- 1Division of Cardiovascular Medicine, Department of Medicine, Jichi Medical University, Tochigi, Japan
- 2Jichi Medical University Center of Excellence, Community Medicine Cardiovascular Research and Development, Tochigi, Japan
Background: Disease-related anorexia-cachexia is associated with poor prognosis of patients with cardiovascular disease (CVD) or cancer. Growth differentiation factor-15 (GDF-15) has emerged as a central regulator of appetite and body weight. However, the exact role of GDF-15 in lean patients has not been elucidated.
Aim: Our aim is to evaluate whether the association of GDF-15 with mortality, including cancer death, differs according to body mass index (BMI) level.
Methods and Results: We collected blood samples from 4,061 patients with CV risk factors who were enrolled in the nationwide practice-based J-HOP (Japan Morning Surge-Home Blood Pressure) study. Serum GDF-15 levels were determined by immunoassay analysis. During a mean follow-up period of 6.6 years, we observed 174 (6.7/1000 person-year) all-cause deaths, 68 (2.6/1000 person-year) cancer deaths, and 56 (2.2/1000 person-year) CV deaths. Patients were stratified according to the cut-points of GDF-15 at 1,200 ng/L and BMI at 22.5 and 25.0 kg/m2. The association between the GDF-15/BMI based study groups and each outcome was evaluated by Cox-proportional hazard models with adjustment for established risk factors. The multivariate Cox regression model showed that patients with elevated GDF-15 (≥1,200 ng/L) and low BMI (<22.5 kg/m2) were significantly associated with increased risk of all outcomes [all-cause death, hazard ratio (HR) 3.15, 95% confidence interval (CI) 1.85–5.34, p < 0.001; cancer death, HR 3.52, 95%CI 1.64–7.57, p = 0.001; CV death, HR 2.88, 95%CI 1.20–6.92, p = 0.018, respectively] compared to a reference group with non-elevated GDF-15 and normal BMI (22.5–25.0 kg/m2). In analyses of a subgroup with low BMI (<22.5 kg/m2), patients with elevated GDF-15 had 4.79-fold increased risk of cancer death and 11-fold greater risk of CV death when compared with patients with non-elevated GDF-15 (<1,200 ng/L) after adjustment for established risk factors.
Conclusion: In patients with CV risk factors, GDF-15 was associated with all-cause, cancer, and CV death. This relationship was especially remarkable in patients with low BMI. The serum GDF-15 levels in patients with low BMI might be a useful marker to identify the potential for anorexia-cachexia associated with CVD and cancer.
Introduction
Cardiovascular disease (CVD) is the main cause of mortality globally, but cancer is a more common cause of death than CVD in some high-income and upper-middle-income countries (1). In these countries, as longevity has increased due to advances in medical care, the number of patients having both CVD and cancer has also increased, and onco-cardiology has emerged as a new discipline for the clinical management of these patients. Recent efforts have focused on disease prevention as well as treatment of cancer therapeutics-related cardiac dysfunction. In addition, there are a number of shared risk factors and common pathologies regulating the prognosis of CVD and cancer (2).
Growth differentiation factor-15 (GDF-15), a member of the transforming growth factor-beta superfamily (3), is expressed in multiple organs in response to tissue stress in many disease states (4, 5). Higher levels of GDF-15 may be useful for the diagnosis and management of CVD (4, 5) and cancer (6, 7) and are a strong predictor of mortality from either disease (8–10). On the other hand, it has been reported that GDF-15 exerts a cardioprotective effect through the activation of anaplastic lymphoma kinase (ALK) receptors and phosphorylation of the Smad signaling pathway (11) and that it has an overall protective role in early cancer (6). Serum GDF-15 levels might be elevated in patients with CVD and cancer compensatorily. Furthermore, emerging evidence indicates that GDF-15 regulates body weight through an effect on the appestat (12–15). GDF-15 forms a co-receptor complex with glial cell-derived neurotrophic factor receptor alpha-like (GFRAL) and rearranged during transfection (RET) and induces an anorexia effect via the appestat (12–15) independently of other well-known appetite-related pathways involving melanocortin-4, leptin, or incretin (13). Weight loss in patients with CVD or cancer—i.e., disease-related anorexia-cachexia—is a well-known phenomenon and clearly associated with poor prognosis. In previous cohorts, patients with chronic kidney disease (16), heart failure (17), and cancer (18) also showed an inverse correlation between serum GDF-15 levels and body mass index (BMI). However, in general outpatients under potential stress, it is not clear whether GDF-15 is a driver of the association between lower BMI and adverse outcome. In this study, we evaluated the prognostic value of GDF-15 and the relationship between GDF-15 and BMI in a large general practice population of patients with CV risk factors.
Methods
Study Design
All subjects were recruited from the Japan Morning Surge-Home Blood Pressure (J-HOP) study (19). The J-HOP study was a nationwide prospective study conducted in Japan that included 4,310 outpatients with any of the following risk factors for CVD: hypertension (clinic systolic blood pressure [SBP] >140 mmHg and/or diastolic blood pressure [DBP] >90 mmHg, or current use of antihypertensive medication), hyperlipidemia (total cholesterol level >240 mg/dl or treated hyperlipidemia), diabetes mellitus (fasting blood sugar ≥126 mg/dL or current use of an antidiabetic drug), glucose intolerance (fasting blood sugar >140 mg/dl at 2 h after a 75-g oral glucose tolerance test), metabolic syndrome (20), chronic kidney disease (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), history of CVD (CAD, stroke, aortic dissection, peripheral artery disease, or congestive heart failure), atrial fibrillation, current smoking, chronic obstructive pulmonary disease, and sleep apnea syndrome (an apnea hypopnea index of >15 events/hour by overnight sleep polysomnography).
