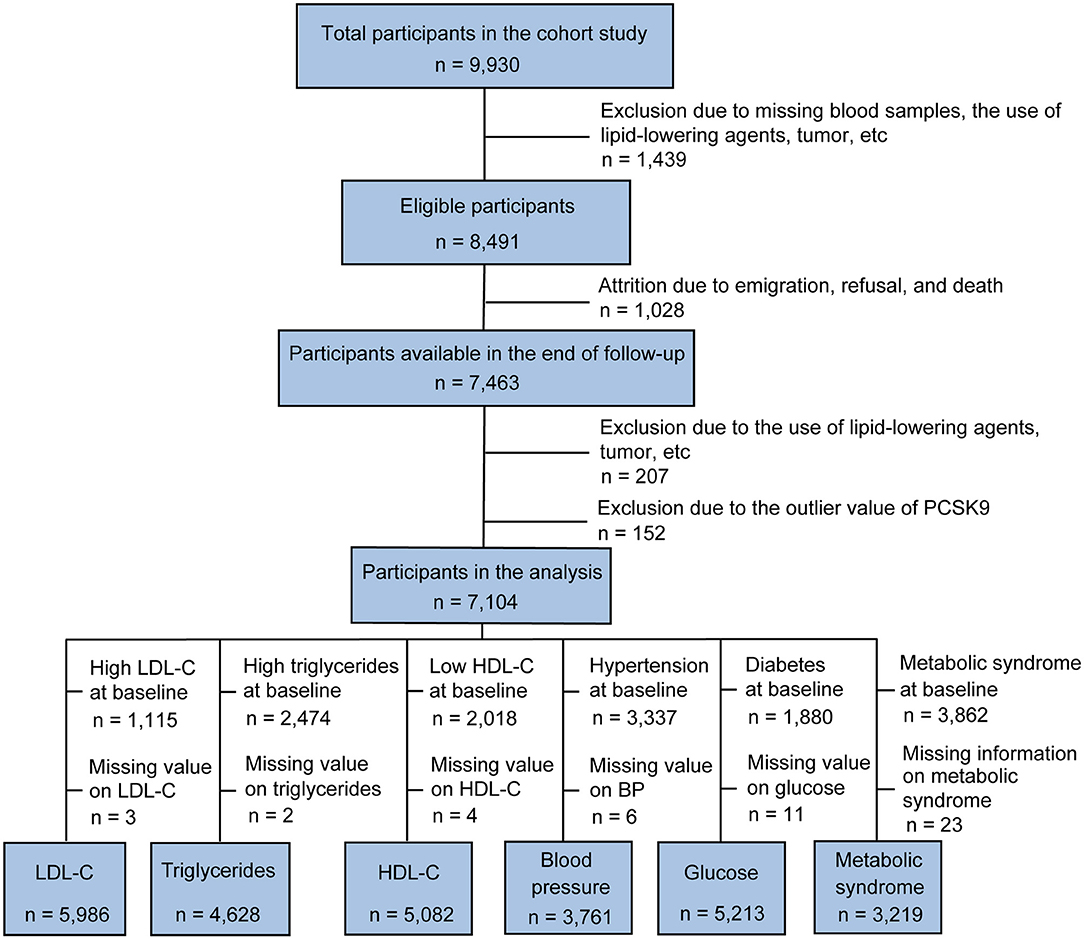
Subjects were recruited from the China Cardiometabolic Disease and Cancer Cohort (4C) Study, a community-based study conducted among 259,657 Chinese individuals aged 40 years and older (14, 15). The 4C study was performed in 25 communities across mainland China. The study design and methods have been described previously in detail (14). The data presented in this article are based on the subsamples from the Chongming District, Shanghai, China. From May to November 2011, a total of 9,930 subjects (40–73 years of age) participated in the baseline survey. In 2014, participants returned for a 3.1-year follow-up investigation. Individuals with the following conditions were excluded from this study: missing blood samples, the use of lipid-lowering agents (such as statins, fibrates, and natural products), tumor, infectious or systematic inflammatory diseases, significant hematologic disorders, thyroid dysfunction, severe liver and/or renal insufficiency, and PCSK9 outliers, which were defined as the extreme value (lower or upper 1% of the distribution) or repeated measurements coefficient of variation >15%, resulting in the inclusion of 7,104 participants (2,273 men and 4,831 women) in the analysis (Figure 1).
The study protocol was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all participants.
Data Collection
A standardized questionnaire was used by trained physicians to collect essential information, including age, sex, lifestyle factors, educational attainment, physical activity, and previous medical history. Physical activity was evaluated based on the short form of the International Physical Activity Questionnaire by adding questions regarding the frequency and duration of moderate and vigorous activities and walking [Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)]. Current smokers or drinkers were defined as subjects who had a regular smoking or drinking status in the past 6 months. Anthropometric measurements, including height, weight, and waist circumference, were collected by certified medical staff using standard protocols. Blood pressure was measured with an automated electronic device (OMRON Model1 Plus; Omron Company, Kyoto, Japan).
Laboratory Measurements
Venous blood samples were obtained after overnight fasting for at least 10 h and were collected in tubes containing EDTA. The blood samples were centrifuged at 4°C and stored at −80°C until analysis. Circulating LDL-cholesterol, HDL-cholesterol, total cholesterol, and triglycerides, were measured with an autoanalyzer (Hitachi 7080; Tokyo, Japan). Venous plasma glucose level was determined by the glucose oxidase method (ADVIA-1650 Chemistry System, Bayer, Leverkusen, Germany). Fasting insulin was measured by RIA (Linco Research, St. Charles, MO). The circulating C-reactive protein was determined by ELISA kit (DY1707, R&D Systems, Minneapolis, MN) as recommended by the manufacturer.
PCSK9 Measurement
The circulating PCSK9 levels were measured in duplicate using a commercial ELISA kit (DY3888, R&D Systems, Minneapolis, MN) according to the manufacturer's instructions and compared with purified human PCSK9 standards.
Definition of Cardiometabolic Risk Factors
A low HDL-cholesterol was defined as HDL-cholesterol <1.0 mmol/L in men or <1.3 mmol/L in women. A high LDL-cholesterol was defined as LDL-cholesterol ≥3.37 mmol/L. A high triglyceride was defined as triglyceride ≥1.7 mmol/L or treatment with a lipid-lowering medication. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or current use of antihypertensive treatment. Incident type 2 diabetes was diagnosed according to the American Diabetes Association 2010 criteria, which is defined as an FPG ≥ 7.0 mmol/L (≥126 mg/dL), 2-h PPG ≥ 11.1 mmol/L (≥ 200 mg/dL), or HbA1c ≥ 6.5% (48 mmol/mol). The metabolic syndrome was defined based on the Joint Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity (16).
Statistical Analysis
The continuous variables with normal distribution are shown as means ± SD, and variables with skewed distribution are expressed as medians (interquartile range) and log-transformed to approximate normality before analysis. Categorical variables are reported as a percentage (%). The subjects were divided into two groups according to sex. For comparisons between groups, we performed an unpaired independent-samples Student t-test for normally distributed continuous variables and a non-parametric Mann-Whitney U test for skewed distributed variables. The Chi-squared tests were applied to compare categorical variables. The Pearson correlation analysis was used to evaluated correlation coefficients between baseline circulating PCSK9 levels and metabolic parameters. Multivariate Cox regression analysis was run to determine the potential association between baseline PCSK9 levels and incidence of cardiometabolic risk factors. We also used restricted cubic splines with five knots at the 5th, 35th, 50th, 65th, and 95th centiles to flexibly model exploring the association of circulating PCSK9 levels on a continuous scale with the incidence of cardiometabolic risk factors. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between PCSK9 and the incidence of cardiometabolic risk factors were generated with the Cox proportional hazards model. In order to minimize the effect of potential confounding factors, covariates were selected based on biologic interest, well-established risk factors for cardiometabolic diseases, or associated exposures and outcomes. Variables showing p < 0.05 in the univariable regression model were entered into the multivariable model. Multivariable adjusted models were used to explore the independent effect of PCSK9 on cardiometabolic risk factors. Data management and statistical analyses were performed with SPSS software (version 25.0) and R version 3.6.1. The significance level was set at p < 0.05, and p-values were provided for two-sided tests.
Results
Baseline Characteristics of Subjects
Table 1 summarizes the baseline characteristics of 7,104 participants who were stratified according to sex. The mean age of the subjects was 56.2 ± 7.5 years, and 32.0% of the participants were males. Circulating PCSK9 levels were higher in women than men (286.7 ± 90.1 ng/mL vs. 276.1 ± 86.4 ng/mL, p < 0.001).
Correlation of Baseline Circulating PCSK9 Levels With Clinical Characteristics
According to the Pearson correlation analysis, circulating PCSK9 levels were significantly and positively correlated with LDL-cholesterol, total cholesterol, and triglycerides (all p < 0.001) both in men and women (Table 2). Moreover, a positive correlation was observed between circulating PCSK9 and age, BMI, waist circumference, fasting plasma glucose, systolic and diastolic blood pressure, HOMA-IR, and C-reactive protein (all p < 0.01) in women only.