The exclusion criteria for the J-HOP Study were a recent history of cardiovascular disease events (within the most recent 6 months), malignancy, current hemodialysis treatment, and chronic inflammatory disease. Patients were recruited for the J-HOP study between 2005 and 2012 and followed up through March 2015 by 75 doctors at 71 institutions (45 primary practices, 22 hospital-based outpatient clinics, and 4 specialized University hospitals). The study protocol was registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN000000894). All participants provided written informed consent, and the Institutional Review Board of Jichi Medical School approved the study.
Laboratory Testing
The J-HOP study personnel collected blood samples from the patients at enrollment after they had completed a fasting period. The blood samples were centrifuged at 3,000 g for 15 min at room temperature. The supernatants were stored at 4°C, sent to a commercial laboratory (SRL Inc., Tokyo), frozen in aliquots, and stored at −80°C in a deep freezer. All routine biochemical analyses were performed within 24 h of sample collection at this single laboratory center.
The patients' GDF-15 levels were measured with an automated platform (Cobas e 411 analyzer; Roche Diagnostics, Indianapolis, IN). The assay has a limit of detection below 400 ng/L, a linear measuring range up to 20,000 ng/L, and an inter-assay imprecision of 2.3% and 1.8% at GDF-15 concentrations of 1,100 and 17,200 ng/L, respectively (Roche Diagnostics; data on file).
Study Groups Based on GDF-15 and BMI
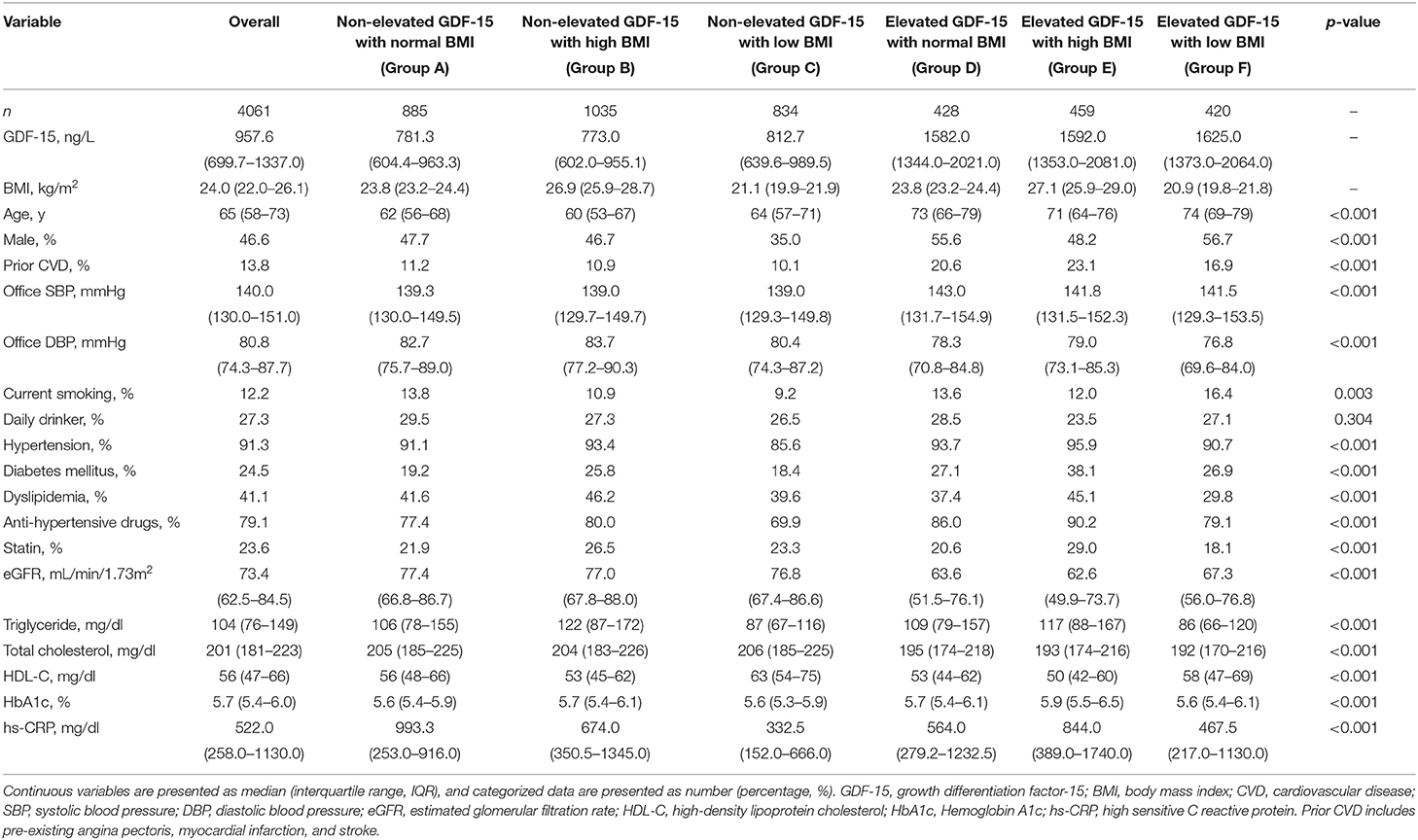
Study participants were categorized into six groups based on a combination of GDF-15 and BMI. For GDF-15, a serum level ≥1,200 ng/L was defined as elevated (21, 22). In accordance with the large-scale Asian cohorts (23, 24), a range of BMI from 22.5 to 25.0 kg/m2 was defined as normal because this range is associated with the lowest risk of mortality. The six GDF-15/BMI-based study groups were thus as follows: Group A, non-elevated GDF-15 (<1200 ng/L) with normal BMI (22.5–25.0 kg/m2); Group B, non-elevated GDF-15 with high BMI (≥25.0 kg/m2); Group C, non-elevated GDF-15 with low BMI (<22.5 kg/m2); Group D, elevated GDF-15 (≥1200 ng/L) with normal BMI; Group E, elevated GDF-15 with high BMI; and Group F, elevated GDF-15 with low BMI.