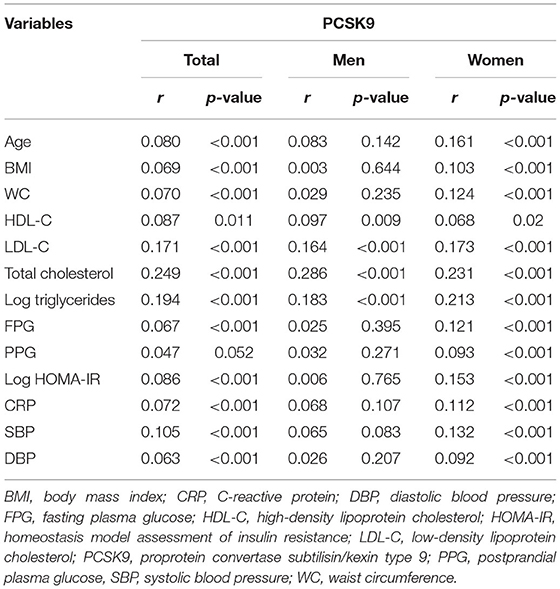
Table 2. Pearson correlation analysis between baseline circulating PCSK9 and clinical characteristics.
Association Between Baseline Circulating PCSK9 Levels and Incidence of Cardiometabolic Risk Factors
Table 3 presents the hazard ratios (HR) for categorized cardiometabolic risk factors per 1-SD increment in baseline circulating PCSK9. Circulating PCSK9 was positively associated with incidence of high LDL-cholesterol both in men (HR 1.33, 95% CI 1.09–1.65, p < 0.001) and women (HR 1.36, 95% CI 1.12–1.69, p < 0.001) after adjustment for age, current smoking status, alcohol consumption, physical activity, educational attainment, BMI, waist circumference, C-reactive protein, fasting plasma glucose, post-loading plasma glucose, HOMA-IR, systolic blood pressure, diastolic blood pressure, and lipid profiles. Furthermore, a positive association was observed between circulating PCSK9 with incidence of high triglycerides (HR 1.31, 95% CI 1.13–1.72, p < 0.001), hypertension (HR 1.28, 95% CI 1.08–1.53, p = 0.011), type 2 diabetes (HR 1.34, 95% CI 1.09–1.76, p = 0.005), and metabolic syndrome (HR 1.30, 95% CI 1.11–1.65, p = 0.009) in women only (Figure 2). And the positive linear dose-response relationship was evident in the cubic spline regression model (Figure 3, p for non-linearity >0.1). No statistically significant association was observed between circulating PCSK9 and incidence of low HDL-C (p > 0.1) both in men and women.
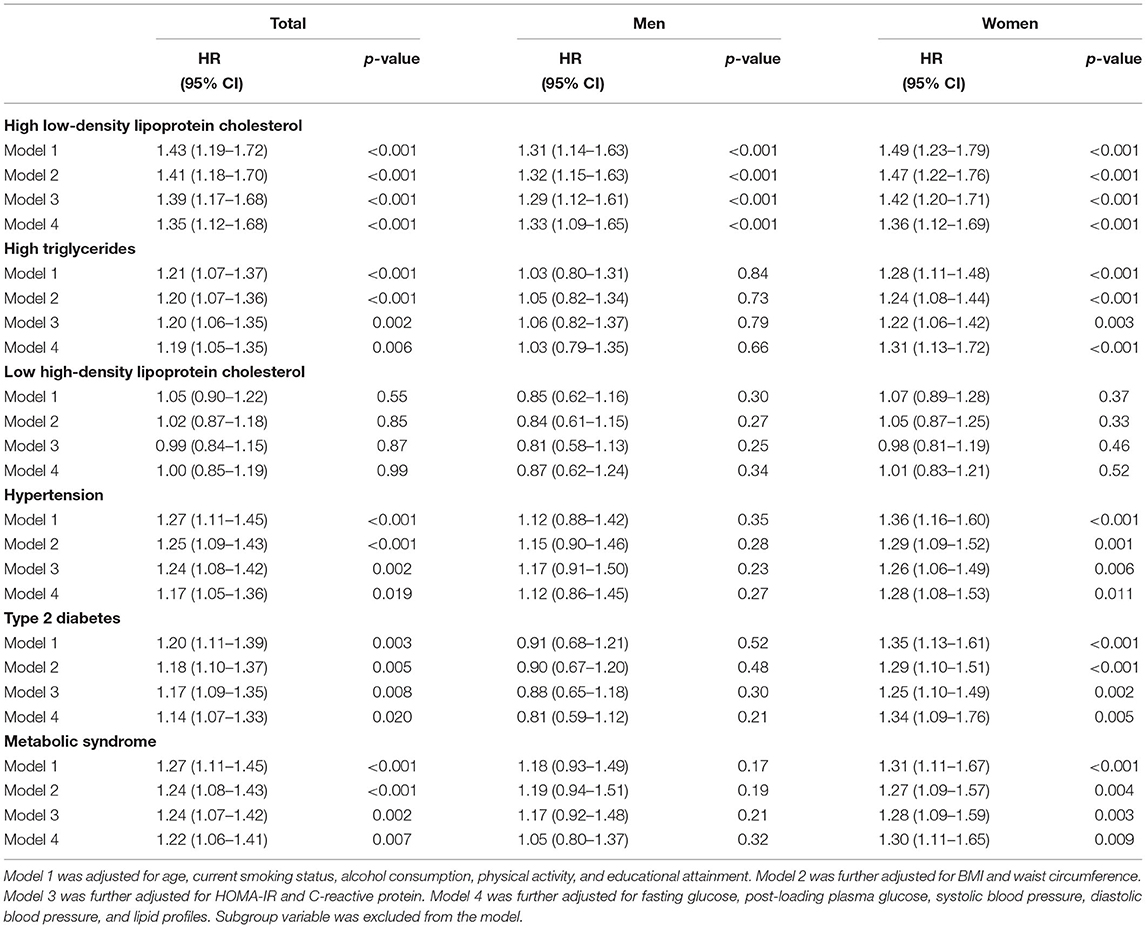
Table 3. Multivariable Cox regression of cardiometabolic risk factors on per 1-SD increment in PCSK9.
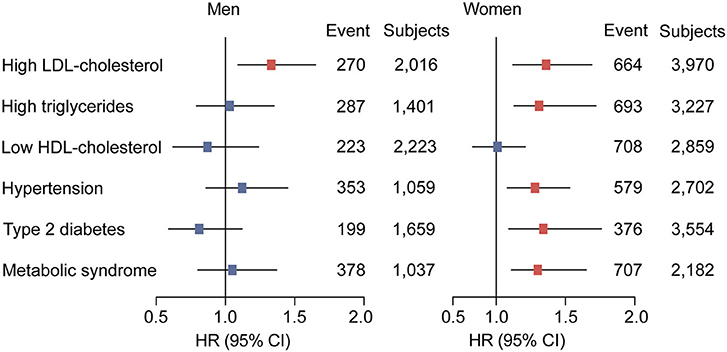
Figure 2. Adjusted hazard ratios (HRs) of cardiometabolic risk factors according to per 1-SD increment in baseline circulating PCSK9 levels in men and women. Model was adjusted for age, current smoking status, alcohol consumption, physical activity, educational attainment, BMI, waist circumference, C-reactive protein, fasting plasma glucose, post-loading plasma glucose, HOMA-IR, systolic blood pressure, diastolic blood pressure, and lipid profiles. Subgroup variable was excluded from the model.
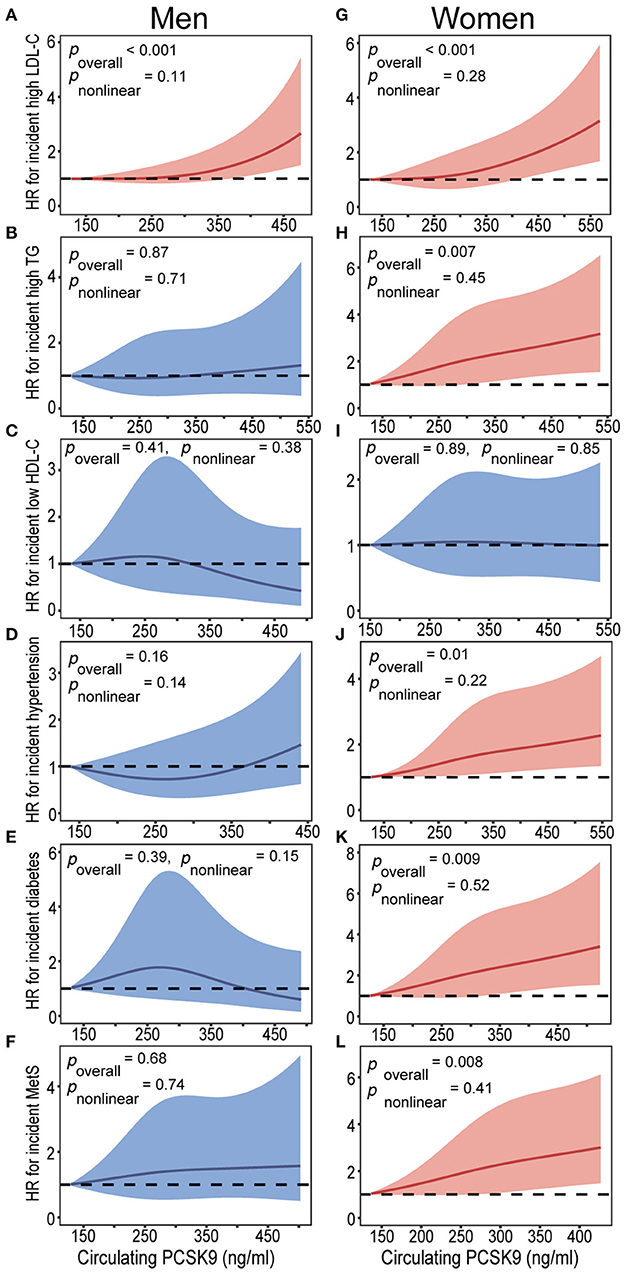
Figure 3. Baseline circulating PCSK9 levels on a continuous scale and incidence of cardiometabolic risk factors in men (A-F) and women (G-L). Cardiometabolic risk factors including high LDL-C (A,G), high triglycerides (B,H), low HDL-C (C,I), hypertension (D,J), type 2 diabetes (E,K), and metabolic syndrome (F,L). Hazard ratios (HRs) are indicated by solid lines and 95% confidence intervals (CIs) by shaded areas. Model was adjusted for age, current smoking status, alcohol consumption, physical activity, educational attainment, BMI, waist circumference, C-reactive protein, fasting plasma glucose, post-loading plasma glucose, HOMA-IR, systolic blood pressure, diastolic blood pressure, and lipid profiles. Subgroup variable was excluded from the model. LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; PCSK9, proprotein convertase subtilisin/kexin type 9.
Discussion
In the present study, we found that subjects with higher circulating PCSK9 had progressively worse cardiometabolic risk profiles in women, including incident high LDL-cholesterol, elevated triglycerides, hypertension, type 2 diabetes, and metabolic syndrome. However, no significant relationship was observed between PCSK9 and cardiometabolic risk factors in males, except incidence of high LDL-cholesterol. To the best of our knowledge, this is the first prospective cohort study to investigate the association between PCSK9 and incidence of cardiometabolic risk factors.
The key and predominantly characterized activity of secreted PCSK9 is enhancing the degradation of the LDL receptor (17), a principal endocytic receptor that mediates the clearance and catabolism of LDL (18). As a consequence of reduced LDL clearance, circulating levels of LDL-cholesterol increasing, which is a well-established risk factor of cardiovascular disease. Moreover, multiple studies reported that PCSK9 is also involved in the degradation of very low-density lipoprotein receptor, apolipoprotein E receptor 2 (ApoER2), LDLR-related protein-1 (LRP1), and fatty acid transporter CD36 (19, 20). Additionally, the human evidence of the PCSK9 (S127R) gain-of-function mutation genetic study revealed that PCSK9 dramatically increased the production rate of apoB (21). Then the effect on apoB resulted in an overproduction of very low-density lipoprotein, intermediate-density lipoprotein, and LDL. Therefore, the observed positive correlation between PCSK9 and intermediate-density lipoprotein, which is the triglyceride-rich LDL subfraction (22), raises the possibility of a significant contribution of intermediate-density lipoprotein to the positive relationship between PCSK9 and triglycerides. Furthermore, PCSK9 increases intestinal triglyceride-rich lipoprotein production and secretion through transcriptional and post-transcriptional mechanisms and then enhances triglycerides accumulation by targeting the intermediate-density lipoprotein receptor in the adipose tissue (23). No significant association was observed between PCSK9 and low HDL-cholesterol, thus, it is reasonable to speculate that PCSK9 may associate with atherosclerotic cardiovascular disease through a pathway not fully overlapping with reduced HDL-cholesterol.
The existence of a significant correlation between triglycerides and glucose metabolism has supported the investigation of a possible involvement of PCSK9 in glucose homeostasis and insulin resistance. In accordance with the previous findings (11, 13), in our female population circulating PCSK9 is positively associated with fasting glucose and insulin resistance, which involved in the initiation and progression of cardiometabolic disease. Previous study also found that high serum PCSK9 is associated with increased risk of new-onset diabetes after transplantation in renal transplant recipients (24). Notably, several studies showed that LDL-cholesterol-lowering PCSK9 (rs11583680, rs11591147, rs2479409, and rs11206510) genetic variants were associated with higher circulating fasting glucose levels and increased risk of type 2 diabetes (25). However, other studies reported that the loss-of-function PCSK9 (p.R46L) genetic variant was not associated with impaired glucose homeostasis in humans (26) and PCSK9 inhibition did not increase the risk of new-onset diabetes, nor did it worsen glycemia (27, 28). Further study is necessary to shed lights on the underlying mechanism of the association between PCSK9 and glucose homeostasis.