Outcome Ascertainment
The outcomes were categorized as follows: (1) all-cause death; (2) cancer death defined as de novo cancer-related mortality; (3) CV death defined as death from cardiovascular disease (i.e., acute myocardial infarction, cerebrovascular attack, heart failure) and including sudden death. The criteria for myocardial infarction included definite electrocardiographic findings (i.e., ST elevation), typical or atypical symptoms together with electrocardiographic findings and abnormal enzymes, or typical symptoms and abnormal cardiac enzymes with or without electrocardiographic findings. Cerebrovascular attack included cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage based on the findings of brain CT or MRI. Transient ischemic attack was not included.
Evidence on the above death outcomes was ascertained by ongoing reports from a general physician at each institute. The cause of death was also ascertained by means of frequent reviews of patients' medical records. When patients failed to come to the hospital, their family members were interviewed by telephone. The end point committee adjudicated all events by reviewing the patients' files and source documents and by requesting more detailed written information from investigators when necessary.
Statistical Analyses
Demographics and other baseline characteristics were compared across the GDF-15/BMI-based study groups. Continuous variables are presented as medians and interquartile ranges (IQRs), and groups were compared using the Kruskal-Wallis test. Categorical variables are presented as counts and percentages, and groups were compared using χ2 tests. GDF-15 values were logarithmically transformed due to skewed distributions. Blood concentrations under the measuring limit of each biomarker were calculated as the half-value of the limit, i.e., GDF-15 at 200 ng/L.
Unadjusted risks of outcomes were assessed across the BMI/biomarker-based study groups using cumulative incidence plots and log-rank tests. We evaluated the association between baseline GDF-15 levels and outcomes by using multivariable Cox proportional hazards models with a log-transformed continuous model and with a dichotomous model (relative to under 1,200 ng/L at GDF-15). Multivariable adjusted analysis was also performed to assess the association between the GDF-15/BMI-based study groups and risk of outcomes. By way of sensitivity analyses, we analyzed the relation of GDF-15 level to outcome in three subgroups based on the cut-point of BMI (22.5 and 25.0 kg/m2). Hazard ratios (HRs) were calculated after a composite risk score, renal function (estimated glomerular filtration rate [eGFR]), daily drinker (as a factor affecting body weight), and inflammation (high sensitive C-reactive protein [hs-CRP]). Composite risk scores are a useful approach for controlling for confounders because of concerns of overfitting the models as a result of the limited number of events among the study participants. The composite risk scores were created in the study population by determining the 10-year predicted probabilities for each outcome using Cox proportional hazards regression models, including the covariates of CV traditional risk factors (i.e. age, sex, current smoking, diabetes mellitus [DM], previous CV event, statin use, anti-hypertensive drug use, total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], office systolic blood pressure [SBP]) (25). We also analyzed with the individual covariates as sensitive analyses and showed the results in Supplementary Material. HRs and 95% confidence intervals (CIs) were expressed per 1 SD increase in GDF-15 level or relative to the reference group, respectively.
We analyzed the additional contribution of GDF-15 beyond traditional risk factors in predicting each outcome by using multiple metrics of biomarker performance, including discrimination (Harrell's c-statistics) (26) and reclassification [net reclassification index [NRI] (27) and integrated discrimination index [IDI] (28)]. Because no established categories exist that guide clinical decisions in relation to risk of cancer death with cardiovascular risk factors, we calculated a category-free NRI from time-dependent models. For the reclassification analyses, we estimated risk at 10 years. The 95%CI of each metric was estimated by using 1,000 bootstrap samples. Two-sided p-values < 0.05 were considered significant. All analyses were performed by using R, version 3.6.0 (The R Foundation for Statistical Computing) and SAS, version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
Among the 4,310 patients who were enrolled in the J-HOP study, the following were excluded: 221 patients whose blood samples were not sufficient for the measurement of the GDF-15 level, and 27 patients whose data were incomplete. The data of the final total of 4,061 patients were included in the analyses.
Table 1 provides the baseline clinical characteristics of the overall population and the patients as divided by the GDF-15/BMI-based study groups. In the overall population, the median age of the patients was 66 years, and there were more females than males. The median concentration of GDF-15 was 957.7 ng/L (IQR 700.0–1337.0 ng/L). The proportion of females was high at 56.6% in patients with GDF-15 <1200 ng/L, whereas the proportion of males was high (53.3%) in those with elevated GDF-15 (p < 0.001 by chi-square test). Age, office SBP, proportion of prior CVD, and smoking rates were higher, and eGFR tended to be low in patients with elevated GDF-15. Most of the patients had hypertension and were taking anti-hypertensive drugs, and the proportions of both hypertension and anti-hypertensive drug use were higher in patients with higher BMI. Higher TG and lower HDL-C concentration were also observed in patients with higher BMI.
GDF-15 Predicted All-Cause, Cancer, and CV Death
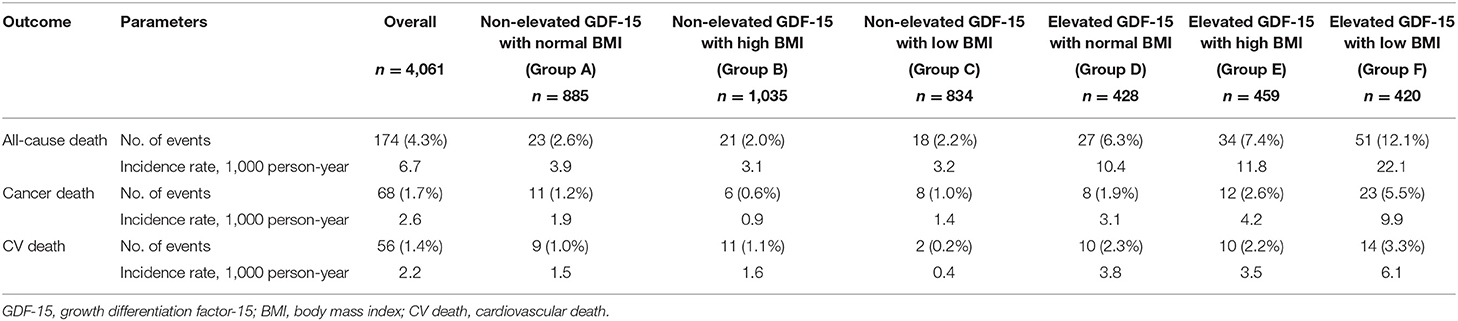
During the mean follow-up of 6.6 ± 3.9 years, there were 174 all-cause deaths (6.7/1,000 person-years), of which 68 (2.6/1,000 person-years) were cancer deaths and 56 (2.2/1,000 person-years) were CV deaths. In patients with non-elevated GDF-15, the incidence rate of outcomes was generally low regardless of the range of BMI. Among patients with elevated GDF-15, the incidence rate was about 2-fold higher in those with low BMI than in those with normal or high BMI (Table 2). The detail cause of death was shown in Supplementary Table 1.
In multivariable Cox proportional hazards models adjusted for traditional risk factors, higher concentrations of GDF-15 modeled as a dichotomous (relative to <1,200 ng/L) and continuous variable (1SD increase) were associated with an increased risk for each outcome (Figure 1). In sensitive analyses, patients with ≥1,200 ng/L of GDF-15 were at a marginally increased risk of CV death compared to those with non-elevated GDF-15 (HR 1.80, 95%CI 0.95–3.44, p = 0.073, Supplementary Figure 1). Not discrimination (c-statistics) but reclassification (NRI and IDI) was significantly improved with the addition of log GDF-15 to the fully adjusted model for all-cause, cancer, and CV death (Table 3). In sensitive analyses adjusted for individual covariates, incorporating log GDF-15 improved discrimination (c-statistics) of the model for each outcome (Supplementary Table 2). These findings suggested that GDF-15 was not only associated with each outcome but was also a sufficiently strong predictor to exhibit an incremental benefit for the model including the traditional risk factors.
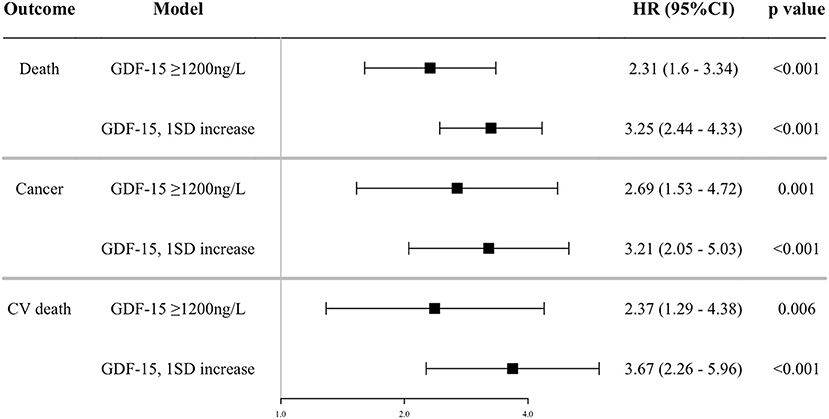
Figure 1. Association between GDF-15 and outcomes. Shown are the results of a Cox proportion hazard analysis by continuous and dichotomous models of GDF-15 and outcomes adjusted for the composite risk score of CV traditional risk factors (age, sex, current smoking, diabetes mellitus, previous CV event, statin use, anti-hypertensive drug use, total cholesterol, high-density lipoprotein cholesterol, office systolic blood pressure), estimated glomerular filtration rate, daily drinker, and high sensitive C reactive protein (log). GDF-15, growth differentiation factor-15; CV death, cardiovascular death; HR, hazard ratio; 95%CI, 95% confidence interval.
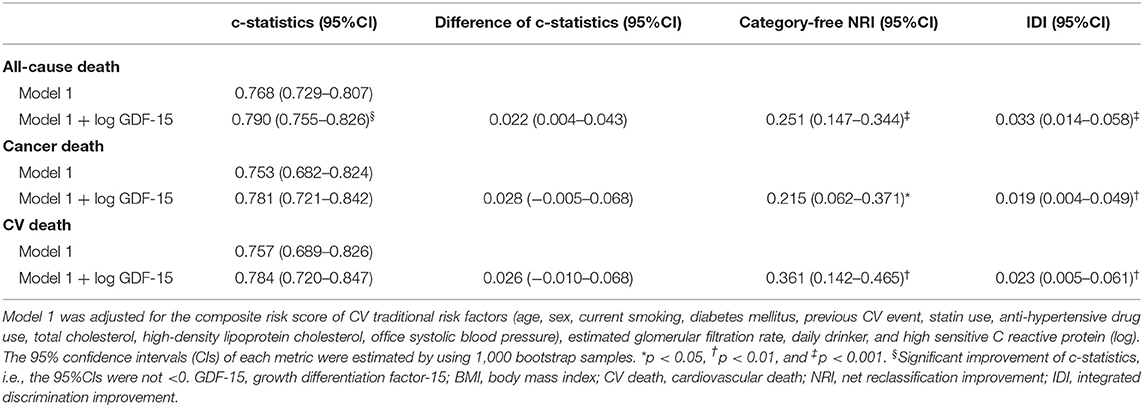
Table 3. Change in risk-predictive metrics by incorporating prognostic biomarkers to the base model.
High Prognostic Value of GDF-15 in Patients With Low BMI
The cumulative Kaplan-Meier plots of each outcome by the GDF-15/BMI-based study groups are shown in Figure 2. In the groups with non-elevated GDF-15 (Groups A, B, and C), the incidence of each outcome was generally low, and patients with elevated GDF-15 and low BMI (Group F) were associated with increased event rates during follow-up. The event rates of patients with elevated GDF-15 and normal or high BMI (Groups D and C) showed an intermediate separation of event curves. Patients with elevated GDF-15 and low BMI (Group F) were at significantly increased risk for each outcome compared with the reference group (Group A) in multivariable Cox proportional hazards models adjusted for traditional risk factors (Figure 3).