A positive correlation was observed between baseline circulating PCSK9 levels and blood pressure in females, which is in line with data obtained in other cross-sectional researches (11, 12). Genetic evidence from the Hypertension Genetic Epidemiology Network (HyperGEN) and the Reasons for Geographic And Racial Differences in Stroke study (REGARDS) studies have shown that PCSK9 variation associated with blood pressure in African Americans (29). However, other investigation failed to identify a relationship between PCSK9 and blood pressure (30). Such an inconsistent association may be explained in part by a limited sample size and cross-sectional study design.
In line with the previous reports (11, 12, 24), we found PCSK9 was positively correlated with both BMI and waist circumference in females, suggesting that PCSK9 may be associated with obesity, especially visceral adiposity, the most prevalent features of the cardiovascular disease. Nevertheless, only a minor reduction of the cardiometabolic risk was yielded with per 1-SD increment in PCSK9 after further adjustment for BMI and waist circumference. Consequently, relationship between circulating PCSK9 and cardiometabolic risk factors in women may not be merely the link of excess adipose tissue.
The most striking finding in the present study was the positive relationship between circulating PCSK9 levels and incidence of high triglycerides, hypertension, type 2 diabetes, and metabolic syndrome only in female subjects. Consistent with the previous studies, correlations between PCSK9 and a variety of clinical characteristics, including age, BMI, LDL-cholesterol, triglycerides, glucose, blood pressure, and C-reactive protein, were either stronger or only present in females (11, 12, 31). Furthermore, evidence from small-scale research (aged 15–26 years) found that obesity and type 2 diabetes were associated with significantly higher levels of PCSK9 in America young women, but not in young men (31). In humans, many studies described circulating PCSK9 levels as being significantly higher in women than men, but differences have also been found in postmenopausal compared to premenopausal women as well as in pregnant compared to non-pregnant women. Therefore, it is reasonable to speculate a possible role for sex hormones in PCSK9 synthesis and/or metabolism. According to evidence from animal and human studies, PCSK9 is regulated by hormones such as estrogen, glucagon, insulin, growth hormone, and thyroid hormone (32). Previous studies reported that circulating PCSK9 concentration are decreased when endogenous estrogens are high in women (33), and pharmacologically elevated estrogen levels have been shown to lower PCSK9 levels both in animals and humans (34, 35). Conversely, several studies indicated that the difference in circulating PCSK9 levels between postmenopausal and premenopausal women appears to be independent of estrogen status (11), and estrogen at physiological concentrations does not affect hepatocyte PCSK9 expression in human (36). Overall, the clear molecular mechanisms underlying the sex differences remain unclear. More intensive investigations are needed to clarify the effect of gender on the association between PCSK9 and a variety of cardiometabolic abnormalities.
There are several limitations of this study. First, the use of a single baseline PCSK9 measurement to predict outcomes was a simplified and practical approach but was unable to assess the relationship between changes in PCSK9 levels and the incidence of metabolic disorders. Second, we did not record the type of diabetes among incident cases, but given that all the participants in our study were at least 40 years old at enrollment, these participants were unlikely to have type 1 diabetes because the fasting glucose was only mildly elevated and insulin levels were high normal. Third, due to the observational nature of the study, we cannot draw the causality from our findings. Thus, further studies are necessary to clarify the underlying mechanisms behind the association of PCSK9 with cardiometabolic dysregulation and to determine whether high PCSK9 level is a cause or a consequence of cardiometabolic abnormalities, or both. Forth, it is not clear whether our findings in middle-aged and elderly Chinese female individuals can be generalized to younger populations or individuals of other ethnicities. In our study, men and women are significantly different for most evaluated parameters. Therefore, we failed to assess its potential impact on differences in results between the two groups. Although we have adjusted for almost all potential confounding factors in the regression models. Further research should be undertaken among diverse groups and to shed lights on the effects of sex on associations of PCSK9 with cardiometabolic risk factors. Fifth, loss or gain of function of PCSK9 gene mutations may have had potential impacts on the statistical results. However, due to the study design, we failed to analyze the PCSK9 mutations status in present study.