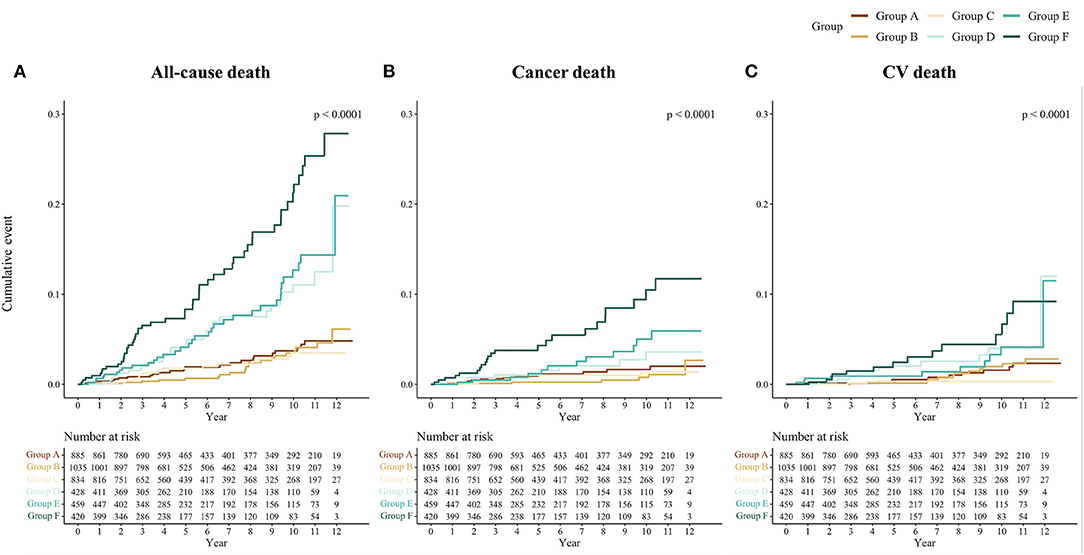
Figure 2. Cumulative incidence by outcomes and the GDF-15/BMI-based groups. Kaplan-Meier curves of the cumulative incidence rates of (A) all-cause, (B) cancer, and (C) CV death by the GDF-15/BMI-based study group are shown. The GDF-15/BMI-based study groups were as follows: Group A, non-elevated GDF-15 (<1,200 ng/L) with normal BMI (22.5–25.0 kg/m2); Group B, non-elevated GDF-15 with high BMI (≥25.0 kg/m2); Group C, non-elevated GDF-15 with low BMI (<22.5 kg/m2); Group D, elevated GDF-15 (≥1,200 ng/L) with normal BMI; Group E, elevated GDF-15 with high BMI; and Group F, elevated GDF-15 with low BMI. GDF-15, growth differentiation factor-15; BMI, body mass index; CV death, cardiovascular death.
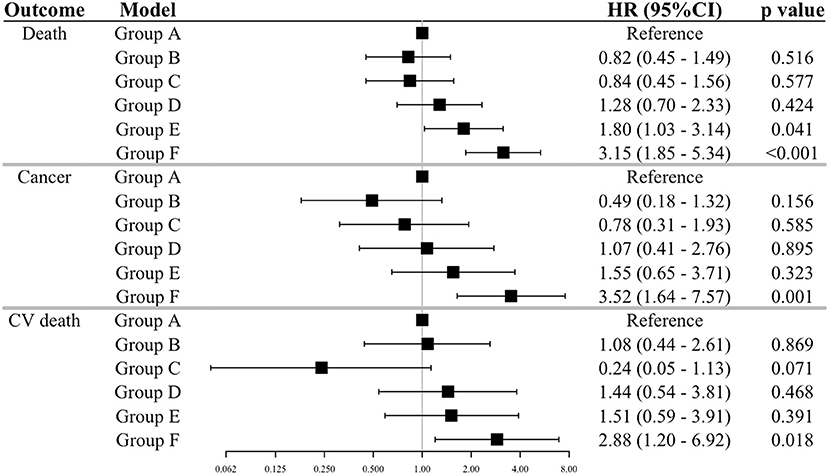
Figure 3. Relations of the GDF-15/BMI-based groups to outcomes. The results of a Cox proportion hazard analysis by the GDF-15/BMI-based group outcomes adjusted for traditional risk factors are shown. The GDF-15/BMI-based groups were as defined in Figure 2. Adjustments of the Cox model are as shown in Figure 1. GDF-15, growth differentiation factor-15; BMI, body mass index; CV death, cardiovascular death.
Next, we analyzed the relationship between GDF-15 and outcomes in each range of BMI (i.e., <22.5, 22.5–25.0, ≥25.0 kg/m2). In the BMI range of <22.5 kg/m2, patients with elevated GDF-15 were associated with increasing hazard for all-cause death and more than a 4-fold increased risk of cancer and CV death as compared with those with non-elevated GDF-15. The relationship between GDF-15 and outcomes was also supported in the groups of high BMI, with a consistently increasing risk of all-cause and cancer death (except CV death) with elevated GDF-15 levels (Figure 4). On the other hand, in the range of normal BMI, elevated GDF-15 levels were not associated with outcomes after adjustment for traditional risk factors (Figure 4). In the sensitive analyses, we got the similar results, however, the association of GDF-15 with the risk of all-cause and cancer death became no longer statistically significant in the groups of high BMI (Supplementary Figure 3).
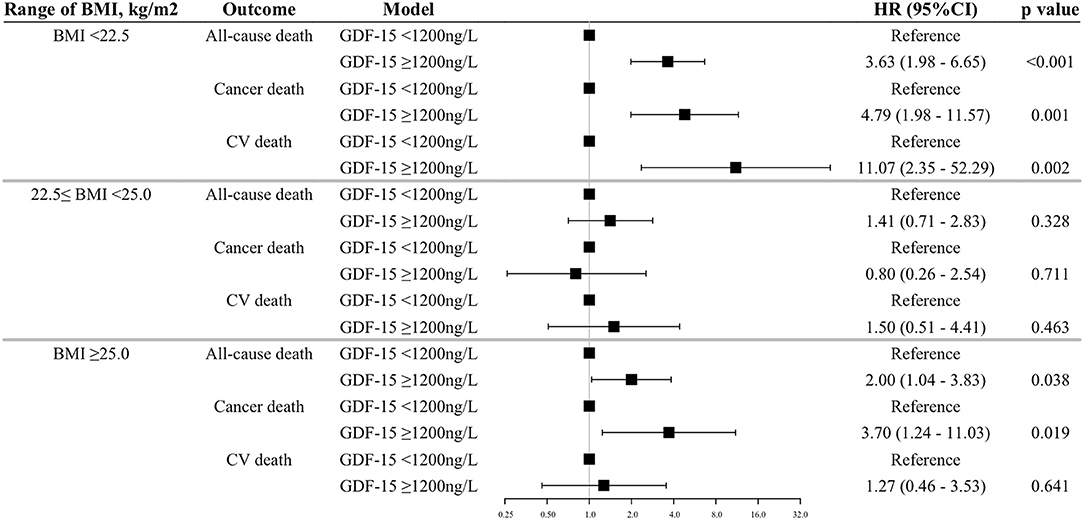
Figure 4. Association between elevated GDF-15 and outcomes in the BMI subgroups. Shown are the results of a Cox proportion hazard analysis by a dichotomous model of GDF-15 and outcomes adjusted for traditional risk factors. The BMI subgroups were stratified based on the cut-off points of 22.5 and 25.0 kg/m2 (i.e., <22.5 as low BMI, 22.5–25.0 as normal BMI, ≥25.0 as high BMI). Adjustments of the Cox model are as shown in Figure 1. GDF-15, growth differentiation factor-15; BMI, body mass index; CV death, cardiovascular death.
We also analyzed the relations of GDF-15 level to outcome in other subgroups based on the BMI cut-off points (21 and 27 kg/m2) from a Japanese cohort study (29). The associations between elevated GDF-15 and the increased risks of outcomes were also limited in the range under 21 kg/m2 of BMI (all-cause death, HR 3.65, 95%CI 1.63–8.21, p = 0.002; cancer death HR 4.86, 95%CI 1.38–17.14, p = 0.014; and CV death, HR 18.35, 95%CI 2.24–150.1, p = 0.007, relative to the reference group with non-elevated GDF-15, respectively). These findings suggested that the high prognostic value of GDF-15 was limited in patients with low BMI.
Discussion
The present results showed that elevated GDF-15 was associated with increased risks of all-cause, cancer, and CV death in outpatients with CV risk factors, and that incorporating GDF-15 to the predictive model for these outcomes improved its performance significantly. In the sub-analyses stratified the range of BMI, elevated GDF-15 levels were associated with increased risks of all-cause and cancer death in the groups of low and high BMI. The relationship between GDF-15 and the risk of CV death was stronger only in the group with BMI <22.5 kg/m2. However, these prognostic values of GDF-15 were not observed in the subgroups with a normal range of BMI (22.5–25.0 kg/m2). These findings suggests that GDF-15 is a strong predictor for all-cause, cancer, and CV death in patients with CV risk factors and especially in those with low BMI.
Our study showed that GDF-15 had a high prognostic value for cancer death in patients with CV risk factors. GDF-15 is a stress-induced cytokine whose expression is induced by transcription factors such as p53 and EGR-1 (30, 31). Although GDF-15 is weakly expressed in the liver, kidney, and prostate, its expression from other tissues increases in the presence of stress or a disease state (4, 5). In common cancers, including breast, colon, prostate, and pancreas cancers, GDF-15 is produced in tumor tissues (6, 7, 32–34). GDF-15 is cleaved from a propeptide by furin-like proteases before its secretion, but this intracellular cleavage from a propeptide does not process efficiently in tumor tissue (6). The 3-h half-life of GDF-15 is prolonged in the circulation, and the serum levels of GDF-15 increase markedly in advanced cancer (7). In this study, patients with cancer were excluded at baseline, so the outcome of cancer death implied that the patients developed malignancy and died of advanced cancer. Given that the mean follow-up period was 6.6 years, patients who died from malignancy might have had undiagnosed cancer at baseline.
This study further found that high GDF-15 levels in patients with low BMI conferred an increased risk for all-cause death, cancer death, and CV death. In a cohort of patients with advanced cancer, serum GDF-15 levels were strongly correlated to weight loss (18), and patients with chronic renal and cardiac failure were also associated with anorexia-cachexia (16, 17). Based on recent developments in GDF-15-related research, the pathological mechanism underlying this association was considered to involve activation of the GFRAL/RET complex and the resulting effect on the appestat (12–15). GDF-15 molecules bind to GFRAL receptors, and this complex combines two RET molecules. Then, thte GDF-GFRAL-RET complex signals downstream effectors including Akt, ERK1/2, and PLCγ, and activates c-Fos in the area postrema (AP) of the hindbrain and solitary nucleus (35, 36). The activation of AP also activates other brain centers such as the hypothalamus, parabrachial nucleus, and amygdala, suggesting effects on complex feeding and emotional behaviors (13, 16). In addition, this physiological mechanism is independent of other pathways which affect feeding, such as melanocortin-4 and leptin receptors (13). These evidences suggest that GDF15-GFRAL-RET is a central regulator of feeding and body weight and a new therapeutic target of both anorexia-cachexia and obesity (14, 36, 37). Body weight loss resulting from elevated GDF-15 might cause poor prognosis in patients with various types of cancer and CVD. In sub-analyses of groups with low BMI, patients with non-elevated GDF-15 levels (group C) were considered to have relatively low risks for anorexia-cachexia and were not associated with poor prognosis. On the other hand, patients with elevated GDF-15 levels (group F), who might be at risk of anorexia-cachexia, had more than 4-fold elevation of risk for cancer and CV death compared with those with non-elevated GDF-15 (Group C). These facts suggest that the serum GDF-15 levels of patients with low BMI would be useful as a marker to identify weight loss sufficient to cause poor prognosis in various diseases.