In summary, our results indicate that subjects with higher circulating PCSK9 had progressively worse cardiometabolic risk profiles in women. These findings highlight the importance of elevated circulating PCSK9 levels in the development of atherosclerotic cardiovascular disease in female individuals. Moreover, based on our study, elevated circulating PCSK9 levels may emerge as novel target of efficacious prevention of cardiovascular disease in women.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JS, XL, and WZ drafted the manuscript and performed the experiments. ZY, QS, LQ, and GN conceived and designed the study. WZ, YN, JF, HZ, and NL recruited the subjects, processed samples, and contributed to the acquisition of data. JS, XL, WZ, YN, and ZY analyzed the data. ZY revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81970669, 81873565, 81670743, and 81370953), the National Key Research and Development Plan Precision Medicine Research (2017YFSF090203), the Shanghai Health System Outstanding Young Talents Training Program (XYQ2013098), the Shanghai Sailing Program (18YF1415800), and the Shanghai Health and Family Planning Commission (21740173).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge the support of the study participants, study staff, and partner organizations participating in the study.
Abbreviations
BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; HR, hazard ratio; LDL, low-density lipoprotein; PCSK9, proprotein convertase subtilisin/kexin type 9; PPG, postprandial plasma glucose; SBP, systolic blood pressure; SD, standard deviation; TG, triglycerides.
References
1. Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. (2003) 100:928–33. doi: 10.1073/pnas.0335507100
2. Seidah NG, Abifadel M, Prost S, Boileau C, Prat A. The proprotein convertases in hypercholesterolemia and cardiovascular diseases: emphasis on proprotein convertase subtilisin/kexin 9. Pharmacol Rev. (2017) 69:33–52. doi: 10.1124/pr.116.012989
3. Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. (2008) 48:646–54. doi: 10.1002/hep.22354
4. Zhang D-W, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. (2007) 282:18602–12. doi: 10.1074/jbc.M702027200
5. Abifadel M, Varret M, Rabès J-P, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. (2003) 34:154–6. doi: 10.1038/ng1161
6. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. (2005) 37:161–5. doi: 10.1038/ng1509
7. Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. (2014) 114:1022–36. doi: 10.1161/CIRCRESAHA.114.301621
8. Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. (2012) 366:1108–18. doi: 10.1056/NEJMoa1105803
9. Dullaart RPF. PCSK9 inhibition to reduce cardiovascular events. N Engl J Med. (2017) 376:1790–1. doi: 10.1056/NEJMe1703138
10. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
11. Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. (2009) 94:2537–43. doi: 10.1210/jc.2009-0141
12. Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Q, et al. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. (2010) 213:632–6. doi: 10.1016/j.atherosclerosis.2010.09.027
13. Baass A, Dubuc G, Tremblay M, Delvin EE, O'Loughlin J, Levy E, et al. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. (2009) 55:1637–45. doi: 10.1373/clinchem.2009.126987
14. Lu J, He J, Li M, Tang X, Hu R, Shi L, et al. Predictive value of fasting glucose, postload glucose, and hemoglobin a on risk of diabetes and complications in Chinese adults. Diabetes Care. (2019) 42:1539–48. doi: 10.2337/dc18-1390
15. Wang T, Lu J, Su Q, Chen Y, Bi Y, Mu Y, et al. Ideal Cardiovascular health metrics and major cardiovascular events in patients with prediabetes and diabetes. JAMA Cardiol. (2019) 4:874–83. doi: 10.1001/jamacardio.2019.2499
16. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
17. Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. (2007) 32:71–7. doi: 10.1016/j.tibs.2006.12.008
18. Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. (1986) 232:34–47. doi: 10.1126/science.3513311
19. Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N, et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. (2008) 283:2363–2372. doi: 10.1074/jbc.M708098200
20. Demers A, Samami S, Lauzier B, Des Rosiers C, Ngo Sock ET, Ong H, et al. PCSK9 induces CD36 degradation and affects long-chain fatty acid uptake and triglyceride metabolism in adipocytes and in mouse liver. Arterioscler Thromb Vasc Biol. (2015) 35:2517–25. doi: 10.1161/ATVBAHA.115.306032