Several large pooled analyses indicated a U-shaped relationship between BMI and increased risks of all-cause, cancer, and CV death (23, 24, 29, 38, 39), and also reported strong associations between lower BMI and death from respiratory disease and between higher BMI and cancer and CV death (24, 38). In this study, the BMI range of <22.5 kg/m2 was associated with an increased risk of all-cause and cancer death compared with that of 22.5–25.0 kg/m2 in a Cox hazard model, however, the BMI range of ≥25.0 kg/m2 was not associated with an increased risk of all-cause, cancer, and CV death (data not shown). This finding may have been related to our study design, which included an older population and a shorter follow-up period compared to previous large cohorts. In particular, patients in this study were undergoing treatment for primary prevention of CVD, which naturally could have decreased the incidence of CV death.
There were some limitations of this study. First, we measured blood GDF-15 levels only one time at the baseline, and thus we could not assess the interaction and fluctuation of GDF-15 levels over time during the progression of each adverse event. Second, the patients in this study were all Japanese, and thus our findings may not be generalizable to other race/ethnic groups. Third, the study outcomes were cancer and CVD mortality, so we could not assess the prognostic value of GDF-15 for the development of cancer and CVD in this study. Additionally, many of the records of cancer death did not mention the primary lesion (Supplementary Table 1), we could not assess which type of cancers developed. Fourth, variables which affected BMI were limited to alcohol usage. It was preferable to add the adjustments related body weight, such as poor nutrition and physical inactivity. This issue might affect the results potentially.
In conclusion, GDF-15 might be a strong predictor for all-cause, cancer, and CV death in patients with CV risk factors. Especially in patients with low BMI, elevated GDF-15 levels were related to higher risks for these outcomes. Because elevated GDF-15 levels could cause body weight loss through effects on the appestat, serum GDF-15 levels might be a useful marker to identify the potential anorexia-cachexia in patients with low BMI.
Data Availability Statement
The datasets generated for this article are not readily available because our group plans to submit articles continuously by using the datasets. Requests to access the datasets should be directed to a2thcmlvQGppY2hpLmFjLmpw.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Jichi Medical School. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KK, SH, and MS contributed to conception and design of the study. SH organized the database. KN performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was financially supported in part by Roche Diagnostics; by a grant from the 21st Century Center of Excellence Project run by Japan's Ministry of Education, Culture, Sports, Science, and Technology (to KK); by a grant from the Foundation for Development of the Community (Tochigi, Japan); by a grant from Omron Healthcare; by a Grant-in-Aid for Scientific Research (B) (21390247) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, 2009 to 2013; and by funds from the MEXT-Supported Program for Strategic Research Foundation at Private Universities, 2011 to 2015, Cooperative Basic and Clinical Research on Circadian Medicine (S1101022).
Conflict of Interest
KK has received research grants and honoraria from Roche Diagnostics, Omron Healthcare and A& D Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.660317/full#supplementary-material
Supplementary Figure 1. Association between GDF-15 and outcomes by Cox proportion hazard analyses adjusted for the individual covariates. Shown are the results of a Cox proportion hazard analysis by continuous and dichotomous models of GDF-15 and outcomes adjusted for age, sex, current smoking, diabetes mellitus, previous CV event, statin use, anti-hypertensive drug use, total cholesterol, high-density lipoprotein cholesterol, office systolic blood pressure, estimated glomerular filtration rate, daily drinker, and high sensitive C reactive protein (log). GDF-15, growth differentiation factor-15; CV death, cardiovascular death; HR, hazard ratio; 95%CI, 95% confidence interval.
Supplementary Figure 2. Relations of the GDF-15/BMI-based groups to outcomes by Cox proportion hazard analyses adjusted for the individual covariates. The results of a Cox proportion hazard analysis by the GDF-15/BMI-based group outcomes adjusted for traditional risk factors are shown. The GDF-15/BMI-based study groups were as follows: Group A, non-elevated GDF-15 (<1,200 ng/L) with normal BMI (22.5–25.0 kg/m2); Group B, non-elevated GDF-15 with high BMI (≥25.0 kg/m2); Group C, non-elevated GDF-15 with low BMI (<22.5 kg/m2); Group D, elevated GDF-15 (≥1,200 ng/L) with normal BMI; Group E, elevated GDF-15 with high BMI; and Group F, elevated GDF-15 with low BMI. Adjustments of the Cox model are as shown in Supplementary Figure 1. GDF-15, growth differentiation factor-15; BMI, body mass index; CV death, cardiovascular death.
Supplementary Figure 3. Association between elevated GDF-15 and outcomes in the BMI subgroups by Cox proportion hazard analyses adjusted for the individual covariates. Shown are the results of a Cox proportion hazard analysis by a dichotomous model of GDF-15 and outcomes adjusted for traditional risk factors. The BMI subgroups were stratified based on the cut-off points of 22.5 and 25.0 kg/m2 (i.e., <22.5 as low BMI, 22.5–25.0 as normal BMI, ≥25.0 as high BMI). Adjustments of the Cox model are as shown in Supplementary Figure 1. GDF-15, growth differentiation factor-15; BMI, body mass index; CV death, cardiovascular death.
Supplementary Table 1. Cause of death.
Supplementary Table 2. Change in risk-predictive metrics by incorporating prognostic biomarkers to the base modeladjusted for the individual covariates.
References
1. Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. (2020) 395:785–94. doi: 10.1016/S0140-6736(19)32007-0
2. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
3. Bootcov M, Bauskin A, Valenzuela S, Moore A, Bansal M, He X, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. (1997) 94:11514–9. doi: 10.1073/pnas.94.21.11514
4. Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. (2015) 2015:490842. doi: 10.1155/2015/490842
5. Wollert KC, Kempf T, Wallentin L. Growth Differentiation Factor 15 as a biomarker in cardiovascular disease. Clin Chem. (2017) 63:140–51. doi: 10.1373/clinchem.2016.255174
6. Bauskin A, Brown D, Junankar S, Rasiah K, Eggleton S, Hunter M, et al. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. (2005) 65:2330–6. doi: 10.1158/0008-5472.CAN-04-3827
7. Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA. (2003) 100:3410–5. doi: 10.1073/pnas.0530278100
8. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. (2011) 123:2101–10. doi: 10.1161/CIRCULATIONAHA.110.979740
9. Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin Chem. (2012) 58:172–82. doi: 10.1373/clinchem.2011.171926
10. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. (2012) 126:1596–604. doi: 10.1161/CIRCULATIONAHA.112.129437
11. Ago T, Sadoshima J. GDF15, a cardioprotective TGF-beta superfamily protein. Circ Res. (2006) 98:294–7. doi: 10.1161/01.RES.0000207919.83894.9d
12. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. (2017) 23:1215–9. doi: 10.1038/nm.4393
13. Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. (2017) 550:255–9. doi: 10.1038/nature24042
14. Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. (2017) 23:1150–7. doi: 10.1038/nm.4392
15. Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. (2017) 23:1158–66. doi: 10.1038/nm.4394
16. Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. (2007) 13:1333–40. doi: 10.1038/nm1677
17. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. (2007) 50:1054–60. doi: 10.1016/j.jacc.2007.04.091
18. Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr., Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. (2003) 97:1211–6. doi: 10.1002/cncr.11178
19. Hoshide S, Kario K, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J-HOP Study. Am J Hypertens. (2014) 27:939–47. doi: 10.1093/ajh/hpt290
20. Definition and the diagnostic standard for metabolic syndrome-Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Nippon Naika Gakkai Zasshi. (2005) 94:794–809.
21. Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. (2007) 53:284–91. doi: 10.1373/clinchem.2006.076828
22. Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell. (2010) 9:1057–64. doi: 10.1111/j.1474-9726.2010.00629.x
23. Zheng W, McLerran D, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. (2011) 364:719–29. doi: 10.1056/NEJMoa1010679
24. Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ. (2013) 347:f5446. doi: 10.1136/bmj.f5446
25. D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. (2008) 117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579
26. Newson R. Comparing the predictive powers of survival models using Harrell'c or Somers' d. Stata J. (2010) 10:339–58. doi: 10.1177/1536867X1001000303
27. Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27:157–72. doi: 10.1002/sim.2929
28. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. (2011) 30:11–21. doi: 10.1002/sim.4085
29. Sasazuki S, Inoue M, Tsuji I, Sugawara Y, Tamakoshi A, Matsuo K, et al. Body mass index and mortality from all causes and major causes in Japanese: results of a pooled analysis of 7 large-scale cohort studies. J Epidemiol. (2011) 21:417–30. doi: 10.2188/jea.JE20100180
30. Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, et al. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem. (2000) 275:20127–35. doi: 10.1074/jbc.M909580199
31. Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. (2004) 279:6883–92. doi: 10.1074/jbc.M305295200
32. Liu T, Bauskin A, Zaunders J, Brown D, Pankhurst S, Russell P, et al. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. (2003) 63:5034–40
33. Koopmann J, Buckhaults P, Brown D, Zahurak M, Sato N, Fukushima N, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. (2004) 10:2386–92. doi: 10.1158/1078-0432.CCR-03-0165
34. Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, et al. Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res. (2006) 12:89–96. doi: 10.1158/1078-0432.CCR-05-1331
35. Tsai VW, Manandhar R, Jorgensen SB, Lee-Ng KK, Zhang HP, Marquis CP, et al. The anorectic actions of the TGFbeta cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS ONE. (2014) 9:e100370. doi: 10.1371/journal.pone.0100370
36. Xiong Y, Walker K, Min X, Hale C, Tran T, Komorowski R, et al. Long-acting MIC-1/GDF15 molecules to treat obesity: evidence from mice to monkeys. Sci Transl Med. (2017) 9:eaan8732. doi: 10.1126/scitranslmed.aan8732
37. Tsai VW, Lin S, Brown DA, Salis A, Breit SN. Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int J Obes (Lond). (2016) 40:193–7. doi: 10.1038/ijo.2015.242
38. Jee S, Sull J, Park J, Lee S, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. (2006) 355:779–87. doi: 10.1056/NEJMoa054017
Keywords: GDF-15, cancer, mortality, cardiovascular disease, onco-cardiology
Citation: Negishi K, Hoshide S, Shimpo M and Kario K (2021) Growth Differentiation Factor 15 Predicts Cancer Death in Patients With Cardiovascular Risk Factors: The J-HOP Study. Front. Cardiovasc. Med. 8:660317. doi: 10.3389/fcvm.2021.660317
Received: 29 January 2021; Accepted: 07 May 2021;
Published: 04 June 2021.
Edited by:
Alberto Milan, Università di Torino, ItalyReviewed by:
Lorenzo Airale, University Hospital of the City of Health and Science of Turin, ItalyGiacomo Pucci, University of Perugia, Italy
Copyright © 2021 Negishi, Hoshide, Shimpo and Kario. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuomi Kario, a2thcmlvQGppY2hpLmFjLmpw
 Keita Negishi
Keita Negishi Satoshi Hoshide
Satoshi Hoshide Masahisa Shimpo1
Masahisa Shimpo1 Kazuomi Kario
Kazuomi Kario