21. Ouguerram K, Chetiveaux M, Zair Y, Costet P, Abifadel M, Varret M, et al. Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler Thromb Vasc Biol. (2004) 24:1448–53. doi: 10.1161/01.ATV.0000133684.77013.88
22. Kwakernaak AJ, Lambert G, Dullaart RPF. Plasma proprotein convertase subtilisin-kexin type 9 is predominantly related to intermediate density lipoproteins. Clin Biochem. (2014) 47:679–82. doi: 10.1016/j.clinbiochem.2014.03.008
23. Baragetti A, Grejtakova D, Casula M, Olmastroni E, Jotti GS, Norata GD, et al. Proprotein Convertase Subtilisin-Kexin type-9 (PCSK9) and triglyceride-rich lipoprotein metabolism: facts and gaps. Pharmacol Res. (2018) 130:1–11. doi: 10.1016/j.phrs.2018.01.025
24. Eisenga MF, Zelle DM, Sloan JH, Gaillard C, Bakker SJL, Dullaart RPF. High serum PCSK9 is associated with increased risk of new-onset diabetes after transplantation in renal transplant recipients. Diabetes Care. (2017) 40:894–901. doi: 10.2337/dc16-2258
25. Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst-Hunter Z, Lyall DM, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. (2017) 5:97–105. doi: 10.1016/S2213-8587(16)30396-5
26. Bonnefond A, Yengo L, Le May C, Fumeron F, Marre M, Balkau B, et al. The loss-of-function PCSK9 p.R46L genetic variant does not alter glucose homeostasis. Diabetologia. (2015) 58:2051–5. doi: 10.1007/s00125-015-3659-8
27. Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. (2019) 7:618–28. doi: 10.1016/S2213-8587(19)30158-5
28. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. (2017) 5:941–50. doi: 10.1016/S2213-8587(17)30313-3
29. Tran NT, Aslibekyan S, Tiwari HK, Zhi D, Sung YJ, Hunt SC, et al. PCSK9 variation and association with blood pressure in African Americans: preliminary findings from the HyperGEN and REGARDS studies. Front Genet. (2015) 6:136. doi: 10.3389/fgene.2015.00136
30. Yang S-H, Du Y, Li S, Zhang Y, Xu R-X, Zhu C-G, et al. Plasma PCSK9 level is unrelated to blood pressure and not associated independently with carotid intima-media thickness in hypertensives. Hypertens Res. (2016) 39:598–605. doi: 10.1038/hr.2016.38
31. Levenson AE, Shah AS, Khoury PR, Kimball TR, Urbina EM, de Ferranti SD, et al. Obesity and type 2 diabetes are associated with elevated PCSK9 levels in young women. Pediatr Diabetes. (2017) 18:755–60. doi: 10.1111/pedi.12490
32. Ferri N, Ruscica M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: insights on insulin resistance, inflammation, and atherogenic dyslipidemia. Endocrine. (2016) 54:588–601. doi: 10.1007/s12020-016-0939-0
33. Persson L, Henriksson P, Westerlund E, Hovatta O, Angelin B, Rudling M. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler Thromb Vasc Biol. (2012) 32:810–4. doi: 10.1161/ATVBAHA.111.242461
34. Ghosh M, Gälman C, Rudling M, Angelin B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res. (2015) 56:463–9. doi: 10.1194/jlr.M055780
35. Fu W, Gao X-P, Zhang S, Dai Y-P, Zou W-J, Yue L-M. 17β-Estradiol Inhibits PCSK9-Mediated LDLR Degradation Through GPER/PLC Activation in HepG2 Cells. Front Endocrinol. (2019) 10:930. doi: 10.3389/fendo.2019.00930
Keywords: proprotein convertase subtilisin kexin type 9, dyslipidemia, hypertension, type 2 diabetes, metabolic syndrome, cardiometabolic risk factors
Citation: Shi J, Li X, Zhang W, Niu Y, Lin N, Zhang H, Ning G, Fan J, Qin L, Su Q and Yang Z (2021) Circulating Proprotein Convertase Subtilisin/Kexin Type 9 Levels and Cardiometabolic Risk Factors: A Population-Based Cohort Study. Front. Cardiovasc. Med. 8:664583. doi: 10.3389/fcvm.2021.664583
Received: 05 February 2021; Accepted: 09 April 2021;
Published: 10 May 2021.
Edited by:
Xiongfei Pan, Vanderbilt University Medical Center, United StatesReviewed by:
Angela Pirillo, Università degli Studi di Milano, ItalyRei Shibata, Nagoya University Hospital, Japan
Copyright © 2021 Shi, Li, Zhang, Niu, Lin, Zhang, Ning, Fan, Qin, Su and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Yang, yangzhen@xinhuamed.com.cn; Li Qin, qinli@xinhuamed.com.cn; Qing Su, suqing@xinhuamed.com.cn
†These authors have contributed equally to this work
 Jie Shi
Jie Shi Xiaoyong Li1†
Xiaoyong Li1† Li Qin
Li Qin Qing Su
Qing Su Zhen Yang
Zhen Yang